Altered Cerebro-Cerebellar Effective Connectivity in New-Onset Juvenile Myoclonic Epilepsy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Magnetic Resonance Imaging Acquisition and Data Preprocessing
2.3. Degree Centrality Analysis
2.4. Effective Connectivity Analysis
2.5. Statistical Analysis
3. Results
3.1. General Data
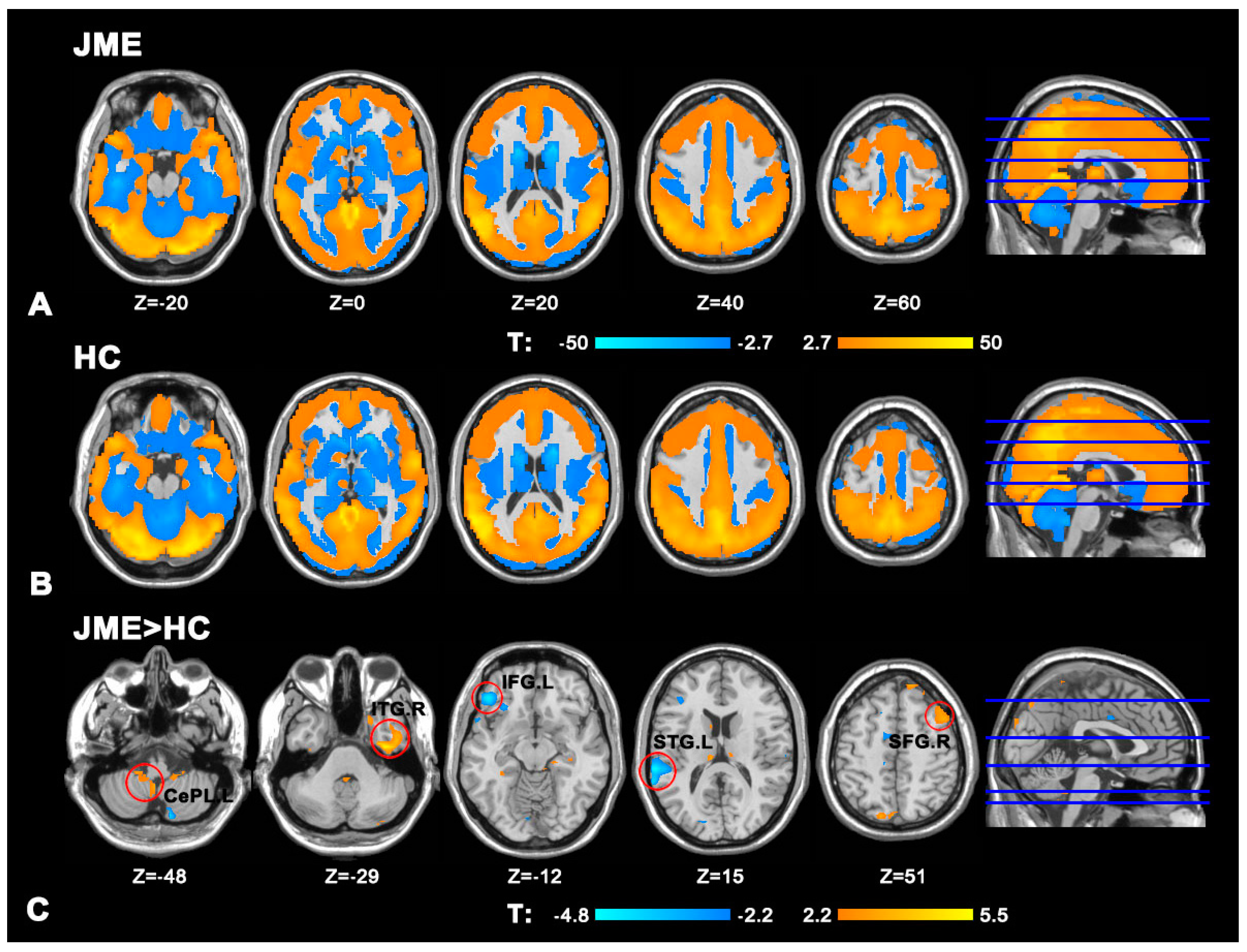
3.2. Degree Centrality Analysis
3.3. Effective Connectivity Analysis
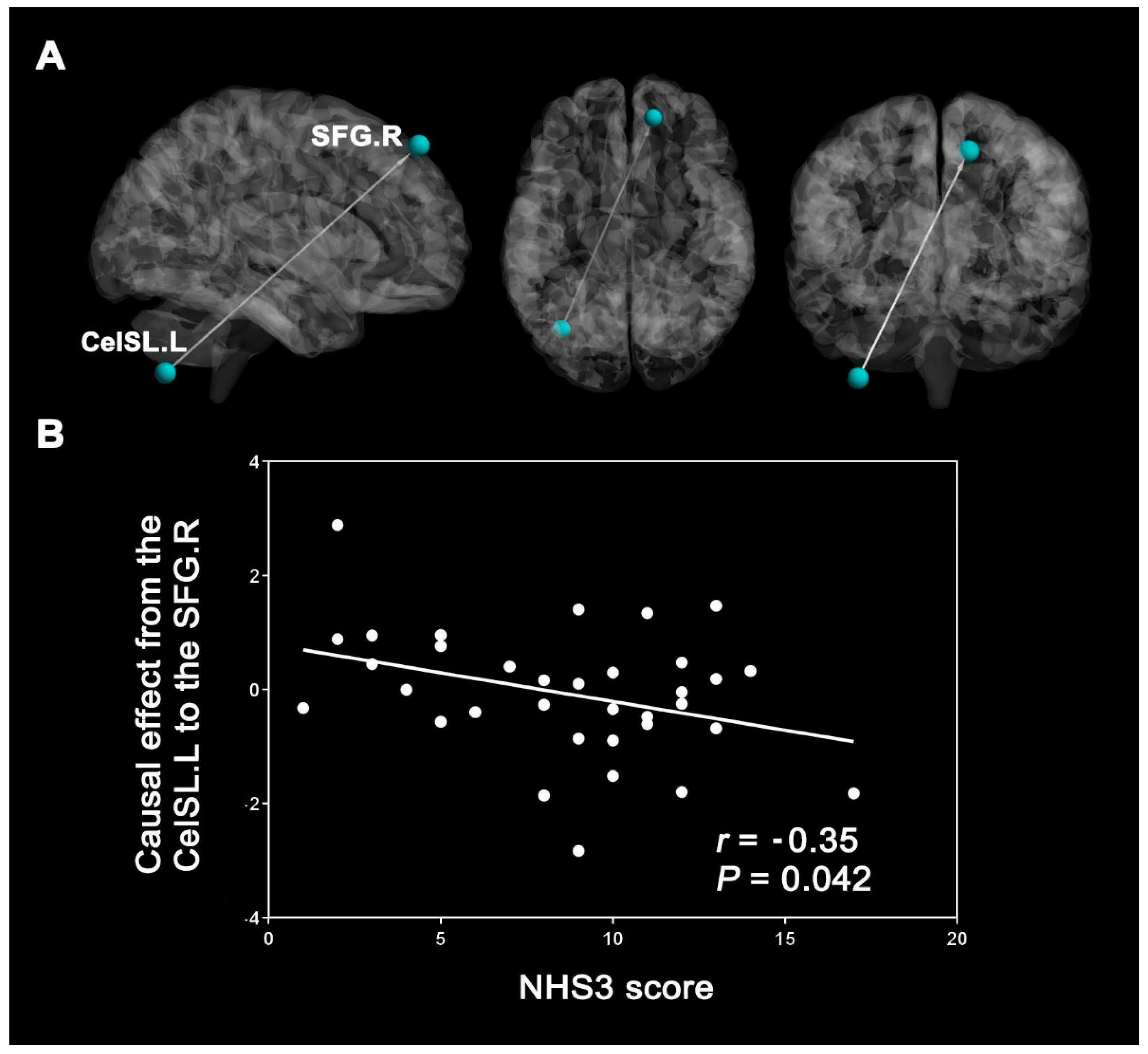
3.4. Correlation Results
4. Discussion
4.1. Analyses of Differences in DC Values between Groups
4.1.1. The Encephalic Region and the Significance of Increased DC Values
4.1.2. The Encephalic Region and the Significance of Decreased DC Values
4.2. Effective Connectivity Analysis
4.3. Correlation Analysis
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| JME | juvenile myoclonic epilepsy |
| EC | effective connectivity |
| HC | healthy control |
| DC | degree centrality |
| GCA | Granger causality analysis |
| CePL.L | left cerebellum posterior lobe |
| ITG.R | right inferior temporal gyrus |
| SFG.R | right superior frontal gyrus |
| IFG.L | left inferior frontal gyrus |
| STG.L | left superior temporal gyrus |
| PreCU.R | right precuneus |
| CeAL.L | left cerebellum anterior lobe |
| CePL.R | right cerebellum posterior lobe |
| CeISL.L | left inferior semi-lunar lobule of cerebellum |
| IGE | idiopathic systemic epilepsy |
| EEG | electroencephalogram |
| GSWD | generalized spike-wave or polyspike-wave discharge |
| rsFC | resting state functional connectivity |
| FC | functional connectivity |
| ICA | independent component analysis |
| ROI | region of interest |
| DEC | dynamic effective connectivity |
| SN | salience network |
| DMN | default mode network |
| NHS3 | National Hospital Seizure Severity Scale |
| GRE-EPI | gradient-echo echo-planar imaging |
| TR | repetition time |
| TE | echo time |
| FOV | field of view |
| MPRAGE | magnetization-prepared rapid gradient-echo |
| FD | frame-wise displacement |
| MNI | Montreal Neurological Institute |
| FWHM | full width at half maximum |
| SMA | supplementary motor area |
| CN | cerebellar nucleus |
| PFC | prefrontal cortex |
| LF | lateral fissure |
| CEN | central control network |
| ECoG | electrocorticographic |
| BOLD | blood oxygen level-dependent |
| DCM | dynamic causal modeling |
| GSW | generalized spike wave |
| GTCS | generalized tonic-clonic seizure |
References
- Lee, H.J.; Park, K.M. Structural and functional connectivity in newly diagnosed juvenile myoclonic epilepsy. Acta Neurol. Scand. 2019, 139, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Janz, D. Epilepsy with impulsive petit mal (juvenile myoclonic epilepsy). Acta Neurol. Scand. 1985, 72, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camfield, C.S.; Striano, P.; Camfield, P.R. Epidemiology of juvenile myoclonic epilepsy. Epilepsy Behav. 2013, 28 (Suppl. 1), S15–S17. [Google Scholar] [CrossRef] [PubMed]
- Vollmar, C.; O’Muircheartaigh, J.; Barker, G.J.; Symms, M.R.; Thompson, P.; Kumari, V.; Duncan, J.S.; Janz, D.; Richardson, M.P.; Koepp, M.J. Motor system hyperconnectivity in juvenile myoclonic epilepsy: A cognitive functional magnetic resonance imaging study. Brain 2011, 134, 1710–1719. [Google Scholar] [CrossRef]
