Phenols Extraction from Sorghum Byproducts: Upcycling Strategies and Food Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Proximate Composition
2.3. Determination of Total Phenolic Content, Flavans, Pigment, and Antioxidant Activity
2.4. Extraction of Soluble Phenolic Compounds by Sorghum Byproducts Using Ultrasound-Assisted Extraction and Optimization Procedure
2.5. Optimization of Antioxidant Activity by UAE Extracts
2.6. Profiling of Phenolic Acids by HPLC-DAD
2.7. Bread-Making Process
2.8. Color Measurements
2.9. Consumer Acceptance
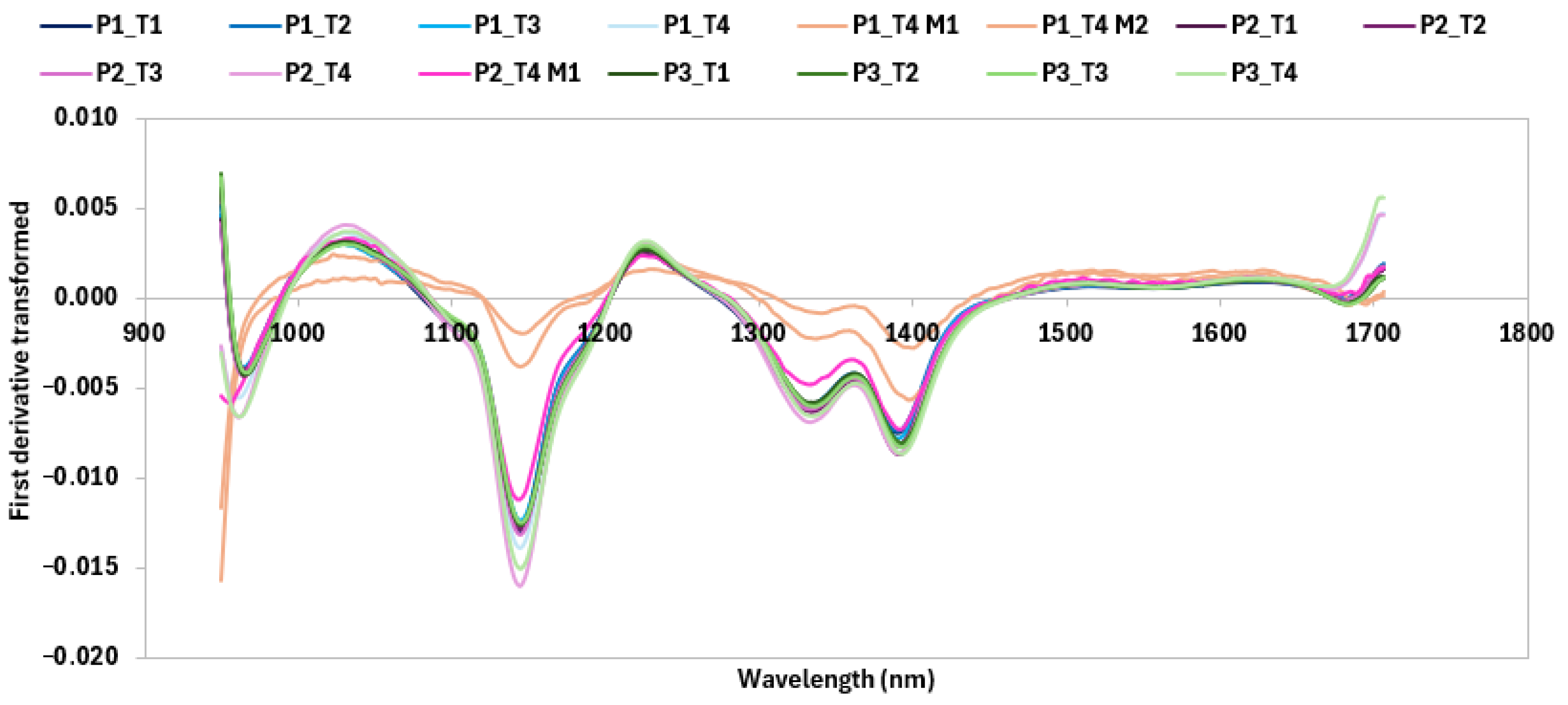
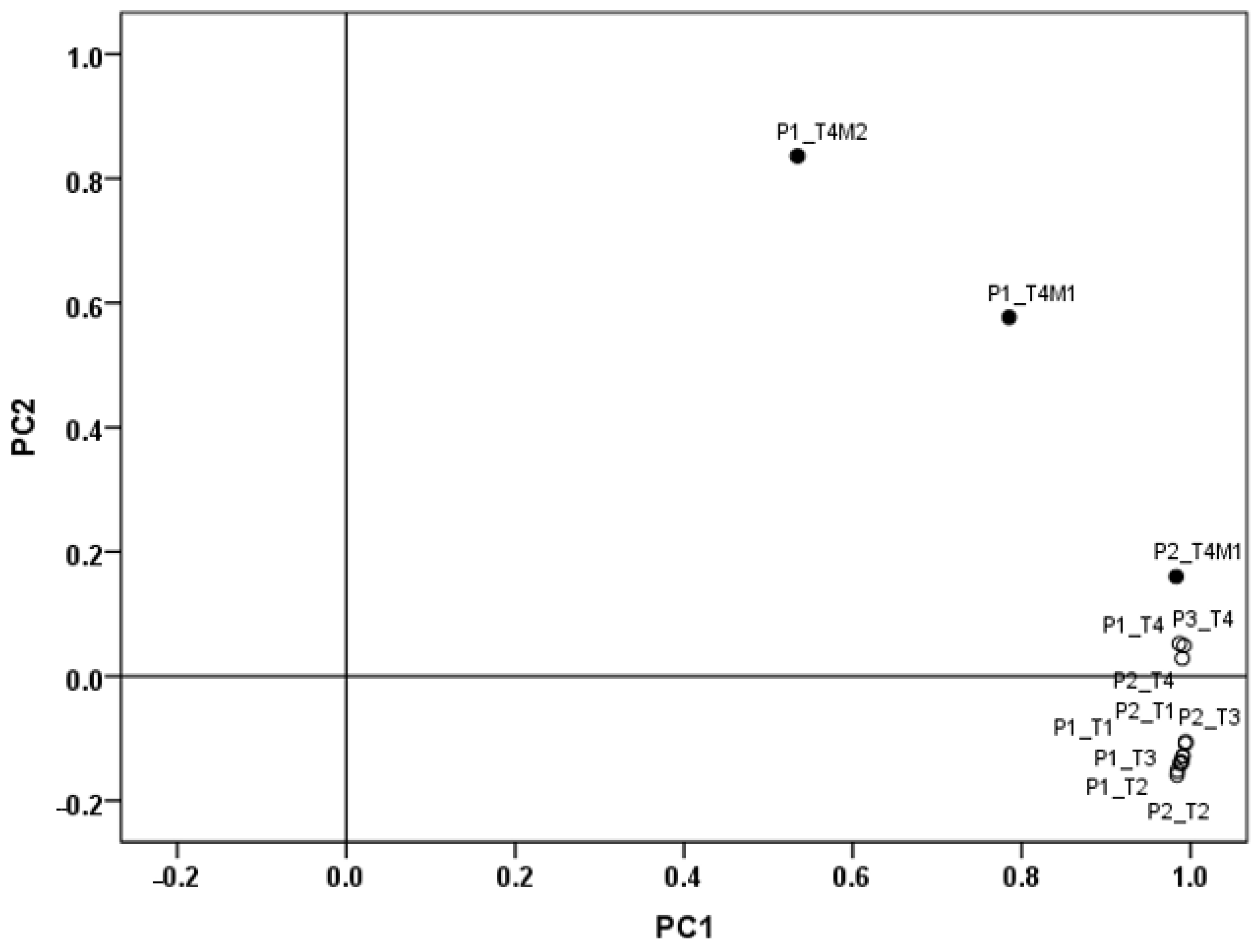
2.10. Evaluation of Raw Materials and Bread Shelf Life by Hyperspectral Images Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Raw Material Characterization
3.1.1. Proximate Composition
3.1.2. Characterization with HSI
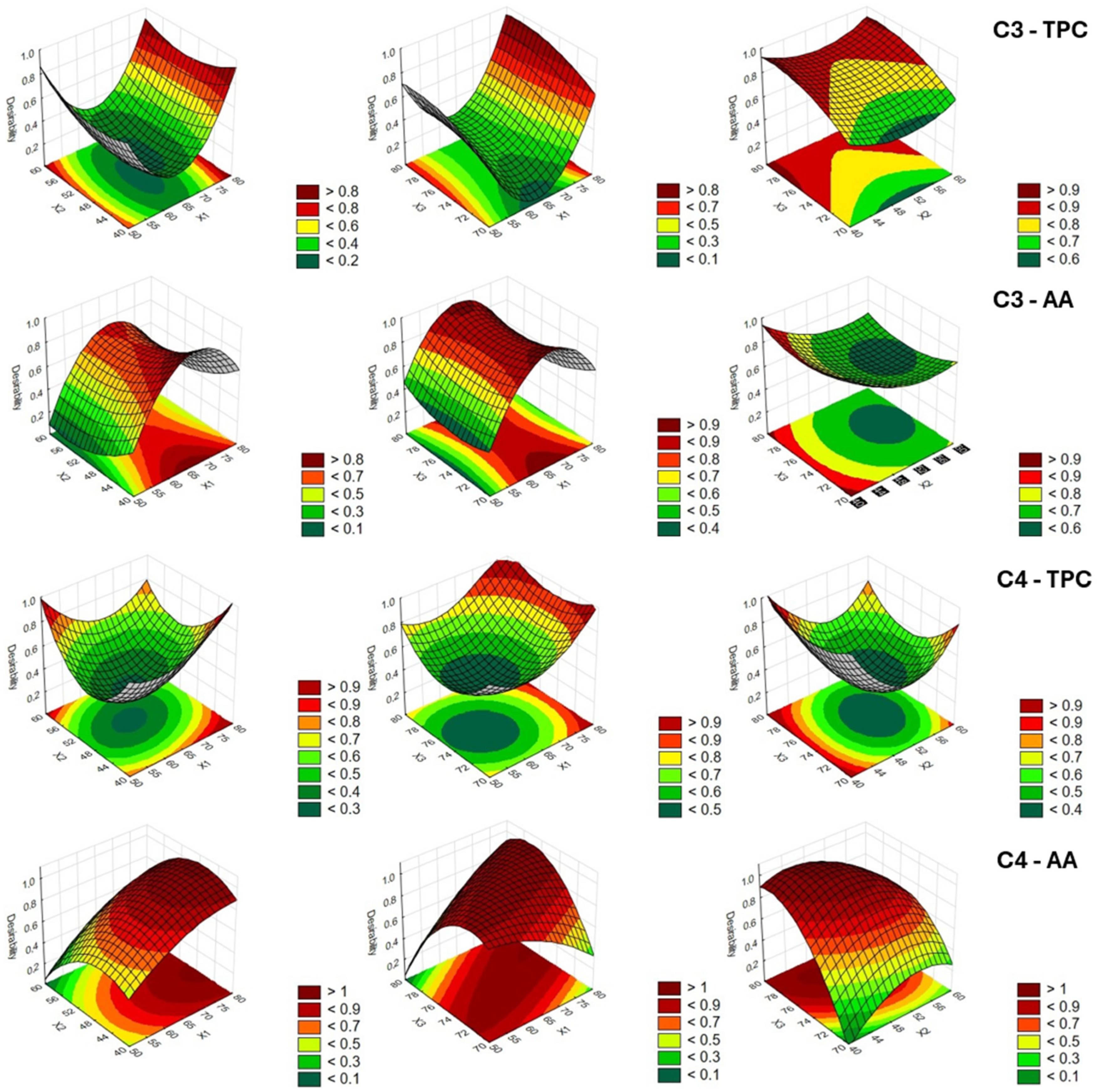
3.2. Optimization of UAE Process Parameters and Validation of the RSM Models
3.3. Characterization of Phenolic Acids Extract by HPLC-DAD
3.4. Effect of Sorghum Stalk Optimal Extract on Bread
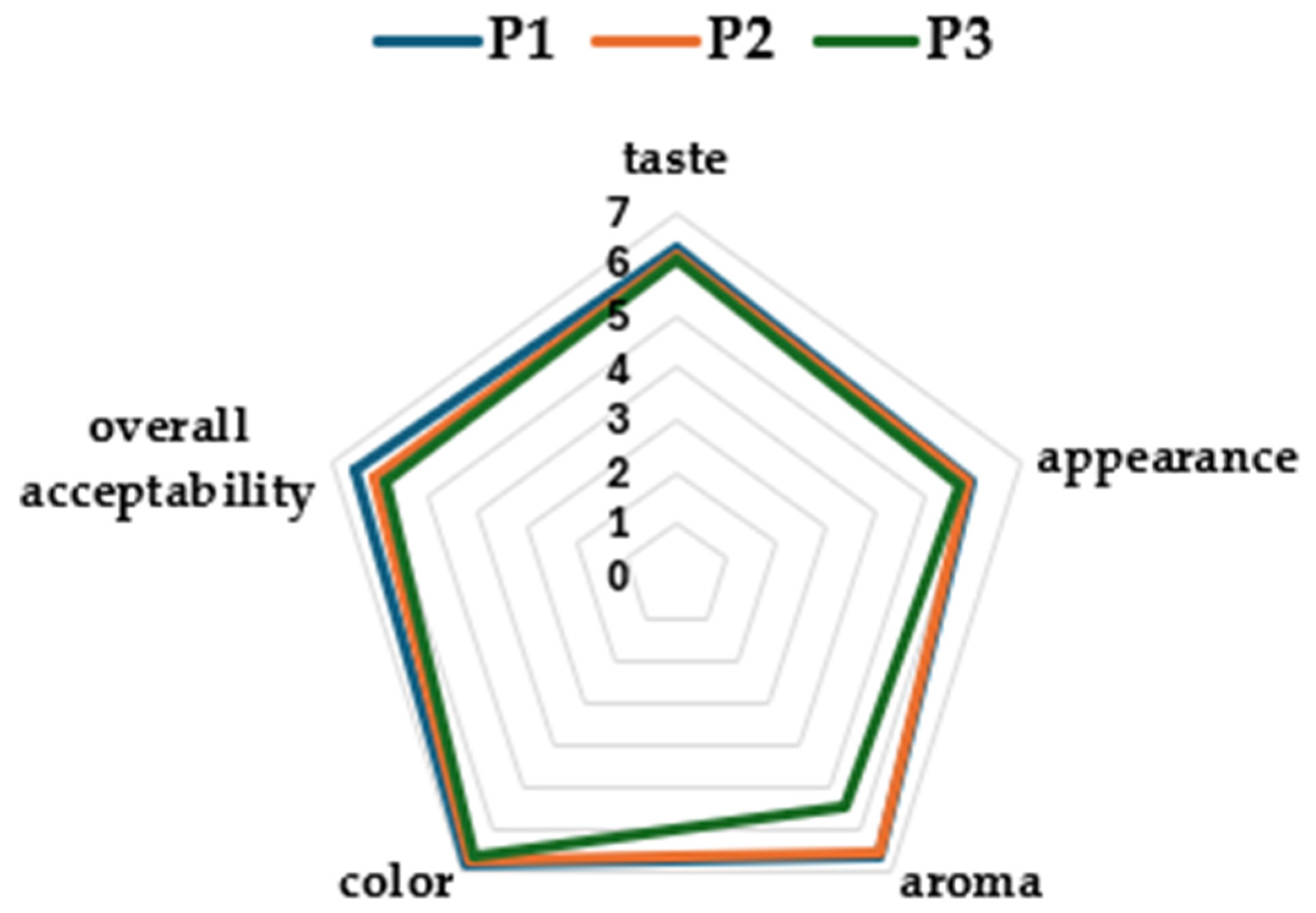
3.5. Sensorial Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.K.; Li, X.L.; Yan, H.L.; Yan, J.C.; Lei, Z.P.; Ren, S.B.; Wang, Z.C.; Kang, S.G.; Shui, H.F. Two-step separation for enriching phenols and esters from base-catalyzed depolymerization of sorghum stalk. Fuel 2022, 314, 122739. [Google Scholar] [CrossRef]
- Tanwar, R.; Panghal, A.; Chaudhary, G.; Kumari, A.; Chhikara, N. Nutritional, phytochemical and functional potential of sorghum: A review. Food Chem. Adv. 2023, 3, 100501. [Google Scholar] [CrossRef]
- Sunoj, V.J.; Prasad, P.V.; Ciampitti, I.A.; Maswada, H.F. Narrowing diurnal temperature amplitude alters carbon tradeoff and reduces growth in C4 crop sorghum. Front. Plant Sci. 2020, 11, 1262. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, M.J.; Webster, M. Current and potential future uses of sorghum to increase nutrient density for human foods. J. Food Sci. 2024, 89, A42–A51. [Google Scholar] [CrossRef]
- Xie, Q.; Xu, Z. Sustainable Agriculture: From Sweet Sorghum Planting and Ensiling to Ruminant Feeding. Mol. Plant 2019, 12, 603–606. [Google Scholar] [CrossRef]
- Meena, K.; Visarada, K.; Meena, D.K. Sorghum bicolor (L.) Moench a multifarious crop -fodder to therapeutic potential and biotechnological applications: A future food for the millennium. Future Foods 2022, 6, 100188. [Google Scholar] [CrossRef]
- Joy, S.P.; Krishnan, C. Modified organosolv pretreatment for improved cellulosic ethanol production from sorghum biomass. Ind. Crops Prod. 2022, 177, 114409. [Google Scholar] [CrossRef]
- De Carvalho, A.P.A.; Silva, V.D.M.; Souza, P.T.F.; Garcia, C.F.; Queiroz, V.A.V.; Junqueira, R.G.; Garcia, M.A.V.T. Sorghum starch: Extraction, characterization and film development. Mater. Today Commun. 2024, 39, 109020. [Google Scholar] [CrossRef]
- Taylor, J.; Duodu, K.G. Sorghum and Millets: Chemistry, Technology, and Nutritional Attributes; Elsevier Ink: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Lestari, E.G.; Herawati, H.; Suarni, S.; Ishaq, M.I.; Syahruddin, K.; Widiastuti, D.; Mulyati, A.H.; Yunita, R.; Haziman, M.L.; Suparjo. Nutritional profiling and selection of promising F3 sorghum genotypes from bioguma and Gando Keta crosses for food security and industrial applications. Food Humanit. 2025, 4, 100574. [Google Scholar] [CrossRef]
- Chhikara, N.; Abdulahi, B.; Munezero, C.; Kaur, R.; Singh, G.; Panghal, A. Exploring the nutritional and phytochemical potential of sorghum in food processing for food security. Nutr. Food Sci. 2018, 49, 318–332. [Google Scholar] [CrossRef]
