Predicting Regional Cerebral Blood Flow Using Voxel-Wise Resting-State Functional MRI
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Arterial Spin Labeling Data Acquisition, Processing, and CBF Extraction
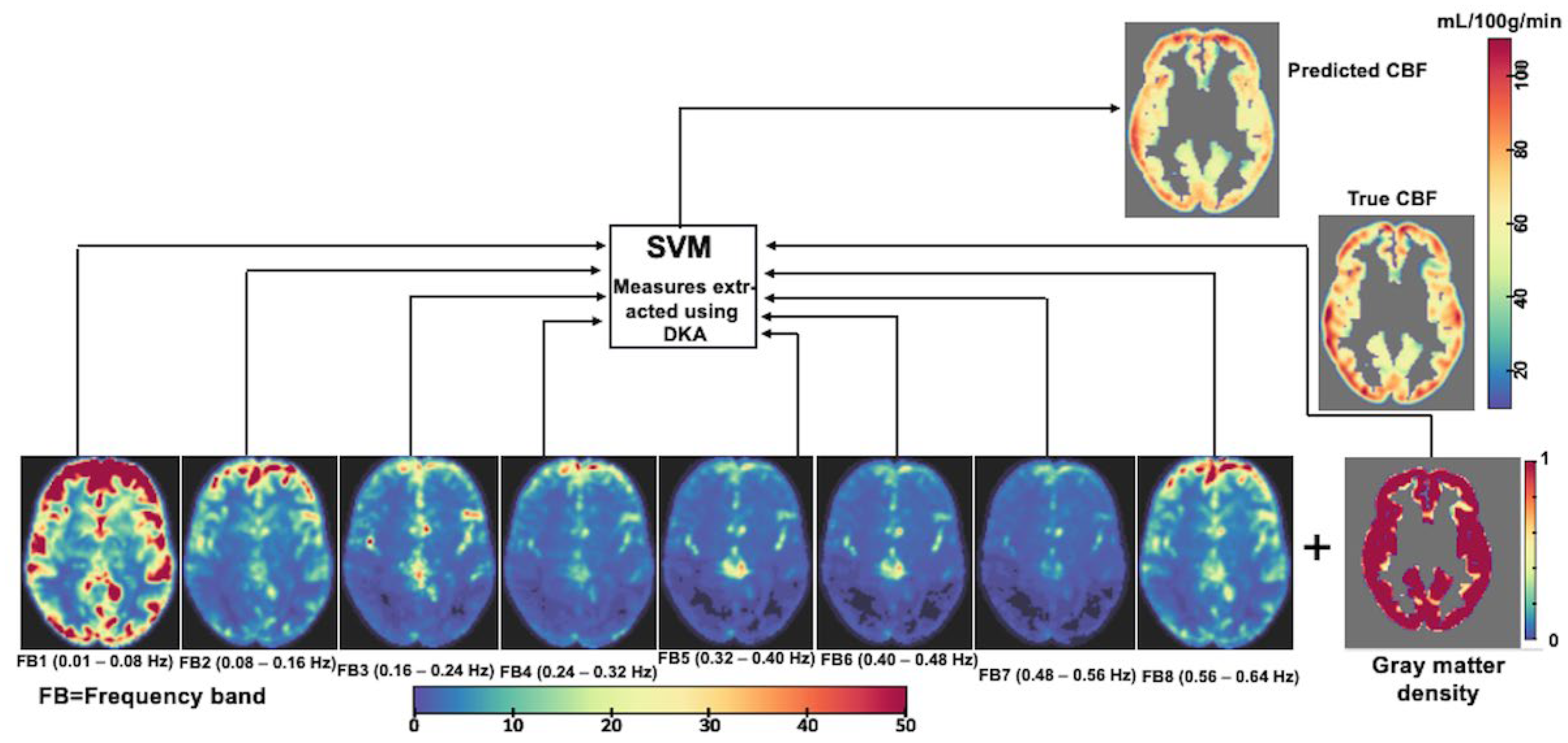
2.3. RsfMRI Data Acquisition, Processing, Time-Series Extraction, and Spectral Features
2.4. Structural MRI Data Collection and Partial Voxel Occupancy Calculation
2.5. SPECT Data Acquisition, Processing, and Analyses
2.6. CBF Prediction Based on Voxel-Wise Cortical rsfMRI Data
2.7. Analysis
3. Results
3.1. Effects of Including PVA on rCBF Prediction in the ACP Sample
3.2. rCBF Differences Between the UKBB MDD Cohort and the Amen Clinics Inc. Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fantini, S.; Sassaroli, A.; Tgavalekos, K.T.; Kornbluth, J. Cerebral blood flow and autoregulation: Current measurement techniques and prospects for noninvasive optical methods. Neurophotonics 2016, 3, 031411. [Google Scholar] [CrossRef]
- Adhikari, B.M.; Dukart, J.; Hipp, J.F.; Forsyth, A.; McMillan, R.; Muthukumaraswamy, S.D.; Ryan, M.C.; Hong, L.E.; Eickhoff, S.B.; Jahandshad, N.; et al. Effects of ketamine and midazolam on resting state connectivity and comparison with ENIGMA connectivity deficit patterns in schizophrenia. Hum. Brain Mapp. 2020, 41, 767–778. [Google Scholar] [CrossRef]
- Adhikari, B.M.; Hong, L.E.; Sampath, H.; Chiappelli, J.; Jahanshad, N.; Thompson, P.M.; Rowland, L.M.; Calhoun, V.D.; Du, X.; Chen, S.; et al. Functional network connectivity impairments and core cognitive deficits in schizophrenia. Hum. Brain Mapp. 2019, 40, 4593–4605. [Google Scholar] [CrossRef]
- Mayberg, H.S.; Brannan, S.K.; Mahurin, R.K.; Jerabek, P.A.; Brickman, J.S.; Tekell, J.L.; Silva, J.A.; McGinnis, S.; Glass, T.G.; Martin, C.C.; et al. Cingulate function in depression a potential predictor of treatment response. NeuroReport 1997, 8, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Mayberg, H.S.; Lewis, P.J.; Regenold, W.; Wagner, H.N. Paralimbic Hypoperfusion in Unipolar Depression. J. Nucl. Med. 1994, 35, 929–934. [Google Scholar] [PubMed]
- Wright, S.; Kochunov, P.; Chiappelli, J.; McMahon, R.P.; Muellerklein, F.; Wijtenburg, S.A.; White, M.G.; Rowland, L.M.; Hong, L.E. Accelerated white matter aging in schizophrenia: Role of white matter blood perfusion. Neurobiol. Aging 2016, 35, 2411–2418. [Google Scholar] [CrossRef]
- Wright, S.N.; Hong, L.E.; Winkler, A.M.; Chiappelli, J.; Nugent, K.; Muellerklein, F.; Du, X.; Rowland, L.M.; Wang, D.J.J.; Kochunov, P. Perfusion shift from white to gray matter may account for processing speed deficits in schizophrenia. Hum. Brain Mapp. 2015, 36, 3793–3804. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.R.; Leggett, R.W. Reference values for resting blood flow to organs of man. Clin. Phys. Physiol. Meas. 1989, 10, 187–217. [Google Scholar] [CrossRef]