- Anderson, J.; Hamandi, K. Understanding juvenile myoclonic epilepsy: Contributions from neuroimaging. Epilepsy Res. 2011, 94, 127–137. [Google Scholar] [CrossRef]
- Pugnaghi, M.; Carmichael, D.W.; Vaudano, A.E.; Chaudhary, U.J.; Benuzzi, F.; Di Bonaventura, C.; Giallonardo, A.T.; Rodionov, R.; Walker, M.C.; Duncan, J.S.; et al. Generalized spike and waves: Effect of discharge duration on brain networks as revealed by BOLD fMRI. Brain Topogr. 2014, 27, 123–137. [Google Scholar] [CrossRef]
- Lee, H.J.; Seo, S.A.; Lee, B.I.; Kim, S.E.; Park, K.M. Thalamic nuclei volumes and network in juvenile myoclonic epilepsy. Acta Neurol. Scand. 2020, 141, 271–278. [Google Scholar] [CrossRef]
- Ekmekci, B.; Bulut, H.T.; Gümüştaş, F.; Yıldırım, A.; Kuştepe, A. The relationship between white matter abnormalities and cognitive functions in new-onset juvenile myoclonic epilepsy. Epilepsy Behav. 2016, 62, 166–170. [Google Scholar] [CrossRef]
- Marcián, V.; Filip, P.; Bareš, M.; Brázdil, M. Cerebellar Dysfunction and Ataxia in Patients with Epilepsy: Coincidence, Consequence, or Cause? Tremor Hyperkinet. Mov. 2016, 6, 376. [Google Scholar] [CrossRef]
- Marcián, V.; Mareček, R.; Koriťáková, E.; Pail, M.; Bareš, M.; Brázdil, M. Morphological changes of cerebellar substructures in temporal lobe epilepsy: A complex phenomenon, not mere atrophy. Seizure 2018, 54, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, L.; Fan, B.; Chen, Z.; Chen, Z.; Lv, C.; Zheng, J. Disruption of Cerebellar-Cerebral Functional Connectivity in Temporal Lobe Epilepsy and the Connection to Language and Cognitive Functions. Front. Neurosci. 2022, 16, 871128. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Pang, X.; Wang, Y.; Li, C.; Long, Q.; Zheng, J. Altered interhemispheric functional homotopy and connectivity in temporal lobe epilepsy based on fMRI and multivariate pattern analysis. Neuroradiology 2021, 63, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Liu, R.; Luo, C.; Jiang, S.; Dong, L.; Peng, R.; Guo, F.; Wang, P. Altered Structural and Functional Connectivity of Juvenile Myoclonic Epilepsy: An fMRI Study. Neural Plast. 2018, 2018, 7392187. [Google Scholar] [CrossRef]
- Garcia-Ramos, C.; Dabbs, K.; Lin, J.J.; Jones, J.E.; Stafstrom, C.E.; Hsu, D.A.; Meyerand, M.E.; Prabhakaran, V.; Hermann, B.P. Progressive dissociation of cortical and subcortical network development in children with new-onset juvenile myoclonic epilepsy. Epilepsia 2018, 59, 2086–2095. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Wenhua, L.; Liu, Y.; Ruan, X.; Chen, X.; Liu, L.; Yu, S.; Chan, R.C.; Wei, X.; Jiang, X. Decreased Subcortical and Increased Cortical Degree Centrality in a Nonclinical College Student Sample with Subclinical Depressive Symptoms: A Resting-State fMRI Study. Front. Hum. Neurosci. 2016, 10, 617. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Feng, Y.; Xu, J.J.; Mao, C.N.; Xia, W.; Ren, J.; Yin, X. Disrupted Brain Functional Network Architecture in Chronic Tinnitus Patients. Front. Aging Neurosci. 2016, 8, 174. [Google Scholar] [CrossRef] [Green Version]
