Innovative Technologies in the Neurorehabilitation of Traumatic Brain Injury: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. PICO Evaluation
2.3. Inclusion and Exclusion Criteria
2.4. Literature Selection
2.5. Study Quality Assessment
3. Results
3.1. Robotic and Virtual Systems for Motor Rehabilitation
3.2. Humanoid Robots
3.3. Virtual Reality Systems for Cognitive Rehabilitation
3.4. Computer-Based Rehabilitative Approach
3.5. Tele-Rehabilitation
3.6. Neuromodulation and Combined Approaches
4. Discussion
| Robotic Device | Short Description | Picture |
|---|---|---|
| Erigo [12,24] | Erigo is a robotic tilt table used in the early stage of recovery after an acute TBI. It allows an early and gradual robotic verticalization combined with a cyclic leg movement in order to stimulate CNS through critical afferent stimuli. The tilt table inclination can be regulated by the therapists, who can control the gradualness of verticalization (i.e., from 45° to 90°) as well as the stepping speed, according to the patients’ needs. In addition, the Erigo device also can improve cardiocirculatory stability through muscle activation, pump function and venous return. | 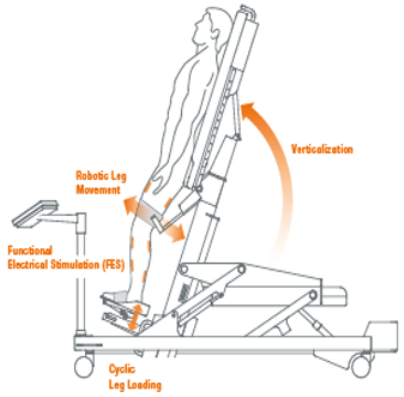 |
| Lokomat [25,26,27] | The Lokomat is a robotic gait-assisted device widely used in the neurorehabilitation of walking. It consists of an external gait orthosis integrated with a computer-controlled linear actuators at each hip and knee joint, in addition to an advanced system of body weight support system and a treadmill. The therapists can control the level of gait assisted-support, the force and the pattern of gait movement. |  |
| GEO-O [25] | GEO SYSTEM is a robotic end effector system that simulates the repetitive training of locomotion in everyday life, such as walking on the flat, ascending and descending stairs. The patient’s feet are secured to the platforms moving in all directions (i.e., upwards, downwards, forwards and backwards) with the assistance of six engines. There is also an Evolution version that also includes the use of an immersive scenario to fully involve the person in rehabilitation therapy in dual-task activities. |  |
| Human-Robot | Short Description | Picture |
| Robot-PEPPER [31] | Pepper is one of the most widely known social humanoid robots, which is used to recognize faces and basic human emotions. This humanoid robot was conceptualised for human interaction, thanks to the conversation and his colourful touchscreen. |  |
| Robot-NAO [55] | Nao is another humanoid robot characterised by its small size, and it is able to interact with adult, adolescent and paediatric patients. It is equipped with sensors that allow it to walk, dance, speak, and recognize faces and objects. It can be used to provide social activities and to recognize emotions, giving sensorial feedback to patients. |  |
| Virtual Reality System | Short Description | Picture |
| BTS Nirvana [38] | BTS NIRVANA is an innovative therapeutic system that assists the rehabilitation process of patients affected by neurological diseases, thanks to its multi-sensorial stimulation. The patients can move or manipulate specific objects in different ways (i.e., balls, flowers, and butterflies) or create specific combinations (i.e., colour-number) with a dynamic involvement in the virtual environment. During the interaction between the patient and the screen, the system produces audio and video feedback (using the sprite activity). The difficulty of exercises increases the base of the number of distractors and reduces the time available for the execution. |  |
| VRRS Virtual Reality Rehabilitation System [33] | Il Khymeia VRRS—Virtual Reality Rehabilitation System—is the most widely used for VR training and teletraining in clinical practice. The VRRS, in fact, is conceptualised with a “central HUB” that can be connected via USB, a series of specialised peripherals fully synchronised and integrated with the system. The VRRS is equipped with exercise modules for cognitive, language, postural, and motor rehabilitation. These virtual exercises can be selected and included in the rehab program by the therapist, who can shape the difficulty in relation to the time of execution and the type of activity. | 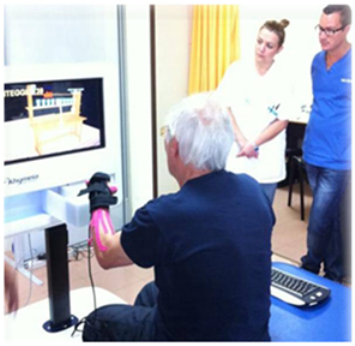 |
| Neuro-DRIVE System [36] | The Neuro-DRIVE system uses a virtual reality driving simulator. It consists of a curved screen in addition to a driving console similar to a typical automobile. Each driving console presents turn signals, gas and brake pedals, a steering wheel, a digital dashboard, and a seat belt. The patient is seated in front of the screen, holding the steering wheel and pushing the pedals while interacting with the virtual environment stimuli. |  |
- (1)
- Patients with a diagnosis of DoC or sTBI need to achieve an early and gradual verticalization in order to avoid deterioration of the autonomic nervous system and bedridden complications [12,24]. For these reasons, the Erigo device could be a useful tool in clinical practice to meet patient necessities. In addition, sTBI could benefit from RAGT through Lokomat, which can assist a passive gait-increasing BWS, always monitoring vital parameters to guarantee a safe and feasible rehabilitative intervention [26].
