Enhancing the Bioactive Properties of Sugarcane Vinegar Through Caesalpinia sappan Extract Supplementation: A Novel Approach for Functional Beverage Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Chemicals
2.2. Plant Materials and Their Preparation
2.3. Starter Culture Preparation of Yeast and Acetic Acid Bacteria
2.4. Vinegar Production from Sugarcane Juice and C. sappan
2.5. Analytical Methods
2.5.1. Volatile Organic Compounds (VOCs)
2.5.2. Antioxidant Activity
2.5.3. Antimicrobial Activity
2.5.4. pH, Total Acidity, and Color
2.5.5. Total Sugar, Total Phenolic Compound, and Ethanol Content
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Raw Materials
3.2. Physicochemical Characteristics of Vinegar Produced from Fusions of Sugarcane Juice and C. sappan Extract
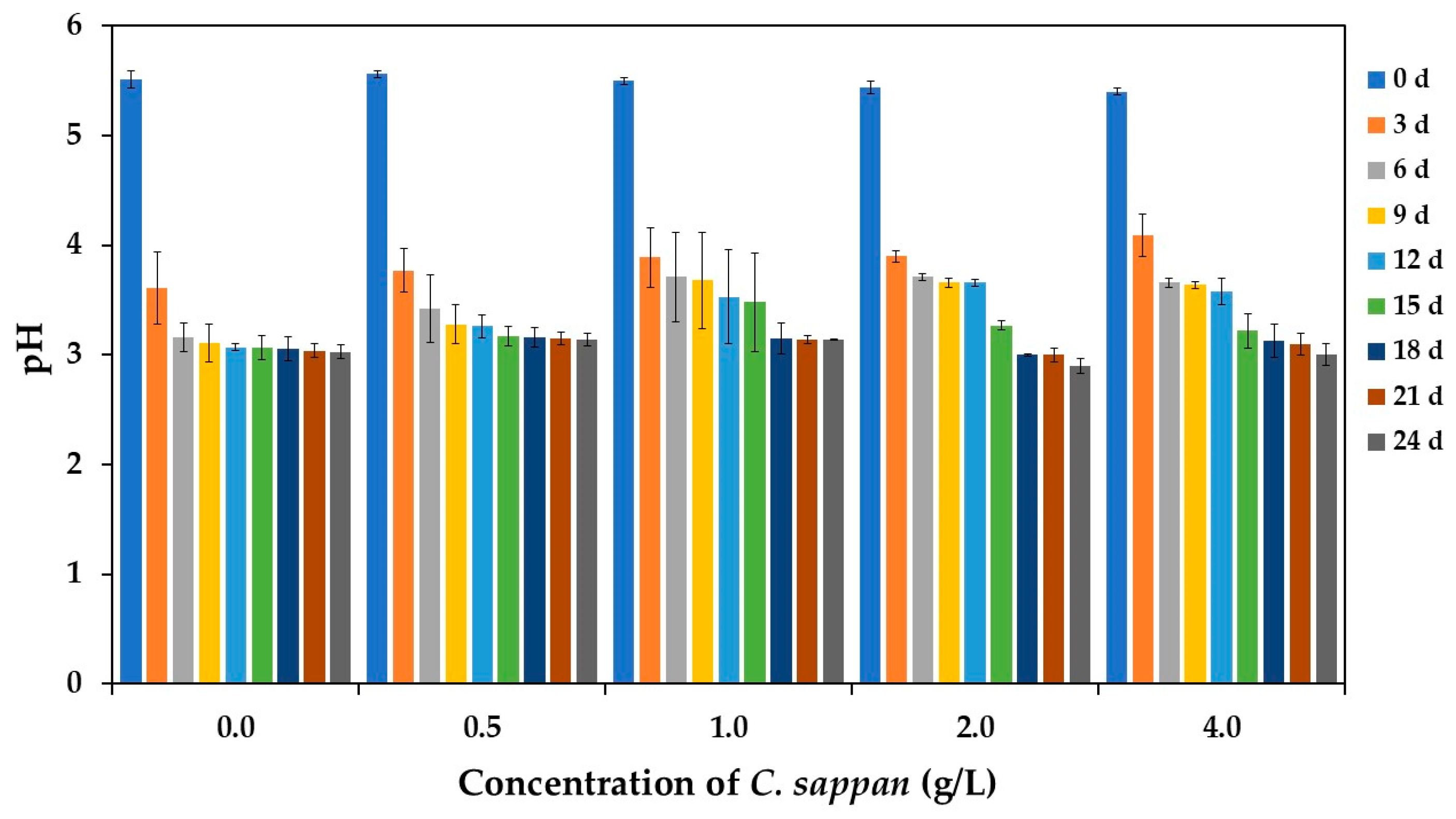
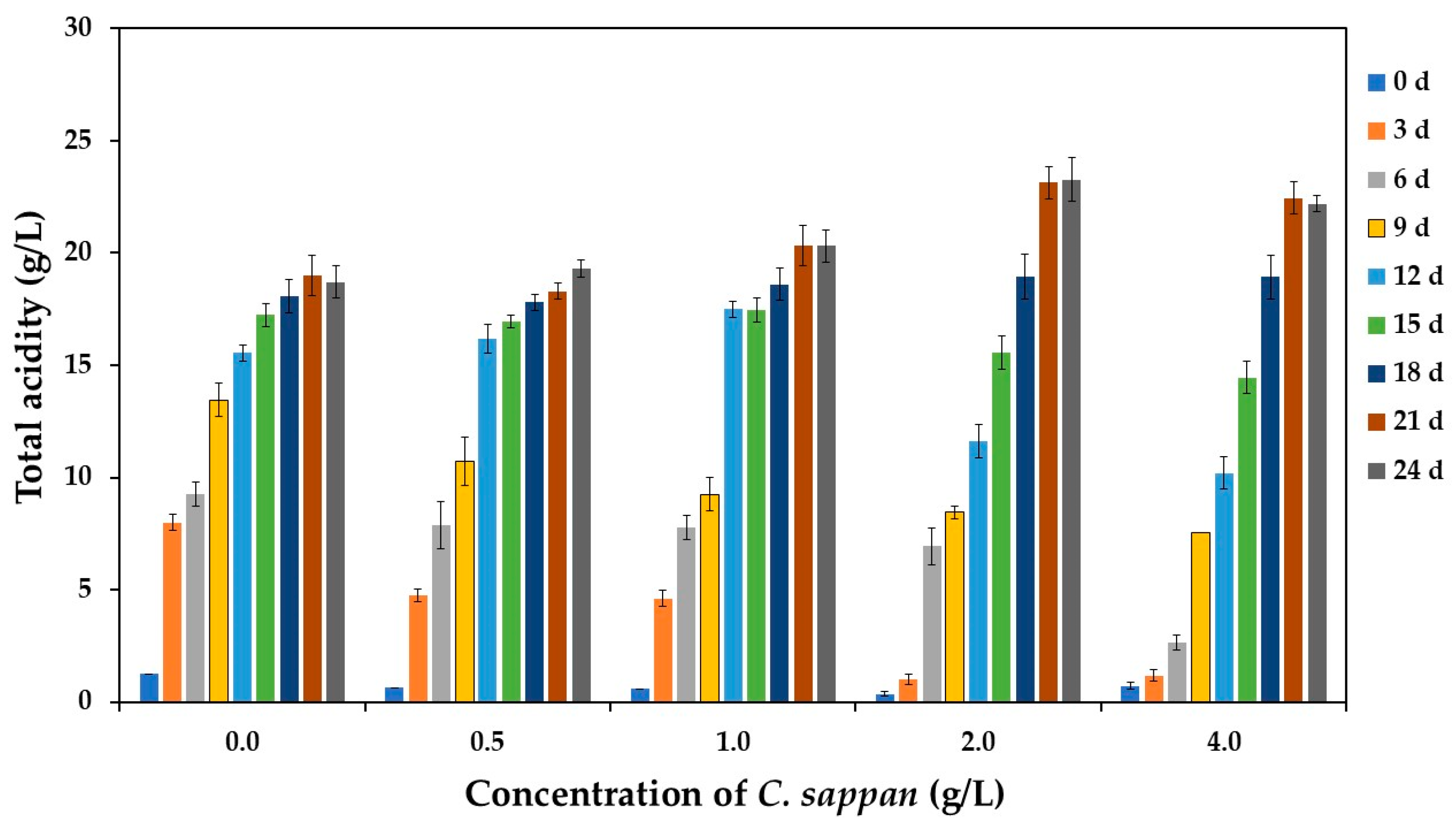
3.2.1. pH and Total Acidity During Fermentation
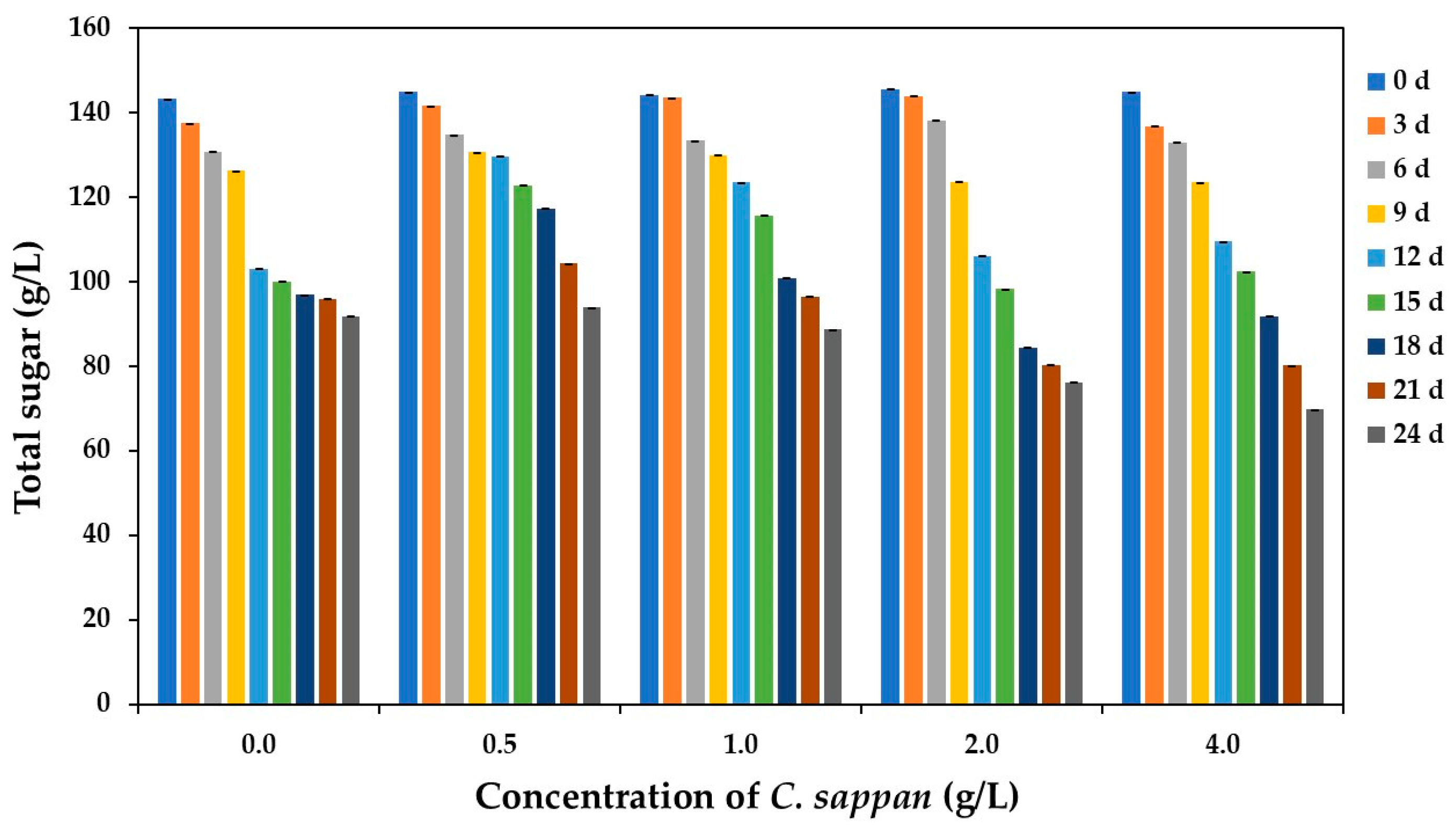
3.2.2. Total Sugar During Fermentation, Ethanol Content, and Color of the Final Products
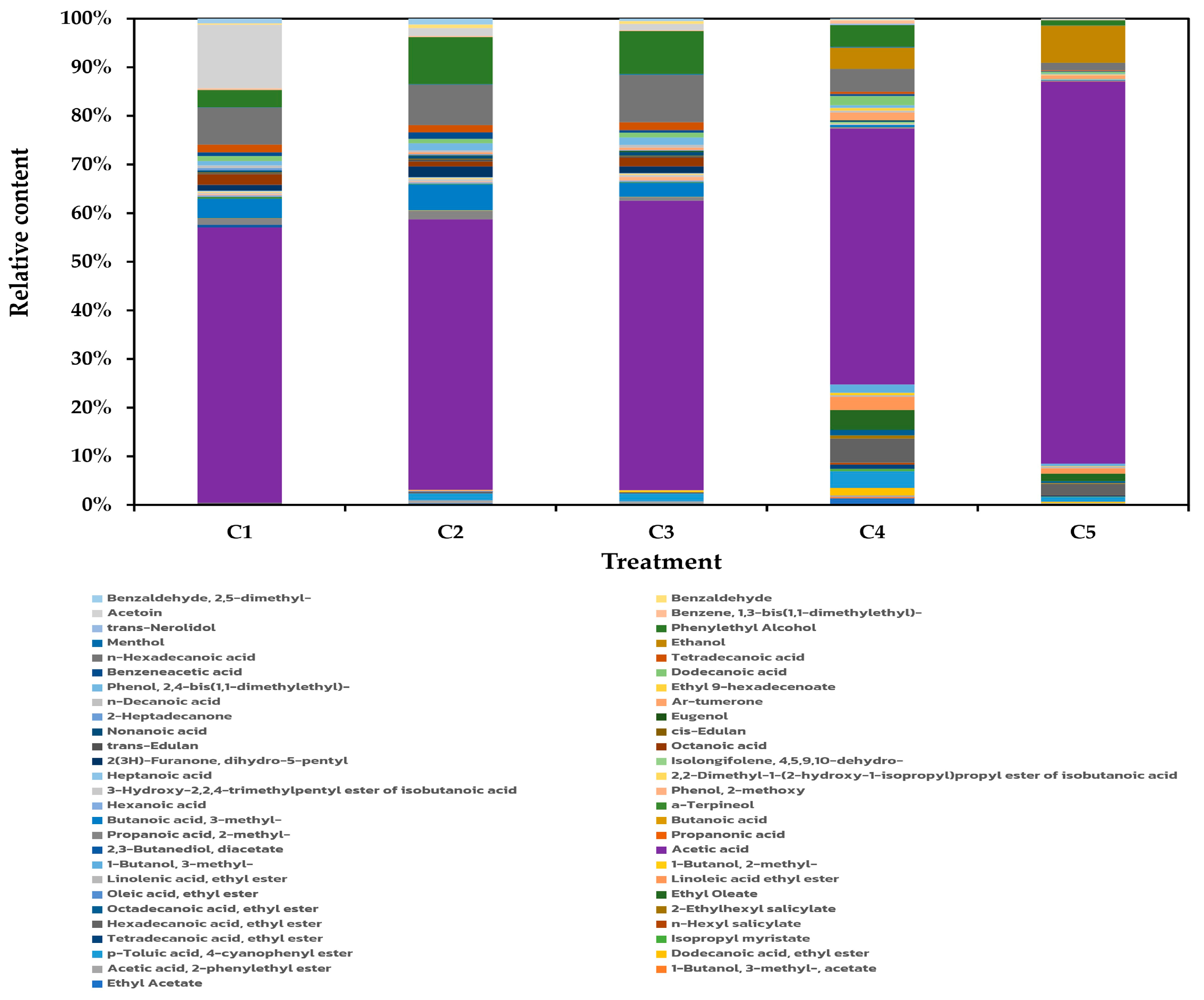
3.2.3. Volatile Organic Compounds (VOCs) in Vinegar Products
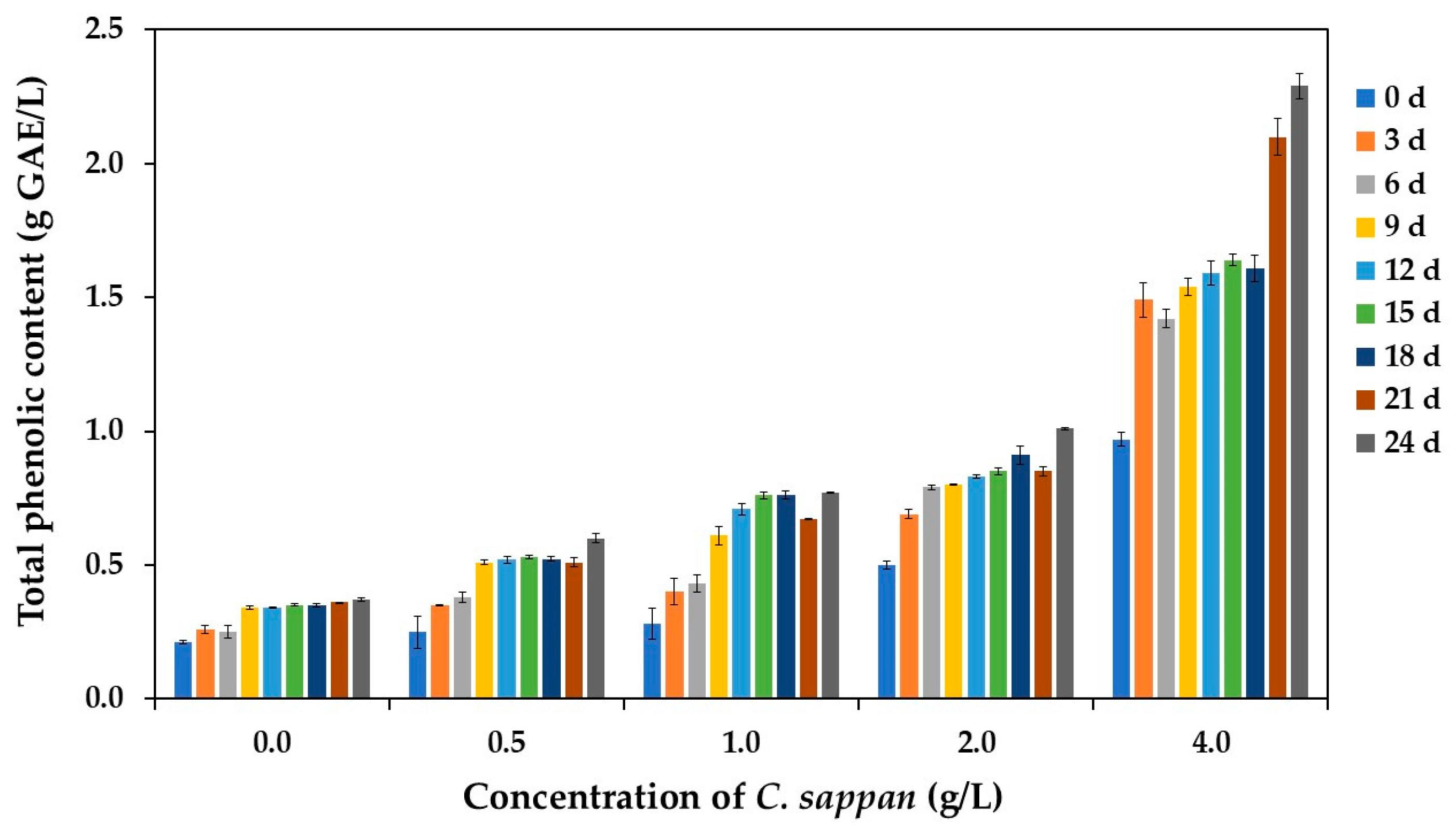
3.2.4. Total Phenolic Content (TPC) in the Vinegar Products
3.2.5. Antioxidant Activity of Vinegar Products
3.2.6. Antimicrobial Activity of Vinegar Products
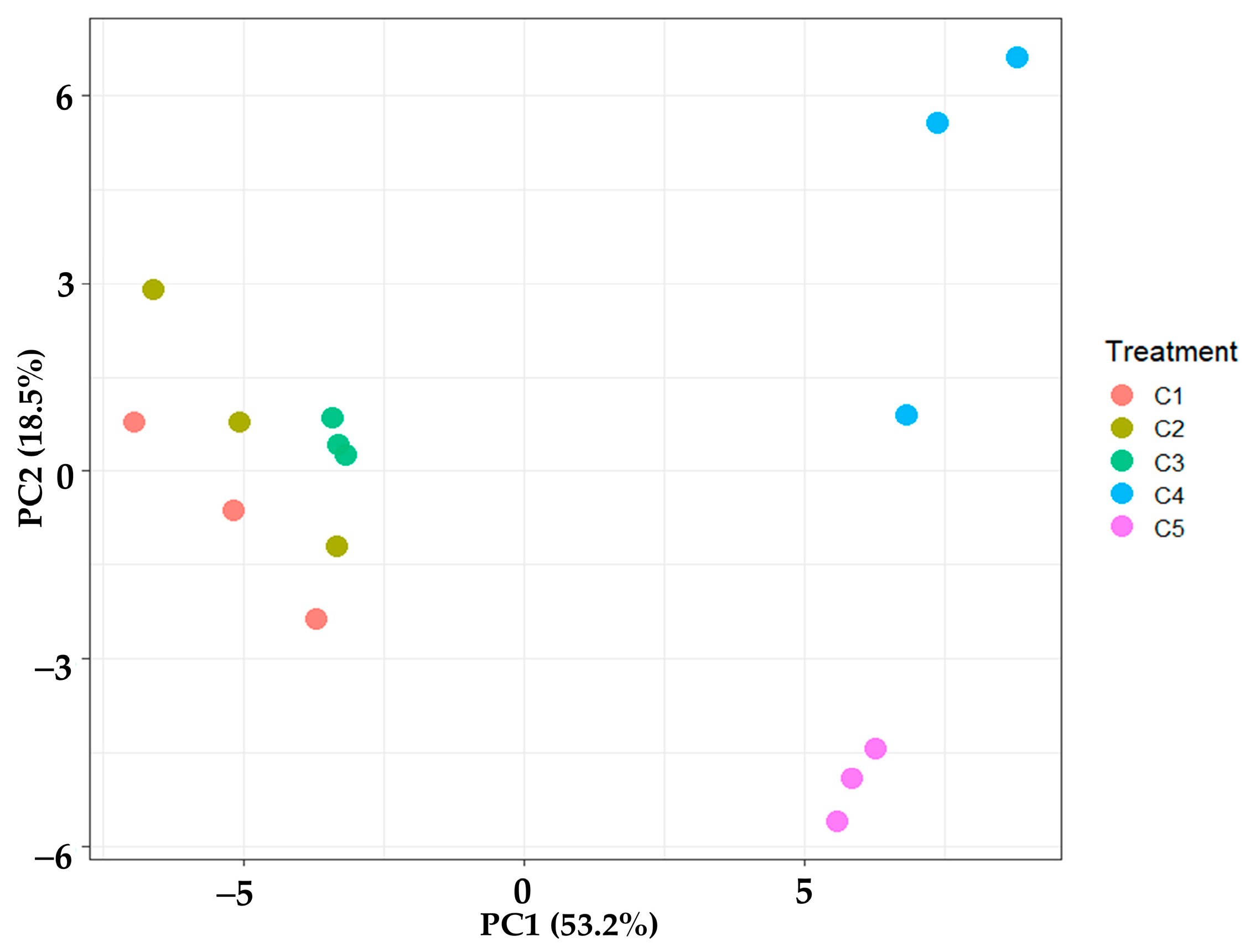