- Friston, K.J. Functional and effective connectivity in neuroimaging: A synthesis. Hum. Brain Mapp. 1994, 2, 56–78. [Google Scholar] [CrossRef]
- Hua, M.; Peng, Y.; Zhou, Y.; Qin, W.; Yu, C.; Liang, M. Disrupted pathways from limbic areas to thalamus in schizophrenia highlighted by whole-brain resting-state effective connectivity analysis. Prog Neuropsychopharmacol. Biol. Psychiatry 2020, 99, 109837. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, G.; Zheng, W.; Shi, J.; Liu, H.; Sun, Y. Altered dynamic effective connectivity of the default mode network in newly diagnosed drug-naive juvenile myoclonic epilepsy. Neuroimage Clin. 2020, 28, 102431. [Google Scholar] [CrossRef]
- Piper, R.J.; Richardson, R.M.; Worrell, G.; Carmichael, D.W.; Baldeweg, T.; Litt, B.; Denison, T.; Tisdall, M.M. Towards network-guided neuromodulation for epilepsy. Brain 2022, 145, 3347–3362. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, J.B.; Suh, S.I. Alteration of cerebello-thalamocortical spontaneous low-frequency oscillations in juvenile myoclonic epilepsy. Acta Neurol. Scand. 2019, 140, 252–258. [Google Scholar] [CrossRef]
- Jiang, S.; Li, X.; Li, Z.; Chang, X.; Chen, Y.; Huang, Y.; Zhang, Y.; Wang, H.; Zuo, X.; Li, X.; et al. Cerebello-cerebral connectivity in idiopathic generalized epilepsy. Eur. Radiol. 2020, 30, 3924–3933. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Zeng, L.L.; Song, Y.; Shen, H.; Fang, P.; Zhang, L.; Xu, L.; Gong, J.; Zhang, Y.C.; Zhang, Y.; et al. Altered cerebellar-cerebral functional connectivity in benign adult familial myoclonic epilepsy. Epilepsia 2016, 57, 941–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streng, M.L.; Krook-Magnuson, E. The cerebellum and epilepsy. Epilepsy Behav. 2021, 121, 106909. [Google Scholar] [CrossRef] [PubMed]
- Engel, J., Jr.; International League Against Epilepsy (ILAE). A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: Report of the ILAE Task Force on Classification and Terminology. Epilepsia 2001, 42, 796–803. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, M.F.; Duncan, J.S.; Sander, J.W. The National Hospital Seizure Severity Scale: A further development of the Chalfont Seizure Severity Scale. Epilepsia 1996, 37, 563–571. [Google Scholar] [CrossRef]
- Chao-Gan, Y.; Yu-Feng, Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front. Syst. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, X.N.; Ehmke, R.; Mennes, M.; Imperati, D.; Castellanos, F.X.; Sporns, O.; Milham, M.P. Network centrality in the human functional connectome. Cereb. Cortex 2012, 22, 1862–1875. [Google Scholar] [CrossRef]
- Buckner, R.L.; Sepulcre, J.; Talukdar, T.; Krienen, F.M.; Liu, H.; Hedden, T.; Andrews-Hanna, J.R.; Sperling, R.A.; Johnson, K.A. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 1860–1873. [Google Scholar] [CrossRef]
- Li, S.; Ma, X.; Huang, R.; Li, M.; Tian, J.; Wen, H.; Lin, C.; Wang, T.; Zhan, W.; Fang, J.; et al. Abnormal degree centrality in neurologically asymptomatic patients with end-stage renal disease: A resting-state fMRI study. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2016, 127, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zhao, Y.; Jin, H.; Jian, J.; Wang, H.; Jin, S.; Ren, H. Abnormal hubs in global network as neuroimaging biomarker in right temporal lobe epilepsy at rest. Front. Psychiatry 2022, 13, 981728. [Google Scholar] [CrossRef] [PubMed]