- Dahunsi, S.O.; Adesulu-Dahunsi, A.T.; Osueke, C.O.; Lawal, A.I.; Olayanju, T.M.A.; Ojediran, J.O.; Izebere, J.O. Biogas generation from Sorghum bicolor stalk: Effect of pretreatment methods and economic feasibility. Energy Rep. 2019, 5, 584–593. [Google Scholar] [CrossRef]
- Kumari, P.K.; Umakanth, A.V.; Narsaiah, T.B.; Uma, A. Exploring anthocyanins, antioxidant capacity and α-glucosidase inhibition in bran and flour extracts of selected sorghum genotypes. Food Biosci. 2021, 41, 100979. [Google Scholar] [CrossRef]
- Pehlivan, U.; Ozdemir, S.; Ozer, H.; Dede, O.H. Fuel properties and incineration behavior of poultry litter blended with sweet sorghum bagasse and pyrolysis oil. J. Environ. Manag. 2024, 357, 120844. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.S.; Maragoni-Santos, C.; Marques de Farias, P.; Marcolongo Gomes Cortat, C.; Chieregato Maniglia, B.; Schmitz Ongaratto, R.; Ferreira, S.; Cavalcante Fai, A.E. Rethinking single-use plastics: Innovations, polices, consumer awareness and market shaping biodegradable solutions in the packaging industry. Trends Food Sci. Technol. 2025, 158, 104906. [Google Scholar] [CrossRef]
- Iadecola, R.; Ciccoritti, R.; Ceccantoni, B.; Bellincontro, A.; Amoriello, T. Optimization of Phenolic Compound Extraction from Brewers’ Spent Grain Using Ultrasound Technologies Coupled with Response Surface Methodology. Sustainability 2022, 14, 3309. [Google Scholar] [CrossRef]
- International Association for Cereal Science and Technology. ICC Standard Methods (Methods No. 105/2, 110/1, 104/1); ICC: Vienna, Austria, 2003. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; AOAC International: Arlington, VA, USA, 1990; Volume II, Section 985.29; p. 1105. [Google Scholar]
- Ciccoritti, R.; Taddei, F.; Gazza, L.; Nocente, F. Influence of kernel thermal pre-treatments on 5-n-alkylresorcinols, polyphenols and antioxidant activity of durum and einkorn wheat. Eur. Food Res. Technol. 2021, 247, 353–362. [Google Scholar] [CrossRef]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Dere, S.; Guneş, T.; Sivaci, R. Spectrophotometric determination of chlorophyll-A, B and total carotenoid contents of some algae species using different solvents. Turk. J. Bot. 1998, 22, 13–18. [Google Scholar]
- Ciccoritti, R.; Ruggiero, G.; Ciorba, R.; Manetti, C.; Amoriello, M.; Amoriello, T. Shelf-life assessment of apricot fruit during cold storage by a portable visible and near-infrared hyperspectral imaging device. Eur. Food Res. Technol. 2025, 251, 545–558. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Amoriello, T.; Mellara, F.; Ruggeri, S.; Ciorba, R.; Ceccarelli, D.; Ciccoritti, R. Artichoke By-Products Valorization for Phenols-Enriched Fresh Egg Pasta: A Sustainable Food Design Project. Sustainability 2022, 14, 14778. [Google Scholar] [CrossRef]
- Moskowitz, H.R. Utilitarian benefits of magnitude estimation scaling for testing product acceptability. In Selected Sensory Methods: Problems and Approaches to Measuring Hedonics; Kuznicki, J.T., Johnson, R.A., Rutkiewic, A.F., Eds.; American Society for Testing and Materials: Philadelphia, PA, USA, 1982. [Google Scholar]
- Amoriello, T.; Ciorba, R.; Ruggiero, G.; Amoriello, M.; Ciccoritti, R. A Performance Evaluation of Two Hyperspectral Imaging Systems for the Prediction of Strawberries’ Pomological Traits. Sensors 2024, 24, 174. [Google Scholar] [CrossRef] [PubMed]
- Amoriello, T.; Mellara, F.; Amoriello, M.; Ciccoritti, R. Evaluation of Nutritional Values of Edible Algal Species Using a Shortwave Infrared Hyperspectral Imaging and Machine Learning Technique. Foods 2024, 13, 2277. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Venktesh Bhat, B.; Shukla, G.P.; Singh, K.K.; Gehrana, D. Variation in carbohydrate and protein fractions, energy, digestibility and mineral concentrations in stover of sorghum cultivars. Trop. Grassl.-Forrajes Trop. 2018, 6, 42–52. [Google Scholar] [CrossRef]
