Heme Oxygenase-1 Overexpression Promotes Uveal Melanoma Progression and Is Associated with Poor Clinical Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Pharmacological Treatments
2.2. Western Blot Analysis
2.3. Real-Time PCR for Gene Expression Analysis
2.4. Clonogenic Assay
2.5. Wound Healing Assay
2.6. Real-Time Cell Proliferation Assay
2.7. Immunofluorescence
2.8. Patients’ Cohort
2.9. Immunohistochemical Analysis
2.10. Statistical Analysis
3. Results
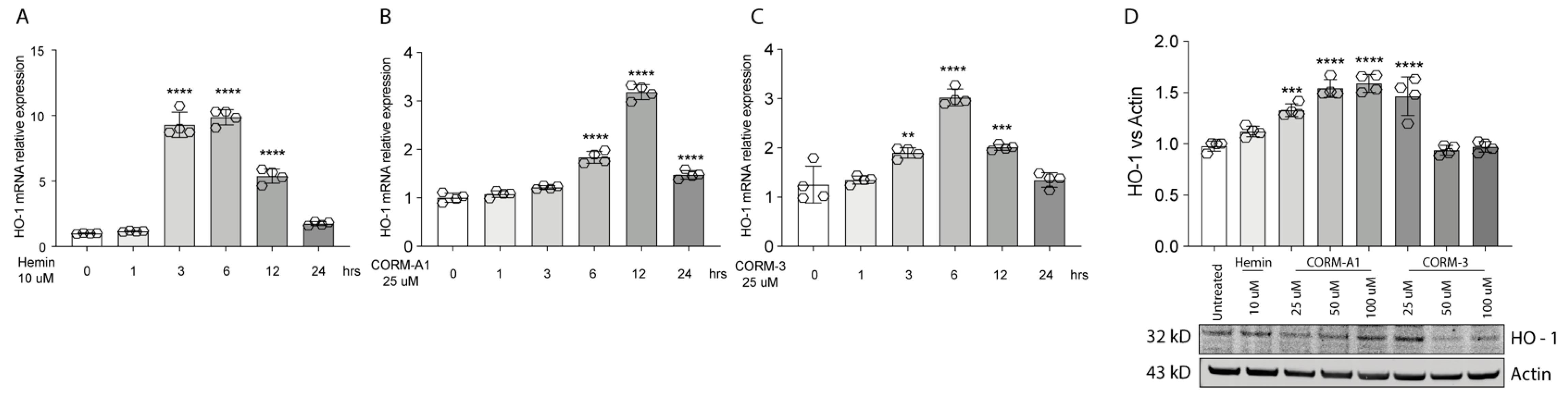
3.1. Hemin and CORMs Induce HO-1 in UM Cells
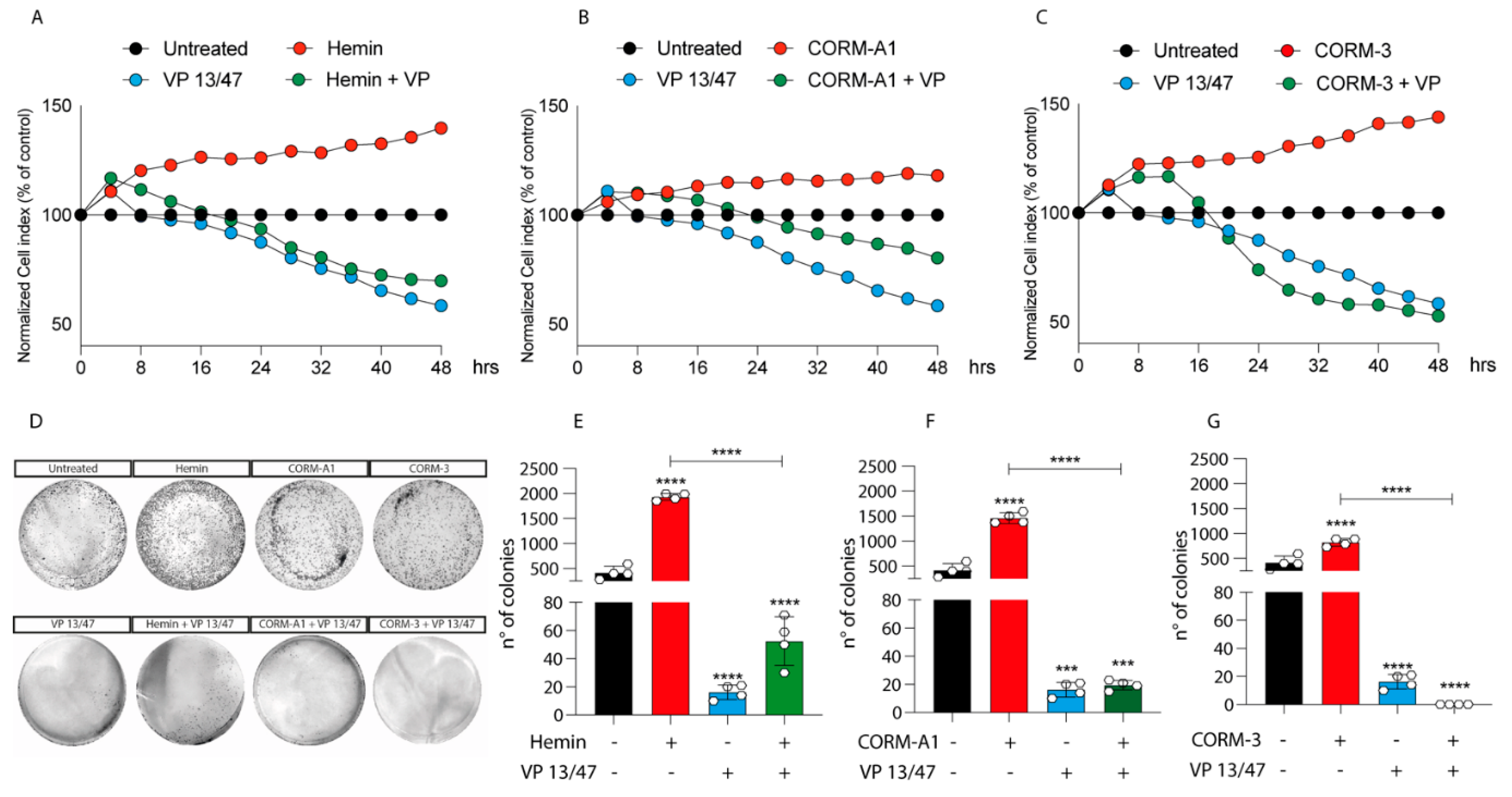
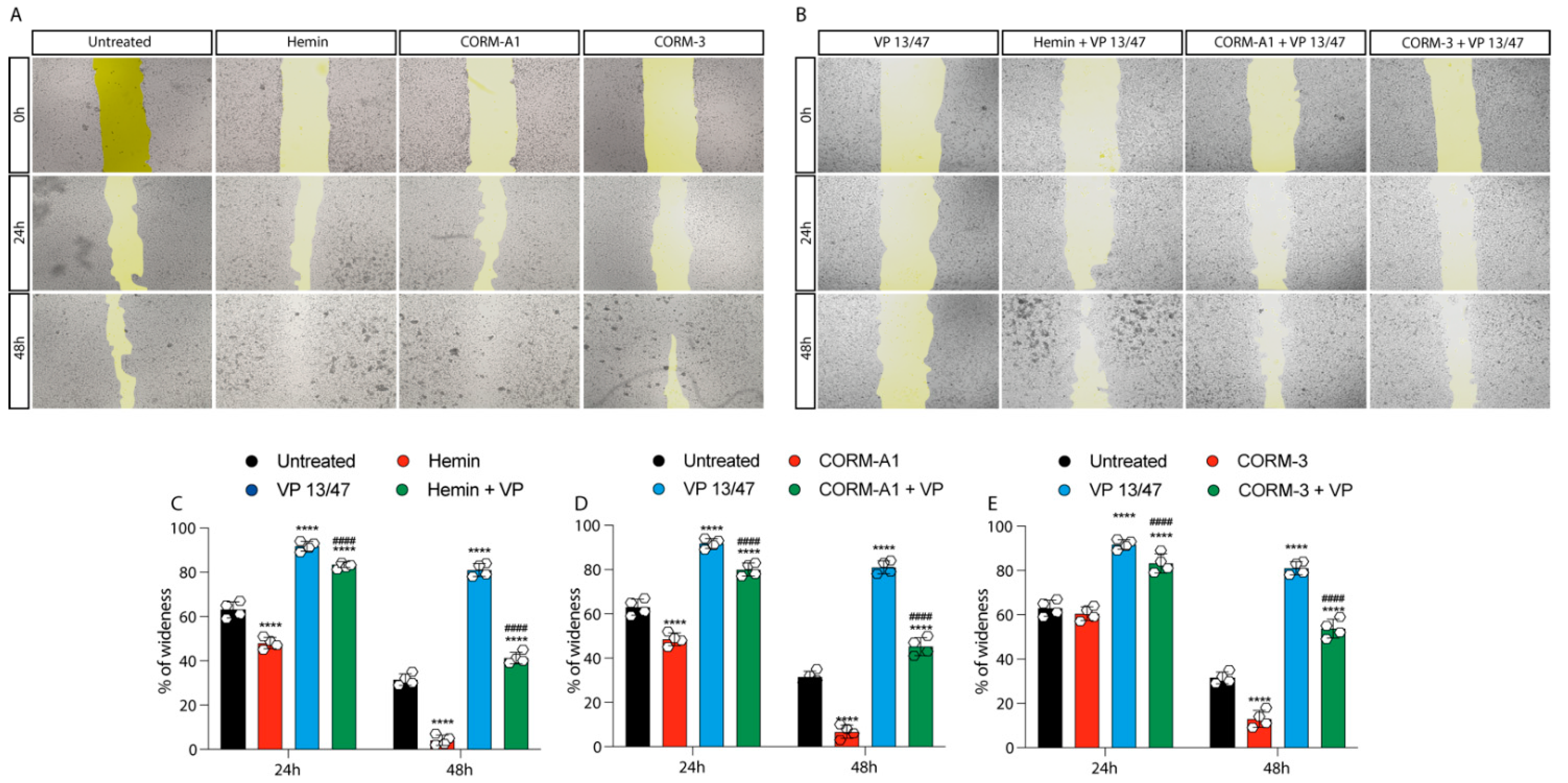
3.2. HO-1 Promotes In Vitro UM Proliferation
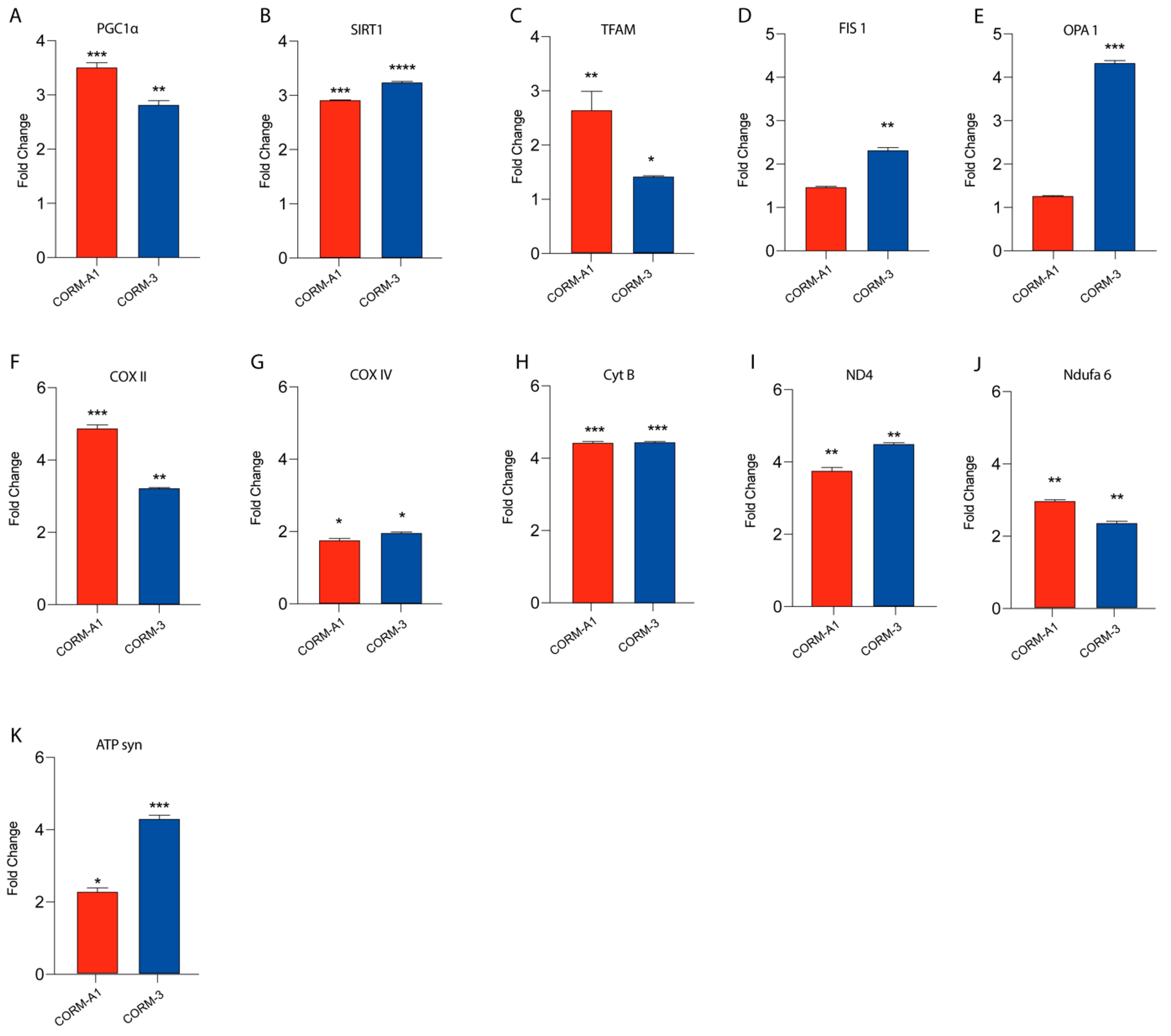
3.3. CORMs Enhance UM Mitochondrial Fitness
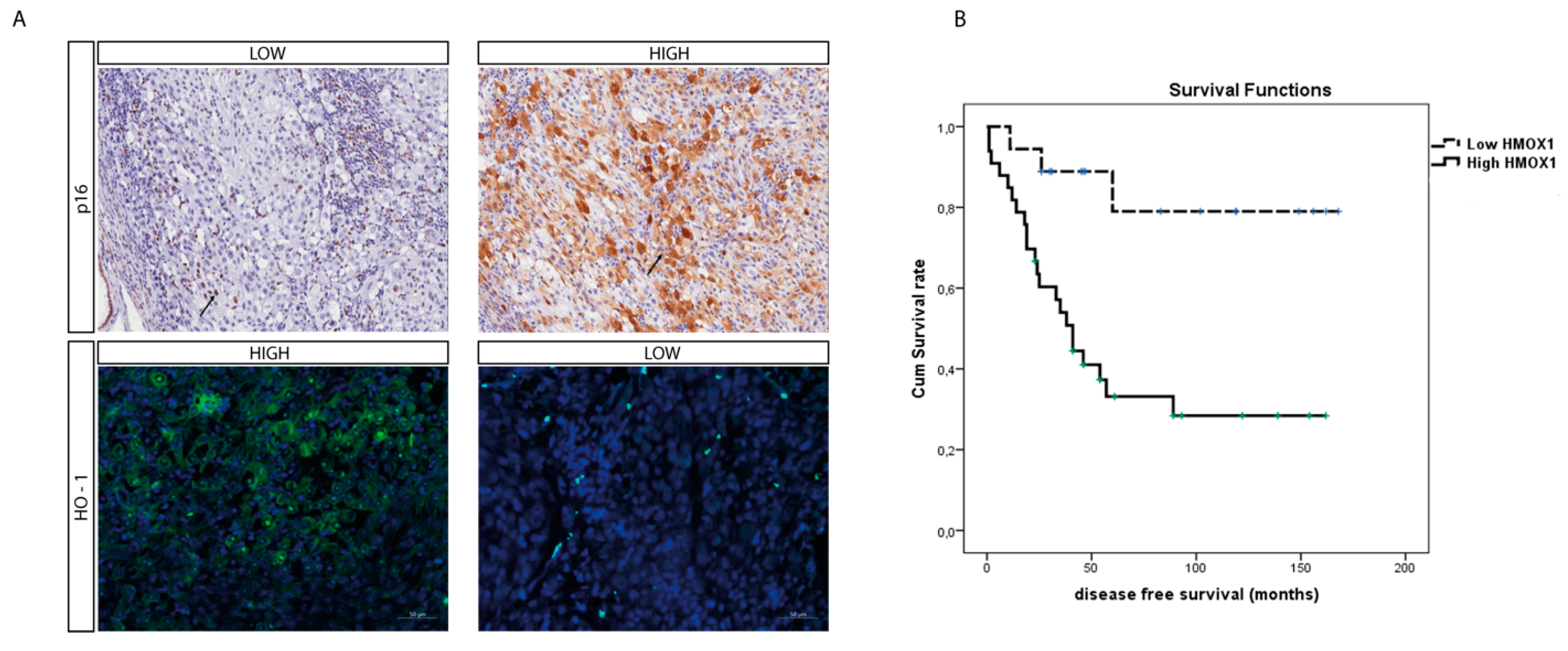
3.4. HO-1 Expression Is a Prognostic Marker of Mortality and Correlates to Disease Stage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grisanti, S.; Tura, A. Uveal melanoma. In Noncutaneous Melanoma; Scott, J.F., Gerstenblith, M.R., Eds.; Codon Publications: Brisbane, QLD, Australia, 2018. [Google Scholar]
- Rantala, E.S.; Hernberg, M.; Kivela, T.T. Overall survival after treatment for metastatic uveal melanoma: A systematic review and meta-analysis. Melanoma Res. 2019, 29, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Tibullo, D.; Barbagallo, I.; Giallongo, C.; La Cava, P.; Parrinello, N.; Vanella, L.; Stagno, F.; Palumbo, G.A.; Li Volti, G.; Di Raimondo, F. Nuclear translocation of heme oxygenase-1 confers resistance to imatinib in chronic myeloid leukemia cells. Curr. Pharm. Des. 2013, 19, 2765–2770. [Google Scholar] [CrossRef]
- Spampinato, M.; Sferrazzo, G.; Pittala, V.; Di Rosa, M.; Vanella, L.; Salerno, L.; Sorrenti, V.; Carota, G.; Parrinello, N.; Raffaele, M.; et al. Non-competitive heme oxygenase-1 activity inhibitor reduces non-small cell lung cancer glutathione content and regulates cell proliferation. Mol. Biol. Rep. 2020, 47, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Tibullo, D.; Barbagallo, I.; Giallongo, C.; Vanella, L.; Conticello, C.; Romano, A.; Saccone, S.; Godos, J.; Di Raimondo, F.; Li Volti, G. Heme oxygenase-1 nuclear translocation regulates bortezomibinduced cytotoxicity and mediates genomic instability in myeloma cells. Oncotarget 2016, 7, 28868–28880. [Google Scholar] [CrossRef] [PubMed]
- Castruccio Castracani, C.; Longhitano, L.; Distefano, A.; Di Rosa, M.; Pittala, V.; Lupo, G.; Caruso, M.; Corona, D.; Tibullo, D.; Li Volti, G. Heme Oxygenase-1 and Carbon Monoxide Regulate Growth and Progression in Glioblastoma Cells. Mol. Neurobiol. 2020, 57, 2436–2446. [Google Scholar] [CrossRef]