- (2)
- Patients affected by moderate TBI may gain balance and coordination thanks to VR exercises, which are also known to promote the enjoyment and active involvement of the patients [28,29,30], even if the evidence found is not sufficient to support its systematic use in TBI clinical practice. Instead, the GE-O system seems to be a valid tool for gait training in moderate TBI patients to improve endurance and walking speed [25].
- (3)
- On the other hand, the C-BT, as well as VR systems, are widely used in severe and moderate TBI patients to train cognitive functions. VRRS allows a specific subdomain training and, thanks to its big screen, provides a wider view of space and facilitates the execution of cognitive tasks [33,38], whereas moderate TBI could take advantage of C-BT, which requires more control in the upper limb [39,40].
- (4)
- Another useful innovative approach is TR; despite the poor evidence in the TBI population, it can be useful in ensuring continuity of care between discharge from the hospital and return to home [42], avoiding travelling costs and geographical barriers for patients living far from metropolitan areas [65,66]. Specifically, the VRRS for TR is a feasible tool for moderate TBI patients that allows therapists to plan a specific training session (i.e., motor, with regard to upper limb, and cognitive) remotely supervised by the Tele-CockPit workstation [42].
- (5)
- Last, NIBS in the moderate to severe TBI population is a promising approach, especially used to stimulate cognitive functions [67]. However, it is still an emerging approach and there is not enough data to confirm its use in clinical practice. Possible future directions could investigate the efficacy of NIBS combined with other technologies such as TR, C-BT and VR.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2019, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Mckee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, D.; Pekic, S.; Stojanovic, M.; Popovic, V. Traumatic brain injury: Neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary 2019, 22, 270–282. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Calabrò, R.S.; Bramanti, P. Cognitive rehabilitation after severe acquired brain injury: Current evidence and future directions. Neuropsychol. Rehabil. 2018, 28, 879–898. [Google Scholar] [CrossRef]
- Bland, D.C.; Zampieri, C.; Damiano, D.L. Effectiveness of physical therapy for improving gait and balance in individuals with traumatic brain injury: A systematic review. Brain Inj. 2011, 25, 664–679. [Google Scholar] [CrossRef]
- Gassert, R.; Dietz, V. Rehabilitation robots for the treatment of sensorimotor deficits: A neurophysiological perspective. J. Neuroeng. Rehabil. 2018, 15, 46. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Cacciola, A.; Bertè, F.; Manuli, A.; Leo, A.; Bramanti, A.; Naro, A.; Milardi, D.; Bramanti, P. Robotic gait rehabilitation and substitution devices in neurological disorders: Where are we now? Neurol. Sci. 2016, 37, 503–514. [Google Scholar] [CrossRef]
- Ferreira, F.M.R.M.; Chaves, M.E.A.; Oliveira, V.C.; Martins, J.S.R.; Vimieiro, C.B.S.; Van Petten, A.M.V.N. Effect of Robot-Assisted Therapy on Participation of People with Limited Upper Limb Functioning: A Systematic Review with GRADE Recommendations. Occup. Ther. Int. 2021, 2021, 6649549. [Google Scholar] [CrossRef]
- Maggio, M.G.; De Luca, R.; Molonia, F.; Porcari, B.; Destro, M.; Casella, C.; Salvati, R.; Bramanti, P.; Calabrò, R.S. Cognitive rehabilitation in patients with traumatic brain injury: A narrative review on the emerging use of virtual reality. J. Clin. Neurosci. 2019, 61, 1–4. [Google Scholar] [CrossRef]
- Shin, H.; Kim, K. Virtual reality for cognitive rehabilitation after brain injury: A systematic review. J. Phys. Ther. Sci. 2015, 27, 2999–3002. [Google Scholar] [CrossRef]
- Oberholzer, M.; Müri, R.M. Neurorehabilitation of Traumatic Brain Injury (TBI): A Clinical Review. Med. Sci. 2019, 7, 47. [Google Scholar] [CrossRef]
- De Luca, R.; Bonanno, M.; Vermiglio, G.; Trombetta, G.; Andidero, E.; Caminiti, A.; Pollicino, P.; Rifici, C.; Calabrò, R.S. Robotic Verticalization plus Music Therapy in Chronic Disorders of Consciousness: Promising Results from a Pilot Study. Brain Sci. 2022, 12, 1045. [Google Scholar] [CrossRef] [PubMed]
- Nudo, R.J. Neural bases of recovery after brain injury. J. Commun. Disord. 2011, 44, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Braun, R.G. Wittenberg GF. Motor Recovery: How Rehabilitation Techniques and Technologies Can Enhance Recovery and Neuroplasticity. Semin. Neurol. 2021, 41, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Semprini, M.; Laffranchi, M.; Sanguineti, V.; Avanzino, L.; De Icco, R.; De Michieli, L.; Chiappalone, M. Technological Approaches for Neurorehabilitation: From Robotic Devices to Brain Stimulation and Beyond. Front. Neurol. 2018, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Müller-Eising, C.; Diliberti, M.L.; Manuli, A.; Parrinello, F.; Rao, G.; Barone, V.; Civello, T. Who Will Pay for Robotic Rehabilitation? The Growing Need for a Cost-effectiveness Analysis. Inno. Clin. Neurosci. 2020, 17, 14–16. [Google Scholar]