- Meng, L.; Hou, W.; Chui, J.; Han, R.; Gelb, A.W. Cardiac Output and Cerebral Blood Flow: The Integrated Regulation of Brain Perfusion in Adult Humans. Anesthesiology 2015, 123, 1198–1208. [Google Scholar] [CrossRef]
- Fox, P.T.; Raichle, M.E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl. Acad. Sci. USA 1986, 83, 1140–1144. [Google Scholar] [CrossRef]
- Fox, P.T.; Raichle, M.E.; Mintun, M.A.; Dence, C. Nonoxidative glucose consumption during focal physiologic neural activity. Science 1988, 241, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Liotti, M.; Mayberg, H.S.; McGinnis, S.; Brannan, S.K.; Jerabek, P.A. Unmasking Disease-Specific Cerebral Blood Flow Abnormalities: Mood Challenge in Patients with Remitted Unipolar Depression. Am. J. Psychiatry 2002, 159, 1830–1840. [Google Scholar] [CrossRef]
- Ingvar, D.H.; Franzén, G. Distribution of cerebral activity in chronic Schizophrenia. Lancet 1974, 304, 1484–1486. [Google Scholar] [CrossRef]
- Gonzalez, S.; Vasavada, M.M.; Njau, S.; Sahib, A.K.; Espinoza, R.; Narr, K.L.; Leaver, A.M. Acute changes in cerebral blood flow after single-infusion ketamine in major depression: A pilot study. Neurol. Psychiatry Brain Res. 2020, 38, 5–11. [Google Scholar] [CrossRef]
- Boisvert, M.; Lungu, O.; Pilon, F.; Dumais, A.; Potvin, S. Regional cerebral blood flow at rest in schizophrenia and major depressive disorder: A functional neuroimaging meta-analysis. Psychiatry Res. Neuroimaging 2023, 335, 111720. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. How to Make More Published Research True. PLoS Med. 2014, 11, e1001747. [Google Scholar] [CrossRef]
- du Sert, O.P.; Unrau, J.; Gauthier, C.J.; Chakravarty, M.; Malla, A.; Lepage, M.; Raucher-Chene, D. Cerebral blood flow in schizophrenia: A systematic review and meta-analysis of MRI-based studies. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2023, 121, 110669. [Google Scholar]
- Moses, W.W. Fundamental Limits of Spatial Resolution in PET. Nucl. Instrum. Methods Phys. Res. A 2011, 648, S236–S240. [Google Scholar] [CrossRef]
- Aguirre, G.K.; Detre, J.A.; Alsop, D.C. Experimental design and the relative sensitivity of BOLD and perfusion fMRI. NeuroImage 2002, 15, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.M.; Hong, L.E.; Zhao, Z.; Wang, D.J.; Thompson, P.M.; Jahanshad, N.; Zhu, A.H.; Holiga, S.; Turner, J.A.; van Erp, T.G.; et al. Cerebral blood flow and cardiovascular risk effects on resting brain regional homogeneity. NeuroImage 2022, 262, 119555. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, B.; Yang, H.; Shan, Y.; Dong, C.; Zang, Y.; Lu, J. The Relationship Among Glucose Metabolism, Cerebral Blood Flow, and Functional Activity: A Hybrid PET/fMRI Study. Mol. Neurobiol. 2021, 58, 2862–2873. [Google Scholar] [CrossRef] [PubMed]