- Zang, Z.X.; Yan, C.G.; Dong, Z.Y.; Huang, J.; Zang, Y.F. Granger causality analysis implementation on MATLAB: A graphic user interface toolkit for fMRI data processing. J. Neurosci. Methods 2012, 203, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Chen, J.; Wen, Z.; Zhang, L. Abnormal functional connectivity and effective connectivity between the default mode network and attention networks in patients with alcohol-use disorder. Acta Radiol. 2021, 62, 251–259. [Google Scholar] [CrossRef]
- van den Heuvel, M.P.; Sporns, O. Network hubs in the human brain. Trends Cogn. Sci. 2013, 17, 683–696. [Google Scholar] [CrossRef]
- Stam, C.J. Modern network science of neurological disorders. Nat. Rev. Neurosci. 2014, 15, 683–695. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Guell, X.; Stoodley, C.J.; Halko, M.A. The Theory and Neuroscience of Cerebellar Cognition. Annu. Rev. Neurosci. 2019, 42, 337–364. [Google Scholar] [CrossRef]
- Ming, X.; Prasad, N.; Thulasi, V.; Elkins, K.; Shivamurthy, V.K.N. Possible contribution of cerebellar disinhibition in epilepsy. Epilepsy Behav. 2021, 118, 107944. [Google Scholar] [CrossRef]
- Eelkman Rooda, O.H.J.; Kros, L.; Faneyte, S.J.; Holland, P.J.; Gornati, S.V.; Poelman, H.J.; Jansen, N.A.; Tolner, E.A.; van den Maagdenberg, A.; De Zeeuw, C.I.; et al. Single-pulse stimulation of cerebellar nuclei stops epileptic thalamic activity. Brain Stimul. 2021, 14, 861–872. [Google Scholar] [CrossRef]
- Gong, J.; Jiang, S.; Li, Z.; Pei, H.; Li, Q.; Yao, D.; Luo, C. Distinct effects of the basal ganglia and cerebellum on the thalamocortical pathway in idiopathic generalized epilepsy. Hum. Brain Mapp. 2021, 42, 3440–3449. [Google Scholar] [CrossRef]
- Pelzer, E.A.; Melzer, C.; Timmermann, L.; von Cramon, D.Y.; Tittgemeyer, M. Basal ganglia and cerebellar interconnectivity within the human thalamus. Brain Struct. Funct. 2017, 222, 381–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nenert, R.; Allendorfer, J.B.; Bebin, E.M.; Gaston, T.E.; Grayson, L.E.; Houston, J.T.; Szaflarski, J.P. Cannabidiol normalizes resting-state functional connectivity in treatment-resistant epilepsy. Epilepsy Behav. 2020, 112, 107297. [Google Scholar] [CrossRef] [PubMed]
- Kros, L.; Eelkman Rooda, O.H.; Spanke, J.K.; Alva, P.; van Dongen, M.N.; Karapatis, A.; Tolner, E.A.; Strydis, C.; Davey, N.; Winkelman, B.H.; et al. Cerebellar output controls generalized spike-and-wave discharge occurrence. Ann. Neurol. 2015, 77, 1027–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Nakahara, K.; Obata, K.; Matsunari, R.; Suzuki-Tsuburaya, R.; Tabata, H.; Kinoshita, M. Hyperperfusion in the cerebellum lobule VIIb in patients with epileptic seizures. BMC Neurol. 2022, 22, 352. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y.; Crepel, V.; Represa, A. Seizures beget seizures in temporal lobe epilepsies: The boomerang effects of newly formed aberrant kainatergic synapses. Epilepsy Curr. 2008, 8, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.H.; Young, I.M.; Conner, A.K.; Glenn, C.A.; Chakraborty, A.R.; Nix, C.E.; Bai, M.Y.; Dhanaraj, V.; Fonseka, R.D.; Hormovas, J.; et al. Anatomy and White Matter Connections of the Inferior Temporal Gyrus. World Neurosurg. 2020, 143, e656–e666. [Google Scholar] [CrossRef]