- Lo, A.C.; Guarino, P.D.; Richards, L.G.; Haselkorn, J.K.; Wittenberg, G.F.; Federman, D.G.; Ringer, R.J.; Wagner, T.H.; Krebs, H.I.; Volpe, B.T.; et al. An economic analysis of robot-assisted therapy for long-term upper-limb impairment after stroke. Stroke 2011, 42, 2630–2632. [Google Scholar] [CrossRef]
- Lo, K.; Stephenson, M.; Lockwood, C. The economic cost of robotic rehabilitation for adult stroke patients: A systematic review. JBI Database System Rev. Implement Rep. 2019, 17, 520–547. [Google Scholar] [CrossRef]
- Nascimento, A.S.; Fagundes, C.V.; Mendes, F.A.D.S.; Leal, J.C. Effectiveness of Virtual Reality Rehabilitation in Persons with Multiple Sclerosis: A Systematic Review and Meta-analysis of Randomised Controlled Trials. Mult. Scler. Relat. Disord. 2021, 54, 103128. [Google Scholar] [CrossRef]
- Truijen, S.; Abdullahi, A.; Bijsterbosch, D.; van Zoest, E.; Conijn, M.; Wang, Y.; Struyf, N.; Saeys, W. Effect of home-based virtual reality training and telerehabilitation on balance in individuals with Parkinson disease, multiple sclerosis, and stroke: A systematic review and meta-analysis. Neurol. Sci. 2022, 43, 2995–3006. [Google Scholar] [CrossRef]
- Mehrholz, J.; Thomas, S.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 2020, 10, CD006185. [Google Scholar] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- De Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Taveggia, G.; Ragusa, I.; Trani, V.; Cuva, D.; Angeretti, C.; Fontanella, M.; Panciani, P.P.; Borboni, A. Robotic tilt table reduces the occurrence of orthostatic hypotension over time in vegetative states. Int. J. Rehabil. Res. 2015, 38, 162–166. [Google Scholar] [CrossRef]
- Esquenazi, A.; Lee, S.; Wikoff, A.; Packel, A.; Toczylowski, T.; Feeley, J. A Comparison of Locomotor Therapy Interventions: Partial-Body Weight-Supported Treadmill, Lokomat, and G-EO Training in People With Traumatic Brain Injury. PMR 2017, 9, 839–846. [Google Scholar] [CrossRef]
- Williams, K.; Christenbury, J.; Niemeier, J.P.; Newman, M.; Pinto, S. Is Robotic Gait Training Feasible in Adults With Disorders of Consciousness? J. Head Trauma Rehabil. 2020, 35, E266–E270. [Google Scholar] [CrossRef]
- Esquenazi, A.; Lee, S.; Packel, A.T.; Braitman, L. A randomized comparative study of manually assisted versus robotic-assisted body weight supported treadmill training in persons with a traumatic brain injury. PMR 2013, 5, 280–290. [Google Scholar] [CrossRef]
- Cuthbert, J.P.; Staniszewski, K.; Hays, K.; Gerber, D.; Natale, A.; O’Dell, D. Virtual reality-based therapy for the treatment of balance deficits in patients receiving inpatient rehabilitation for traumatic brain injury. Brain Inj. 2014, 28, 181–188. [Google Scholar] [CrossRef]
- Ustinova, K.I.; Perkins, J.; Leonard, W.A.; Hausbeck, C.J. Virtual reality game-based therapy for treatment of postural and co-ordination abnormalities secondary to TBI: A pilot study. Brain Inj. 2014, 28, 486–495. [Google Scholar] [CrossRef]
- Tefertiller, C.; Hays, K.; Natale, A.; O’Dell, D.; Ketchum, J.; Sevigny, M.; Eagye, C.B.; Philippus, A.; Harrison-Felix, C. Results From a Randomized Controlled Trial to Address Balance Deficits After Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2019, 1409–1416. [Google Scholar] [CrossRef]
- Corallo, F.; Maresca, G.; Formica, C.; Bonanno, L.; Bramanti, A.; Parasporo, N.; Giambò, F.M.; De Cola, M.C.; Lo Buono, V. Humanoid Robot Use in Cognitive Rehabilitation of Patients with Severe Brain Injury: A Pilot Study. J. Clin. Med. 2022, 11, 2940. [Google Scholar] [CrossRef] [PubMed]
- Dvorkin, A.Y.; Ramaiya, M.; Larson, E.B.; Zollman, F.S.; Hsu, N.; Pacini, S.; Shah, A.; Patton, J.L. A “virtually minimal” visuo-haptic training of attention in severe traumatic brain injury. J. Neuroeng. Rehabil. 2013, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Bonanno, M.; Rifici, C.; Pollicino, P.; Caminiti, A.; Morone, G.; Calabrò, R.S. Does Non-Immersive Virtual Reality Improve Attention Processes in Severe Traumatic Brain Injury? Encouraging Data from a Pilot Study. Brain Sci. 2022, 12, 1211. [Google Scholar] [CrossRef]
- Alashram, A.R.; Annino, G.; Padua, E.; Romagnoli, C.; Mercuri, N.B. Cognitive rehabilitation post traumatic brain injury: A systematic review for emerging use of virtual reality technology. J. Clin. Neurosci. 2019, 66, 209–219. [Google Scholar] [CrossRef]
