Altered Functional Connectivity and Complexity in Major Depressive Disorder after Musical Stimulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
- (1)
- Age matching with depression patients.
- (2)
- In good physical and mental health, with no history of mental illness.
- (3)
- A PHQ-9 questionnaire score of less than 4.
- (4)
- Exclusion of individuals with chronic diseases.
- (5)
- Cognitively normal, no history of major depression, schizophrenia, bipolar disorder, or drug abuse, and no medication that may affect cognition and walking.
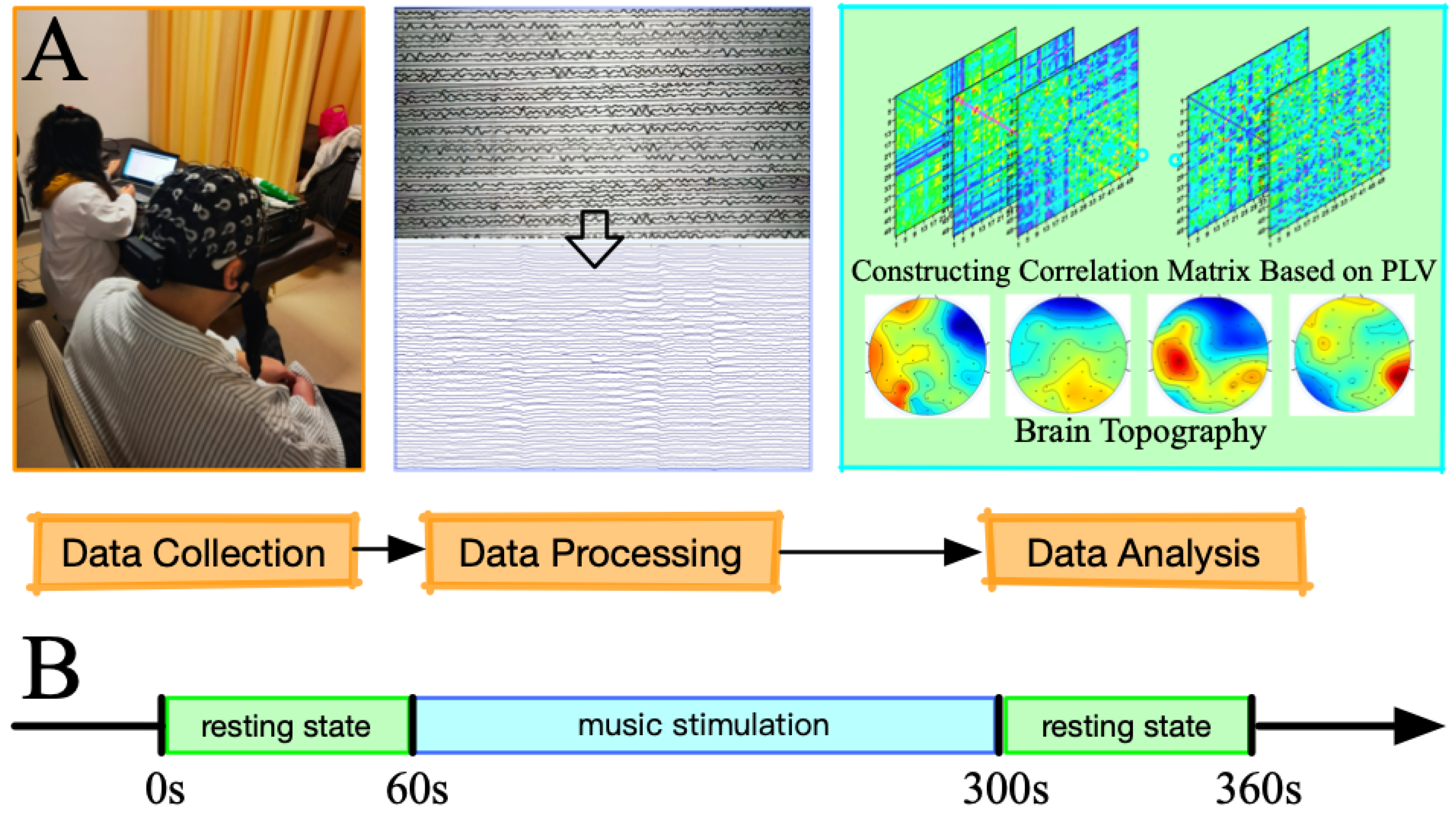
2.2. EEG Data Acquisition and Preprocessing
2.3. Experimental Paradigm
2.4. Data Analysis
2.4.1. Phase Locking Value
2.4.2. Network Characteristics
3. Results
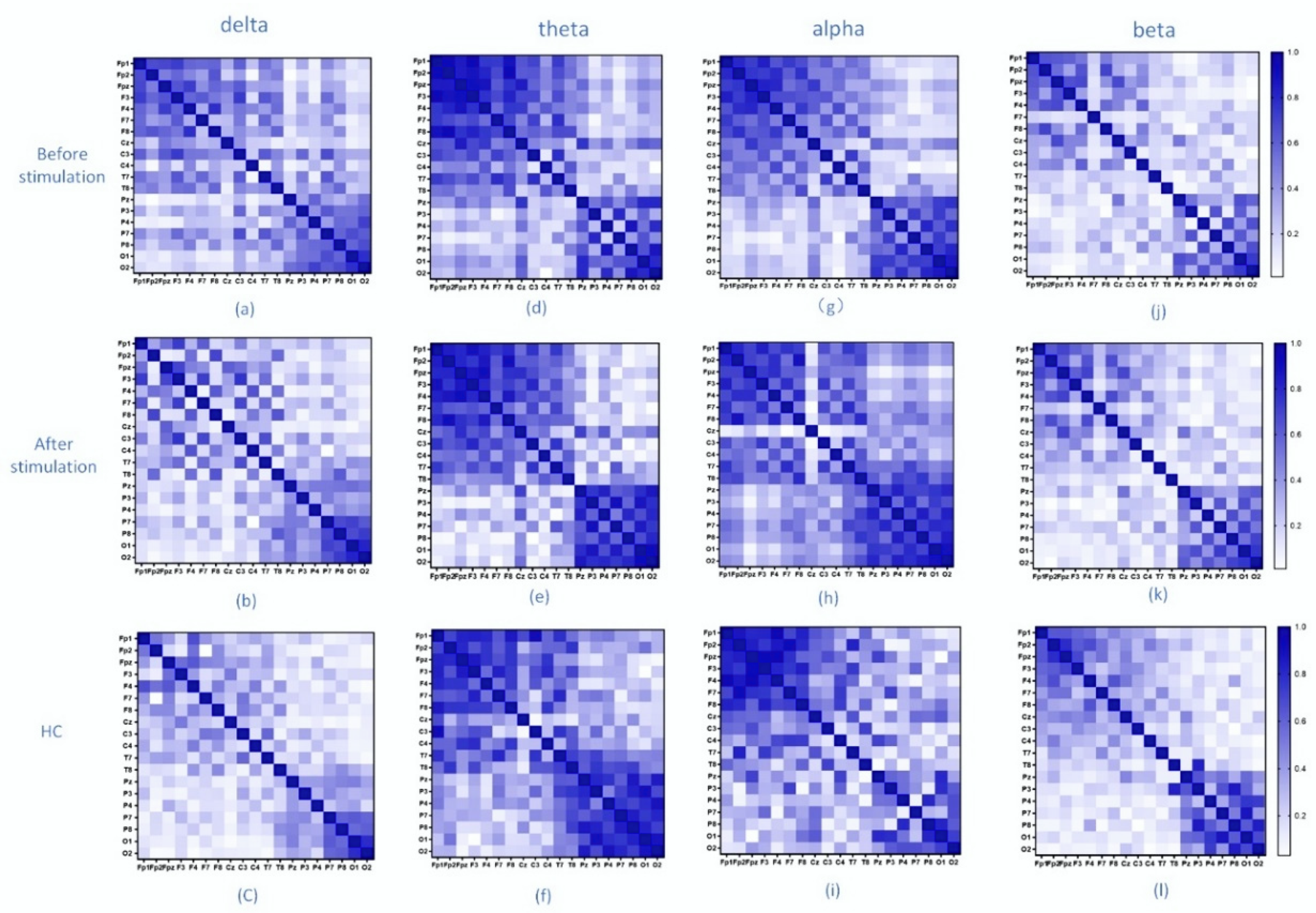
3.1. Network Analysis
3.2. Network Properties
3.3. Classification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major Depressive Disorder. Nat. Rev. Dis. Primers 2016, 2, 16065. [Google Scholar] [CrossRef] [Green Version]
- Ventriglio, A.; Bhugra, D.; Sampogna, G.; Luciano, M.; de Berardis, D.; Sani, G.; Fiorillo, A. From Dysthymia to Treatment-Resistant Depression: Evolution of a Psychopathological Construct. Int. Rev. Psychiatry 2020, 32, 471–476. [Google Scholar] [CrossRef]
- Nystrom, C.; Matousek, M.; Hallstrom, T. Relationships between EEG and Clinical Characteristics in Major Depressive Disorder. Acta Psychiatr. Scand. 1986, 73, 390–394. [Google Scholar] [CrossRef]
- Fava, M.; Kendler, K.S. Major Depressive Disorder. Neuron 2000, 28, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Perna, G.; Daccò, S.; Alciati, A.; Cuniberti, F.; de Berardis, D.; Caldirola, D. Childhood Maltreatment History for Guiding Personalized Antidepressant Choice in Major Depressive Disorder: Preliminary Results from a Systematic Review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 107, 110208. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. Editorial of Special Issue “Crosstalk between Depression, Anxiety, and Dementia: Comorbidity in Behavioral Neurology and Neuropsychiatry”. Biomedicines 2021, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Paluska, S.A.; Schwenk, T.L. Physical Activity and Mental Health. Sports Med. 2000, 29, 167–180. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Spekker, E.; Polyák, H.; Tóth, F.; Vécsei, L. Mitochondrial Impairment: A Common Motif in Neuropsychiatric Presentation? The Link to the Tryptophan–Kynurenine Metabolic System. Cells 2022, 11, 2607. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; DiPaula, B.A.; Lee, H.Y.; Cooke, C.E. Failure to Fill Electronically Prescribed Antidepressant Medications: A Retrospective Study. Prim. Care Companion CNS Disord. 2011, 13, 26352. [Google Scholar] [CrossRef] [Green Version]