- Miafo, A.P.T.; Koubala, B.B.; Kansci, G.; Muralikrishna, G. Free sugars and non-starchpolysaccharides phenolic acid complexes from bran, spent grain and sorghum seeds. J. Cereal Sci. 2019, 87, 124–131. [Google Scholar] [CrossRef]
- Ayala-Soto, F.E.; Serna-Saldívar, S.O.; Welti-Chanes, J.; Gutierrez-Uribe, J.A. Phenolic compounds, antioxidant capacity and gelling properties of glucoarabinoxylans from three types of Sorghum brans. J. Cereal Sci. 2015, 65, 277–284. [Google Scholar] [CrossRef]
- Stefaniak, T.R.; Dahlberg, J.A.; Bean, B.W.; Dighe, N.; Wolfrum, E.J.; Rooney, W.L. Variation in biomass composition components among forage, biomass, sorghum-sudangrass, and sweet sorghum types. Crop Sci. 2012, 52, 1949–1954. [Google Scholar] [CrossRef]
- Galassi, E.; Taddei, F.; Ciccoritti, R.; Nocente, F.; Gazza, L. Biochemical and technological characterization of two C4 gluten-free cereals: Sorghum bicolor and Eragrostis tef. Cereal Chem. 2020, 97, 65–73. [Google Scholar] [CrossRef]
- Huang, H.; Hu, X.; Tian, J.; Jiang, X.; Sun, T.; Luo, H.; Huang, D. Rapid and nondestructive prediction of amylose and amylopectin contents in sorghum based on hyperspectral imaging. Food Chem. 2021, 359, 129954. [Google Scholar] [CrossRef]
- Yang, L.; Gao, H.; Meng, L.; Fu, X.; Du, X.; Wu, D.; Huang, L. Nondestructive measurement of pectin polysaccharides using hyperspectral imaging in mulberry fruit. Food Chem. 2021, 334, 127614. [Google Scholar] [CrossRef]
- Zhang, H.; Ge, Y.; Xie, X.; Atefi, A.; Wijewardane, N.K.; Thapa, S. High throughput analysis of leaf chlorophyll content in sorghum using RGB, hyperspectral, and fluorescence imaging and sensor fusion. Plant Methods 2022, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yan, C.; Jiang, X.; Shen, F.; You, J.; Fang, Y. Classification of aflatoxin B1 naturally contaminated peanut using visible and near-infrared hyperspectral imaging by integrating spectral and texture features. Infrared Phys. Technol. 2021, 114, 103652. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Chen, Y.; Luo, W.; Huang, Y.; Tao, D.; Liu, X. Non- destructive determination of fat and moisture contents in Salmon (Salmo salar) fillets using near-infrared hyperspectral imaging coupled with spectral and textural features. J. Food Compos. Anal. 2020, 92, 103567. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, J.; Han, L.; Ma, X.; Hu, X.; Lin, T.; Chen, M. Determination of malathion content in sorghum grains using hyperspectral imaging technology combined with stacked machine learning models. J. Food Compos. Anal. 2024, 135, 106635. [Google Scholar] [CrossRef]
- Huang, H.; Hu, X.; Tian, J.; Peng, X.; Luo, H.; Huang, D.; Wang, H. Rapid and nondestructive determination of sorghum purity combined with deep forest and near-infrared hyperspectral imaging. Food Chem. 2022, 377, 131981. [Google Scholar] [CrossRef]
- Hu, X.; Dai, M.; Peng, J.; Zeng, J.; Tian, J.; Chen, M. Rapid sorghum variety identification by hyperspectral imaging combined with super-depth-of-field microscopy. J. Food Compos. Anal. 2025, 137, 106930. [Google Scholar] [CrossRef]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef]
- Zulkifli, M.Z.A.; Benjamin, M.A.Z.; Rosdan, M.D.E.M.; Saini, A.; Rusdi, N.A.; Awang, M.A. Optimisation of yield, flavonoids, and antioxidant activity via ultrasound-assisted extraction of bamboo leaves from Dinochloa sublaevigata S. Dransf. (Wadan) in Sabah, Malaysia. Adv. Bamboo Sci. 2025, 10, 100128. [Google Scholar] [CrossRef]