- Kwong, K.K.; Belliveau, J.W.; A Chesler, D.; E Goldberg, I.; Weisskoff, R.M.; Poncelet, B.P.; Kennedy, D.N.; E Hoppel, B.; Cohen, M.S.; Turner, R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc. Nadl. Acad. Sci. USA 1992, 89, 5675–5679. [Google Scholar] [CrossRef]
- Ogawa, S.; Tank, D.W.; Menon, R.; Ellermann, J.M.; Kim, S.G.; Merkle, H.; Ugurbil, K. Intrinsic signal changes accompying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 1992, 89, 5951–5955. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Menon, R.; Tank, D.; Kim, S.; Merkle, H.; Ellermann, J.; Ugurbil, K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging: A comparison of signal characteristics with a biophysical model. Biophys. J. 1993, 64, 803–812. [Google Scholar] [CrossRef]
- Lv, Y.; Margulies, D.S.; Craddock, R.C.; Long, X.; Winter, B.; Gierhake, D.; Endres, M.; Villringer, K.; Fiebach, J.; Villringer, A. Identifying the perfusion deficit in acute stroke with resting-state functional magnetic resonance imaging. Ann. Neurol. 2013, 73, 136–140. [Google Scholar] [CrossRef]
- Detre, J.A.; Wang, J. Technical aspects and utility of fMRI using BOLD and ASL. Clin. Neurophysiol. 2002, 113, 621–634. [Google Scholar] [CrossRef]
- Tong, Y.; Frederick, B. Tracking cerebral blood flow in BOLD fMRI using recursively generated regressors. Hum. Brain Mapp. 2014, 35, 5471–5485. [Google Scholar] [CrossRef]
- Yan, S.; Qi, Z.; An, Y.; Zhang, M.; Qian, T.; Lu, J. Detecting perfusion deficit in Alzheimer’s disease and mild cognitive impairment patients by resting-state fMRI. J. Magn. Reson. Imaging 2019, 49, 1099–1104. [Google Scholar] [CrossRef]
- Chand, G.B.; Habes, M.; Dolui, S.; A Detre, J.; A Wolk, D.; Davatzikos, C. Estimating regional cerebral blood flow using resting-state functional MRI via machine learning. J. Neurosci. Methods 2020, 331, 108528. [Google Scholar] [CrossRef]
- Dukart, J. When structure affects function--the need for partial volume effect correction in functional and resting state magnetic resonance imaging studies. PLoS ONE 2014, 9, e114227. [Google Scholar] [CrossRef] [PubMed]
- Yanase, D.; Matsunari, I.; Yajima, K.; Chen, W.; Fujikawa, A.; Nishimura, S.; Matsuda, H.; Yamada, M. Brain FDG PET study of normal aging in Japanese: Effect of atrophy correction. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, V.; Pietrini, P.; Furey, M.L.; E Alexander, G.; Millet, P.; Bokde, A.L.; Teichberg, D.; Schapiro, M.B.; Horwitz, B.; I Rapoport, S. Resting state brain glucose metabolism is not reduced in normotensive healthy men during aging, after correction for brain atrophy. Brain Res. Bull. 2004, 63, 147–154. [Google Scholar] [CrossRef]
