Global Neural Activities Changes under Human Inhibitory Control Using Translational Scenario
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
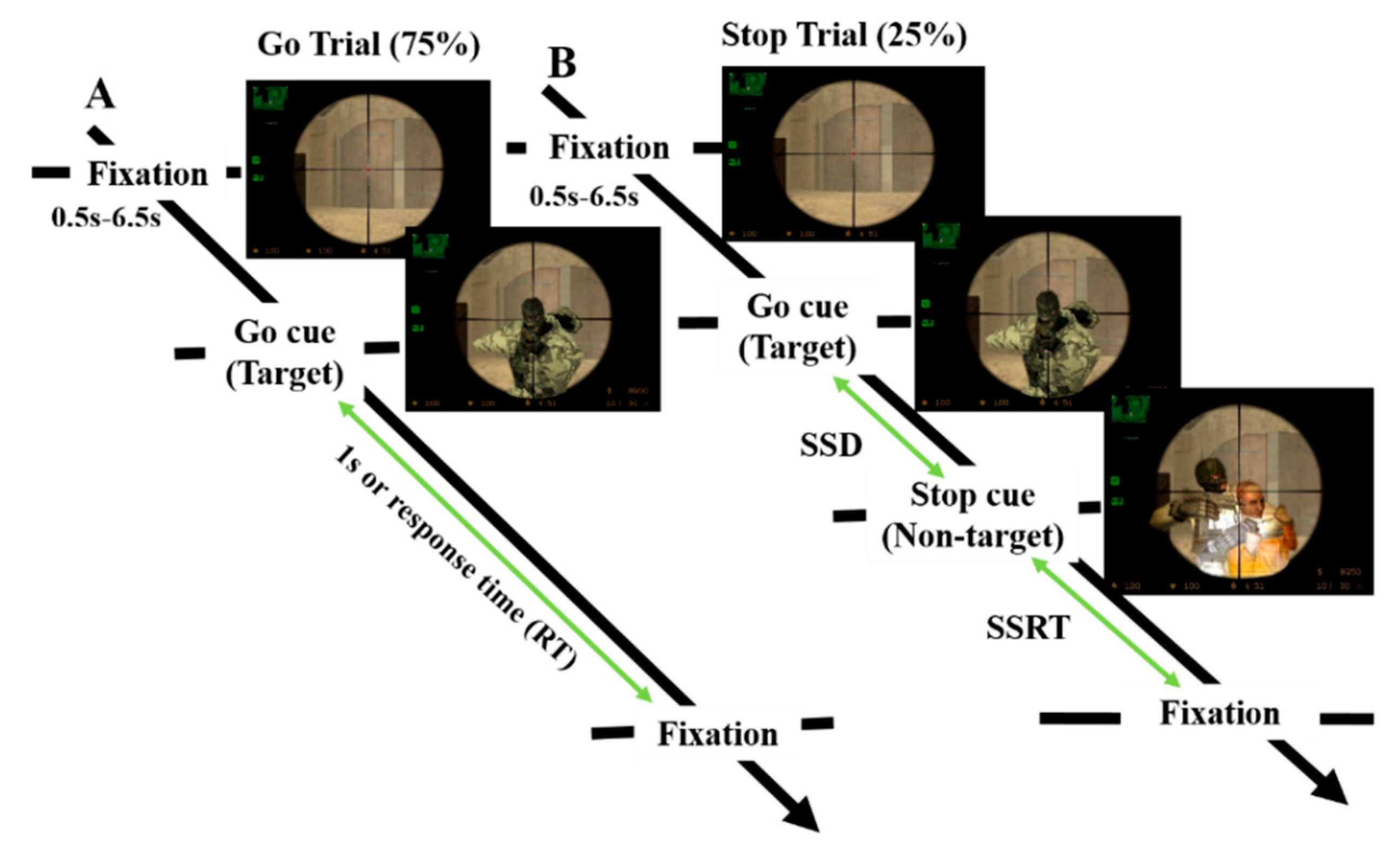
2.2. Experimental Design
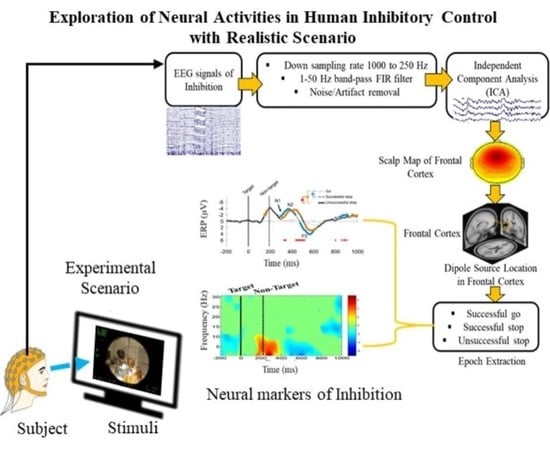
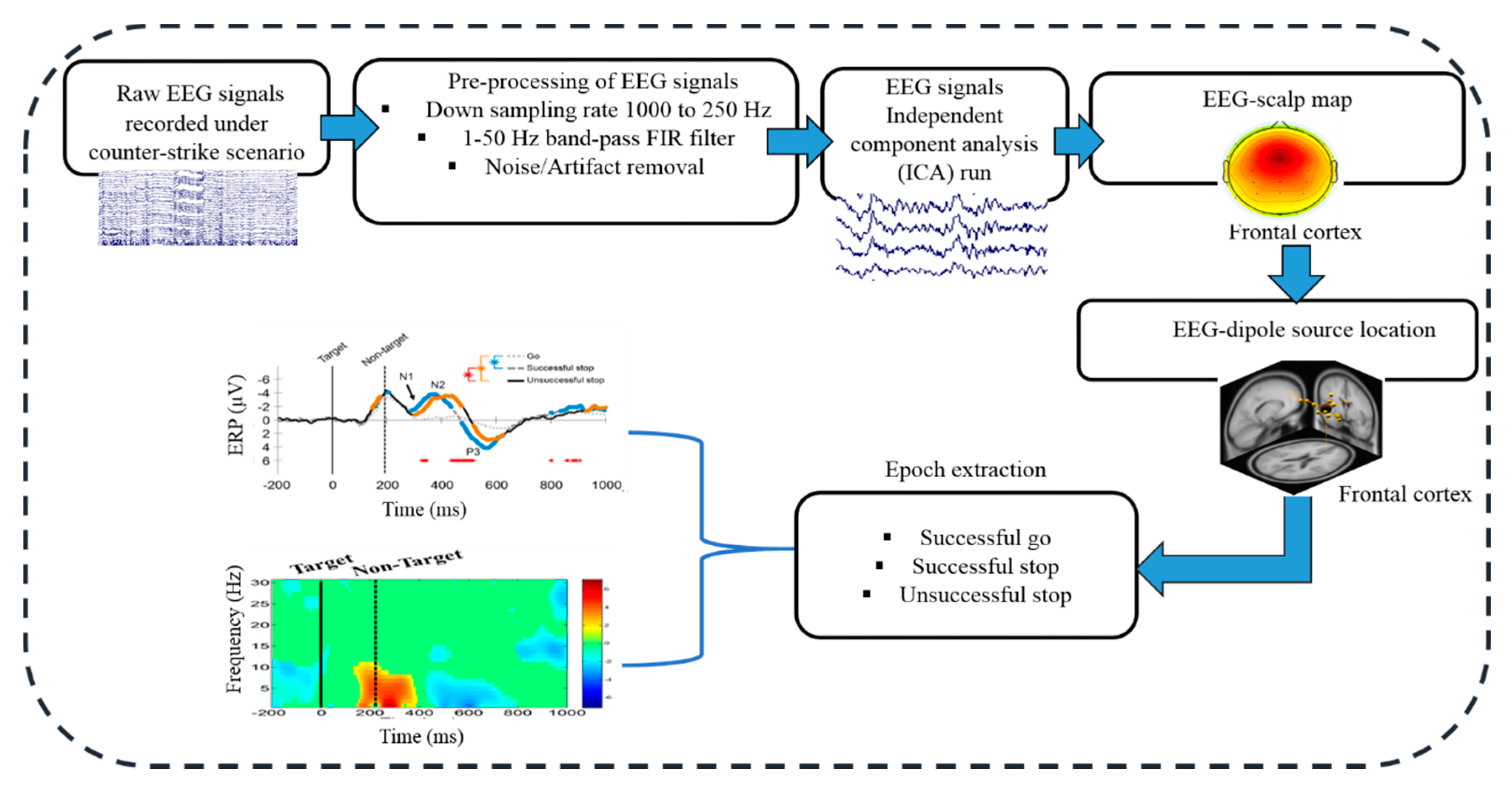
2.3. Acquisition and Preprocessing of EEG Signals
2.4. Independent Component Analysis (ICA)
2.5. Behavioral Analysis
2.6. Event-Related Spectral Perturbation (ERSP) Analysis
2.7. Statistical Analysis
3. Results
3.1. Behavioral Results
3.2. Electroencephalography (EEG) Results
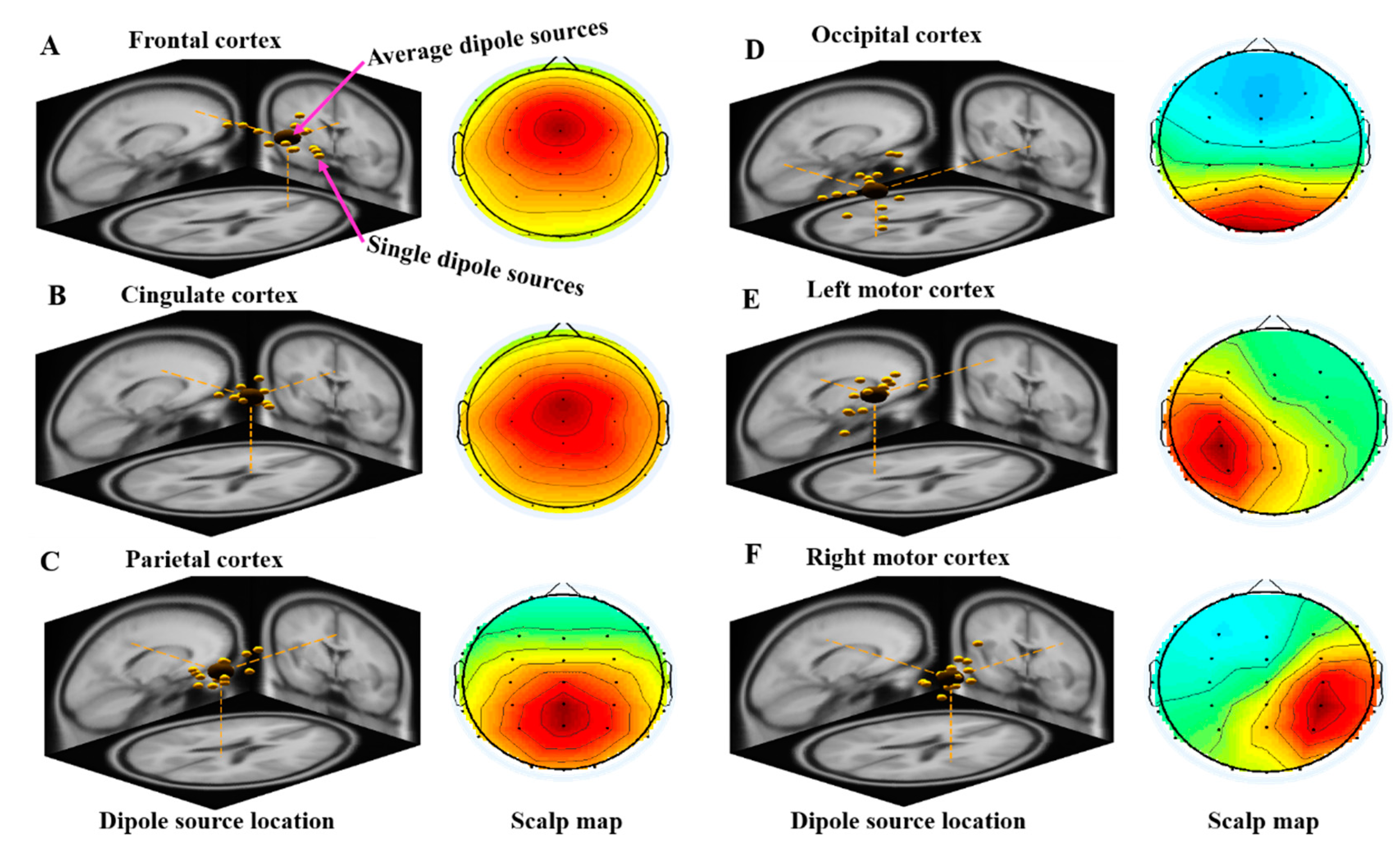
3.2.1. Independent Component Scalp Map and Location of Dipole Sources
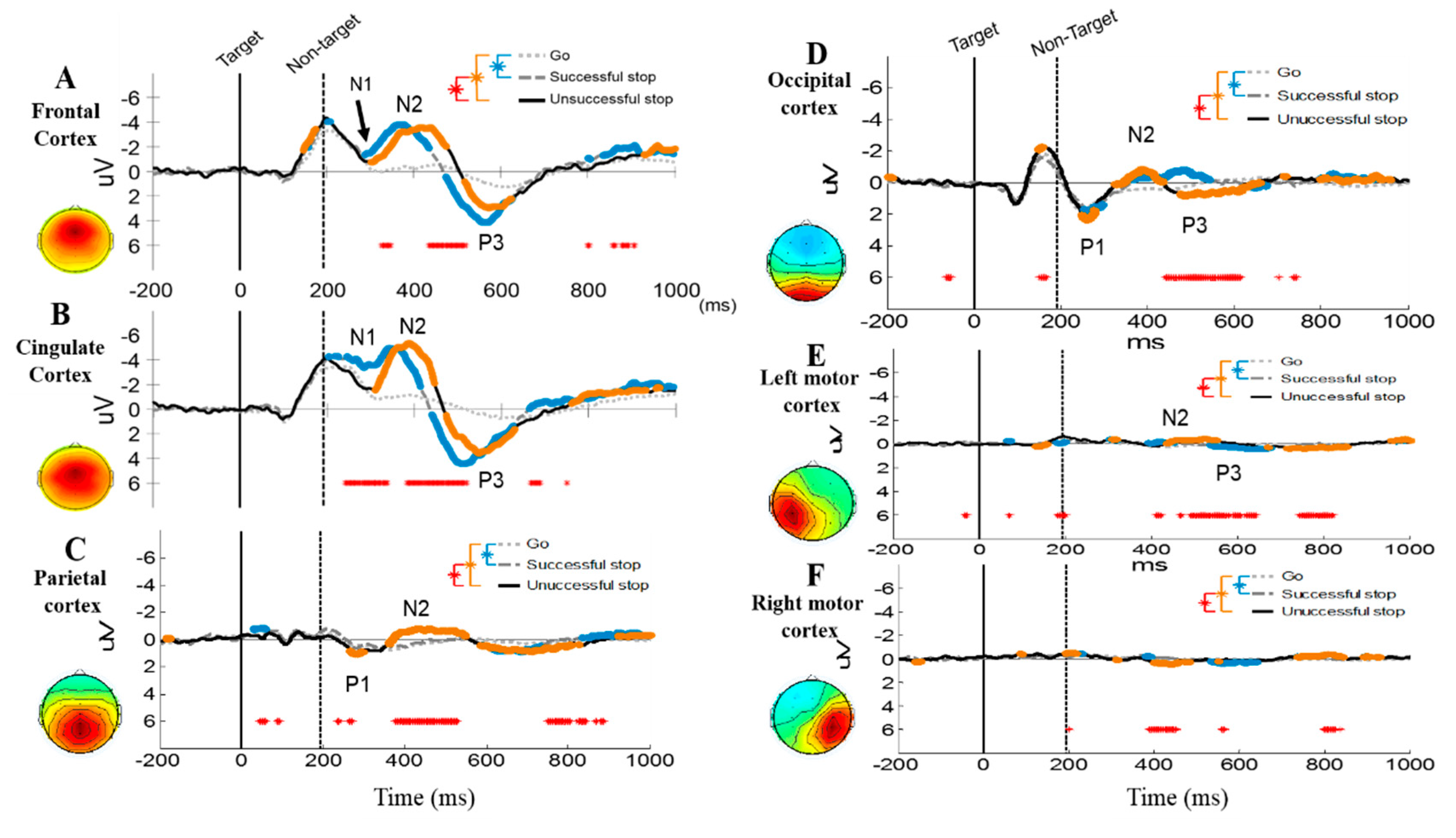
3.2.2. Event-Related Potentials (ERP) under Human Inhibition
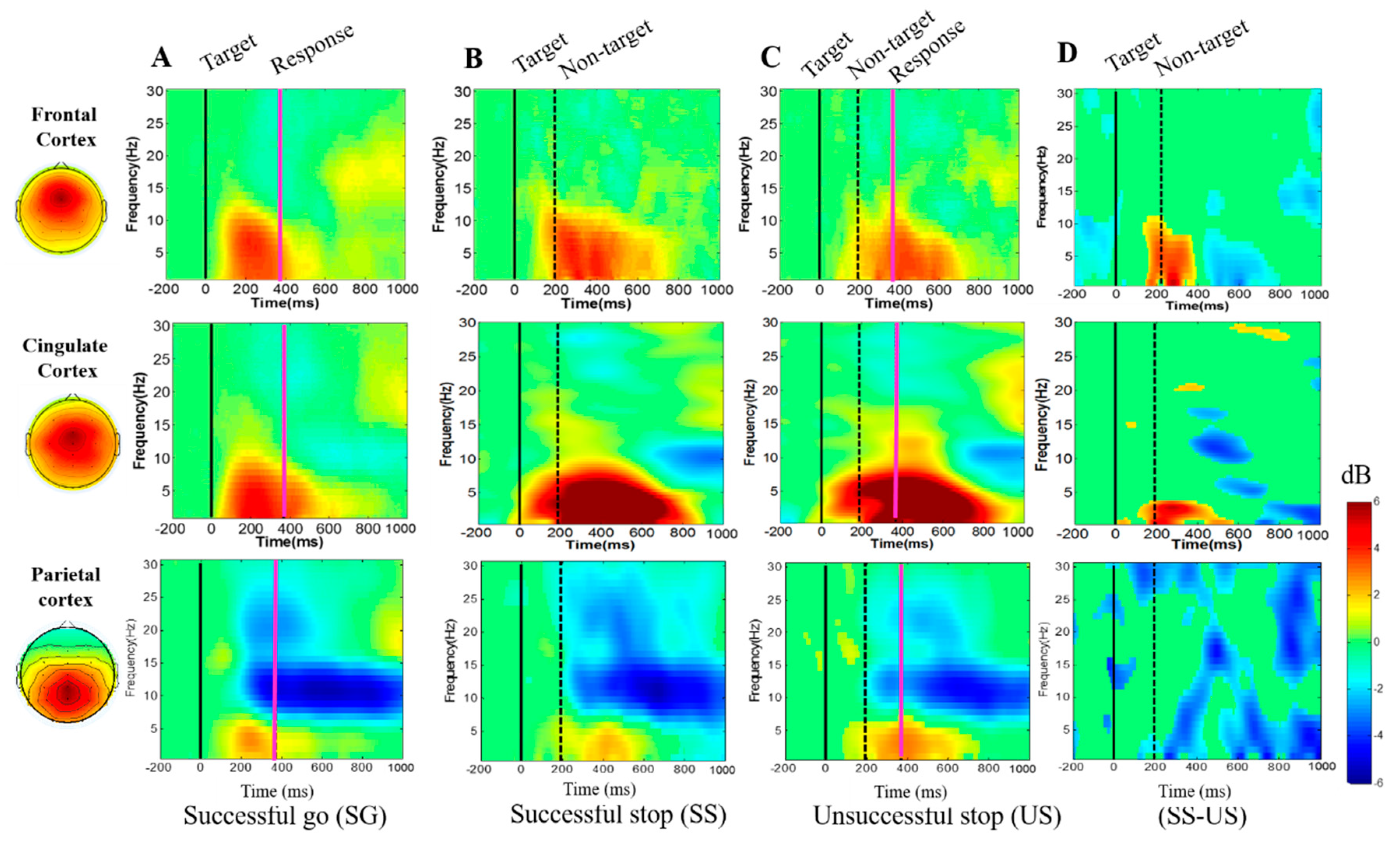
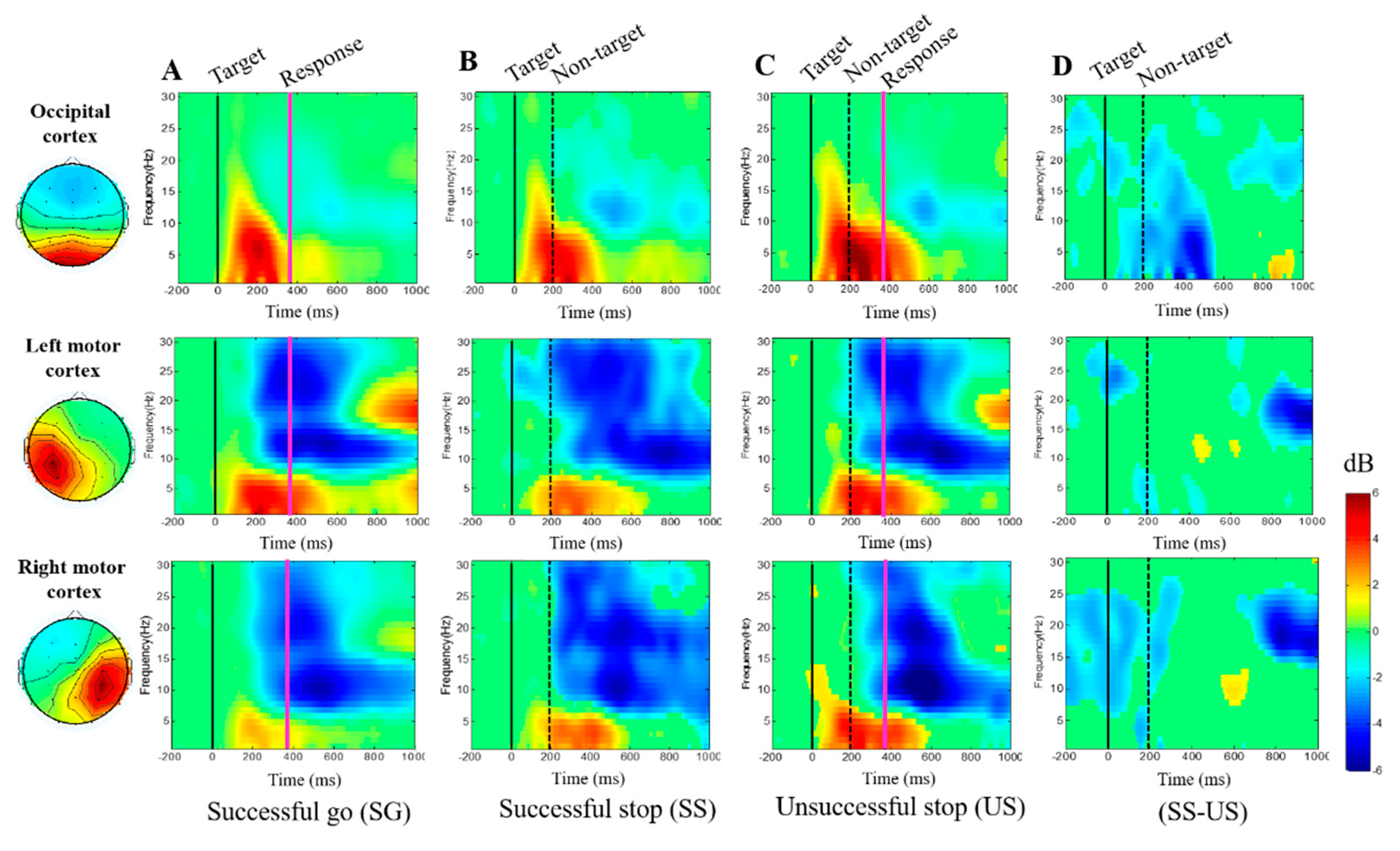
3.2.3. Event-Related Spectral Perturbation (ERSP) during Human Inhibition
4. Discussion
4.1. EEG-ERP N2 and P3 Waves under Human Inhibitory Control
4.2. EEG-Power Spectral Changes under Inhibitory Control
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lappin, J.S.; Eriksen, C.W. Use of a delayed signal to stop a visual reaction-time response. J. Exp. Psychol. 1966, 72, 805–811. [Google Scholar] [CrossRef]
- Logan, G.D.; Burkell, J. Dependence and independence in responding to double stimulation: A comparison of stop, change and dual-task paradigms. J. Exp. Psychol. Hum. Percept. Perform. 1986, 12, 549–563. [Google Scholar] [CrossRef]
- Logan, G.D.; Cowan, W.B. On the ability to inhibit thought and action: A theory of an act of control. Psychol. Rev. 1984, 91, 295–327. [Google Scholar] [CrossRef]