- Aalbers, S.; Fusar-Poli, L.; Freeman, R.E.; Spreen, M.; Ket, J.C.; Vink, A.C.; Maratos, A.; Crawford, M.; Chen, X.-J.; Gold, C. Music Therapy for Depression. Cochrane Database Syst. Rev. 2017, 2017, CD004517. [Google Scholar] [CrossRef] [PubMed]
- Gartlehner, G.; Gaynes, B.N.; Amick, H.R.; Asher, G.N.; Morgan, L.C.; Coker-Schwimmer, E.; Forneris, C.; Boland, E.; Lux, L.J.; Gaylord, S.; et al. Comparative Benefits and Harms of Antidepressant, Psychological, Complementary, and Exercise Treatments for Major Depression: An Evidence Report for a Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2016, 164, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Erkkilä, J.; Punkanen, M.; Fachner, J.; Ala-Ruona, E.; Pöntiö, I.; Tervaniemi, M.; Vanhala, M.; Gold, C. Individual Music Therapy for Depression: Randomised Controlled Trial. Br. J. Psychiatry 2011, 199, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Frisch, A. Symbol and Structure: Music Therapy for the Adolescent Psychiatric Inpatient. Music Ther. 1990, 9, 16–34. [Google Scholar] [CrossRef] [Green Version]
- Partesotti, E.; Peñalba, A.; Manzolli, J. Digital Instruments and Their Uses in Music Therapy. Nord. J. Music Ther. 2018, 27, 399–418. [Google Scholar] [CrossRef]
- Hanser, S.B. A Music Therapy Strategy for Depressed Older Adults in the Community. J. Appl. Gerontol. 1990, 9, 283–298. [Google Scholar] [CrossRef]
- Deng, J.; Chen, Y.; Zeng, W.; Luo, X.; Li, Y. Brain Response of Major Depressive Disorder Patients to Emotionally Positive and Negative Music. J. Mol. Neurosci. 2022, 72, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Bodner, E.; Iancu, I.; Gilboa, A.; Sarel, A.; Mazor, A.; Amir, D. Finding Words for Emotions: The Reactions of Patients with Major Depressive Disorder towards Various Musical Excerpts. Arts Psychother. 2007, 34, 142–150. [Google Scholar] [CrossRef]
- Mahato, S.; Paul, S. Electroencephalogram (EEG) Signal Analysis for Diagnosis of Major Depressive Disorder (MDD): A Review. In Nanoelectronics, Circuits and Communication Systems; Nath, V., Mandal, J.K., Eds.; Springer: Singapore, 2019; pp. 323–335. [Google Scholar]
- Koelsch, S. A Neuroscientific Perspective on Music Therapy. Ann. N. Y. Acad. Sci. 2009, 1169, 374–384. [Google Scholar] [CrossRef]
- Geipel, J.; Koenig, J.; Hillecke, T.K.; Resch, F. Short-Term Music Therapy Treatment for Adolescents with Depression—A Pilot Study. Arts Psychother. 2022, 77, 101874. [Google Scholar] [CrossRef]