- Smith, D.J.; Nicholl, B.I.; Cullen, B.; Martin, D.; Ul-Haq, Z.; Evans, J.; Gill, J.M.R.; Roberts, B.; Gallacher, J.; Mackay, D.; et al. Prevalence and characteristics of probable major depression and bipolar disorder within UK biobank: Cross-sectional study of 172,751 participants. PLoS ONE 2013, 8, e75362. [Google Scholar] [CrossRef]
- Chappell, M.A.; Groves, A.R.; MacIntosh, B.J.; Donahue, M.J.; Jezzard, P.; Woolrich, M.W. Partial volume correction of multiple inversion time arterial spin labeling MRI data. Magn. Reson. Med. 2011, 65, 1173–1183. [Google Scholar] [CrossRef]
- Adhikari, B.M.; Jahanshad, N.; Shukla, D.; Glahn, D.C.; Blangero, J.; Reynolds, R.C.; Cox, R.W.; Fieremans, E.; Veraart, J.; Novikov, D.S.; et al. Heritability estimates on resting state fMRI data using ENIGMA analysis pipeline. Pac. Symp. Biocomput. 2018, 23, 307–318. [Google Scholar]
- Adhikari, B.M.; Jahanshad, N.; Shukla, D.; Glahn, D.C.; Blangero, J.; Fox, P.T.; Reynolds, R.C.; Cox, R.W.; Fieremans, E.; Veraart, J.; et al. Comparison of heritability estimates on resting state fMRI connectivity phenotypes using the ENIGMA analysis pipeline. Hum. Brain Mapp. 2018, 39, 4893–4902. [Google Scholar] [CrossRef]
- Kochunov, P.; Lancaster, J.L.; Glahn, D.C.; Purdy, D.; Laird, A.R.; Gao, F.; Fox, P. Retrospective motion correction protocol for high-resolution anatomical MRI. Hum. Brain Mapp. 2006, 27, 957–962. [Google Scholar] [CrossRef]
- Glasser, M.F.; Sotiropoulos, S.N.; Wilson, J.A.; Coalson, T.S.; Fischl, B.; Andersson, J.L.; Xu, J.; Jbabdi, S.; Webster, M.; Polimeni, J.R.; et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 2013, 80, 105–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 2001, 20, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-T. A method for attenuation correction in radionuclide computed tomography. IEEE Trans. Nucl. Sci. 1978, 25, 638–643. [Google Scholar] [CrossRef]
- Meyer, D.; Dimitriadou, E.; Hornik, K.; Weingessel, A.; Leisch, F.; Chang, C.C.; Lin, C.C. e1071: Misc Functions of the Department of Statistics, Probability Theory Group, (Formerly: E1071), TU Wien. R package version 1.7-1. 2023. [Google Scholar]
- Ding, X.; Yang, F.; Cao, J. Random radial basis function kernel-based support vector machine. J. Frankl. Inst. 2021, 358, 10121–10140. [Google Scholar] [CrossRef]
- Amen, D.G.; Meysami, S.; Raji, C.A.; George, N. Patterns of Regional Cerebral Blood Flow as a Function of Age Throughout the Lifespan. J. Alzheimers Dis. 2018, 65, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Chun, J.W.; Seok, J.H.; Yun, M.; Oh, M.K. Cortical surface-based analysis of 18F-FDG PET: Measured metabolic abnormalities in schizophrenia are affected by cortical structural abnormalities. NeuroImage 2006, 31, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Mayberg, H. Depression and frontal-subcortical circuits: Focus on prefrontal-limbic interactions. In Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders; Lichter, D.G., Cummings, J.L., Eds.; The Guilford Press: New York, NY, USA, 2001; pp. 177–206. [Google Scholar]