- Cullen, K.R.; Gee, D.G.; Klimes-Dougan, B.; Gabbay, V.; Hulvershorn, L.; Mueller, B.A.; Camchong, J.; Bell, C.J.; Houri, A.; Kumra, S.; et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci. Lett. 2009, 460, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Zhou, S.Y.; Nakamura, K.; Tanino, R.; Furuichi, A.; Kido, M.; Kawasaki, Y.; Noguchi, K.; Seto, H.; Kurachi, M.; et al. A follow-up MRI study of the fusiform gyrus and middle and inferior temporal gyri in schizophrenia spectrum. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1957–1964. [Google Scholar] [CrossRef]
- Martino, J.; Gabarrós, A.; Deus, J.; Juncadella, M.; Acebes, J.J.; Torres, A.; Pujol, J. Intrasurgical mapping of complex motor function in the superior frontal gyrus. Neuroscience 2011, 179, 131–142. [Google Scholar] [CrossRef]
- Niendam, T.A.; Laird, A.R.; Ray, K.L.; Dean, Y.M.; Glahn, D.C.; Carter, C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012, 12, 241–268. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Smallwood, J.; Spreng, R.N. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 2014, 1316, 29–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggs, R.G.; Khan, A.B.; Chakraborty, A.R.; Abraham, C.J.; Anderson, C.D.; Karas, P.J.; Bonney, P.A.; Palejwala, A.H.; Conner, A.K.; O’Donoghue, D.L.; et al. Anatomy and White Matter Connections of the Superior Frontal Gyrus. Clin. Anat. 2020, 33, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Dehaene, S.; Changeux, J.P. Experimental and theoretical approaches to conscious processing. Neuron 2011, 70, 200–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doucet, G.E.; He, X.; Sperling, M.; Sharan, A.; Tracy, J.I. Frontal gray matter abnormalities predict seizure outcome in refractory temporal lobe epilepsy patients. Neuroimage Clin. 2015, 9, 458–466. [Google Scholar] [CrossRef]
- Jiang, S.; Luo, C.; Gong, J.; Peng, R.; Ma, S.; Tan, S.; Ye, G.; Dong, L.; Yao, D. Aberrant Thalamocortical Connectivity in Juvenile Myoclonic Epilepsy. Int. J. Neural Syst. 2018, 28, 1750034. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Hu, J.; Wang, Z.; You, R.; Cao, D. Basal ganglia stroke is associated with altered functional connectivity of the left inferior temporal gyrus. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2022, 32, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, J.D.; Oya, H.; Kawasaki, H.; Volkov, I.O.; Severson, M.A., 3rd; Howard, M.A., 3rd; Brugge, J.F. Functional connections within the human inferior frontal gyrus. J. Comp. Neurol. 2007, 503, 550–559. [Google Scholar] [CrossRef]
- Briggs, R.G.; Chakraborty, A.R.; Anderson, C.D.; Abraham, C.J.; Palejwala, A.H.; Conner, A.K.; Pelargos, P.E.; O’Donoghue, D.L.; Glenn, C.A.; Sughrue, M.E. Anatomy and white matter connections of the inferior frontal gyrus. Clin. Anat. 2019, 32, 546–556. [Google Scholar] [CrossRef]