3.2.7. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kara, M.; Assouguem, A.; Al Kamaly, O.M.; Benmessaoud, S.; Imtara, H.; Mechchate, H.; Hano, C.; Zerhouni, A.R.; Bahhou, J. The impact of apple variety and the production methods on the antibacterial activity of vinegar samples. Molecules 2021, 26, 5437. [Google Scholar] [CrossRef] [PubMed]
- Salbe, A.D.; Johnston, C.S.; Buyukbse, A.; Tsitouras, P.D.; Harman, S.M. Vinegar lacks antiglycemic action on enteral carbohydrate absorption in human subjects. Nutr. Res. 2009, 29, 846–849. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, production, composition and health benefits of vinegars: A review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef]
- Zheng, F.J.; Lin, B.; Yang, Y.X.; Fang, X.C.; Verma, K.K.; Chen, G.L. Efficacy and functionality of sugarcane original vinegar on mice. Front. Microbiol. 2023, 14, 1224666. [Google Scholar]
- Abdali, Y.E.; Saghrouchni, H.; Kara, M.; Mssilou, I.; Allali, A.; Bin Jardan, A.; Kafkas, N.E.; El-Assri, E.M.; Nafidi, H.A.; Bourhia, M.; et al. Exploring the bioactive compounds in some apple vinegar samples and their biological activities. Plants 2023, 12, 3850. [Google Scholar] [CrossRef]
- Grand View Research. Available online: https://www.grandviewresearch.com/industry-analysis/vinegar-market-report (accessed on 31 March 2025).