- Han, Y.; Wang, J.; Zhao, Z.; Min, B.; Lu, J.; Li, K.; He, Y.; Jia, J. Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: A resting-state fMRI study. NeuroImage 2011, 55, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Y.; Jiang, T.; Zhou, B.; An, N.; Dai, H.; Wang, P.; Niu, Y.; Wang, L.; Zhang, X. Altered spontaneous activity in Alzheimer’s disease and mild cognitive impairment revealed by Regional Homogeneity. NeuroImage 2012, 59, 1429–1440. [Google Scholar] [CrossRef]


| Name of Region | Abbreviation | Original Model | Corrected Model | Z-Value of Difference (Significance) |
|---|---|---|---|---|
| Banks of superior temporal sulcus | BSTS | 0.26 (p = 1.2 × 10−2) | 0.42 (p = 3.2 × 10−5) | 1.21 (p = 1.9 × 10−1) |
| Caudal anterior cingulate | CACG | 0.16 (p = 1.1 × 10−1) | 0.38 (p = 2.0 × 10−4) | 1.64 (p = 1.0 × 10−1) |
| Caudal middle frontal gyrus | CMFG | 0.13 (p = 1.7 × 10−1) | 0.41 (p = 4.6 × 10−5) | 2.11 (p = 4.3 × 10−2) |
| Cuneus | CU | 0.16 (p = 1.2 × 10−1) | 0.46 (p = 2.7 × 10−6) | 2.38 (p = 2.3 × 10−2) |
| Entorhinal cortex | EC | 0.07 (p = 3.2 × 10−1) | 0.59 (p = 2.8 × 10−10) | 4.22 (p = 5.4 × 10−5) |
| Fusiform gyrus | FG | 0.13 (p = 1.8 × 10−1) | 0.58 (p = 7.4 × 10−10) | 3.70 (p = 4.3 × 10−4) |
| Inferior parietal gyrus | IPG | 0.24 (p = 2.0 × 10−2) | 0.36 (p = 3.4 × 10−4) | 0.95 (p = 2.5 × 10−1) |
| Inferior temporal gyrus | ITG | 0.14 (p = 1.5 × 10−1) | 0.49 (p = 5.2 × 10−7) | 2.74 (p = 9.3 × 10−3) |
| Isthmus cingulate gyrus | ICG | 0.17 (p = 8.8 × 10−2) | 0.43 (p = 1.7 × 10−5) | 1.97 (p = 5.7 × 10−2) |
| Lateral occipital gyrus | LOG | 0.16 (p = 1.0 × 10−1) | 0.45 (p = 5.2 × 10−6) | 2.23 (p = 3.3 × 10−2) |
| Lateral orbito-frontal gyrus | LOFG | 0.09 (p = 2.7 × 10−1) | 0.49 (p = 5.8 × 10−7) | 3.10 (p = 3.3 × 10−3) |
| Lingual gyrus | LG | 0.11 (p = 2.1 × 10−1) | 0.51 (p = 1.4 × 10−7) | 3.13 (p = 3.0 × 10−3) |
| Medial orbito-frontal gyrus | MOFG | 0.09 (p = 2.6 × 10−1) | 0.42 (p = 2.3 × 10−5) | 2.50 (p = 1.8 × 10−2) |
| Middle temporal gyrus | MTG | 0.16 (p = 1.1 × 10−1) | 0.34 (p = 8.5 × 10−4) | 1.35 (p = 1.6 × 10−1) |
| Para hippocampal gyrus | PHIG | 0.09 (p = 2.6 × 10−1) | 0.55 (p = 5.2 × 10−9) | 3.70 (p = 4.3 × 10−4) |
| Para central gyrus | PaCG | 0.07 (p = 3.0 × 10−1) | 0.49 (p = 4.7 × 10−7) | 3.23 (p = 2.2 × 10−3) |
| Pars-opercularis | POP | 0.19 (p = 6.8 × 10−2) | 0.38 (p = 2.1 × 10−4) | 1.43 (p = 1.4 × 10−1) |
| Pars-orbitalis | POR | 0.10 (p = 2.3 × 10−1) | 0.33 (p = 1.1 × 10−3) | 1.70 (p = 9.5 × 10−2) |