- Aulisio, M.C.; Han, D.Y.; Glueck, A.C. Virtual reality gaming as a neurorehabilitation tool for brain injuries in adults: A systematic review. Brain Inj. 2020, 34, 1322–1330. [Google Scholar] [CrossRef]
- Ettenhofer, M.L.; Guise, B.; Brandler, B.; Bittner, K.; Gimbel, S.I.; Cordero, E.; Nelson Schmitt, S.; Williams, K.; Cox, D.; Roy, M.J. Neurocognitive Driving Rehabilitation in Virtual Environments (NeuroDRIVE): A pilot clinical trial for chronic traumatic brain injury. NeuroRehabilitation 2019, 44, 531–544. [Google Scholar] [CrossRef]
- Jacoby, M.; Averbuch, S.; Sacher, Y.; Katz, N.; Weiss, P.L.; Kizony, R. Effectiveness of executive functions training within a virtual supermarket for adults with traumatic brain injury: A pilot study. IEEE Trans. Neural. Syst. Rehabil. Eng. 2013, 21, 182–188. [Google Scholar] [CrossRef]
- De Luca, R.; Maggio, M.G.; Maresca, G.; Latella, D.; Cannavò, A.; Sciarrone, F.; Lo Voi, E.; Accorinti, M.; Bramanti, P.; Calabrò, R.S. Improving Cognitive Function after Traumatic Brain Injury: A Clinical Trial on the Potential Use of the Semi-Immersive Virtual Reality. Behav. Neurol. 2019, 2019, 9268179. [Google Scholar] [CrossRef]
- Lebowitz, M.S.; Dams-O’Connor, K.; Cantor, J.B. Feasibility of computerized brain plasticity-based cognitive training after traumatic brain injury. J. Rehabil. Res. Dev. 2012, 49, 1547–1556. [Google Scholar] [CrossRef]
- Zickefoose, S.; Hux, K.; Brown, J.; Wulf, K. Let the games begin: A preliminary study using attention process training-3 and Lumosity brain games to remediate attention deficits following traumatic brain injury. Brain Inj. 2013, 27, 707–716. [Google Scholar] [CrossRef]
- Ownsworth, T.; Arnautovska, U.; Beadle, E.; Shum, D.H.K.; Moyle, W. Efficacy of Telerehabilitation for Adults With Traumatic Brain Injury: A Systematic Review. J. Head Trauma Rehabil. 2018, 33, E33–E46. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Maggio, M.G.; Naro, A.; Portaro, S.; Cannavò, A.; Calabrò, R.S. Can patients with severe traumatic brain injury be trained with cognitive telerehabilitation? An inpatient feasibility and usability study. J. Clin. Neurosci. 2020, 79, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Raso, M.G.; Arcuri, F.; Liperoti, S.; Mercurio, L.; Mauro, A.; Cusato, F.; Romania, L.; Serra, S.; Pignolo, L.; Tonin, P. Telemonitoring of Patients With Chronic Traumatic Brain Injury: A Pilot Study. Front. Neurol. 2021, 12, 598777. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.K.; Kim, D.Y.; Paik, N.J. Transcranial direct current stimulation of the left prefrontal cortex improves attention in patients with traumatic brain injury: A pilot study. J. Rehabil. Med. 2012, 44, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Shanmugalingam, A.; McIntyre, A.; Burhan, A.M. The Effect of Non-Invasive Brain Stimulation (NIBS) on Executive Functioning, Attention and Memory in Rehabilitation Patients with Traumatic Brain Injury: A Systematic Review. Diagnostics 2021, 11, 627. [Google Scholar] [CrossRef]
- Sacco, K.; Galetto, V.; Dimitri, D.; Geda, E.; Perotti, F.; Zettin, M.; Geminiani, G.C. Concomitant use of transcranial direct current stimulation and computer-assisted training for the rehabilitation of attention in traumatic brain injured patients: Behavioral and neuroimaging results. Front. Behav. Neurosci 2016, 10, 57. [Google Scholar] [CrossRef]
- Ulam, F.; Shelton, C.; Richards, L.; Davis, L.; Hunter, B.; Fregni, F.; Higgins, K. Cumulative effects of transcranial direct current stimulation on EEG oscillations and attention/working memory during subacute neurorehabilitation of traumatic brain injury. Clin. Neurophysiol. 2015, 126, 486–496. [Google Scholar] [CrossRef]
- Leśniak, M.; Polanowska, K.; Seniów, J.; Członkowska, A. Effects of repeated anodal tDCS coupled with cognitive training for patients with severe traumatic brain injury. J. Head Trauma Rehabil. 2014, 29, E20–E29. [Google Scholar] [CrossRef]
- Riener, R.; Lünenburger, L.; Colombo, G. Human-centered robotics applied to gait training and assessment. J. Rehabil. Res. Dev. 2006, 43, 679–694. [Google Scholar] [CrossRef]
- Lünenburger, L.; Colombo, G.; Riener, R.; Dietz, V. Biofeedback in gait training with the robotic orthosis Lokomat. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2007, 2004, 4888–4891. [Google Scholar] [CrossRef]
- Santamaria, V.; Luna, T.; Khan, M.; Agrawal, S. The robotic Trunk-Support-Trainer (TruST) to measure and increase postural workspace during sitting in people with spinal cord injury. Spinal Cord Ser. Cases 2020, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Hornby, T.G.; Reisman, D.S.; Ward, I.G.; Scheets, P.L.; Miller, A.; Haddad, D.; Fox, E.J.; Fritz, N.E.; Hawkins, K.; Henderson, C.E. Clinical Practice Guideline to Improve Locomotor Function Following Chronic Stroke, Incomplete Spinal Cord Injury, and Brain Injury. J. Neurol. Phys. Ther. 2020, 44, 49–100. [Google Scholar] [CrossRef] [PubMed]