- Yılmaz, M.; Karaaslan, M.; Vardin, H. Optimization of extraction parameters on the isolation of phenolic compounds from sour cherry (Prunus cerasus L.) pomace. J. Food Sci. Technol. 2015, 52, 2851–2859. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound-assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Cui, J.; Zhang, H.; Duan, Y.; Zhang, D.; Cai, M.; Chen, G. Ultrasound assisted extraction of polyphenolic compounds from red sorghum (Sorghum bicolor L.) bran and their biological activities and polyphenolic compositions. Ind. Crops Prod. 2018, 112, 296–304. [Google Scholar] [CrossRef]
- Barros, F.; Dykes, L.; Awika, J.M.; Rooney, L.W. Accelerated solvent extraction of phenolic compounds from sorghum brans. J. Cereal Sci. 2013, 58, 305–312. [Google Scholar] [CrossRef]
- García-Oms, S.; Sánchez-Bonet, D.; Belda-Antolí, M.; Padrón-Sanz, C.; Lloris-Carsi, J.M.; Cejalvo-Lapeña, D. Optimisation of a green ultrasound-assisted extraction (UAE) methodology for obtaining maximum antioxidant activity from red algae and determination of the co-extracted compounds. J. Appl. Phycol. 2024, 36, 1433–1444. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Virot, M.; Tomao, V.; Le Bourvellec, C.; Renard, C.M.C.G.; Chemat, F. Towards the industrial production of antioxidants from food processing by-products with ultrasound-assisted extraction. Ultrason. Sonochem. 2010, 17, 1066–1074. [Google Scholar] [CrossRef]
- Manzoor, M.; Singh, J.; Gani, A.; Noor, N. Valorization of natural colors as health-promoting bioactive compounds: Phytochemical profile, extraction techniques, and pharmacological perspectives. Food Chem. 2021, 362, 130141. [Google Scholar] [CrossRef]
- Das, A.K.; Adsare, S.R.; Das, M.; Kulthe, P.S. Advanced techniques in extraction of phenolics from cereals, pulses, fruits, and vegetables. In Plant Secondary Metabolites, Volume Two; Apple Academic Press: New York, NY, USA, 2017; pp. 53–102. [Google Scholar]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochemistry 2021, 70, 105325. [Google Scholar] [CrossRef]
- Sethi, S.; Kaur, L.; Nath, P.; Yadav, D.N. Bioactive compounds and phytonutrients from cereals. In Plant-Based Bioactive Compounds and Food Ingredients; Apple Academic Press: New York, NY, USA, 2023; pp. 155–205. [Google Scholar]
- Hong, S.; Pangloli, P.; Perumal, R.; Cox, S.; Noronha, L.E.; Dia, V.P.; Smolensky, D. A Comparative Study on Phenolic Content, Antioxidant Activity and Anti-Inflammatory Capacity of Aqueous and Ethanolic Extracts of Sorghum in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Antioxidants 2020, 9, 1297. [Google Scholar] [CrossRef]
- Dia, V.P.; Pangloli, P.; Jones, L.; McClure, A.; Patel, A. Phytochemical concentrations and biological activities of Sorghum bicolor alcoholic extracts. Food Funct. 2016, 7, 3410–3420. [Google Scholar] [CrossRef]
- Moraes, É.A.; da Silva Marineli, R.; Lenquiste, S.A.; Steel, C.J.; de Menezes, C.B.; Queiroz, V.A.V.; Júnior, M.R.M. Sorghum flour fractions: Correlations among polysaccharides, phenolic compounds, antioxidant activity and glycemic index. Food Chem. 2015, 180, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Sohounhloue, D.C. Optimization of the Extraction of Sorghum’s Polyphenols for Industrial Production by Membrane Processes. Res. J. Recent Sci. 2012, 1, 2502. [Google Scholar]
- Denardi-Souza, T.; Luz, C.; Mañes, J.; Badiale-Furlong, E.; Meca, G. Antifungal effect of phenolic extract of fermented rice bran with Rhizopus oryzae and its potential use in loaf bread shelf life extension. J. Sci. Food Agric. 2018, 98, 5011–5018. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Ma, J.; Cheng, K.W.; Jiang, Y.; Chen, F.; Wang, M. The effects of grape seed extract fortification on the antioxidant activity and quality attributes of bread. Food Chem. 2010, 119, 49–53. [Google Scholar] [CrossRef]