- Yasin, S.; Hussain, S.A.; Aslan, S.; Raza, I.; Muzammel, M.; Othmani, A. EEG Based Major Depressive Disorder and Bipolar Disorder Detection Using Neural Networks:A Review. Comput. Methods Programs Biomed. 2021, 202, 106007. [Google Scholar] [CrossRef]
- Shao, X.; Sun, S.; Li, J.; Kong, W.; Zhu, J.; Li, X.; Hu, B. Analysis of Functional Brain Network in MDD Based on Improved Empirical Mode Decomposition with Resting State EEG Data. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1546–1556. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A.; Kähkönen, S. Functional Connectivity in the Brain—Is It an Elusive Concept? Neurosci. Biobehav. Rev. 2005, 28, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, X.; Zhu, J.; Wang, Y.; La, R.; Zhang, X.; Wei, L.; Hu, B. Graph Theory Analysis of Functional Connectivity in Major Depression Disorder with High-Density Resting State EEG Data. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Porta-Casteràs, D.; Cano, M.; Camprodon, J.A.; Loo, C.; Palao, D.; Soriano-Mas, C.; Cardoner, N. A Multimetric Systematic Review of FMRI Findings in Patients with MDD Receiving ECT. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 108, 110178. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, W.; Ali, S.S.A.; Yasin, M.A.M.; Malik, A.S. A Machine Learning Framework Involving EEG-Based Functional Connectivity to Diagnose Major Depressive Disorder (MDD). Med. Biol. Eng. Comput. 2018, 56, 233–246. [Google Scholar] [CrossRef]
- Ebneabbasi, A.; Mahdipour, M.; Nejati, V.; Li, M.; Liebe, T.; Colic, L.; Leutritz, A.L.; Vogel, M.; Zarei, M.; Walter, M.; et al. Emotion Processing and Regulation in Major Depressive Disorder: A 7T Resting-State FMRI Study. Hum. Brain Mapp. 2021, 42, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, C.; Wang, X.; Xu, J.; Chang, Y.; Ristaniemi, T.; Cong, F. Functional Connectivity of Major Depression Disorder Using Ongoing EEG during Music Perception. Clin. Neurophysiol. 2020, 131, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, F.; La Porta, F.; Petrone, V.; Battaglia, S.; Orlandi, S.; Ippolito, G.; Romei, V.; Piperno, R.; Lullini, G. Accuracy of EEG Biomarkers in the Detection of Clinical Outcome in Disorders of Consciousness after Severe Acquired Brain Injury: Preliminary Results of a Pilot Study Using a Machine Learning Approach. Biomedicines 2022, 10, 1897. [Google Scholar] [CrossRef] [PubMed]
- Mercadié, L.; Caballe, J.; Aucouturier, J.-J.; Bigand, E. Effect of Synchronized or Desynchronized Music Listening during Osteopathic Treatment: An EEG Study. Psychophysiology 2014, 51, 52–59. [Google Scholar] [CrossRef]
- Kim, J.; André, E. Emotion Recognition Based on Physiological Changes in Music Listening. IEEE Trans. Pattern Anal. Mach. Intell. 2008, 30, 2067–2083. [Google Scholar] [CrossRef]