- Turolla, A.; Venneri, A.; Farina, D.; Cagnin, A.; Cheung, V. Rehabilitation Induced Neural Plasticity after Acquired Brain Injury. Neural. Plast. 2018, 6565418. [Google Scholar] [CrossRef]
- Retel Helmrich, I.; Lingsma, H.F.; Turgeon, A.F.; Yamal, J.M.; Steyerberg, E.W. Prognostic Research in Traumatic Brain Injury: Markers, Modeling, and Methodological Principles. J. Neurotrauma 2021, 38, 2502–2513. [Google Scholar] [CrossRef]
- Assad-Uz-Zaman, M.; Rasedul Islam, M.; Miah, S.; Rahman, M.H. NAO robot for cooperative rehabilitation training. J. Rehabil. Assist. Technol. Eng. 2019, 6, 2055668319862151. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.K.; Fountain, M.K.; Hood, A.F.; Verduzco-Gutierrez, M. Upper Limb Motor Improvement after Traumatic Brain Injury: Systematic Review of Interventions. Neurorehabil. Neural. Repair 2022, 36, 17–37. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Naro, A.; Russo, M.; Leo, A.; De Luca, R.; Balletta, T.; Buda, A.; La Rosa, G.; Bramanti, A.; Bramanti, P. The role of virtual reality in improving motor performance as revealed by EEG: A randomized clinical trial. J. Neuroeng. Rehabil. 2017, 14, 53. [Google Scholar] [CrossRef]
- Hao, J.; Xie, H.; Harp, K.; Chen, Z.; Siu, K.C. Effects of Virtual Reality Intervention on Neural Plasticity in Stroke Rehabilitation: A Systematic Review. Arch. Phys. Med. Rehabil. 2022, 103, 523–541. [Google Scholar] [CrossRef]
- Highland, K.B.; Kruger, S.E.; Roy, M.J. If You Build It, They Will Come, But What Will Wounded Warriors Experience? Presence in the CAREN. Stud. Health Technol. Inform. 2015, 219, 23–27. [Google Scholar] [PubMed]
- Sessoms, P.H.; Gottshall, K.R.; Collins, J.D.; Markham, A.E.; Service, K.A.; Reini, S.A. Improvements in gait speed and weight shift of persons with traumatic brain injury and vestibular dysfunction using a virtual reality computer-assisted rehabilitation environment. Mil. Med. 2015, 180, 143–149. [Google Scholar] [CrossRef]
- Lippa, S.M.; Rosen, K.B.; Delpy, K.B.; Pape, M.M.; Kruger, S.E. Overground and Virtual Reality Gait Speed Are Associated With Atypical Symptom Reporting in Active Duty Service Members With a History of Mild to Moderate Traumatic Brain Injury. J. Head Trauma Rehabil. 2021, 37, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Fetta, J.; Starkweather, A.; Gill, J.M. Computer-Based Cognitive Rehabilitation Interventions for Traumatic Brain Injury: A Critical Review of the Literature. J. Neurosci. Nurs. 2017, 49, 235–240. [Google Scholar] [CrossRef]
- Subbarao, B.S.; Stokke, J.; Martin, S.J. Telerehabilitation in Acquired Brain Injury. Phys. Med. Rehabil. Clin. N. Am. 2021, 32, 223–238. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Bramanti, A.; Garzon, M.; Celesti, A.; Russo, M.; Portaro, S.; Naro, A.; Manuli, A.; Tonin, P.; Bramanti, P. Telerehabilitation in individuals with severe acquired brain injury: Rationale, study design, and methodology. Medicine 2018, 97, e13292. [Google Scholar] [CrossRef] [PubMed]
- Bramanti, A.; Manuli, A.; Calabrò, R.S. Stroke Telerehabilitation in Sicily: A Cost-Effective Approach to Reduce Disability? Innov. Clin. Neurosci. 2018, 15, 11–15. [Google Scholar]
- Lindberg, B.; Nilsson, C.; Zotterman, D.; Söderberg, S.; Skär, L. Using Information and Communication Technology in Home Care for Communication between Patients, Family Members, and Healthcare Professionals: A Systematic Review. Int. J. Telemed. Appl. 2013, 2013, 461829. [Google Scholar] [CrossRef]
- Stagg, C.J.; Lin, R.L.; Mezue, M.; Segerdahl, A.; Kong, Y.; Xie, J.; Tracey, I. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. J. Neurosci. 2013, 33, 11425–11431. [Google Scholar] [CrossRef]
- De Munter, L.; Polinder, S.; Havermans, R.J.M.; Steyerberg, E.W.; de Jongh, M.A.C. Prognostic factors for recovery of health status after injury: A prospective multicentre cohort study. BMJ Open 2021, 11, e038707. [Google Scholar] [CrossRef]

| Reference | Sample Size (Subjects Affected) | Study Design | Severity | Robotic Device/Advanced Approach | Outcome Measure | Intensity of Duration Training | Major Findings | PEDro Score |
|---|---|---|---|---|---|---|---|---|
| [12] (De Luca et al., 2022) | 16 | Pilot study | MCS (9 plus and 7 minus) | Erigo device for verticalization plus music stimulation | CRS-r; LCF; FIM; FCS; TCT; | Three times a week, for about 8 consecutive weeks, each session lasting about 45 min | In the experimental group, significant changes were found in patients’ awareness, global functional outcome, and non-verbal skills, while the control group, showed improvements in the individual scores without reaching a statistical significance. | 6 |