- Lusted, L.B. Signal detectability and medical decision-making. Science 1971, 171, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, X.; Nisar, M.F.; Lin, M.; Zhong, J.L. Heme Oxygenases: Cellular Multifunctional and Protective Molecules against UV-Induced Oxidative Stress. Oxid. Med. Cell. Longev. 2019, 2019, 5416728. [Google Scholar] [CrossRef]
- Goswami, B.; Rajappa, M.; Sharma, M.; Sharma, A. Inflammation: Its role and interplay in the development of cancer, with special focus on gynecological malignancies. Int. J. Gynecol. Cancer 2008, 18, 591–599. [Google Scholar] [CrossRef]
- Abe, M. Guest editorial: Understanding the pathogenesis and the evolving treatment paradigm for multiple myeloma in the era of novel agents. Int. J. Hematol. 2011, 94, 307–309. [Google Scholar] [CrossRef][Green Version]
- Meister, S.; Schubert, U.; Neubert, K.; Herrmann, K.; Burger, R.; Gramatzki, M.; Hahn, S.; Schreiber, S.; Wilhelm, S.; Herrmann, M.; et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007, 67, 1783–1792. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Ara, G.; Herbst, R.; Palombella, V.J.; Adams, J. The proteasome inhibitor PS-341 in cancer therapy. Clin. Cancer Res. 1999, 5, 2638–2645. [Google Scholar] [PubMed]
- Richardson, P.G.; Baz, R.; Wang, M.; Jakubowiak, A.J.; Laubach, J.P.; Harvey, R.D.; Talpaz, M.; Berg, D.; Liu, G.; Yu, J.; et al. Phase 1 study of twice-weekly ixazomib, an oral proteasome inhibitor, in relapsed/refractory multiple myeloma patients. Blood 2014, 124, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Salerno, L.; Pittala, V.; Romeo, G.; Modica, M.N.; Siracusa, M.A.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Tibullo, D.; Sorrenti, V. Evaluation of novel aryloxyalkyl derivatives of imidazole and 1,2,4-triazole as heme oxygenase-1 (HO-1) inhibitors and their antitumor properties. Bioorg. Med. Chem. 2013, 21, 5145–5153. [Google Scholar] [CrossRef]
- Was, H.; Cichon, T.; Smolarczyk, R.; Lackowska, B.; Mazur-Bialy, A.; Mazur, M.; Szade, A.; Dominik, P.; Mazan, M.; Kotlinowski, J.; et al. Effect of Heme Oxygenase-1 on Melanoma Development in Mice-Role of Tumor-Infiltrating Immune Cells. Antioxidants 2020, 9, 1223. [Google Scholar] [CrossRef]
- Vanella, L.; Barbagallo, I.; Tibullo, D.; Forte, S.; Zappala, A.; Li Volti, G. The non-canonical functions of the heme oxygenases. Oncotarget 2016, 7, 69075–69086. [Google Scholar] [CrossRef]
- van der Kooij, M.K.; Speetjens, F.M.; van der Burg, S.H.; Kapiteijn, E. Uveal Versus Cutaneous Melanoma; Same Origin, Very Distinct Tumor Types. Cancers 2019, 11, 845. [Google Scholar] [CrossRef] [PubMed]