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.; Jekle, M.; Becker, T. Development of fibre-enriched wheat breads: Impact of recovered agroindustrial by-products on physicochemical properties of dough and bread characteristics. Eur. Food Res. Technol. 2017, 243, 1973–1988. [Google Scholar] [CrossRef]
- Oliviero, T.; Fogliano, V. Food design strategies to increase vegetable intake: The case of vegetable enriched pasta. Trends Food Sci. Technol. 2016, 51, 58–64. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J.; Li, Y. Extracts of sorghum bran, grape seed, and green tea: Chromatographic comparison of phenolic profiles and mitigation effect on acrylamide in antioxidant-fortified bread. Food Chem. Adv. 2022, 1, 100082. [Google Scholar] [CrossRef]
- Bojnanská, T.; Kolesárová, A.; Cech, M.; Tancinová, D.; Urminská, D. Extracts with Nutritional Potential and Their Influence on the Rheological Properties of Dough and Quality Parameters of Bread. Foods 2024, 13, 382. [Google Scholar] [CrossRef]
- Legan, J.D. Mould Spoilage of Bread: The Problem and Some Solutions. Int. Biodeterior. Biodegrad. 1993, 32, 33–53. [Google Scholar] [CrossRef]
- Pateras, I. Bread Spoilage and Staling. In Technology of Breadmaking; Springer Ebooks: Boston, MA, USA, 1999; pp. 240–261. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. Ecology of Fungal Food Spoilage. In Fungi Food Spoilage; Springer: Cham, Switzerland, 2022; pp. 3–12. [Google Scholar]
- Alisaac, E.; Behmann, J.; Rathgeb, A.; Karlovsky, P.; Dehne, H.W.; Mahlein, A.K. Assessment of Fusarium Infection and Mycotoxin Contamination of Wheat Kernels and Flour Using Hyperspectral Imaging. Toxins 2019, 11, 556. [Google Scholar] [CrossRef]
- Alpers, T.; Kerpes, R.; Frioli, M.; Nobis, A.; Hoi, K.I.; Bach, A.; Becker, T. Impact of storing condition on staling and microbial spoilage behavior of bread and their contribution to prevent food waste. Foods 2021, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tain, X.; Yu, Q.; Hu, W.; Chen, J.; Zhou, L. Sweet sorghum stalks extract has antimicrobial activity. Ind. Crops Prod. 2021, 170, 113746. [Google Scholar] [CrossRef]
- Birhanu, S. Potential benefits of sorghum [Sorghum bicolor (L.) Moench] on human health: A review. Int. J. Food Eng. Technol. 2021, 5, 8–18. [Google Scholar] [CrossRef]
- Magdoleen, G.; Daffalla Hussien, M. Phytochemical screening and antibacterial activities of Sorghum bicolor leaves derived from in vitro culture. GSC Biol. Pharm. Sci. 2020, 10, 65–72. [Google Scholar]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Y.; Chen, H.; Liu, H.; Yu, Q.; Han, L. Isolation and identification of polyphenols from fresh sweet sorghum stems and their antibacterial mechanism against foodborne pathogens. Front. Bioeng. Biotechnol. 2022, 9, 770726. [Google Scholar] [CrossRef]
- Hiroyuki, H.; Tanimoto, K.; Tamura, Y.; Mizutani, K.; Kinoshita, T. Mode of Antibacterial Action of Retrochalcones from Glycyrrhiza Inflata. Phytochemistry 1998, 48, 125–129. [Google Scholar]
- Ziani, I.; Bouakline, H.; El Guerraf, A.; El Bachiri, A.; Fauconnier, M.L.; Sher, F. Integrating AI and advanced spectroscopic techniques for precision food safety and quality control. Trends Food Sci. Technol. 2025, 156, 104850. [Google Scholar] [CrossRef]
- Saleem, Z.; Hussain Khan, M.; Ahmad, M.; Sohaib, A.; Ayaz, H.; Mazzara, M. Prediction of Microbial Spoilage and Shelf-Life of Bakery Products through Hyperspectral Imaging. IEEE Access 2020, 8, 176986–176996. [Google Scholar] [CrossRef]