| Pars-triangularis | PTR | 0.12 (p = 2.0 × 10−1) | 0.29 (p = 4.7 × 10−3) | 1.28 (p = 1.8 × 10−1) |
| Pericalcarine | PCAL | 0.16 (p = 1.2 × 10−1) | 0.48 (p = 9.1 × 10−7) | 2.55 (p = 1.5 × 10−2) |
| Postcentral gyrus | PoCG | 0.09 (p = 2.6 × 10−1) | 0.42 (p = 2.3 × 10−5) | 2.51 (p = 1.7 × 10−2) |
| Posterior cingulate gyrus | PCG | 0.18 (p = 8.0 × 10−2) | 0.39 (p = 1.0 × 10−4) | 1.62 (p = 1.0 × 10−1) |
| Precentral gyrus | PrCG | 0.09 (p = 2.7 × 10−1) | 0.46 (p = 2.9 × 10−6) | 2.86 (p = 6.7 × 10−3) |
| Precuneus | PCU | 0.18 (p = 7.7 × 10−2) | 0.42 (p = 2.6 × 10−5) | 1.84 (p = 7.0 × 10−2) |
| Rostral anterior cingulate gyrus | RACG | 0.17 (p = 9.6 × 10−2) | 0.36 (p = 3.9 × 10−4) | 1.45 (p = 1.4 × 10−1) |
| Rostral middle frontal gyrus | RMFG | 0.09 (p = 2.7 × 10−1) | 0.31 (p = 2.6 × 10−3) | 1.62 (p = 1.1 × 10−1) |
| Superior frontal gyrus | SFG | 0.09 (p = 2.8 × 10−1) | 0.45 (p = 5.0 × 10−6) | 2.79 (p = 8.1 × 10−3) |
| Superior parietal gyrus | SPG | 0.17 (p = 9.8 × 10−2) | 0.49 (p = 6.6 × 10−7) | 2.51 (p = 1.7 × 10−2) |
| Superior temporal gyrus | STG | 0.15 (p = 1.3 × 10−1) | 0.35 (p = 5.7 × 10−4) | 1.50 (p = 1.3 × 10−1) |
| Supramarginal gyrus | SMG | 0.23 (p = 2.8 × 10−2) | 0.40 (p = 7.4 × 10−5) | 1.33 (p = 1.7 × 10−1) |
| Frontal pole | FP | 0.12 (p = 1.9 × 10−1) | 0.53 (p = 3.6 × 10−8) | 3.23 (p = 2.1 × 10−3) |
| Temporal pole | TP | 0.15 (p = 1.4 × 10−1) | 0.50 (p = 2.5 × 10−7) | 2.79 (p = 8.0 × 10−3) |
| Transverse temporal gyrus | TTG | 0.18 (p = 7.2 × 10−2) | 0.27 (p = 8.7 × 10−3) | 0.65 (p = 3.2 × 10−1) |
| Insula | IN | 0.18 (p = 7.7 × 10−2) | 0.42 (p = 2.3 × 10−5) | 1.87 (p = 7.0 × 10−2) |
| Region | Abbreviation | MDD Effect Size (UKBB) | MDD Effect Size (Amen Clinics Inc.) |
|---|---|---|---|
| Banks of superior temporal sulcus | BSTS | −0.28 (p < 10−16) | −0.27 (p = 0.01) |
| Caudal anterior cingulate | CACG | −0.34 (p < 10−16) | −0.55 (p = 6 × 10−7) |
| Caudal middle frontal gyrus | CMFG | −0.10 (p = 4 × 10−5) | −0.29 (p = 0.009) |
| Cuneus | CU | −0.24 (p < 10−16) | −0.33 (p = 0.002) |
| Entorhinal cortex | EC | −0.13 (p = 3 × 10−8) | −0.03 (p = 0.4) |
| Fusiform gyrus | FG | −0.27 (p < 10−16) | −0.46 (p = 3 × 10−5) |
| Inferior parietal gyrus | IPG | −0.20 (p < 10−16) | −0.45 (p = 3 × 10−5) |
| Inferior temporal gyrus | ITG | −0.25 (p < 10−16) | −0.27 (p = 0.02) |
| Isthmus cingulate gyrus | ICG | −0.39 (p < 10−16) | −0.41 (p = 2 × 10−4) |
| Lateral occipital gyrus | LOG | −0.24 (p < 10−16) | −0.37 (p = 1 × 10−3) |
| Lateral orbito-frontal gyrus | LOFG | −0.30 (p < 10−16) | −0.70 (p = 2 × 10−10) |
| Lingual gyrus | LG | −0.19 (p = 10−16) | −0.18 (p = 0.09) |
| Medial orbito-frontal gyrus | MOFG | −0.29 (p < 10−16) | −0.58 (p = 1 × 10−7) |
| Middle temporal gyrus | MTG | −0.32 (p < 10−16) | −0.64 (p = 6 × 10−9) |