- Tanamool, V.; Chantarangsee, M.; Soemphol, W. Simultaneous vinegar fermentation from a pineapple by-product using the co-inoculation of yeast and thermotolerant acetic acid bacteria and their physiochemical properties. 3 Biotech 2020, 10, 115. [Google Scholar] [CrossRef]
- Boondaeng, A.; Kasemsumran, S.; Ngowsuwan, K.; Vaithanomsat, P.; Apiwatanapiwat, W.; Trakunjae, C.; Janchai, P.; Jungtheerapanich, S.; Niyomvong, N. Comparison of the chemical properties of pineapple vinegar and mixed pineapple and dragon fruit vinegar. Fermentation 2022, 8, 597. [Google Scholar] [CrossRef]
- Plioni, I.; Bekatorou, A.; Terpou, A.; Mallouchos, A.; Plessas, S.; Koutinas, A.A.; Katechaki, E. Vinegar production from Corinthian currants finishing side-stream: Development and comparison of methods based on immobilized acetic acid bacteria. Foods 2021, 10, 3133. [Google Scholar] [CrossRef]
- Zhang, C.; He, Y.; Jiang, Y.C.; Jia, Y.L.; Zhang, W.; Wen, L.K. Optimization of fermentation process and composition analysis of Gastrodia elata vinegar. Food Res. Dev. 2022, 43, 154–161. [Google Scholar]
- Tian, Y.; Xia, T.; Qiang, X.; Zhao, Y.; Li, S.; Wang, Y.; Zheng, Y.; Yu, J.; Wang, J.; Wang, M. Nutrition, bioactive components, and hepatoprotective activity of fruit vinegar produced from Ningxia wolfberry. Molecules 2022, 27, 4422. [Google Scholar] [CrossRef]
- Qiang, X.; Zhao, M.; Xia, T.; Wang, Q.; Yu, J.; Song, Y.; Zhang, H.; Qiao, C.; Wang, M. The functional components and hepatic protective mechanism of wolfberry vinegar by mixed-culture fermentation. Foods 2025, 14, 1278. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, Foreign Agricultural Service. Sugar: World Markets and Trade; USDA, Foreign Agricultural Service: Washington, DC, USA, 2024; pp. 2–4.
- Sankhla, S.; Chaturvedi, A.; Kuna, A.; Dhanlakshmi, K. Preservation of sugarcane juice using hurdle technology. Sugar Tech 2012, 14, 26–39. [Google Scholar] [CrossRef]
- Makur, M.M.; Duraisamy, R.; Birhanu, T. Clarifying capacity of eco-friendly nano cao and okra (Abelmoschus esculentus) extract on the processing of sugarcane juice: A review. Int. Res. J. Sci. Technol. 2019, 1, 21–30. [Google Scholar] [CrossRef]
- Akter, N.; Linkon, K.M.M.R.; Akter, M.; Hossain, M.A.; Rahman, M.M.; Khan, T.; Alim, M.A.; Esrafil, M. Assessment of physico-chemical and microbial quality of sugarcane juice sold by street vendors in three regions of Bangladesh. Food Nutr. Sci. 2024, 15, 263–276. [Google Scholar] [CrossRef]
- Badami, S.; Moorkoth, S. Caesalpinia sappan—A medicinal and dye yielding plant. Nat. Prod. Radiance 2004, 3, 75–82. [Google Scholar]
- Thanayutsiri, T.; Patrojanasophon, P.; Opanasopit, P.; Ngawhirunpat, T.; Laiwattanapaisal, W.; Rojanarata, T. Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles. Green Process. Synth. 2023, 12, 20228109. [Google Scholar] [CrossRef]
- Mekala, K.; Radha, R. A review on sappan wood—A therapeutic dye yielding tree. Res. J. Pharmacogn. Phytochem. 2015, 7, 227. [Google Scholar] [CrossRef]
- Saini, R.; Dhiman, N.K. Natural anti-inflammatory and anti-allergy agents: Herbs and botanical ingredients. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2022, 21, 90–114. [Google Scholar] [CrossRef]
- Vij, T.; Anil, P.P.; Shams, R.; Dash, K.K.; Kalsi, R.; Pandey, V.K.; Harsányi, E.; Shaikh, A.M. A comprehensive review on bioactive compounds found in Caesalpinia sappan. Molecules 2023, 28, 6247. [Google Scholar] [CrossRef]
- Soemphol, W.; Saichana, N.; Yakuchi, T.; Adachi, O.; Matsushita, K.; Toyama, H. Characterization of genes involved in D-sorbitol oxidation in thermotolerant Gluconobacter frateurii. Biosci. Biotechnol. Biochem. 2012, 76, 1497–1505. [Google Scholar] [CrossRef]
- Tejedor-Calvo, E.; Garcia-Barreda, S.; Sánchez, S.; Morales, D.; Soler-Rivas, C.; Ruiz-Rodriguez, A.; Sanz, M.A.; Garcia, A.P.; Morte, A.; Marco, P. Supercritical CO2 extraction method of aromatic compounds from truffles. LWT Food Sci. Technol. 2021, 150, 111954. [Google Scholar] [CrossRef]
- Kitwetcharoen, H.; Chamnipa, N.; Thanonkeo, S.; Klanrit, P.; Tippayawat, P.; Klanrit, P.; Klanrit, P.; Yamada, M.; Thanonkeo, P. Enhancing kombucha functionality: Utilizing dried pineapple peels and cores as an alternative ingredient for improved antioxidant and antimicrobial properties. LWT Food Sci. Technol. 2025, 216, 117358. [Google Scholar] [CrossRef]
- Phung, L.T.; Kitwetcharoen, H.; Chamnipa, N.; Boonchot, N.; Thanonkeo, S.; Tippayawat, P.; Klanrit, P.; Yamada, M.; Thanonkeo, P. Changes in the chemical compositions and biological properties of kombucha beverages made from black teas and pineapple peels and cores. Sci. Rep. 2023, 13, 7859. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Wong, L.F.; Lianto, F.S.; Wong, S.K.; Lim, K.K.; Joe, C.E.; Lim, T.Y. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008, 109, 477–483. [Google Scholar] [CrossRef]