- Bressler, S.L.; Menon, V. Large-scale brain networks in cognition: Emerging methods and principles. Trends Cogn. Sci. 2010, 14, 277–290. [Google Scholar] [CrossRef]
- Micheli, C.; Kaping, D.; Westendorff, S.; Valiante, T.A.; Womelsdorf, T. Inferior-frontal cortex phase synchronizes with the temporal-parietal junction prior to successful change detection. Neuroimage 2015, 119, 417–431. [Google Scholar] [CrossRef]
- Nakae, T.; Matsumoto, R.; Kunieda, T.; Arakawa, Y.; Kobayashi, K.; Shimotake, A.; Yamao, Y.; Kikuchi, T.; Aso, T.; Matsuhashi, M.; et al. Connectivity Gradient in the Human Left Inferior Frontal Gyrus: Intraoperative Cortico-Cortical Evoked Potential Study. Cereb Cortex 2020, 30, 4633–4650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Davis, C.; Thomas, A.M.; Economo, M.N.; Abrego, A.M.; Svoboda, K.; De Zeeuw, C.I.; Li, N. A cortico-cerebellar loop for motor planning. Nature 2018, 563, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.L.; Bajada, C.J.; Rice, G.E.; Cloutman, L.L.; Lambon Ralph, M.A. An emergent functional parcellation of the temporal cortex. Neuroimage 2018, 170, 385–399. [Google Scholar] [CrossRef]
- Jo, H.J.; Kenney-Jung, D.L.; Balzekas, I.; Welker, K.M.; Jones, D.T.; Croarkin, P.E.; Benarroch, E.E.; Worrell, G.A. Relationship Between Seizure Frequency and Functional Abnormalities in Limbic Network of Medial Temporal Lobe Epilepsy. Front. Neurol. 2019, 10, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.K.; Karunanayaka, P.; Privitera, M.D.; Holland, S.K.; Szaflarski, J.P. Semantic association investigated with functional MRI and independent component analysis. Epilepsy Behav. 2011, 20, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Amiri, S.; Mehvari-Habibabadi, J.; Mohammadi-Mobarakeh, N.; Hashemi-Fesharaki, S.S.; Mirbagheri, M.M.; Elisevich, K.; Nazem-Zadeh, M.R. Graph theory application with functional connectivity to distinguish left from right temporal lobe epilepsy. Epilepsy Res. 2020, 167, 106449. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Liu, J.; Qin, L.; Pang, X.; Zheng, J. Disruption and lateralization of cerebellar–cerebral functional networks in right temporal lobe epilepsy: A resting-state fMRI study. Epilepsy Behav. 2019, 96, 80–86. [Google Scholar] [CrossRef]
- Schmahmann, J.D. The cerebellum and cognition. Neurosci. Lett. 2019, 688, 62–75. [Google Scholar] [CrossRef]
- Oscarsson, O. Functional Organization of the Spino- and Cuneocerebellar Tracts. Physiol. Rev. 1965, 45, 495–522. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Ko, R.; MacMore, J. The human basis pontis: Motor syndromes and topographic organization. Brain 2004, 127, 1269–1291. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Yu, L.; Fan, B.; Wang, M.; Jiang, B.; Su, Y.; Li, P.; Zheng, J. Disturbance of functional and effective connectivity of the salience network involved in attention deficits in right temporal lobe epilepsy. Epilepsy Behav. 2021, 124, 108308. [Google Scholar] [CrossRef]