- Cevoli, C.; Gianotti, A.; Troncoso, R.; Fabbri, A. Quality evaluation by physical tests of a traditional Italian flat bread Piadina during storage and shelf-life improvement with sourdough and enzymes. Eur. Food Res. Technol. 2015, 240, 1081–1089. [Google Scholar] [CrossRef]
- Mehdizadeh, S.A.; Noshad, M.; Nouri, S.F. Non-destructive Detection of Bread Staleness Using Hyperspectral Images. Innov. Food Technol. 2023, 10, 299–317. [Google Scholar]
- Karl, T.; Harre, F.; Warneke, C.; de Gouw, J.; Grayless, C.; Fall, R. Senescing grass crops as regional sources of reactive volatile organic compounds. J. Geophys. Res. 2005, 110, D15302. [Google Scholar] [CrossRef]
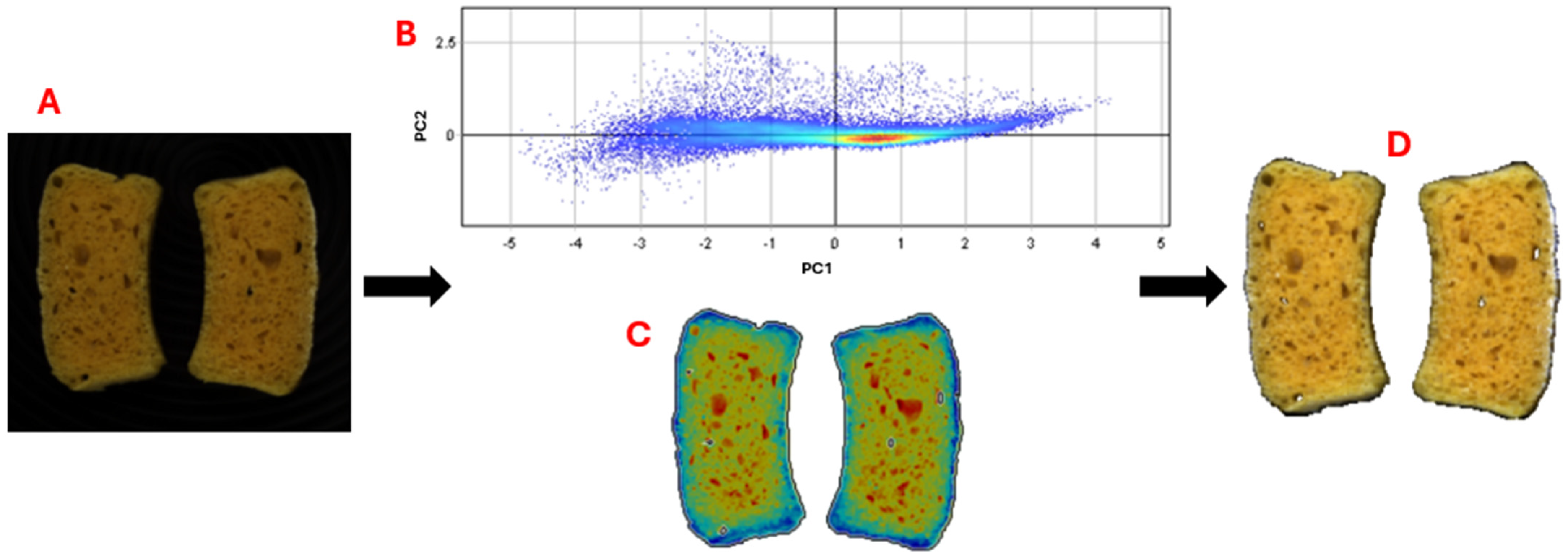
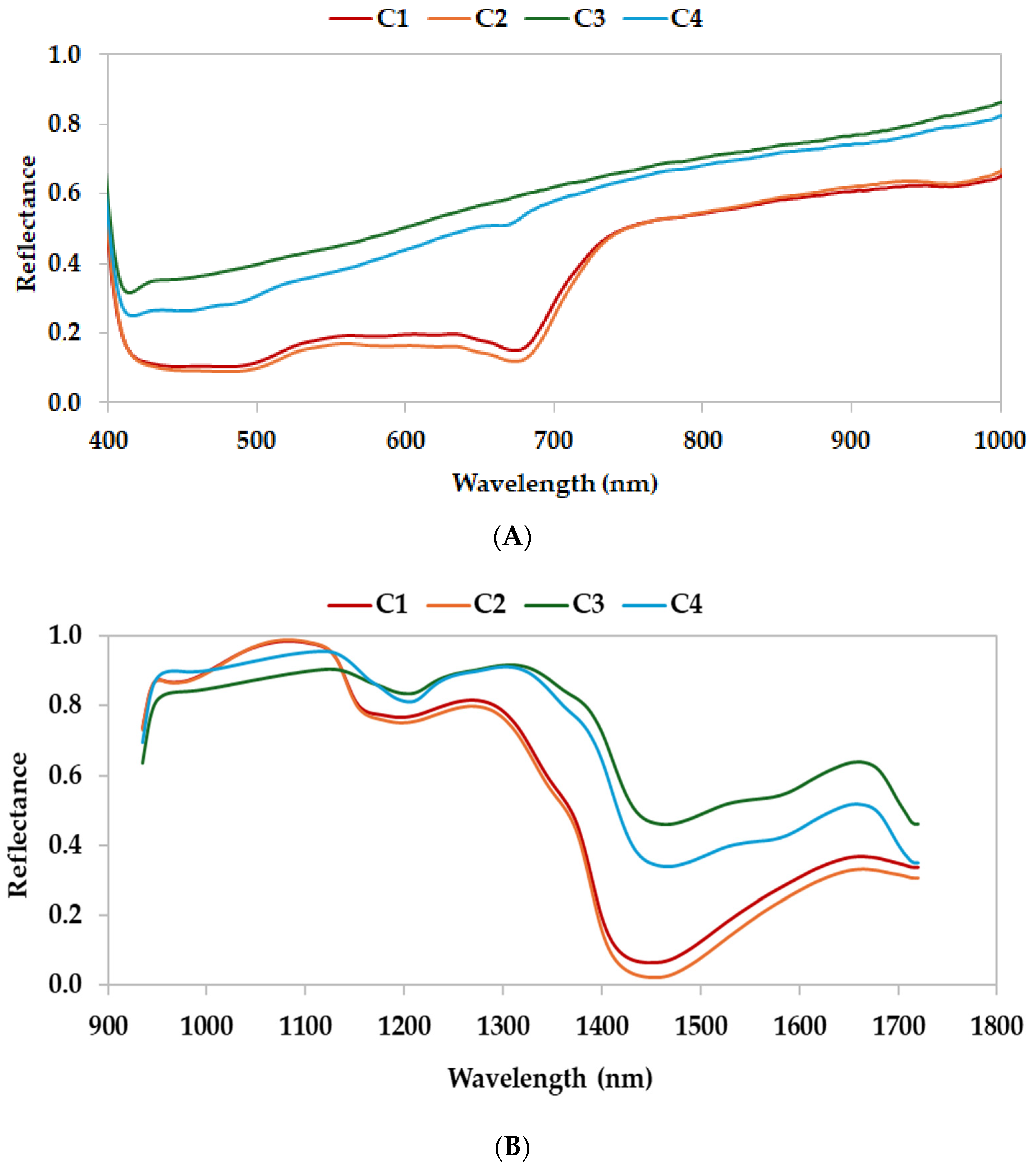

| Sample | Type of Waste | Type of Farm |
|---|---|---|
| C1 | Stalk | Organic |
| C2 | Stalk | Integrated |
| C3 | Debranning products | Integrated |
| C4 | Debranning products | Organic |
| Run | X1 | X2 | X3 | X1 (%) | X2 (min) | X3 (°C) |
|---|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 50 | 40 | 75 |
| 2 | 1 | −1 | 0 | 80 | 40 | 75 |
| 3 | −1 | 1 | 0 | 50 | 60 | 75 |
| 4 | 1 | 1 | 0 | 80 | 60 | 75 |
| 5 | −1 | 0 | −1 | 50 | 50 | 70 |
| 6 | 1 | 0 | −1 | 80 | 50 | 70 |
| 7 | −1 | 0 | 1 | 50 | 50 | 80 |
| 8 | 1 | 0 | 1 | 80 | 50 | 80 |
| 9 | 0 | −1 | −1 | 65 | 40 | 70 |
| 10 | 0 | 1 | −1 | 65 | 60 | 70 |
| 11 | 0 | −1 | 1 | 65 | 40 | 80 |
| 12 | 0 | 1 | 1 | 65 | 60 | 80 |
| 13 | 0 | 0 | 0 | 65 | 50 | 75 |
| 14 | 0 | 0 | 0 | 65 | 50 | 75 |
| 15 | 0 | 0 | 0 | 65 | 50 | 75 |
| Sample | Moisture (g 100 g−1 FW) | Protein (g 100 g−1 DW) | Ash (g 100 g−1 DW) | TDF (g 100 g−1 DW) |
|---|---|---|---|---|
| C1 | 63.1 ± 0.5 b | 8.1 ± 0.2 b | 8.48 ± 0.03 b | 60.4 ± 1.4 a |
| C2 | 69.6 ± 0.8 a | 9.9 ± 0.2 a | 9.03 ± 0.01 a | 61.7 ± 0.3 a |
| C3 | 11.4 ± 0.3 c | 10.5 ± 0.1 d | 2.64 ± 0.01 d | 32.1 ± 0.7 b |
| C4 | 11.7 ± 0.3 c | 13.5 ± 0.1 c | 4.00 ± 0.02 c | 29.8 ± 0.4 c |
| C1 | C2 | C3 | C4 | |||||
|---|---|---|---|---|---|---|---|---|
| Run | TPCexp | TPCRSM | TPCexp | TPCRSM | TPCexp | TPCRSM | TPCexp | TPCRSM |
| 1 | 326 ± 2 | 324 | 234 ± 1 | 234 | 226 ± 3 | 227 | 257 ± 1 | 256 |
| 2 | 340 ± 4 | 343 | 228 ± 1 | 229 | 234 ± 4 | 233 | 288 ± 4 | 285 |
| 3 | 326 ± 1 | 323 | 248 ± 3 | 247 | 230 ± 4 | 231 | 278 ± 1 | 281 |
| 4 | 323 ± 6 | 324 | 256 ± 3 | 255 | 233 ± 1 | 232 | 259 ± 4 | 259 |
| 5 | 291 ± 6 | 292 | 235 ± 1 | 235 | 219 ± 5 | 218 | 265 ± 1 | 263 |
| 6 | 294 ± 3 | 292 | 256 ± 1 | 259 | 221 ± 4 | 222 | 260 ± 2 | 260 |
| 7 | 344 ± 2 | 346 | 264 ± 1 | 260 | 227 ± 2 | 226 | 253 ± 1 | 253 |
| 8 | 368 ± 5 | 366 | 241 ± 2 | 240 | 229 ± 1 | 230 | 262 ± 1 | 264 |
| 9 | 286 ± 8 | 286 | 250 ± 3 | 249 | 205 ± 1 | 205 | 265 ± 2 | 268 |
| 10 | 302 ± 1 | 302 | 267 ± 1 | 266 | 206 ± 3 | 206 | 277 ± 6 | 277 |
| 11 | 377 ± 2 | 376 | 249 ± 1 | 250 | 212 ± 1 | 212 | 275 ± 2 | 275 |
| 12 | 340 ± 3 | 340 | 270 ± 2 | 271 | 214 ± 2 | 214 | 267 ± 2 | 264 |
| 13 | 330 ± 1 | 330 | 252 ± 4 | 252 | 208 ± 1 | 208 | 221 ± 1 | 221 |
| 14 | 330 ± 1 | 330 | 252 ± 4 | 252 | 208 ± 1 | 208 | 221 ± 1 | 221 |
| 15 | 330 ± 1 | 330 | 252 ± 4 | 252 | 208 ± 1 | 208 | 221 ± 1 | 221 |
| C1 | C2 | C3 | C4 | |||||
|---|---|---|---|---|---|---|---|---|
| Sum of Square | p-Value | Sum of Square | p-Value | Sum of Square | p-Value | Sum of Square | p-Value | |
| X1 | 370.01 | <0.0001 | 0.20 | 0.8614 | 63.37 | 0.0015 | 57.20 | 0.0138 |
| X2 | 369.48 | 0.1711 | 1529.25 | <0.0001 | 11.98 | 0.1261 | 2.88 | 0.5518 |
| X3 | 16,562.61 | <0.0001 | 59.53 | 0.0064 | 242.57 | <0.0001 | 34.26 | 0.0497 |
| X12 | 25.31 | 0.8805 | 770.88 | <0.0001 | 2659.80 | <0.0001 | 2692.59 | <0.0001 |
| X22 | 0.29 | <0.0001 | 0.01 | 0.9776 | 125.43 | <0.0001 | 6711.89 | <0.0001 |