| [24] (Taveggia et al., 2015) | 8 | RCT | VS and MCS | Erigo device with a constant monitoring of Blood pressure and rate with SOMNOscreen plus, a polygraphy device | LCF, CRS-r; blood pressure; heart rate | Each training was performed in the morning in appropriate physical therapy room. Sessions were repeated three times a week for 24 sessions. | Training with the Erigo device, thanks to the integrated cycling movements, decreased the cardiovascular distress in MCS and VS patients with hemodynamic instability. | 3 |
| [25] (Esquenazi et al., 2017) | 22 | Randomised, prospective, pilot study | Moderate TBI | G-EO; Lokomat; manual assisted BWSTT | SSV; MV; spatiotemporal asymmetry ratio; 6MWT; MSIS | The authors administered 18 sessions of gait training for 6 to 8 weeks, generally 3 times per week. Each session lasted up to 75 min | Gait training rehabilitation using G-EO, Lokomat, or PBWSTT in individuals with chronic TBI improved SSV. MV was improved only in Lokomat and BWSTT groups; patients treated with G-EO and PBWSTT significantly increased their endurance (6MWT) in the post-training | 6 |
| [26] (Williams et al., 2020) | 4 | Clinical Trial | MCS (lack of functional communication or ability to consistently follow commands) | RAGT with Lokomat | HR; MAP; SaO2; ABS; FLACC; CRS-r; MAS; RLA | 5 to 20 min, over 1- to 2-week periods. | Authors suggested that RAGT could be used in DoC due to TBI, as a safe and feasible intervention, as a part of a physical therapy program in adults with DoC due to TBI. However, they reported that two patients had experienced adverse symptoms such as pain from harness placement and increased somnolence. | 1 |
| [27] (Esquenazi et al., 2013) | 16 | Randomised prospective study | Moderate TBI | RATT and MATT | SSV;MV; spatiotemporal asymmetry ratio; 6MWT; MSIS | Each training lasted 45 min, 3 times a week with either RATT or MATT for a total of 18 training sessions. | Both patient groups showed significant improvement in their motor function. However, no training technique appears to be superior to the other, between the two intervention groups. | 4 |
| [28] (Cuthbert et al., 2014) | 20 | Randomised controlled trial | Moderate TBI | Nintendo Wii (virtual reality gaming system) | PACES; BBS; FGA | 8 min of Wii Fit balance board games and 7 min of Wii Sport games, 4-times per week | The authors presented that the administration of Wii balance board is more useful to informally assess or treat static balance alteration rather than for dynamic balance. | 2 |
| [29] (Ustinova et al., 2014) | 15 | Pilot study | Moderate TBI | interactive, customised VR games and scenarios, utilising an Xbox Kinect sensor | BBS; FGA; KAS; FRT | Patients underwent 15 sessions, lasting 50–55 min, scheduled 2–3 times a week over 5–6 consecutive week | This study showed that patients affected by chronic impairments due to TBI increased their postural stability, gait, arm movement and coordination, thanks to VR training. | 4 |
| [30] (Teterfiller et al., 2019) | 63 | Randomised Controlled Trial | Moderate to severe TBI | Xbox Kinect games | CB&MS; BESTest; ABC; PART-O | Each session was scheduled 3–4 times per week for 12 weeks, lasting for 30 min. | No statistical significance between the two groups was achieved; however, both treatment groups presented better balance responses to these interventions in chronic TBI patients. | 6 |
| [31] (Corallo et al., 2022) | 12 | Pilot study | Severe TBI | Human-Robot Pepper | LCF; MMSE; SIB; BDI I-II; HAM-A; FIM; EQ-5D | Each session was scheduled for 3 times a week for 8 weeks, lasting for 60 min. | Results demonstrated that the experimental group presented better improvements in mood symptoms and in QoL than controls, unlike their cognitive performance that did not register any improvement. | 5 |
| [32] (Dvorkin et al., 2013) | 21 | Pilot study | Severe TBI | VRROOM system (Virtual Reality and Robotics Optical Operations Machine) | Kinematic analysis of arm movements; RLA | Patients were evaluated for two consecutive days about 3 conditions (no haptic feedback, a break-through force, and haptic nudge) lasting for 12 successive, 4-min blocks. | Patients well-tolerated the Visuo-haptic environments that promoted motor functions in TBI population. | 4 |
| [33] (De Luca et al., 2022) | 30 | Pilot study | Severe TBI | VRRS | MoCA; AM; TMT; HRS-D | Training session was organised for 3 times a week for 8 weeks (i.e., 24 sessions lasting for 45 min each). | The authors found great improvements in cognitive performance and mood symptoms in both two groups, especially in the experimental group, which received training using the VRRS; improvements were registered in each specific attention (i.e., visual attention, task switching, visual search speed, etc.). | 5 |