- Logan, G.D. On the ability to inhibit complex movements: A stop-signal study of typewriting. J. Exp. Psychol. Hum. Percept. Perform. 1982, 8, 778–792. [Google Scholar] [CrossRef]
- Logan, G.D. Executive control of thought and action. Acta Psychol. 1985, 60, 193–210. [Google Scholar] [CrossRef]
- Band, G.P.H.; Van Der Molen, M.W.; Logan, G.D. Horse-race model simulations of the stop-signal procedure. Acta Psychol. 2003, 112, 105–142. [Google Scholar] [CrossRef]
- Tabu, H.; Mima, T.; Aso, T.; Takahashi, R.; Fukuyama, H. Functional relevance of pre-supplementary motor areas for the choice to stop during Stop signal task. Neurosci. Res. 2011, 70, 277–284. [Google Scholar] [CrossRef]
- Tabu, H.; Mima, T.; Aso, T.; Takahashi, R.; Fukuyama, H. Common inhibitory prefrontal activation during inhibition of hand and foot responses. NeuroImage 2012, 59, 3373–3378. [Google Scholar] [CrossRef]
- Verbruggen, F.; Logan, G.D. Response inhibition in the stop-signal paradigm. Trends Cogn. Sci. 2008, 12, 418–424. [Google Scholar] [CrossRef]
- Kok, A.; Ramautar, J.R.; De Ruiter, M.B.; Band, G.P.H.; Ridderinkhof, K.R. ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology 2004, 41, 9–20. [Google Scholar] [CrossRef]
- Mostofsky, S.H.; Schafer, J.G.B.; Abrams, M.T.; Goldberg, M.C.; Flower, A.A.; Boyce, A.; Courtney, S.M.; Calhoun, V.D.; Kraut, M.A.; Denckla, M.B.; et al. fMRI evidence that the neural basis of response inhibition is task-dependent. Cogn. Brain Res. 2003, 17, 419–430. [Google Scholar] [CrossRef]
- Rubia, K.; Russella, T.; Overmeyera, S.; Brammer, M.J.; Bullmore, E.T.; Sharmaa, T.; Simmonsa, A.; Williams, S.C.; Giampietro, V.; Andrew, C.M.; et al. Mapping motor inhibition: Conjunctive brain activations across different versions of go/no-go and stop tasks. NeuroImage 2001, 13, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Rubia, K.; Smith, A.B.; Brammer, M.J.; Taylor, E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage 2003, 20, 351–358. [Google Scholar] [CrossRef]