| X32 | 136.81 | 0.0035 | 381.20 | <0.0001 | 53.27 | 0.0031 | 2828.28 | <0.0001 |
| X1 X2 | 144.05 | 0.0028 | 88.13 | 0.0014 | 16.05 | 0.0794 | 1299.16 | <0.0001 |
| X1 X3 | 213.18 | 0.0005 | 976.22 | <0.0001 | 0.58 | 0.7290 | 97.75 | 0.0021 |
| X2 X3 | 1392.16 | <0.0001 | 10.02 | 0.2262 | 0.40 | 0.7728 | 207.11 | <0.0001 |
| Residual | 264.66 | 128.50 | 93.99 | 136.98 | ||||
| Lack of fit | 69.57 | 0.1308 | 39.35 | 0.0941 | 11.79 | 0.5044 | 55.02 | 0.0999 |
| Pure error | 195.09 | 89.15 | 82.21 | 81.96 | ||||
| Total SS | 19,475.60 | 3943.94 | 3281.72 | 12,582.01 | ||||
| R2 | 0.986 | 0.968 | 0971 | 0.9875 | ||||
| Radj2 | 0.981 | 0.954 | 0.958 | 0.9819 | ||||
| AAD | ||||||||
| C1 | C2 | C3 | C4 | |||||
|---|---|---|---|---|---|---|---|---|
| Run | AAexp | AARSM | AAexp | AARSM | AAexp | AARSM | AAexp | AARSM |
| 1 | 20.2 ± 0.1 | 20.4 | 26.2 ± 0.4 | 26.4 | 20.9 ± 1.3 | 21.0 | 27.9 ± 0.1 | 28.0 |
| 2 | 20.7 ± 0.4 | 20.6 | 20.1 ± 0.1 | 20.1 | 22.3 ± 1.0 | 22.5 | 28.8 ± 0.7 | 28.7 |
| 3 | 23.9 ± 0.1 | 24.1 | 22.0 ± 0.8 | 22.1 | 18.6 ± 0.5 | 18.5 | 26.6 ± 0.5 | 26.7 |
| 4 | 19.1 ± 0.1 | 19.0 | 22.3 ± 0.3 | 22.2 | 20.7 ± 0.8 | 20.6 | 28.5 ± 0.9 | 28.4 |
| 5 | 25.6 ± 0.3 | 25.3 | 26.5 ± 0.4 | 26.2 | 19.2 ± 0.9 | 19.2 | 29.6 ± 0.4 | 29.7 |
| 6 | 24.5 ± 0.3 | 24.5 | 19.7 ± 0.7 | 19.7 | 21.9 ± 1.4 | 21.9 | 25.6 ± 0.2 | 25.9 |
| 7 | 25.2 ± 0.9 | 25.2 | 23.4 ± 0.3 | 23.4 | 19.7 ± 1.7 | 19.8 | 24.1 ± 0.9 | 23.9 |
| 8 | 20.8 ± 0.7 | 21.1 | 23.5 ± 0.1 | 23.7 | 20.8 ± 0.3 | 20.8 | 30.2 ± 0.7 | 30.1 |
| 9 | 22.1 ± 0.7 | 22.3 | 23.2 ± 0.2 | 23.3 | 26.7 ± 0.2 | 26.6 | 25.9 ± 0.9 | 25.7 |
| 10 | 22.4 ± 0.3 | 22.6 | 24.5 ± 0.4 | 24.7 | 24.3 ± 1.0 | 24.4 | 28.0 ± 0.4 | 27.8 |
| 11 | 20.0 ± 0.4 | 19.9 | 26.6 ± 0.3 | 26.4 | 26.5 ± 0.5 | 26.4 | 27.7 ± 0.2 | 27.9 |
| 12 | 21.8 ± 0.1 | 21.6 | 22.8 ± 0.1 | 22.7 | 24.0 ± 0.8 | 24.1 | 23.8 ± 0.4 | 24.1 |
| 13 | 22.6 ± 0.3 | 22.6 | 22.4 ± 0.6 | 22.4 | 23.4 ± 0.2 | 23.4 | 30.5 ± 0.2 | 30.5 |
| 14 | 22.6 ± 0.3 | 22.6 | 22.4 ± 0.6 | 22.4 | 23.4 ± 0.2 | 23.4 | 30.5 ± 0.2 | 30.5 |
| 15 | 22.6 ± 0.3 | 22.6 | 22.4 ± 0.6 | 22.4 | 23.4 ± 0.2 | 23.4 | 30.5 ± 0.2 | 30.5 |
| C1 | C2 | C3 | C4 | |||||
|---|---|---|---|---|---|---|---|---|
| Sum of Square | p-Value | Sum of Square | p-Value | Sum of Square | p-Value | Sum of Square | p-Value | |
| X1 | 24.54 | <0.0001 | 36.27 | <0.0001 | 13.20 | 0.0001 | 5.98 | <0.0001 |
| X2 | 4.29 | 0.0001 | 5.21 | <0.0001 | 19.12 | <0.0001 | 2.90 | 0.0030 |
| X3 | 11.71 | <0.0001 | 1.38 | 0.0133 | 0.26 | 0.5124 | 2.56 | 0.0049 |
| X12 | 1.32 | 0.0160 | 1.18 | 0.0201 | 110.66 | <0.0001 | 4.67 | 0.0006 |
| X22 | 29.54 | <0.0001 | 2.83 | 0.0009 | 8.45 | 0.0010 | 23.91 | <0.0001 |
| X32 | 7.59 | <0.0001 | 11.00 | <0.0001 | 5.42 | 0.0060 | 41.28 | <0.0001 |
| X1 X2 | 14.06 | <0.0001 | 19.97 | <0.0001 | 0.28 | 0.4967 | 0.58 | 0.1462 |
| X1 X3 | 5.58 | <0.0001 | 23.81 | <0.0001 | 1.39 | 0.1363 | 51.29 | <0.0001 |
| X2 X3 | 1.02 | 0.0315 | 13.12 | <0.0001 | 0.01 | 0.9269 | 17.47 | <0.0001 |
| Residual | 3.80 | 3.74 | 11.50 | 5.10 | ||||
| Lack of fit | 0.90 | 0.1925 | 0.54 | 0.4324 | 0.33 | 0.9153 | 0.78 | 0.4066 |
| Pure error | 2.90 | 3.20 | 11.16 | 4.32 | ||||
| Total SS | 106.48 | 120.64 | 178.62 | 148.20 | ||||
| R2 | 0.964 | 0.969 | 0.936 | 0.966 | ||||
| Radj2 | 0.948 | 0.955 | 0.907 | 0.950 | ||||
| AAD | ||||||||
| Sample | Response | X1 (%) | X2 (min) | X3 (°C) |
|---|---|---|---|---|
| C1 | TPC | 80.0 | 40 | 80.0 |
| AA | 50.0 | 55 | 80.0 | |
| C2 | TPC | 65.0 | 60 | 80.0 |
| AA | 57.5 | 40 | 80.0 | |
| C3 | TPC | 80.0 | 40 | 77.5 |
| AA | 65.0 | 40 | 70.0 | |
| C4 | TPC | 80.0 | 40 | 80.0 |
| AA | 80.0 | 50 | 77.5 |