| [34] (Alasharam et al., 2019) | 9 studies | Systematic review | Moderate to Severe | Semi-Immersive and non-immersive virtual systems | Memory (Digit Span); Executive Functioning (WSCT and London Tower); Attention | Ten sessions, 3–4 times per week, 45 min, 2 times per week for 6 weeks, 30–45 min in duration, received 12 sessions of 20–25 min, received 8 sessions, 2 times per week for 4 weeks, 60 min in duration | This systematic review concluded that VR training intervention may improve memory and executive function as an aspect of cognitive function in patients with TBI. However, weak evidence was found about the positive effect of VR intervention on attention post-TBI. | n/A |
| [35] (Ausilio et al., 2020) | 12 studies | Systematic review | Moderate to severe | CAREN, VROOM, Kinect sensors, VMall | Gait; Balance; Cognition | Two times—6 weeks, 2 times—2 months, 10 sessions, 12 sessions, 12 sessions over 4 weeks | VR training has been shown to have a potentially beneficial role in gait and balance deficits in patients with TBI. However, the evidence about the use of VR systems in the treatment of upper limb functioning is still limited in comparison. Finally, the use of VR for cognitive rehabilitation is widely supported, especially in TBI patients. | n/A |
| [36] (Ettenhofer et al., 2019) | 11 | Pilot study | Moderate to severe | NeuroDRIVE | VR Driving Assessment; TMT; WAIS-IV; COWATLA; CVLT-II; Grooved Pegboard; NSI; BDI; Epworth Sleepiness Scale; FSS; SF-36; Satisfaction with Life Scale | Six 90-min sessions (9 h total) conducted over a four-week period | The NeuroDRIVE intervention could be a valuable and useful tool to train cognitive functioning; although it seems that this innovative intervention does not produce improvements in driving abilities, measured with the VR driving assessment. | 2 |
| [37] (Jacoby et al., 2013) | 12 | Pilot study | Moderate to severe | VMall, virtual supermarket environment— is operated via GestureTek’s Interactive Rehabilitation and Exercise System (IREX) video capture system | EFPT; MET-SV | Ten 45-min treatment sessions, 3–4 times per week via the VMall environment | Both kinds of treatment, through VR and not, showed improvements in the IADL activities. Standard OT (without VR) showed better outcomes in daily activities. | 3 |
| [38] (De Luca et al. 2019) | 100 | Clinical trial | Moderate TBI | BTS-Nirvana | MoCA; HRS-D; HRS-A; TMT; VS; FAB; WEIGL | Total of 24 1 h sessions (3 times a week for 8 weeks) | Each treatment produced improvements in cognitive functioning in addition to mood. However, the authors found that only the experimental group presented significantly better results in cognitive flexibility and shifting skills (TMT B-A) and in selective attention/visual research (VS). | 4 |
| [39] (Lebowitz et al., 2012) | 10 | Pilot study | Moderate to severe | Cortex with InSight | ANAM4, a validated, computerized neuropsychological battery that takes about 30 min to administer; CFQ; FrSBe | At home 40 min/d, 5 d/wk for 6 wk | The authors concluded that the use of PC software could be a promising and well-tolerated therapy for TBI patients. Only fatigue was reported by the participants, as a side effect. | 2 |
| [40] (Zickefoose et al. 2013) | 4 | Pilot study | Severe TBI | Attention Process Training-3 (APT-3) and Lumosity™ (2010) Brain Games | WAB-r; NAB, and a designed measure to inform about patients’ perceptions regarding the two intervention programmes | Twenty treatment sessions within a 1-month period, with each session lasting 30 min. | The four patients showed progressive improvements in reaching new levels of difficulty on the tasks during the APT-3 and Lumosity training. | 1 |
| [41] (Ownsworth et al., 2018) | 13 studies | Systematic review | Moderate to severe | Telerehabilitation about the feasibility and/or efficacy of telephone-based (10 studies) and Internet-based (3 studies) interventions | Functional status and depressive symptoms, global composite of functioning, Self-report measures assessed mood, behaviour. Family caregivers reported everyday memory problems, goals, and strategy use | Eight-week in-hospital cognitive rehabilitation program, the 8 × 30-min telephone counselling sessions | Telephone-based interventions were shown to be a promising tool to allow access to specific remote interventions for people with TBI. However, this intervention was limited to short-term outcomes. On the other hand, internet-based intervention studies were focused on the feasibility of web systems. | n/A |
| [42] (De Luca et al., 2020) | 10 | Feasibility and usability study | Severe TBI | Telerehabilitation System VRRS | IMI and SUS | Six training sessions, provided 3 times per week for two weeks, each session lasting about one hour | The telerehabilitation system showed to have good usability, in addition to its advantages, such as facilitating hospital discharge, and optimising motivation during the training. | 1 |