- Mukherjee, S.; Pawar, N.; Kulkarni, O.; Nagarkar, B.; Thopte, S.; Bhujbal, A.; Pawar, P. Evaluation of free-radical quenching properties of standard Ayurvedic formulation Vayasthapana Rasayana. BMC Complement. Altern. Med. 2011, 11, 38. [Google Scholar] [CrossRef]
- Battikh, H.; Chaieb, K.; Bakhrouf, A.; Ammar, E. Antibacterial and antifungal activities of black and green kombucha teas. J. Food Biochem. 2013, 37, 231–236. [Google Scholar] [CrossRef]
- Osiripun, V.; Apisittiwong, T. Polyphenol and antioxidant activities of kombucha fermented from different teas and fruit juices. J. Curr. Sci. Technol. 2021, 11, 188–196. [Google Scholar]
- Nielsen, S.S. Total carbohydrate by phenol-sulfuric acid method. In Food Analysis Laboratory Manual; Food Science Text Series; Springer: Cham, Switzerland, 2017; pp. 137–141. [Google Scholar]
- Jakubczyk, K.; Kałduńska, J.; Kochman, J.; Janda, K. Chemical profile and antioxidant activity of the kombucha beverage derived from white, green, black and red tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Duarte-Almeida, J.M.; Novoa, A.V.; Linares, A.F.; Lajolo, F.M.; Genovese, M.I. Antioxidant activity of phenolics compounds from sugar cane (Saccharum officinarum L.) juice. Plant Foods Human Nutri. 2006, 61, 187–192. [Google Scholar] [CrossRef]
- Chen, G.L.; Zheng, F.J.; Lin, B.; Lao, S.B.; He, J.; Huang, Z.; Zeng, Y.; Sun, J.; Verma, K.K. Phenolic and volatile compounds in the production of sugarcane vinegar. ACS Omega 2020, 5, 30587–30595. [Google Scholar] [CrossRef]
- Rodrigues, N.P.; Brochier, B.; de Medeiros, J.K.; Marczak, L.D.F.; Mercali, G.D. Phenolic profile of sugarcane juice: Effects of harvest season and processing by ohmic heating and ultrasound. Food Chem. 2021, 347, 129058. [Google Scholar] [CrossRef]
- Eggleston, G. Positive aspects of cane sugar and sugar cane derived products in food and nutrition. J. Agric. Food Chem. 2018, 66, 4007–4012. [Google Scholar] [CrossRef]
- Arsiningtyas, I.S. Antioxidant profile of heartwood and sapwood of Caesalpinia sappan L. tree’s part grown in Imogiri nature preserve, Yogyakarta. IOP Conf. Ser. Earth Environ. Sci. 2021, 810, 012040. [Google Scholar] [CrossRef]
- Septiyani, R.; Wikandari, R.; Santoso, U.; Raharjo, S. Brazilin content, color stability, and antioxidant activity of sappan wood (Caesalpinia sappan L.) traditional drink by different blanching and drying methods. Trends Sci. 2024, 21, 8535. [Google Scholar] [CrossRef]
- Berenguer, M.; Vegara, S.; Barrajón, E.; Saura, D.; Valero, M.; Martí, N. Physicochemical characterization of pomegranate wines fermented with three different Saccharomyces cerevisiae yeast strains. Food Chem. 2016, 190, 848–855. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. Value-added tea (Camellia sinesis) as a functional food using the kombucha ‘tea fungus’. Chiang Mai J. Sci. 2018, 45, 136–146. [Google Scholar]
- Tan, M.; Caro, Y.; Shum-Cheong-Sing, A.; Robert, L.; François, J.M.; Petit, T. Evaluation of mixed-fermentation of Saccharomyces cerevisiae with Saprochaete suaveolens to produce natural fruity beer from industrial wort. Food Chem. 2021, 346, 128804. [Google Scholar] [CrossRef]
- Ezenekwe, C.; Ekwegbalu, E.; Orji-Udezuka, A.C.; Obi, C.P.; Ezemba, A.S.; Osuala, O.J.; Anakwenze, V.N.; Ezemba, C.C. Production and physicochemical evaluation of vinegar produced from pineapple and pawpaw fruits with their peels. Asian J. Microbiol. Biotechnol. 2021, 6, 1–10. [Google Scholar]
- Sakti, A.S.; Saputri, F.C.; Muním, A. Optimization of choline chloride-glycerol based natural deep eutectic solvent for extraction bioactive substances from Cinnamomum burmannii barks and Caesalpinia sappan heartwoods. Heliyon. 2019, 5, e02915. [Google Scholar] [CrossRef]
- Walker, G.M. Yeast Physiology and Biotechnology; John Wiley and Sons: Chichester, NY, USA, 1998. [Google Scholar]
- Kanchanarach, W.; Theeragool, G.; Yakushi, T.; Toyama, H.; Adachi, O.; Matsushita, K. Characterization of thermotolerant Acetobacter pasteurianus strains and their quinoprotein alcohol dehydrogenase. Appl. Microbiol. Biotechnol. 2010, 85, 741–751. [Google Scholar] [CrossRef]
- Nagaraju, S.; Thippeswamy, T.G.; Ravi Kumar, H. Evaluating the lipid profile and mineral composition of the seed oil of Caesalpinia sappan L. (Caesalpiniaceae). In Natural Product Experiments in Drug Discovery; Springer: New York, NY, USA, 2022; pp. 75–86. [Google Scholar]
- Blanco, C.A.; Andres-Iglesias, C.; Montero, O. Low-alcohol beers: Flavor compounds, defects, and improvement strategies. Crit. Rev. Food Sci. Nutr. 2016, 56, 1379–1388. [Google Scholar] [CrossRef]
- Brányik, T.; Silva, D.P.; Baszczyňski, M.; Lehnert, R.; Almeida e Silva, J.B. A review of methods of low alcohol and alcohol-free beer production. J. Food Eng. 2012, 108, 493–506. [Google Scholar] [CrossRef]
- Okaru, A.O.; Lachenmeier, D.W. Defining no and low (NoLo) alcohol products. Nutrients 2022, 14, 3873. [Google Scholar] [CrossRef]