- Giallongo, S.; Di Rosa, M.; Caltabiano, R.; Longhitano, L.; Reibaldi, M.; Distefano, A.; Lo Re, O.; Amorini, A.M.; Puzzo, L.; Salvatorelli, L.; et al. Loss of macroH2A1 decreases mitochondrial metabolism and reduces the aggressiveness of uveal melanoma cells. Aging 2020, 12, 9745–9760. [Google Scholar] [CrossRef] [PubMed]
- Longhitano, L.; Tibullo, D.; Vicario, N.; Giallongo, C.; La Spina, E.; Romano, A.; Lombardo, S.; Moretti, M.; Masia, F.; Coda, A.R.D.; et al. IGFBP-6/sonic hedgehog/TLR4 signalling axis drives bone marrow fibrotic transformation in primary myelofibrosis. Aging 2021, 13, 25055–25071. [Google Scholar] [CrossRef]
- Broggi, G.; Falzone, L.; Fallico, M.; Russo, A.; Reibaldi, M.; Longo, A.; Avitabile, T.; De Pasquale, R.; Puzzo, L.; Foti, P.V.; et al. Prognostic Value of the Immunohistochemical Expression of Serine and Arginine-Rich Splicing Factor 1 (SRSF1) in Uveal Melanoma: A Clinico-Pathological and Immunohistochemical Study on a Series of 85 Cases. Appl. Sci. 2021, 11, 7874. [Google Scholar] [CrossRef]
- Piombino, E.; Broggi, G.; Barbareschi, M.; Castorina, S.; Parenti, R.; Bartoloni, G.; Salvatorelli, L.; Magro, G. Wilms’ Tumor 1 (WT1): A Novel Immunomarker of Dermatofibrosarcoma Protuberans-An Immunohistochemical Study on a Series of 114 Cases of Bland-Looking Mesenchymal Spindle Cell Lesions of the Dermis/Subcutaneous Tissues. Cancers 2021, 13, 252. [Google Scholar] [CrossRef]
- Longhitano, L.; Castracani, C.C.; Tibullo, D.; Avola, R.; Viola, M.; Russo, G.; Prezzavento, O.; Marrazzo, A.; Amata, E.; Reibaldi, M.; et al. Sigma-1 and Sigma-2 receptor ligands induce apoptosis and autophagy but have opposite effect on cell proliferation in uveal melanoma. Oncotarget 2017, 8, 91099–91111. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Yang, C.C.; Hsiao, L.D.; Chen, S.Y.; Yang, C.M. Heme Oxygenase-1 Induction by Carbon Monoxide Releasing Molecule-3 Suppresses Interleukin-1beta-Mediated Neuroinflammation. Front. Mol. Neurosci. 2017, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.S.; Soares, N.L.; Vieira, M.; Gramsbergen, J.B.; Vieira, H.L. Carbon Monoxide Releasing Molecule-A1 (CORM-A1) Improves Neurogenesis: Increase of Neuronal Differentiation Yield by Preventing Cell Death. PLoS ONE 2016, 11, e0154781. [Google Scholar] [CrossRef] [PubMed]
- Amata, E.; Marrazzo, A.; Dichiara, M.; Modica, M.N.; Salerno, L.; Prezzavento, O.; Nastasi, G.; Rescifina, A.; Romeo, G.; Pittala, V. Heme Oxygenase Database (HemeOxDB) and QSAR Analysis of Isoform 1 Inhibitors. ChemMedChem 2017, 12, 1873–1881. [Google Scholar] [CrossRef]