- Hampshire, A.; Chamberlain, S.R.; Monti, M.M.; Duncan, J.; Owen, A.M. The role of the right inferior frontal gyrus: Inhibition and attentional control. NeuroImage 2010, 50, 1313–1319. [Google Scholar] [CrossRef]
- Nakata, H.; Sakamoto, K.; Ferretti, A.; Perrucci, M.G.; Del Gratta, C.; Kakigi, R.; Romani, G.L. Somato-motor inhibitory processing in humans: An event-related functional MRI study. NeuroImage 2008, 39, 1858–1866. [Google Scholar] [CrossRef]
- Lansbergen, M.M.; Schutter, D.J.; Kenemans, J.L. Subjective impulsivity and baseline EEG in relation to stopping performance. Brain Res. 2007, 1148, 161–169. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- De Jong, R.; Coles, M.G.H.; Logan, G.D.; Gratton, G. In search of the point of no return: The control of response processes. J. Exp. Psychol. Hum. Percep. Perform. 1990, 16, 164–182. [Google Scholar] [CrossRef]
- Jong, D.R.; Coles, M.G.H.; Logan, G.D. Strategies and mechanisms in nonselective and selective inhibitory motor control. J. Exp. Psychol. Hum. Percep. Perform. 1995, 21, 498–511. [Google Scholar] [CrossRef]
- Naito, E.; Matsumura, M. Movement-related potentials associated with motor inhibition under different preparatory states during performance of two visual stop signal paradigms in humans. Neuropsychology 1996, 34, 565–573. [Google Scholar] [CrossRef]
- Gennady, G.K.; Evgenij, A.L.; Alexander, N.S. A failure to stop and attention fluctuations: An evoked oscillations study of the stop-signal paradigm. Clin. Neurophysiol. 2008, 119, 556–567. [Google Scholar]