- Han, D.; Ma, T.; Sun, S.; Zhang, Y.; Song, L. Brazilin Inhibits the Inflammatory Immune Response Induced by LPS in THP-1 Cells; Research Square Platform LLC: Durham, NC, USA, 2023. [Google Scholar]
- Ubeda, C.; Callejón, R.M.; Hidalgo, C.; Torija, M.J.; Mas, A.; Troncoso, A.M.; Morales, M.L. Determination of major volatile compounds during the production of fruit vinegars by static headspace gas chromatography–mass spectrometry method. Food Res. Int. 2011, 44, 259–268. [Google Scholar] [CrossRef]
- Su, M.S.; Chien, P.J. Aroma impact components of rabbit eye blueberry (Vaccinium ashei) vinegars. Food Chem. 2010, 119, 923–928. [Google Scholar] [CrossRef]
- Roda, A.; Lucini, L.; Torchio, F.; Dordoni, R.; De Faveri, D.M.; Lambri, M. Metabolite profiling and volatiles of pineapple wine and vinegar obtained from pineapple waste. Food Chem. 2017, 29, 734–742. [Google Scholar] [CrossRef]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- Malbasa, R.V.; Loncar, E.S.; Vitas, J.S.; Canadanovic-Brunet, J.M. Influence of starter cultures on the antioxidant activity of kombucha beverage. Food Chem. 2011, 127, 1727–1731. [Google Scholar] [CrossRef]
- Bhadania, M.; Joshi, H.; Patel, P.; Kulkarni, V.H. Protective effect of menthol on β-amyloid peptide induced cognitive deficits in mice. Eur. J. Pharmacol. 2012, 68, 50–54. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef]
- Marrelli, M.; Statti, G.A.; Tundis, R.; Menichini, F.; Conforti, F. Fatty acids, coumarins and polyphenolic compounds of Ficus carica L. cv. Dottato: Variation of bioactive compounds and biological activity of aerial parts. Nat. Prod. Res. 2014, 28, 271–274. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.; Liu, M.; Zhang, C.; Shao, J.; Hou, X.; Tian, J.; Cui, Q. A comprehensive review on phytochemistry, bioactivities, toxicity studies, and clinical studies on Ficus carica Linn. leaves. Biomed. Pharmacol. 2021, 137, 111393. [Google Scholar] [CrossRef]
- Park, S.Y.; Jin, M.L.; Kim, Y.H.; Kim, Y.; Lee, S.J. Anti-inflammatory effects of aromatic-turmerone through blocking of NF kappa B, JNK, and p38 MAPK signaling pathways in amyloid beta-stimulated microglia. Int. Immunopharmacol. 2012, 14, 13–20. [Google Scholar] [CrossRef]
- Li, Y.L.; Du, Z.Y.; Li, P.H.; Yan, L.; Zhou, W.; Tang, Y.D.; Liu, G.R.; Fang, Y.X.; Zhang, K.; Dong, C.Z.; et al. Aromatic-turmerone ameliorates imiquimod-induced psoriasis-like inflammation of BALB/c mice. Int. Immunopharmacol. 2018, 64, 319–325. [Google Scholar] [CrossRef]
- Takamoto, Y.; Kishi, C.; Ehira, H.; Matsui, N.; Yamaguchi, T.; Yoshioka, Y.; Matsumura, S.; Moriyama, T.; Zaima, N. Inhaled turmerones can be incorporated in the organs via pathways different from oral administration and can affect weight-gain of mice. Sci. Rep. 2022, 12, 11039. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological properties and prospects for the application of eugenol-A review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Leal, J.M.; Suárez, L.V.; Jayabalan, R.; Oros, J.H.; Escalante-Aburto, A. A review on health benefits of kombucha nutritional compounds and metabolites. CyTA J. Food 2018, 16, 390–399. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Y.; Xu, X.; Wang, M.; Kou, L. Determination of the total phenolic content in wine samples using potentiometric method based on permanganate ion as an indicator. Molecules 2019, 24, 3279. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Kitwetcharoen, H.; Phung, L.T.; Klanrit, P.; Thanonkeo, S.; Tippayawat, P.; Yamada, M.; Thanonkeo, P. Kombucha healthy drink—Recent advances in production, chemical composition and health benefits. Fermentation 2023, 9, 48. [Google Scholar] [CrossRef]
- Asfar, A.M.I.A.; Asfar, A.M.I.T. Polyphenol in sappan wood (Caesalpinia sappan L.) extract results of ultrasonic-assisted solvent extraction. AIP Conf. Proc. 2023, 2719, 030006. [Google Scholar]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Antioxidant activities, phenolic profiles, and organic acid contents of fruit vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef]
- Ozturk, I.; Caliskan, O.; Tornuk, F.; Ozcan, N.; Yalcin, H.; Baslar, M.; Sagdic, O. Antioxidant, antimicrobial, mineral, volatile, physicochemical and microbiological characteristics of traditional home-made Turkish vinegars. LWT Food Sci. Technol. 2015, 63, 144–151. [Google Scholar] [CrossRef]
- Hasan, A.N.; Antony, U.; Bala, G.V.; Prabha, D.T.; Kavya, C.; Malik, A.F. Study on production vinegar from apple. IOP Conf. Ser. Earth Environ. Sci. 2021, 761, 012125. [Google Scholar] [CrossRef]
- Yohana, W.; Wihardja, R.; Sufiawati, I.; Khaerunnisa, D.F. Decreasing respiratory rate, blood pressure and pulse after drinking secang in prehypertension patients. J. Adv. Med. Dental Sci. Res. 2019, 7, 34–36. [Google Scholar]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbial dynamics between yeasts and acetic acid bacteria in kombucha: Impacts on the chemical composition of the beverage. Foods 2020, 9, 963. [Google Scholar] [CrossRef]