- Juslin, P.N.; Harmat, L.; Eerola, T. What Makes Music Emotionally Significant? Exploring the Underlying Mechanisms. Psychol. Music 2014, 42, 599–623. [Google Scholar] [CrossRef]
- Juslin, P.N.; Västfjäll, D. Emotional Responses to Music: The Need to Consider Underlying Mechanisms. Behav. Brain Sci. 2008, 31, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Xue, J. EEG Analysis with Wavelet Transform under Music Perception Stimulation. J. Healthc. Eng. 2021, 2021, e9725762. [Google Scholar] [CrossRef]
- Wang, T.; Tang, J.; Wang, C.; Yang, D.; Li, J.; Kong, W.; Xi, X. Effect of Music Stimuli on Corticomuscular Coupling and the Brain Functional Connectivity Network. Biomed. Signal Process. Control 2023, 79, 104264. [Google Scholar] [CrossRef]
- Roh, T.; Song, K.; Cho, H.; Shin, D.; Yoo, H.-J. A Wearable Neuro-Feedback System With EEG-Based Mental Status Monitoring and Transcranial Electrical Stimulation. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, S.; Arns, M. EEG Biomarkers in Major Depressive Disorder: Discriminative Power and Prediction of Treatment Response. Int. Rev. Psychiatry 2013, 25, 604–618. [Google Scholar] [CrossRef] [PubMed]
- Khambhati, A.N.; Shafi, A.; Rao, V.R.; Chang, E.F. Long-Term Brain Network Reorganization Predicts Responsive Neurostimulation Outcomes for Focal Epilepsy. Sci. Transl. Med. 2021, 13, eabf6588. [Google Scholar] [CrossRef]
- Pulvermüller, F.; Tomasello, R.; Henningsen-Schomers, M.R.; Wennekers, T. Biological Constraints on Neural Network Models of Cognitive Function. Nat. Rev. Neurosci. 2021, 22, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fang, J.; Liu, J.; Rong, P.; Jorgenson, K.; Park, J.; Lang, C.; Hong, Y.; Zhu, B.; Kong, J. Frequency-Dependent Functional Connectivity of the Nucleus Accumbens during Continuous Transcutaneous Vagus Nerve Stimulation in Major Depressive Disorder. J. Psychiatr. Res. 2018, 102, 123–131. [Google Scholar] [CrossRef]
- He, H.; Yang, M.; Duan, M.; Chen, X.; Lai, Y.; Xia, Y.; Shao, J.; Biswal, B.B.; Luo, C.; Yao, D. Music Intervention Leads to Increased Insular Connectivity and Improved Clinical Symptoms in Schizophrenia. Front. Neurosci. 2018, 11, 744. [Google Scholar] [CrossRef]
- Fachner, J.; Gold, C.; Erkkilä, J. Music Therapy Modulates Fronto-Temporal Activity in Rest-EEG in Depressed Clients. Brain Topogr 2013, 26, 338–354. [Google Scholar] [CrossRef]
- Sporns, O. Network Analysis, Complexity, and Brain Function. Complexity 2002, 8, 56–60. [Google Scholar] [CrossRef]