- Pfefferbaum, A.; Ford, J.M.; Weller, B.J.; Kopell, B.S. ERPs to response production and inhibition. Electroencephalogr. Clin. Neurophysiol. 1985, 60, 423–434. [Google Scholar] [CrossRef]
- Kok, A. Effects of degradation of visual stimulation on components of the event-related potential (ERP) in go/nogo reaction tasks. Biol. Psychol. 1986, 3, 21–38. [Google Scholar] [CrossRef]
- Dimoska, A.; Johnstone, S.J.; Barry, R.J. The auditory-evoked N2 and P3 components in the stop-signal task: Indices of inhibition, response-conflict or error-detection? Brain Cogn. 2006, 62, 98–112. [Google Scholar] [CrossRef]
- Chikara, R.K.; Su, K.; Ko, L.W. Classification of Brain Activities under Response Inhibition using Functional Connectivity. In Proceedings of the International Conference on Technologies and Applications of Artificial Intelligence (TAAI), Kaohsiung, Taiwan, 21–23 November 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Huster, R.J.; Enriquez-Geppert, S.; Lavallee, C.F.; Falkenstein, M.; Herrmann, C.S. Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. Int. J. Psychophysiol. 2013, 87, 217–233. [Google Scholar] [CrossRef]
- Gennari, S.P.; Millman, R.; Hymers, M.; Mattys, S.L. Anterior paracingulate and cingulate cortex mediates the effects of cognitive load on speech sound discrimination. NeuroImage 2018, 178, 735–743. [Google Scholar] [CrossRef]
- Drevets, W.C.; Savitz, J.; Trimble, M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008, 13, 663–681. [Google Scholar] [CrossRef]
- Adams, R.; David, A.S. Patterns of anterior cingulate activation in schizophrenia: A selective review. Neuropsychiatr. Dis. Treat. 2007, 3, 87–101. [Google Scholar] [CrossRef]
- Ko, L.-W.; Shih, Y.-C.; Chikara, R.K.; Chuang, Y.-T.; Chang, E.C. Neural mechanisms of inhibitory response in a battlefield scenario: A simultaneous fMRI-EEG study. Front. Hum. Neurosci. 2016, 10, 185. [Google Scholar] [CrossRef]