| Para hippocampal gyrus | PHIG | −0.28 (p < 10−16) | −0.28 (p = 0.01) |
| Para central gyrus | PaCG | −0.25 (p < 10−16) | −0.41 (p = 2 × 10−4) |
| Pars-opercularis | POP | −0.37 (p < 10−16) | −0.67 (p = 1 × 10−9) |
| Pars-orbitalis | POR | −0.31 (p < 10−16) | −0.27 (p = 0.02) |
| Pars-triangularis | PTR | −0.35 (p < 10−16) | −0.69 (p = 4 × 10−10) |
| Pericalcarine | PCAL | −0.10 (p = 1 × 10−5) | −0.03 (p = 0.4) |
| Postcentral gyrus | PoCG | −0.25 (p < 10−16) | −0.32 (p = 0.004) |
| Posterior cingulate gyrus | PCG | −0.17 (p = 1 × 10−14) | 0.19 (p = 0.08) |
| Precentral gyrus | PrCG | −0.31 (p < 10−16) | −0.26 (p = 0.02) |
| Precuneus | PCU | −0.24 (p < 10−16) | −0.37 (p = 8 × 10−4) |
| Rostral anterior cingulate gyrus | RACG | −0.03 (p = 0.2) | −0.005 (p = 0.4) |
| Rostral middle frontal gyrus | RMFG | −0.30 (p < 10−16) | −0.56 (p = 3 × 10−7) |
| Superior frontal gyrus | SFG | −0.38 (p < 10−16) | −0.42 (p = 2 × 10−4) |
| Superior parietal gyrus | SPG | −0.17 (p = 3 × 10−14) | −0.29 (p = 0.008) |
| Superior temporal gyrus | STG | −0.31 (p < 10−16) | −0.67 (p = 1 × 10−9) |
| Supramarginal gyrus | SMG | −0.25 (p < 10−16) | −0.51 (p = 2 × 10−6) |
| Frontal pole | FP | −0.06 (p = 0.008) | 0.06 (p = 0.3) |
| Temporal pole | TP | −0.24 (p < 10−16) | −0.46 (p = 3 × 10−5) |
| Transverse temporal gyrus | TTG | −0.14 (p = 1 × 10−8) | −0.36 (p = 1 × 10−3) |
| Insula | IN | −0.34 (p < 10−16) | −0.39 (p = 4 × 10−4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, H.; Adhikari, B.M.; Pan, Y.; Keator, D.B.; Amen, D.; Gao, S.; Ma, Y.; Thompson, P.M.; Jahanshad, N.; Turner, J.A.; et al. Predicting Regional Cerebral Blood Flow Using Voxel-Wise Resting-State Functional MRI. Brain Sci. 2025, 15, 908. https://doi.org/10.3390/brainsci15090908
Ke H, Adhikari BM, Pan Y, Keator DB, Amen D, Gao S, Ma Y, Thompson PM, Jahanshad N, Turner JA, et al. Predicting Regional Cerebral Blood Flow Using Voxel-Wise Resting-State Functional MRI. Brain Sciences. 2025; 15(9):908. https://doi.org/10.3390/brainsci15090908
Chicago/Turabian StyleKe, Hongjie, Bhim M. Adhikari, Yezhi Pan, David B. Keator, Daniel Amen, Si Gao, Yizhou Ma, Paul M. Thompson, Neda Jahanshad, Jessica A. Turner, and et al. 2025. "Predicting Regional Cerebral Blood Flow Using Voxel-Wise Resting-State Functional MRI" Brain Sciences 15, no. 9: 908. https://doi.org/10.3390/brainsci15090908
APA StyleKe, H., Adhikari, B. M., Pan, Y., Keator, D. B., Amen, D., Gao, S., Ma, Y., Thompson, P. M., Jahanshad, N., Turner, J. A., van Erp, T. G. M., Milad, M. R., Soares, J. C., Calhoun, V. D., Dukart, J., Hong, L. E., Ma, T., & Kochunov, P. (2025). Predicting Regional Cerebral Blood Flow Using Voxel-Wise Resting-State Functional MRI. Brain Sciences, 15(9), 908. https://doi.org/10.3390/brainsci15090908