- Goldrick, C.; Palanga, L.; Tang, B.; Mealy, G.; Crown, J.; Horgan, N.; Kennedy, S.; Walsh, N. Hindsight: Review of Preclinical Disease Models for the Development of New Treatments for Uveal Melanoma. J. Cancer 2021, 12, 4672–4685. [Google Scholar] [CrossRef]
- Tan, Q.; Qin, Q.; Huang, Z.; Lian, B.; Mo, Q.; Wei, C. Predictive and prognostic effect of HO-1 expression in breast cancer patients undergoing neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2022, 193, 393–403. [Google Scholar] [CrossRef]
- Sacerdoti, D.; Colombrita, C.; Ghattas, M.H.; Ismaeil, E.F.; Scapagnini, G.; Bolognesi, M.; Li Volti, G.; Abraham, N.G. Heme oxygenase-1 transduction in endothelial cells causes downregulation of monocyte chemoattractant protein-1 and of genes involved in inflammation and growth. Cell. Mol. Biol. 2005, 51, 363–370. [Google Scholar]
- Araujo, J.A.; Zhang, M.; Yin, F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharmacol. 2012, 3, 119. [Google Scholar] [CrossRef]
- Ientile, R.; Campisi, A.; Raciti, G.; Caccamo, D.; Curro, M.; Cannavo, G.; Li Volti, G.; Macaione, S.; Vanella, A. Cystamine inhibits transglutaminase and caspase-3 cleavage in glutamate-exposed astroglial cells. J. Neurosci. Res. 2003, 74, 52–59. [Google Scholar] [CrossRef]
- Salomone, F.; Li Volti, G.; Vitaglione, P.; Morisco, F.; Fogliano, V.; Zappala, A.; Palmigiano, A.; Garozzo, D.; Caporaso, N.; D’Argenio, G.; et al. Coffee enhances the expression of chaperones and antioxidant proteins in rats with nonalcoholic fatty liver disease. Transl. Res. 2014, 163, 593–602. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Y.; Bian, C.; Nisar, M.F.; Wang, M.; Hu, X.; Diao, Q.; Nian, W.; Wang, E.; Xu, W.; et al. Heme oxygenase 1 facilitates cell proliferation via the B-Raf-ERK signaling pathway in melanoma. Cell Commun. Signal. 2019, 17, 3. [Google Scholar] [CrossRef]
- Sorrenti, V.; Mazza, F.; Campisi, A.; Vanella, L.; Li Volti, G.; Di Giacomo, C. High glucose-mediated imbalance of nitric oxide synthase and dimethylarginine dimethylaminohydrolase expression in endothelial cells. Curr. Neurovasc. Res. 2006, 3, 49–54. [Google Scholar] [CrossRef]
- Kushida, T.; LiVolti, G.; Goodman, A.I.; Abraham, N.G. TNF-alpha-mediated cell death is attenuated by retrovirus delivery of human heme oxygenase-1 gene into human microvessel endothelial cells. Transplant. Proc. 2002, 34, 2973–2978. [Google Scholar] [CrossRef]
- Jasmer, K.J.; Hou, J.; Mannino, P.; Cheng, J.; Hannink, M. Heme oxygenase promotes B-Raf-dependent melanosphere formation. Pigment. Cell Melanoma Res. 2020, 33, 850–868. [Google Scholar] [CrossRef] [PubMed]