- Chikara, R.K.; Chang, E.C.; Lu, Y.-C.; Lin, D.-S.; Lin, C.-T.; Ko, L.-W. Monetary reward and punishment to response inhibition modulate activation and synchronization within the inhibitory brain network. Front. Hum. Neurosci. 2018, 12, 27. [Google Scholar] [CrossRef]
- Chikara, R.K.; Ko, L.-W. Modulation of the visual to auditory human inhibitory brain network: An EEG dipole source localization study. Brain Sci. 2019, 9, 216. [Google Scholar] [CrossRef]
- Mishkin, M.; Ungerleider, L.G. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav. Brain Res. 1982, 6, 57–77. [Google Scholar] [CrossRef]
- Goodale, M.A.; Milner, A.D. Separate visual pathways for perception and action. Trends Neurosci. 1992, 15, 20–25. [Google Scholar] [CrossRef]
- Logan, G.D.; Cowan, W.B.; Davis, K.A. On the ability to inhibit simple and choice reaction time responses: A model and a method. J. Exp. Psychol. Hum. Percep. Perform 1984, 10, 275–291. [Google Scholar] [CrossRef]
- Jung, T.-P.; Makeig, S.; McKeown, M.J.; Bell, A.J.; Lee, T.-W.; Sejnowski, T. Imaging brain dynamics using independent component analysis. Proc. IEEE 2001, 89, 1107–1122. [Google Scholar] [CrossRef]
- Aron, A.R.; Robbins, T.; Poldrack, R.A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004, 8, 170–177. [Google Scholar] [CrossRef]
- Van Boxtel, G.; Van Der Molen, M.W.; Jennings, J.; Brunia, C.H. A psychophysiological analysis of inhibitory motor control in the stop-signal paradigm. Boil. Psychol. 2001, 58, 229–262. [Google Scholar] [CrossRef]