| [43] (Raso et al., 2021) | 22 | Pilot study | VS and MCS | Advanced video conferencing telehealth system for controlling neurological patients at home. | CSR-r, LCF, WHIM, NCS. | 24 h/d, 7 d/wk for monitoring all basic care activities. | Telehealthcare system demonstrated to be not inferior to usual in-person care, to manage DoC due to TBI. | 7 |
| [44] (Kang et al., 2012) | 9 | Double-blinded pilot study | Moderate to Severe | Anodal transcranial direct current stimulation | Computerised contrast reaction time task and numeric rating scale describing levels of attention, fatigue, task difficulty, and sleep quality | tDCS stimulation was delivered for 20 min | The authors demonstrated that the application of an excitatory anodal tDCS on the left DLPFC was associated with shortened reaction times in TBI patients, while the sham stimulation had no discernible effects. This study concluded that NIBS could be used to stimulate attention in TBI people. | 6 |
| [45] (Hara et al., 2021) | 5 studies | Systematic review | Moderate to severe | rTMS and tDCS | rTMS, target symptoms included attention (n = 2), memory (n = 1), and executive function (n = 2) tDCS studies, target symptoms included cognition (n = 2), attention (n = 3), memory (n = 3), working memory (n = 3), and executive function (n = 1) | 10 Hz 110% MT 2000 pulses/session, 1 Hz 100% MT 2000 pulses/session, 2 mA/35 cm2 × 20 min. Two anodes, 1 mA/25 cm2 × 20 min, Anodal electrode, 1 mA/10 min/current density = 0.028 mA/cm2, Anodal tDCS | In this systematic review, DLPFC was chosen as the preferential stimulation site in all included studies. In some cases, authors registered improvements in comparison with the control group. However, which method is more effective between rTMS or tDCS remains unknown. In conclusion, the authors pointed out that NIBS is more likely to produce improvements when it is combined with other rehabilitative approaches. | n/A |
| [46] (Sacco et al., 2016) | 32 | Pilot study | Severe TBI | tDCS stimulation was performed using a HDCstim device (Newronika srl, Milan, Italy). | TEA; RBANS; BDI; AES | Ten sessions, each session included 20 min of tDCS stimulation followed by 30 min of cognitive training | The proposed treatment produced promising positive results in Divided Attention. In addition, the association between tDCS and computer-based training may have allowed a neural reorganisation, reducing the patients’ cognitive effort. | 4 |
| [47] (Ulam et al., 2015) | 26 | Randomised, double-blind study | Moderate to severe | Anodal tDCS on EEG oscillations | Digit Span; WAIS IV | 20 min sessions of 1 mA anodal stimulation to the left dorsolateral prefrontal cortex (F3, cathode placed at right supraorbital site, Fp2) were provided on 10 consecutive days | In this study, the authors concluded that the use of tDCS can be an emerging tool for the treatment of the neuropsychological sequelae due to TBI, also in the subacute stage of recovery. | 3 |
| [48] (Lesniak et al., 2014) | 23 | Pilot randomised controlled trial | Severe TBI | Transcranial direct current stimulation (A-tDCS) of the left dorsolateral prefrontal cortex | RAVLT; PRM; PASAT; SSP; RVP; EBIQ | A-tDCS (10 min; 1 mA; in the DLPFC, followed by rehabilitative cognitive training daily for 15 days. | The use of tDCS increased the score of most outcome measures, including an auditory verbal memory test, 2 working memory tests, and an attention test, registering a positive response to stimulation, although there were no statistical differences between the experimental and control groups. | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanno, M.; De Luca, R.; De Nunzio, A.M.; Quartarone, A.; Calabrò, R.S. Innovative Technologies in the Neurorehabilitation of Traumatic Brain Injury: A Systematic Review. Brain Sci. 2022, 12, 1678. https://doi.org/10.3390/brainsci12121678
Bonanno M, De Luca R, De Nunzio AM, Quartarone A, Calabrò RS. Innovative Technologies in the Neurorehabilitation of Traumatic Brain Injury: A Systematic Review. Brain Sciences. 2022; 12(12):1678. https://doi.org/10.3390/brainsci12121678
Chicago/Turabian StyleBonanno, Mirjam, Rosaria De Luca, Alessandro Marco De Nunzio, Angelo Quartarone, and Rocco Salvatore Calabrò. 2022. "Innovative Technologies in the Neurorehabilitation of Traumatic Brain Injury: A Systematic Review" Brain Sciences 12, no. 12: 1678. https://doi.org/10.3390/brainsci12121678
APA StyleBonanno, M., De Luca, R., De Nunzio, A. M., Quartarone, A., & Calabrò, R. S. (2022). Innovative Technologies in the Neurorehabilitation of Traumatic Brain Injury: A Systematic Review. Brain Sciences, 12(12), 1678. https://doi.org/10.3390/brainsci12121678