- Mammone, N.; de Salvo, S.; Bonanno, L.; Ieracitano, C.; Marino, S.; Marra, A.; Bramanti, A.; Morabito, F.C. Brain Network Analysis of Compressive Sensed High-Density EEG Signals in AD and MCI Subjects. IEEE Trans. Ind. Inform. 2019, 15, 527–536. [Google Scholar] [CrossRef]
- Behrouzi, T.; Hatzinakos, D. Graph Variational Auto-Encoder for Deriving EEG-Based Graph Embedding. Pattern Recognit. 2022, 121, 108202. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duc, N.T.; Lee, B. Microstate Functional Connectivity in EEG Cognitive Tasks Revealed by a Multivariate Gaussian Hidden Markov Model with Phase Locking Value. J. Neural Eng. 2019, 16, 026033. [Google Scholar] [CrossRef]
- Power, J.D.; Fair, D.A.; Schlaggar, B.L.; Petersen, S.E. The Development of Human Functional Brain Networks. Neuron 2010, 67, 735–748. [Google Scholar] [CrossRef] [Green Version]
- Stam, C.J.; de Haan, W.; Daffertshofer, A.; Jones, B.F.; Manshanden, I.; van Cappellen van Walsum, A.M.; Montez, T.; Verbunt, J.P.A.; de Munck, J.C.; van Dijk, B.W.; et al. Graph Theoretical Analysis of Magnetoencephalographic Functional Connectivity in Alzheimer’s Disease. Brain 2009, 132, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Wu, Q.; Kuang, W.; Huang, X.; He, Y.; Gong, Q. Disrupted Brain Connectivity Networks in Drug-Naive, First-Episode Major Depressive Disorder. Biol. Psychiatry 2011, 70, 334–342. [Google Scholar] [CrossRef]
- Singh, M.K.; Kesler, S.R.; Hadi Hosseini, S.M.; Kelley, R.G.; Amatya, D.; Hamilton, J.P.; Chen, M.C.; Gotlib, I.H. Anomalous Gray Matter Structural Networks in Major Depressive Disorder. Biol. Psychiatry 2013, 74, 777–785. [Google Scholar] [CrossRef]
- Fraiman, D.; Saunier, G.; Martins, E.F.; Vargas, C.D. Biological Motion Coding in the Brain: Analysis of Visually Driven EEG Functional Networks. PLoS ONE 2014, 9, e84612. [Google Scholar] [CrossRef]
- Vecchio, F.; Miraglia, F.; Marra, C.; Quaranta, D.; Vita, M.G.; Bramanti, P.; Rossini, P.M. Human Brain Networks in Cognitive Decline: A Graph Theoretical Analysis of Cortical Connectivity from EEG Data. J. Alzheimer’s Dis. 2014, 41, 113–127. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Y.; Lin, P.; Wang, J. Small-World Brain Functional Networks in Children with Attention-Deficit/Hyperactivity Disorder Revealed by EEG Synchrony. Clin. EEG Neurosci. 2015, 46, 183–191. [Google Scholar] [CrossRef]
- Brumbaugh-Smith, J.; Shier, D. An Empirical Investigation of Some Bicriterion Shortest Path Algorithms. Eur. J. Oper. Res. 1989, 43, 216–224. [Google Scholar] [CrossRef]
- Tatar, A.B. Biometric Identification System Using EEG Signals. Neural Comput. Appl. 2022, 1–15. [Google Scholar] [CrossRef]