- Sharp, D.J.; Bonnelle, V.; De Boissezon, X.; Beckmann, C.F.; James, S.G.; Patel, M.C.; Mehta, M.A. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc. Natl. Acad. Sci. USA 2010, 107, 6106–6111. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Monographs on Statistics & Applied Probability; Chapman Hall/CRC: Boca Raton, FL, USA, 1994; Volume 1, 456p. [Google Scholar]
- Makeig, S.; Debener, S.; Onton, J.; Delorme, A. Mining event-related brain dynamics. Trends Cogn. Sci. 2004, 8, 204–210. [Google Scholar] [CrossRef]
- Andrés, P. Frontal cortex as the central executive of working memory: Time to revise our view. Cortex 2003, 39, 871–895. [Google Scholar] [CrossRef]
- Aron, A.R. The neural basis of inhibition in cognitive control. Neuroscientist 2007, 13, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed]
- Stuphorn, V.; Schall, J.D. Executive control of countermanding saccades by the supplementary eye field. Nat. Neurosci. 2006, 9, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Ladefoged, P.; Silverstein, R.; Papçun, G. Interruptibility of speech. J. Acoust. Soc. Am. 1973, 54, 1105–1108. [Google Scholar] [CrossRef]
- Aron, A.R.; Poldrack, R.A. Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. J. Neurosci. 2006, 26, 2424–2433. [Google Scholar] [CrossRef]
- Chikara, R.K.; Lo, W.-C.; Ko, L.-W. Exploration of brain connectivity during human inhibitory control using inter-trial coherence. Sensors 2020, 20, 1722. [Google Scholar] [CrossRef]
- Nigg, J.T. Is ADHD a disinhibitory disorder. Psychol. Bull. 2001, 127, 571–598. [Google Scholar] [CrossRef]
- Schachar, R.; Logan, G.D. Impulsivity and inhibitory control in normal development and childhood psychopathology. Dev. Psychol. 1990, 26, 710–720. [Google Scholar] [CrossRef]
- Gauggel, S.; Rieger, M.; Feghoff, T.-A. Inhibition of ongoing responses in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 539–544. [Google Scholar] [CrossRef]