- Nitti, M.; Piras, S.; Marinari, U.M.; Moretta, L.; Pronzato, M.A.; Furfaro, A.L. HO-1 Induction in Cancer Progression: A Matter of Cell Adaptation. Antioxidants 2017, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, R.; Lanteri, R.; Li Destri, G.; Caltabiano, R.; Vanella, L.; Lanzafame, S.; Di Cataldo, A.; Li Volti, G.; Di Giacomo, C. Beneficial effects of rutin and L-arginine coadministration in a rat model of liver ischemia-reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G664–G670. [Google Scholar] [CrossRef]
- Huang, S.M.; Lin, C.; Lin, H.Y.; Chiu, C.M.; Fang, C.W.; Liao, K.F.; Chen, D.R.; Yeh, W.L. Brain-derived neurotrophic factor regulates cell motility in human colon cancer. Endocr. Relat. Cancer 2015, 22, 455–464. [Google Scholar] [CrossRef]
- Chang, Y.J.; Chen, W.Y.; Huang, C.Y.; Liu, H.H.; Wei, P.L. Glucose-regulated protein 78 (GRP78) regulates colon cancer metastasis through EMT biomarkers and the NRF-2/HO-1 pathway. Tumour Biol. 2015, 36, 1859–1869. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Ma, C. A Novel Ferroptosis-Associated Gene Signature to Predict Prognosis in Patients with Uveal Melanoma. Diagnostics 2021, 11, 219. [Google Scholar] [CrossRef]
- Torrisi, F.; Alberghina, C.; D’Aprile, S.; Pavone, A.M.; Longhitano, L.; Giallongo, S.; Tibullo, D.; Di Rosa, M.; Zappala, A.; Cammarata, F.P.; et al. The Hallmarks of Glioblastoma: Heterogeneity, Intercellular Crosstalk and Molecular Signature of Invasiveness and Progression. Biomedicines 2022, 10, 806. [Google Scholar] [CrossRef] [PubMed]
| Primer | Forward (5’→3’) | Reverse (5’→3’) | Accession Number |
|---|---|---|---|
| HMOX1 | AAGACTGCGTTCCTGCTCAA | GGGCAGAATCTTGCACTTTGT | NM_002133.3 |
| PGC1alpha | ATGAAGGGTACTTTTCTGCCCC | GGTCTTCACCAACCAGAGCA | NM_001330751.2 |
| SIRT1 | AGGCCACGGATAGGTCCATA | GTGGAGGTATTGTTTCCGGC | NM_012238.5 |
| TFAM | CCGAGGTGGTTTTCATCTGT | AGTCTTCAGCTTTTCCTGCG | NM_003201.3 |
| FIS1 | CTGCTCCCCTGAGATTCGTC | AGCCACAGCCCCGTTTTATT | NM_016068.3 |
| OPA1 | CTGTGGCCTGGATAGCAGAA | GCGAGGCTGGTAGCCATATT | NM_001354663.2 |
| COX IV | CAGCTCTCGGAAGCGTTGTA | GATAACGAGCGCGGTGAAAC | NM_001318802.2 |
| CyT B | TCTTGCACGAAACGGGATCA | TGATTGGCTTAGTGGGCGAA | MZ475297.1 |
| ND4 | ACAAGCTCCATCTGCCTACGACAA | TTATGAGAATGACTGCGCCGGTGA | MZ475297.1 |
| Ndufa 6 | GATGCTTTGGCAAGATGGCG | GTCCCGACTGAAAATGGGCT | NM_002490.6 |
| ATP synthase | CCGCCTTCCGCGGTATAATC | ATGTACGCGGGCAATACCAT | NM_001001937.2 |
| Beta Actin | CCTTTGCCGATCCGCCG | AACATGATCTGGGTCATCTTCTCGC | NM_001101.5 |
| Sex | Age (yrs) | Location | Thickness (mm) | Largest Diameter (mm) | Cell Type | Extrascleral Extension | Pathological T Stage | DFS (Months) | Follow-Up (Months) | HO-1 | p16 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IS | ES | IRS (High ≥ 6; Low < 6) | IS | ES | IRS (High ≥ 6; Low < 6) | ||||||||||||