- Cervantes, J.; Garcia-Lamont, F.; Rodríguez-Mazahua, L.; Lopez, A. A Comprehensive Survey on Support Vector Machine Classification: Applications, Challenges and Trends. Neurocomputing 2020, 408, 189–215. [Google Scholar] [CrossRef]
- Zulfiqar, H.; Yuan, S.-S.; Huang, Q.-L.; Sun, Z.-J.; Dao, F.-Y.; Yu, X.-L.; Lin, H. Identification of Cyclin Protein Using Gradient Boost Decision Tree Algorithm. Comput. Struct. Biotechnol. J. 2021, 19, 4123–4131. [Google Scholar] [CrossRef]
- Mucherino, A.; Papajorgji, P.J.; Pardalos, P.M. K-Nearest Neighbor Classification. In Data Mining in Agriculture; Mucherino, A., Papajorgji, P.J., Pardalos, P.M., Eds.; Springer Optimization and Its Applications; Springer: New York, NY, USA, 2009; pp. 83–106. ISBN 978-0-387-88615-2. [Google Scholar]
- Belgiu, M.; Drăguţ, L. Random Forest in Remote Sensing: A Review of Applications and Future Directions. ISPRS J. Photogramm. Remote Sens. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- Van der Vinne, N.; Vollebregt, M.A.; van Putten, M.J.A.M.; Arns, M. Frontal Alpha Asymmetry as a Diagnostic Marker in Depression: Fact or Fiction? A Meta-Analysis. NeuroImage Clin. 2017, 16, 79–87. [Google Scholar] [CrossRef]
- Roh, S.-C.; Kim, J.S.; Kim, S.; Kim, Y.; Lee, S.-H. Frontal Alpha Asymmetry Moderated by Suicidal Ideation in Patients with Major Depressive Disorder: A Comparison with Healthy Individuals. Clin. Psychopharmacol. Neurosci. 2020, 18, 58–66. [Google Scholar] [CrossRef]



| MDD (n = 8) | HC (n = 8) | p | |||
|---|---|---|---|---|---|
| Average | SD | Average | SD | ||
| age | 30.85 | 7.5 | 27.65 | 8.6 | 0.89 |
| gender | 6 male/2 female | 8 male | |||
| PHQ-9 | 15.42 | 5.32 | 2.44 | 0.92 | 0.00 |
| GAD-7 | 11.62 | 6.50 | 2.19 | 3.74 | 0.00 |
| Classifier | Accuracy | Precision | Recall |
|---|---|---|---|
| KNN | 81.25% | 75% | 68.75% |
| SVM | 93.75% | 87.5% | 93.75% |
| DT | 68.75% | 62.5% | 62.5% |
| RF | 75% | 68.75% | 75% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, P.; Dai, J.; Wang, T.; Li, H.; Ma, C.; Xi, X. Altered Functional Connectivity and Complexity in Major Depressive Disorder after Musical Stimulation. Brain Sci. 2022, 12, 1680. https://doi.org/10.3390/brainsci12121680
Qiu P, Dai J, Wang T, Li H, Ma C, Xi X. Altered Functional Connectivity and Complexity in Major Depressive Disorder after Musical Stimulation. Brain Sciences. 2022; 12(12):1680. https://doi.org/10.3390/brainsci12121680
Chicago/Turabian StyleQiu, Pintao, Jinxiao Dai, Ting Wang, Hangcheng Li, Cunbin Ma, and Xugang Xi. 2022. "Altered Functional Connectivity and Complexity in Major Depressive Disorder after Musical Stimulation" Brain Sciences 12, no. 12: 1680. https://doi.org/10.3390/brainsci12121680