| Sample | Extraction Method | TPC (mg GAE 100 g−1 FW) | TFC (mg CE 100 g−1 FW) | ChA (μg 100 g−1 FW) | ChB (μg 100 g−1 FW) | YPG (g 100 g−1 FW) | AA (mg TE 100 g−1 FW) |
|---|---|---|---|---|---|---|---|
| C1 | TM | 185 ± 1 b | 12 ± 1 b | 2882 ± 210 b | 2493 ± 120 b | 2.00 ± 0.02 b | 20.5 ± 0.8 b |
| UAE | 382 ± 3 a | 20 ± 2 a | 3514 ± 62 a | 2899 ± 42 a | 6.8 ± 0.3 a | 26.0 ± 0.4 a | |
| C2 | TM | 163 ± 8 b | 11 ± 1 b | 2012 ± 154 b | 1600 ± 85 b | 1.60 ± 0.04 b | 22.0 ± 1.0 b |
| UAE | 270 ± 2 a | 18 ± 2 a | 3510 ± 150 a | 2642 ± 165 a | 5.8 ± 0.4 a | 26.6 ± 0.3 a | |
| C3 | TM | 203 ± 2 b | 1.0 ± 0.1 b | 222 ± 12 b | 339 ± 22 b | 1.78 ± 0.04 b | 16.5 ± 0.8 b |
| UAE | 295 ± 3 a | 1.6 ± 0.2 a | 459 ± 25 a | 606 ± 46 a | 1.92 ± 0.03 a | 26.1 ± 0.2 a | |
| C4 | TM | 162 ± 6 b | 0.9 ± 0.1 b | 323 ± 15 b | 425 ± 31 b | 2.00 ± 0.05 b | 16.4 ± 0.3 b |
| UAE | 241 ± 2 a | 1.5 ± 0.2 a | 545 ± 19 a | 778 ± 55 a | 2.28 ± 0.09 a | 27.1 ± 0.3 a |
| Sample | Gallic Acid (μg/mL) | Vanillic Acid (μg/mL) | 4-Coumaric Acid (μg/mL) | Ferulic Acid (μg/mL) | Caffeic Acid (μg/mL) | Syringic Acid (μg/mL) | 3-Cumaric Acid (μg/mL) | Sinapic Acid (μg/mL) |
|---|---|---|---|---|---|---|---|---|
| C1 | ||||||||
| UAE | 14.6 ± 0.9 a | 1.72 ± 0.08 a | 3.9 ± 0.3 a | 3.1 ± 0.1 a | - | - | - | - |
| TM | tr | tr | 0.17 ± 0.02 b | 0.52 ± 0.03 b | - | - | - | - |
| C2 | ||||||||
| UAE | 19.0±1.5 a | 1.05 ± 0.01 a | 4.1 ± 0.8 a | 3.9 ± 0.2 a | - | - | - | - |
| TM | tr | Tr | 0.14 ± 0.03 b | 0.59 ± 0.05 b | - | - | - | - |
| C3 | ||||||||
| UAE | - | 0.10 ± 0.01 b | 0.48 ± 0.02 a | 0.41 ± 0.06 a | 1.84 ± 0.07 a | 0.72 ± 0.04 a | 0.64 ± 0.03 a | 0.10 ± 0.04 a |
| TM | - | 0.20 ± 0.06 a | 0.47 ± 0.06 a | 0.24 ± 0.05 b | 1.67 ± 0.04 b | Tr | 0.12 ± 0.03 b | 0.18 ± 0.05 a |
| C4 | ||||||||
| UAE | - | 0.20 ± 0.07 a | 0.30 ± 0.05 a | 0.6 ± 0.1 a | 2.38 ± 0.01 a | 0.70 ± 0.10 a | 0.97 ± 0.01 a | 0.10 ± 0.02 a |
| TM | - | 0.21 ± 0.08 a | 0.41 ± 0.02 a | 0.3 ± 0.1 b | 2.01 ± 0.09 b | 0.10 ± 0.01 b | tr | tr |
| Bread | Crust | Crumb | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |
| P1 | 57.27 ± 0.85 a | 7.33 ± 1.89 a | 28.25 ± 1.55 b | 58.33 ± 2.16 b | −6.08 ± 4.75 b | 11.34 ± 0.30 c |
| P2 | 59.45 ± 1.20 a | 5.49 ± 5.03 a | 32.34 ± 1.70 a | 61.36 ± 2.21 ab | 2.15 ± 4.35 a | 14.54 ± 0.89 b |
| P3 | 56.80 ± 2.41 a | 8.72 ± 3.04 a | 29.38 ± 1.19 b | 62.46 ± 0.89 a | 0.48 ± 0.22 a | 17.46 ± 1.72 a |
| Bread | TPC (mg GAE 100 g−1 FW) | AA (mg TE 100 g−1 FW) |
|---|---|---|
| P1 | 23.6 ± 0.9 c | 3.90 ± 0.09 c |
| P2 | 27.2 ± 0.6 b | 4.40 ± 0.12 b |
| P3 | 30.0 ± 0.8 a | 7.00 ± 0.10 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoriello, T.; Mellara, F.; Ciorba, R.; Ceccarelli, D.; Amoriello, M.; Taddei, F.; Ciccoritti, R. Phenols Extraction from Sorghum Byproducts: Upcycling Strategies and Food Applications. Antioxidants 2025, 14, 668. https://doi.org/10.3390/antiox14060668
Amoriello T, Mellara F, Ciorba R, Ceccarelli D, Amoriello M, Taddei F, Ciccoritti R. Phenols Extraction from Sorghum Byproducts: Upcycling Strategies and Food Applications. Antioxidants. 2025; 14(6):668. https://doi.org/10.3390/antiox14060668
Chicago/Turabian StyleAmoriello, Tiziana, Francesco Mellara, Roberto Ciorba, Danilo Ceccarelli, Monica Amoriello, Federica Taddei, and Roberto Ciccoritti. 2025. "Phenols Extraction from Sorghum Byproducts: Upcycling Strategies and Food Applications" Antioxidants 14, no. 6: 668. https://doi.org/10.3390/antiox14060668
APA StyleAmoriello, T., Mellara, F., Ciorba, R., Ceccarelli, D., Amoriello, M., Taddei, F., & Ciccoritti, R. (2025). Phenols Extraction from Sorghum Byproducts: Upcycling Strategies and Food Applications. Antioxidants, 14(6), 668. https://doi.org/10.3390/antiox14060668