| f | 29 | ch | 14.20 | 16.20 | mixed | N | pT2a | 168 | 168 | 1 | 1 | 1 | L | 2 | 4 | 8 | H |
| f | 30 | ch/cb | 12.05 | 9.20 | spindle | N | pT2a | 162 | 162 | 1 | 1 | 1 | L | 3 | 3 | 9 | H |
| f | 55 | ch | 9.80 | 13.90 | spindle | N | pT2a | 162 | 162 | 2 | 3 | 6 | H | 2 | 2 | 4 | L |
| m | 68 | ch | 12.80 | 20.10 | mixed | N | pT2b | 156 | 156 | 2 | 1 | 2 | L | 2 | 3 | 6 | H |
| m | 64 | ch | 7.70 | 11.50 | epit | N | pT2a | 154 | 154 | 2 | 2 | 4 | H | 1 | 3 | 3 | L |
| f | 36 | ch | 5.81 | 12.70 | spindle | N | pT2b | 149 | 149 | 0 | 0 | 0 | L | 2 | 2 | 4 | L |
| m | 58 | ch | 13.10 | 14.30 | mixed | N | pT2a | 139 | 139 | 2 | 4 | 8 | H | 2 | 2 | 4 | L |
| m | 63 | ch | 3.30 | 11.70 | spindle | N | pT2a | 122 | 122 | 2 | 2 | 4 | H | 2 | 1 | 2 | L |
| m | 74 | ch/cb | 10.04 | 16.10 | spindle | N | pT2b | 119 (†) | 119 (†) | 0 | 0 | 0 | L | 3 | 3 | 9 | H |
| m | 62 | ch | 6.32 | 10.00 | epit | N | pT2b | 119 | 119 | 2 | 1 | 2 | L | 2 | 4 | 8 | H |
| m | 73 | ch | 9.70 | 11.30 | mixed | N | pT1a | 102 (†) | 102 (†) | 1 | 1 | 1 | L | 2 | 2 | 4 | L |
| m | 80 | ch | 9.24 | 17.70 | epit | N | pT2a | 93 (†) | 93 (†) | 2 | 3 | 6 | H | 2 | 2 | 4 | L |
| f | 78 | ch | 8.07 | 10.70 | mixed | N | pT2a | 89 | 89 | 2 | 3 | 6 | H | 1 | 2 | 2 | L |
| m | 81 | ch | 7.38 | 9.86 | mixed | N | pT2a | 83 (†) | 83 (†) | 2 | 1 | 2 | L | 2 | 1 | 2 | L |
| f | 57 | ch | 10.00 | 11.24 | mixed | N | pT2a | 61 | 61 | 3 | 3 | 9 | H | 3 | 3 | 9 | H |
| m | 66 | ch/cb | 13.50 | 17.50 | epit | N | pT3a | 54 | 54 | 2 | 2 | 4 | H | 2 | 2 | 4 | L |
| m | 74 | ch | 8.02 | 14.61 | epit | N | pT3b | 47 | 47 | 1 | 1 | 1 | L | 3 | 4 | 12 | H |
| f | 52 | ch | 4.96 | 9.92 | spindle | N | pT3a | 46 | 46 | 1 | 1 | 1 | L | 3 | 2 | 6 | H |
| m | 79 | ch | 12.28 | 14.95 | mixed | N | pT2a | 46 | 46 | 2 | 3 | 6 | H | 3 | 3 | 9 | H |
| m | 67 | ch | 7.8 | 11.3 | spindle | N | pT2a | 45 | 45 | 2 | 1 | 2 | L | 2 | 3 | 6 | H |
| m | 64 | ch | 9.30 | 15.17 | mixed | N | pT2b | 41 | 41 | 2 | 2 | 4 | H | 3 | 3 | 9 | H |
| m | 19 | ch | 9.77 | 14.76 | mixed | N | pT3b | 31 | 31 | 2 | 1 | 2 | L | 3 | 3 | 9 | H |
| m | 73 | ch | 15.89 | 18.00 | mixed | N | pT2a | 30 | 30 | 1 | 1 | 1 | L | 2 | 3 | 6 | H |
| f | 81 | ch/cb | 8.12 | 11.37 | mixed | N | pT3a | 26 | 26 | 2 | 1 | 2 | L | 2 | 4 | 8 | H |
| f | 80 | ch | 14.61 | 14.30 | epit | N | pT2b | 26 | 26 | 2 | 3 | 6 | H | 2 | 2 | 4 | L |
| f | 69 | ch | 8.73 | 11.38 | mixed | N | pT2a | 23 | 23 | 2 | 1 | 2 | L | 3 | 3 | 9 | H |