- Utevsky, A.V.; Smith, D.V.; Huettel, S.A. Precuneus is a functional core of the default-mode network. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 932–940. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Jiang, S.; Zhang, Q.; Dong, L.; Jia, X.; He, H.; Yao, Y.; Yang, H.; Zhang, T.; Luo, C.; et al. BOLD-fMRI activity informed by network variation of scalp EEG in juvenile myoclonic epilepsy. Neuroimage Clin. 2019, 22, 101759. [Google Scholar] [CrossRef]
- Husbani, M.A.R.; Shuhada, J.M.; Hamid, A.I.A.; Suardi, K.P.S.; Abdullah, M.S.; Latif, A.Z.A.; Yusof, A.N. Effective connectivity between precuneus and supramarginal gyrus in healthy subjects and temporal lobe epileptic patients. Med. J. Malays. 2021, 76, 360–368. [Google Scholar]
- Di, X.; Biswal, B.B. Identifying the default mode network structure using dynamic causal modeling on resting-state functional magnetic resonance imaging. Neuroimage 2014, 86, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Vaudano, A.E.; Laufs, H.; Kiebel, S.J.; Carmichael, D.W.; Hamandi, K.; Guye, M.; Thornton, R.; Rodionov, R.; Friston, K.J.; Duncan, J.S.; et al. Causal hierarchy within the thalamo-cortical network in spike and wave discharges. PLoS ONE 2009, 4, e6475. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, B.; Hu, W.; Zhang, C.; Wang, X.; Wang, Y.; Liu, C.; Mo, J.; Sang, L.; Ma, Y.; et al. Effective connectivity among the hippocampus, amygdala, and temporal neocortex in epilepsy patients: A cortico-cortical evoked potential study. Epilepsy Behav. 2021, 115, 107661. [Google Scholar] [CrossRef]
- Park, K.M.; Kim, S.E.; Shin, K.J.; Ha, S.Y.; Park, J.; Kim, T.H.; Mun, C.W.; Lee, B.I.; Kim, S.E. Effective connectivity in temporal lobe epilepsy with hippocampal sclerosis. Acta Neurol. Scand. 2017, 135, 670–676. [Google Scholar] [CrossRef]
- Cook, C.J.; Hwang, G.; Mathis, J.; Nair, V.A.; Conant, L.L.; Allen, L.; Almane, D.N.; Birn, R.; DeYoe, E.A.; Felton, E.; et al. Effective Connectivity within the Default Mode Network in Left Temporal Lobe Epilepsy: Findings from the Epilepsy Connectome Project. Brain Connect. 2019, 9, 174–183. [Google Scholar] [CrossRef]
- Sokolov, A.A.; Erb, M.; Gharabaghi, A.; Grodd, W.; Tatagiba, M.S.; Pavlova, M.A. Biological motion processing: The left cerebellum communicates with the right superior temporal sulcus. Neuroimage 2012, 59, 2824–2830. [Google Scholar] [CrossRef]
- Abbasi, B.; Goldenholz, D.M. Machine learning applications in epilepsy. Epilepsia 2019, 60, 2037–2047. [Google Scholar] [CrossRef]
- Gotman, J.; Grova, C.; Bagshaw, A.; Kobayashi, E.; Aghakhani, Y.; Dubeau, F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc. Natl. Acad. Sci. USA 2005, 102, 15236–15240. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Zhang, Q.; Yang, F.; Weng, Y.; Xie, X.; Hao, J.; Qi, R.; Gumenyuk, V.; Stufflebeam, S.M.; Bernhardt, B.C.; et al. Cortico-striato-thalamo-cerebellar networks of structural covariance underlying different epilepsy syndromes associated with generalized tonic-clonic seizures. Hum. Brain Mapp. 2021, 42, 1102–1115. [Google Scholar] [CrossRef]
- Wagner, M.J.; Luo, L. Neocortex-Cerebellum Circuits for Cognitive Processing. Trends Neurosci. 2020, 43, 42–54. [Google Scholar] [CrossRef]