- Osuala, O.J.; Ezemba, C.C.; Chude, C.O.; Ezemba, A.S.; Anaukwu, C. Antimicrobial analysis of traditional and industrial produced vinegar. Int. J. BioSci. Technol. 2021, 14, 28–43. [Google Scholar] [CrossRef]
- Srinivasan, R.; Karthik, S.; Mathivanan, K.; Baskaran, R.; Karthikeyan, M.; Gopi, M.; Govindasamy, C. In vitro antimicrobial activity of Caesalpinia sappan L. Asian Pac. J. Trop. Biomed. 2012, 2, S136–S139. [Google Scholar] [CrossRef]
- Garrido, A.; Atencio, L.A.; Bethancourt, R.; Bethancourt, A.; Guzmán, H.; Gutiérrez, M.; Durant-Archibold, A.A. Antibacterial activity of volatile compounds produced by the octocoral-associated bacteria Bacillus sp. BO53 and Pseudoalteromonas sp. GA327. Antibiotics 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Yagnik, D.; Serafin, V.; Shah, A.J. Antimicrobial activity of apple cider vinegar against Escherichia coli, Staphylococcus aureus and Candida albicans; Downregulating cytokine and microbial protein expression. Sci. Rep. 2018, 8, 1732. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, T.S.; Nour El-Deen, M.M.; Rokaya, M.E.; Sherif, M.M. Evaluation of the antibacterial effect of apple vinegar as a root canal irrigant using endovac irrigation system. Al-Azhar Dent. J. Girls 2019, 6, 53–59. [Google Scholar] [CrossRef][Green Version]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Lyoussi, B.; Oumokhtar, B.; Abdellaoui, A. Phytochemistry, antioxidant and antibacterial activities of two Moroccan Teucrium polium L. subspecies: Preventive approach against Nosocomial infections. Arab. J. Chem. 2020, 13, 3866–3874. [Google Scholar] [CrossRef]


| Sample | Part of Wood | TPC (mg GAE/g DW) | DPPH Radical Scavenging Activity (mg TE/g DW) | IC50 (ppm) | Reference |
|---|---|---|---|---|---|
| Stem | Heartwood | 44.69 ± 0.72 | 44.40 ± 0.75 | 140.00 | This study |
| Stem | Sapwood | 4.43 ± 0.08 | - | 8.50 | [37] |
| Heartwood | 8.25 ± 0.18 | - | 7.10 | ||
| Branch | Sapwood | 7.77 ± 0.06 | - | 24.40 | [37] |
| Heartwood | 4.87 ± 0.12 | - | 15.60 | ||
| Stem | Heartwood | 213.12 ± 0.87 | - | 98.99 | [38] |
| Treatments | Ethanol Content (% v/v) | Color 1 | ||
|---|---|---|---|---|
| Lightness | Green–Red Color | Blue–Yellow Color | ||
| C1 | 0.05 ± 0.00 d | 94.74 ± 1.39 a | −1.14 ± 0.03 a | 12.72 ± 0.79 d |
| C2 | 0.09 ± 0.01 c | 94.27 ± 1.86 a | −2.09 ± 0.33 b | 22.32 ± 0.08 cd |
| C3 | 0.15 ± 0.02 b | 94.10 ± 0.93 a | −3.00 ± 0.08 c | 34.76 ± 8.36 c |
| C4 | 0.10 ± 0.00 c | 92.88 ± 0.16 ab | −4.64 ± 0.17 d | 54.86 ± 0.79 b |
| C5 | 0.18 ± 0.01 a | 90.09 ± 0.93 b | −4.90 ± 0.59 d | 87.72 ± 7.22 a |
| Bacterial Strain | Acetic Acid 1 | dH2O 2 | Diameter of Inhibition Zone (mm) 3 | ||||
|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | |||
| Gram-negative bacteria | |||||||
| Escherichia coli | 26.4 ± 1.1 ab | 0 | 7.6 ± 0.7 a | 10.8 ± 0.4 a | 14.4 ± 0.8 a | 17.0 ± 1.0 a | 23.4 ± 1.1 a |
| Salmonella enterica | 22.6 ± 0.8 c | 0 | 6.6 ± 0.5 a | 8.7 ± 0.4 b | 10.7 ± 0.6 b | 13.6 ± 0.6 b | 16.3 ± 0.5 b |
| Enterobacter aerogenes | 23.3 ± 1.2 bc | 0 | 6.9 ± 0.4 a | 8.8 ± 0.8 b | 10.4 ± 1.2 b | 13.4 ± 0.7 b | 17.5 ± 1.1 b |
| Gram-positive bacteria | |||||||
| Bacillus cereus | 24.1 ± 1.8 bc | 0 | 8.4 ± 1.0 a | 10.9 ± 0.6 a | 14.5 ± 0.7 a | 18.3 ± 0.8 a | 22.3 ± 0.7 a |
| Staphylococcus aureus | 27.5 ± 0.9 a | 0 | 7.3 ± 0.7 a | 9.4 ± 0.9 ab | 14.4 ± 1.0 a | 18.2 ± 0.7 a | 23.3 ± 0.7 a |
| Enterococcus faecalis | 22.9 ± 0.8 c | 0 | 8.1 ± 0.6 a | 11.2 ± 0.9 a | 14.3 ± 0.9 a | 18.5 ± 0.7 a | 23.1 ± 0.8 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klanrit, P.; Kitwetcharoen, H.; Vichitphan, K.; Vichitphan, S.; Thanonkeo, S.; Yamada, M.; Thanonkeo, P. Enhancing the Bioactive Properties of Sugarcane Vinegar Through Caesalpinia sappan Extract Supplementation: A Novel Approach for Functional Beverage Development. Antioxidants 2025, 14, 590. https://doi.org/10.3390/antiox14050590
Klanrit P, Kitwetcharoen H, Vichitphan K, Vichitphan S, Thanonkeo S, Yamada M, Thanonkeo P. Enhancing the Bioactive Properties of Sugarcane Vinegar Through Caesalpinia sappan Extract Supplementation: A Novel Approach for Functional Beverage Development. Antioxidants. 2025; 14(5):590. https://doi.org/10.3390/antiox14050590
Chicago/Turabian StyleKlanrit, Preekamol, Haruthairat Kitwetcharoen, Kanit Vichitphan, Sukanda Vichitphan, Sudarat Thanonkeo, Mamoru Yamada, and Pornthap Thanonkeo. 2025. "Enhancing the Bioactive Properties of Sugarcane Vinegar Through Caesalpinia sappan Extract Supplementation: A Novel Approach for Functional Beverage Development" Antioxidants 14, no. 5: 590. https://doi.org/10.3390/antiox14050590
APA StyleKlanrit, P., Kitwetcharoen, H., Vichitphan, K., Vichitphan, S., Thanonkeo, S., Yamada, M., & Thanonkeo, P. (2025). Enhancing the Bioactive Properties of Sugarcane Vinegar Through Caesalpinia sappan Extract Supplementation: A Novel Approach for Functional Beverage Development. Antioxidants, 14(5), 590. https://doi.org/10.3390/antiox14050590