| Sex | Age (yrs) | Location | Thickness (mm) | Largest Diameter (mm) | Cell Type | Extrascleral Extension | Pathological T Stage | DFS (Months) | Follow-Up (Months) | HO-1 | p16 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IS | ES | IRS (High ≥ 6; Low < 6) | IS | ES | IRS (High ≥ 6; Low < 6) | ||||||||||||
| m | 76 | ch | 13.7 | 17.1 | mixed | N | pT2a | 14 | 119 | 2 | 1 | 2 | L | 3 | 3 | 9 | H |
| f | 50 | ch | 7.36 | 15.6 | epit | N | pT2a | 41 | 111 | 2 | 3 | 6 | H | 2 | 2 | 4 | L |
| m | 48 | ch/cb | 15.34 | 14.3 | mixed | N | pT4b | 41 | 105 (†) | 2 | 4 | 8 | H | 2 | 2 | 4 | L |
| f | 57 | ch/cb | 13.6 | 19 | epit | N | pT2b | 6 | 96 | 2 | 2 | 4 | H | 2 | 4 | 8 | H |
| f | 51 | ch | 9.42 | 19.03 | mixed | N | pT3a | 25 | 96 | 3 | 3 | 9 | H | 1 | 2 | 2 | L |
| f | 84 | ch | 11.7 | 17.4 | mixed | N | pT2b | 89 | 91 (†) | 2 | 4 | 8 | H | 2 | 1 | 2 | L |
| f | 74 | ch | 11.35 | 18.5 | mixed | N | pT4a | 19 | 87 | 3 | 3 | 9 | H | 2 | 2 | 4 | L |
| f | 66 | ch/cb | 8.95 | 15.4 | mixed | N | pT2b | 12 | 84 | 3 | 4 | 12 | H | 2 | 2 | 4 | L |
| m | 69 | ch | 7.21 | 15.8 | mixed | N | pT2a | 54 | 81 (†) | 3 | 3 | 9 | H | 3 | 1 | 3 | L |
| m | 71 | ch | 11.69 | 16.63 | mixed | N | pT3a | 57 | 81 (†) | 2 | 4 | 8 | H | 2 | 2 | 4 | L |
| f | 54 | ch/cb | 9.76 | 11.5 | mixed | N | pT2b | 46 | 74 (†) | 3 | 3 | 9 | H | 2 | 3 | 6 | H |
| f | 64 | ch/cb | 3 | 6 | epit | N | pT1b | 60 | 73 (†) | 2 | 2 | 4 | H | 2 | 2 | 4 | L |
| m | 73 | ch | 9.6 | 15 | epit | N | pT3a | 10 | 60 | 2 | 4 | 8 | H | 2 | 2 | 4 | L |
| f | 74 | ch/cb | 15.93 | 19.7 | mixed | N | pT2a | 35 | 53 (†) | 1 | 3 | 3 | L | 1 | 2 | 2 | L |
| m | 62 | ch | 13.68 | 14.7 | mixed | N | pT3a | 38 | 51 (†) | 2 | 4 | 8 | H | 3 | 2 | 6 | H |
| f | 74 | ch | 5.7 | 12.1 | spindle | N | pT2a | 24 | 50 (†) | 2 | 4 | 8 | H | 1 | 2 | 2 | L |
| f | 85 | ch/cb | 7.3 | 12.5 | epit | Y | pT3d | 26 | 49 (†) | 2 | 4 | 8 | H | 2 | 2 | 4 | L |
| m | 71 | ch | 13.14 | 17.1 | epit | N | pT3a | 33 | 46 (†) | 2 | 4 | 8 | H | 2 | 2 | 4 | L |
| f | 76 | ch | 10.81 | 12.52 | mixed | N | pT3a | 23 | 42 | 1 | 1 | 1 | L | 3 | 4 | 12 | H |
| m | 70 | ch | 9.87 | 11.16 | epit | N | pT3a | 19 | 37 (†) | 2 | 4 | 8 | H | 1 | 3 | 3 | L |
| f | 60 | ch | 8.25 | 16.5 | epit | N | pT2a | 11 | 25 (†) | 2 | 2 | 4 | H | 2 | 3 | 6 | H |
| m | 81 | ch | 13.9 | 12 | mixed | N | pT3a | 18 | 19 (†) | 2 | 4 | 8 | H | 2 | 2 | 4 | L |
| f | 48 | ch | 10.71 | 12.86 | epit | N | pT3a | 1 | 10 (†) | 2 | 1 | 2 | L | 3 | 3 | 6 | H |
| m | 78 | ch | 16.58 | 16.59 | epit | N | pT4a | 2 | 3 (†) | 2 | 4 | 8 | H | 2 | 4 | 8 | H |
| m | 72 | ch/cb | 13.3 | 15.4 | mixed | N | pT3b | 1 | 1 (†) | 2 | 4 | 8 | H | 2 | 1 | 2 | L |
| 3 | 3 | 9 | H | ||||||||||||||