- Danielson, N.B.; Guo, J.N.; Blumenfeld, H. The default mode network and altered consciousness in epilepsy. Behav. Neurol. 2011, 24, 55–65. [Google Scholar] [CrossRef]
- Schmahmann, J.D. Dysmetria of thought: Clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn. Sci. 1998, 2, 362–371. [Google Scholar] [CrossRef]
- Pervaiz, U.; Vidaurre, D.; Woolrich, M.W.; Smith, S.M. Optimising network modelling methods for fMRI. Neuroimage 2020, 211, 116604. [Google Scholar] [CrossRef]

| Characteristics | JME (n = 34) (Mean ± SD) | HCs (n = 34) (Mean ± SD) | p Value |
|---|---|---|---|
| Age (years) | 17.38 ± 4.73 | 19.15 ± 3.46 | 0.21 a |
| Handedness (right/left) | 34/0 | 34/0 | 0.99 b |
| Sex (males/females) | 17/17 | 11/23 | 0.14 b |
| NHS3 score | 8.65 ± 3.92 | - | - |
| Mean FD | 0.12 ± 0.05 | 0.11 ± 0.04 | 0.45 a |
| Anatomical Regions | Abbr. | BA | MNI Coordinates | Cluster Size | Maximal t Value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| JME >HCs | |||||||
| Left cerebellum posterior lobe (Cerebellar Tonsil) | CePL.L | - | −3 | −51 | −48 | 48 | 3.54 |
| Right inferior temporal gyrus | ITG.R | 20 | 39 | −12 | −29 | 133 | 5.42 |
| Right superior frontal gyrus | SFG.R | 8 | 15 | 48 | 51 | 64 | 4.11 |
| JME < HCs | |||||||
| Left inferior frontal gyrus | IFG.L | 47 | −45 | 36 | −12 | 213 | −4.47 |
| Left superior temporal gyrus | STG.L | 40 | −63 | −33 | 15 | 234 | −4.72 |
| Anatomical Regions | Abbr. | BA | MNI Coordinates | Cluster Size | Maximal t Value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Seed region: CePL.L | |||||||
| X→Y | |||||||
| Right precuneus | PreCU.R | 7 | 21 | −63 | 42 | 51 | 3.66 |
| Seed region: ITG.R | |||||||
| Y→X | |||||||
| Left cerebellum anterior lobe (Culmen) | CeAL.L | - | −9 | −39 | −12 | 39 | 4.37 |
| Seed region: SFG.R | |||||||
| Y→X | |||||||
| left inferior semi-lunar lobule of cerebellum | CeISL.L | - | −36 | −69 | −54 | 44 | 4.16 |
| Seed region: IFG.L | |||||||
| Y→X | |||||||
| Right cerebellum posterior lobe (Pyramis) | CePL.R | - | 3 | −75 | −33 | 43 | 4.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Liu, G.; Zhang, P.; Wang, J.; Huang, W.; Jiang, Y.; Zheng, Y.; Han, N.; Zhang, Z.; Zhang, J. Altered Cerebro-Cerebellar Effective Connectivity in New-Onset Juvenile Myoclonic Epilepsy. Brain Sci. 2022, 12, 1658. https://doi.org/10.3390/brainsci12121658
Ma L, Liu G, Zhang P, Wang J, Huang W, Jiang Y, Zheng Y, Han N, Zhang Z, Zhang J. Altered Cerebro-Cerebellar Effective Connectivity in New-Onset Juvenile Myoclonic Epilepsy. Brain Sciences. 2022; 12(12):1658. https://doi.org/10.3390/brainsci12121658
Chicago/Turabian StyleMa, Laiyang, Guangyao Liu, Pengfei Zhang, Jun Wang, Wenjing Huang, Yanli Jiang, Yu Zheng, Na Han, Zhe Zhang, and Jing Zhang. 2022. "Altered Cerebro-Cerebellar Effective Connectivity in New-Onset Juvenile Myoclonic Epilepsy" Brain Sciences 12, no. 12: 1658. https://doi.org/10.3390/brainsci12121658
APA StyleMa, L., Liu, G., Zhang, P., Wang, J., Huang, W., Jiang, Y., Zheng, Y., Han, N., Zhang, Z., & Zhang, J. (2022). Altered Cerebro-Cerebellar Effective Connectivity in New-Onset Juvenile Myoclonic Epilepsy. Brain Sciences, 12(12), 1658. https://doi.org/10.3390/brainsci12121658