- Chamberlain, S.R.; Fineberg, N.A.; Blackwell, A.D.; Robbins, T.W.; Sahakian, B.J. Motor inhibition and cognitive flexibility in obsessive–compulsive disorder and trichotillomania. Am. J. Psychiatry 2006, 163, 1282–1284. [Google Scholar] [CrossRef]
- Chikara, R.K.; Komarov, O.; Ko, L.W. Neural signature of event-related N200 and P300 modulation in parietal lobe during human response inhibition. Int. J. Comput. Biol. Drug Des. 2018, 11, 171–182. [Google Scholar] [CrossRef]
- Munro, G.E.; Dywan, J.; Harris, G.T.; McKee, S.; Unsal, A.; Segalowitz, S.J. Response inhibition in psychopathy: The frontal N2 and P3. Neurosci. Lett. 2007, 418, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Chikara, R.K.; Ko, L.-W. Neural Activities Classification of Human Inhibitory Control Using Hierarchical Model. Sensors 2019, 19, 3791. [Google Scholar] [CrossRef] [PubMed]
- Kirmizi-Alsana, E.; Bayraktaroglua, Z.; Gurvitb, H.; Keskina, Y.H.; Emreb, M.; Demiralpa, T. Comparative analysis of event-related potentials during go/no-go and CPT: Decomposition of response inhibition and sustained attention. Brain Res. 2006, 1104, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Harmony, T.; Alba, A.; Marroquin, J.L.; González-Frankenberger, B. Time-frequency-topographic analysis of induced power and synchrony of EEG signals during a Go/No-Go task. Int. J. Psychophysiol. 2009, 71, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Nigbur, R.; Ivanova, G.; Stürmer, B. Theta power as a marker for cognitive interference. Clin. Neurophysiol. 2011, 122, 2185–2194. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Neuper, C.; Flotzinger, D.; Pregenzer, M. EEG-based discrimination between imagination of right and left hand movement. Electroencephalogr. Clin. Neurophysiol. 1997, 103, 642–651. [Google Scholar] [CrossRef]