| Sex m-f | Age (yrs) | Location | Thickness | Largest diameter | Cell Type | Extrascleral Extension | Pathological T Stage | DFS (months) | Follow-Up (months) | HO-1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 51) | 27–24 | 69 (19–85) | ch 39 ch/cb 12 | 9.8 (5.7–14.2) | 14.6 (9.2–20.1) | Epith: 16 Spindle: 8 Mixed: 27 | No: 50 Yes: 1 | pT1a: 1 pT1b: 1 pT2a: 21 pT2b: 10 pT3a: 11 pT3b: 2 pT3d: 1 pT4a: 2 pT4b: 2 | 41 (1–168) | 73 (1–168) | 4 (0–12) |
| Metastasis free (n = 26) | 16–10 | 67 (19–81) | ch 22 ch/cb 4 | 9.5 (3.3–15.9) | 13.3 (9.2–20.1) | Epith: 6 Spindle: 7 Mixed:13 | No: 26 | pT1a: 1 pT2a: 15 pT2b: 6 pT3a: 2 pT3b: 2 | 86 (23–168) | 86 4 death (23–168) | 2 (0–9) |
| Metastasis (n = 25) | 11–14 | 71 (48–85) | ch 17 ch/cb 8 | 10.8 (3–16.9) | 15.4 (6.0–19.7) | Epith: 10 Spindle: 1 Mixed: 14 | No: 24 Yes: 1 | pT1b: 1 pT2a: 6 pT2b: 4 pT3a: 9 pT3b: 1 pT3d: 1 pt4a: 2 pT4b: 1 | 24 (1–63) | 60 17 death (1–119) | 8 (1–12) |
| p (metastasis free vs. metastasis) | 0.820 * | 0.506 | 0.392 * | 0.102 * | 0.859 * | 0.490 | 0.064 * | 0.001 * | 0.0973 * | <0.001* |
| Metastasis (n = 25) | Metastasis free (n = 26) | |
|---|---|---|
| Low | 4 (16.0%) * | 14 (53.8%) |
| High | 21 (84.0%) | 11 (42.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longhitano, L.; Broggi, G.; Giallongo, S.; Failla, M.; Puzzo, L.; Avitabile, T.; Tibullo, D.; Distefano, A.; Pittalà, V.; Reibaldi, M.; et al. Heme Oxygenase-1 Overexpression Promotes Uveal Melanoma Progression and Is Associated with Poor Clinical Outcomes. Antioxidants 2022, 11, 1997. https://doi.org/10.3390/antiox11101997
Longhitano L, Broggi G, Giallongo S, Failla M, Puzzo L, Avitabile T, Tibullo D, Distefano A, Pittalà V, Reibaldi M, et al. Heme Oxygenase-1 Overexpression Promotes Uveal Melanoma Progression and Is Associated with Poor Clinical Outcomes. Antioxidants. 2022; 11(10):1997. https://doi.org/10.3390/antiox11101997
Chicago/Turabian StyleLonghitano, Lucia, Giuseppe Broggi, Sebastiano Giallongo, Maria Failla, Lidia Puzzo, Teresio Avitabile, Daniele Tibullo, Alfio Distefano, Valeria Pittalà, Michele Reibaldi, and et al. 2022. "Heme Oxygenase-1 Overexpression Promotes Uveal Melanoma Progression and Is Associated with Poor Clinical Outcomes" Antioxidants 11, no. 10: 1997. https://doi.org/10.3390/antiox11101997
APA StyleLonghitano, L., Broggi, G., Giallongo, S., Failla, M., Puzzo, L., Avitabile, T., Tibullo, D., Distefano, A., Pittalà, V., Reibaldi, M., Zanghì, G. N., Longo, A., Russo, A., Caltabiano, R., Volti, G. L., & Musso, N. (2022). Heme Oxygenase-1 Overexpression Promotes Uveal Melanoma Progression and Is Associated with Poor Clinical Outcomes. Antioxidants, 11(10), 1997. https://doi.org/10.3390/antiox11101997