- Solis-Escalante, T.; Müller-Putz, G.R.; Pfurtscheller, G.; Neuper, C. Cue-induced beta rebound during withholding of overt and covert foot movement. Clin. Neurophysiol. 2012, 123, 1182–1190. [Google Scholar] [CrossRef]
- Cunnington, R.; Windischberger, C.; Deecke, L.; Moser, E. The Preparation and execution of self-initiated and externally-triggered movement: A study of event-related fMRI. NeuroImage 2002, 15, 373–385. [Google Scholar] [CrossRef]
- Ko, L.-W.; Chikara, R.K.; Lee, Y.-C.; Lin, W.-C. Exploration of user’s mental state changes during performing brain–computer interface. Sensors 2020, 20, 3169. [Google Scholar] [CrossRef]
- Banis, S.; Geerligs, L.; Lorist, M.M. Acute stress modulates feedback processing in men and women: Differential effects on the feedback-related negativity and theta and beta power. PLoS ONE 2014, 9, e95690. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikara, R.K.; Ko, L.-W. Global Neural Activities Changes under Human Inhibitory Control Using Translational Scenario. Brain Sci. 2020, 10, 640. https://doi.org/10.3390/brainsci10090640
Chikara RK, Ko L-W. Global Neural Activities Changes under Human Inhibitory Control Using Translational Scenario. Brain Sciences. 2020; 10(9):640. https://doi.org/10.3390/brainsci10090640
Chicago/Turabian StyleChikara, Rupesh Kumar, and Li-Wei Ko. 2020. "Global Neural Activities Changes under Human Inhibitory Control Using Translational Scenario" Brain Sciences 10, no. 9: 640. https://doi.org/10.3390/brainsci10090640
APA StyleChikara, R. K., & Ko, L.-W. (2020). Global Neural Activities Changes under Human Inhibitory Control Using Translational Scenario. Brain Sciences, 10(9), 640. https://doi.org/10.3390/brainsci10090640