Potential of Solar Collectors for Clean Thermal Energy Production in Smart Cities using Nanofluids: Experimental Assessment and Efficiency Improvement
Abstract
:1. Introduction
2. Experimental
2.1. Preparation, Quality Tests and Physical Properties
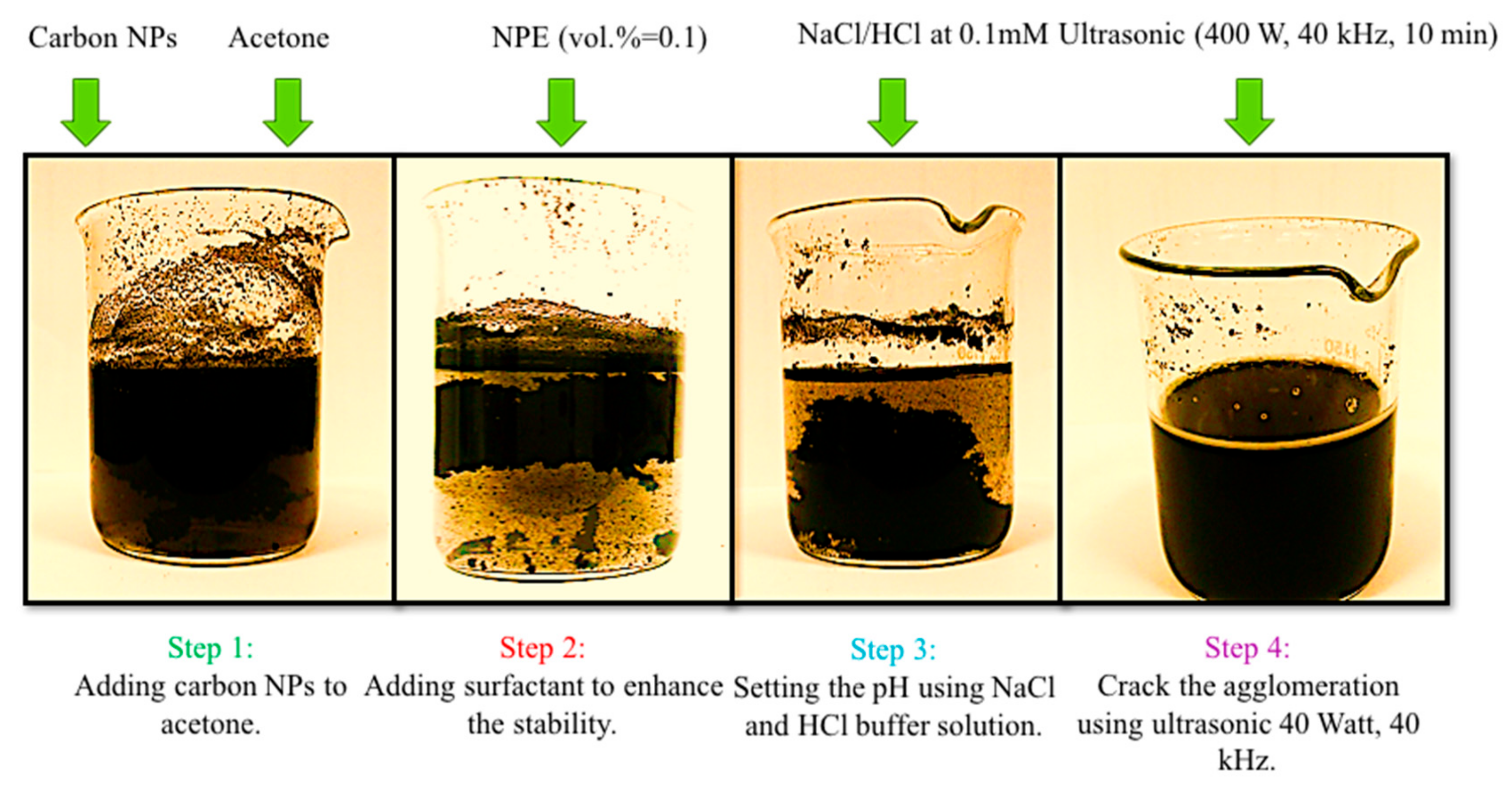
- Disperse the desire mass of carbon nanoparticles into acetone using magnetic stirrer at 250 rpm;
- Add an anionic surfactant, Nonyl Phenol Ethoxilate (NPE) (at vol.% = 0.1 of total prepared nanofluid);
- Set pH of the nanofluid to a value, in which the highest zeta potential was observed. After setting the pH value of each sample, to measure the zeta potential, the sample was assessed with a zeta sizer digital light scattering device manufactured by Malvern CO (Malvern, UK). So, the measurement was carried out after the pH was regulated using the buffer solution.
- Sonicate the nanofluid to disperse the particles uniformly; inside the working fluid.
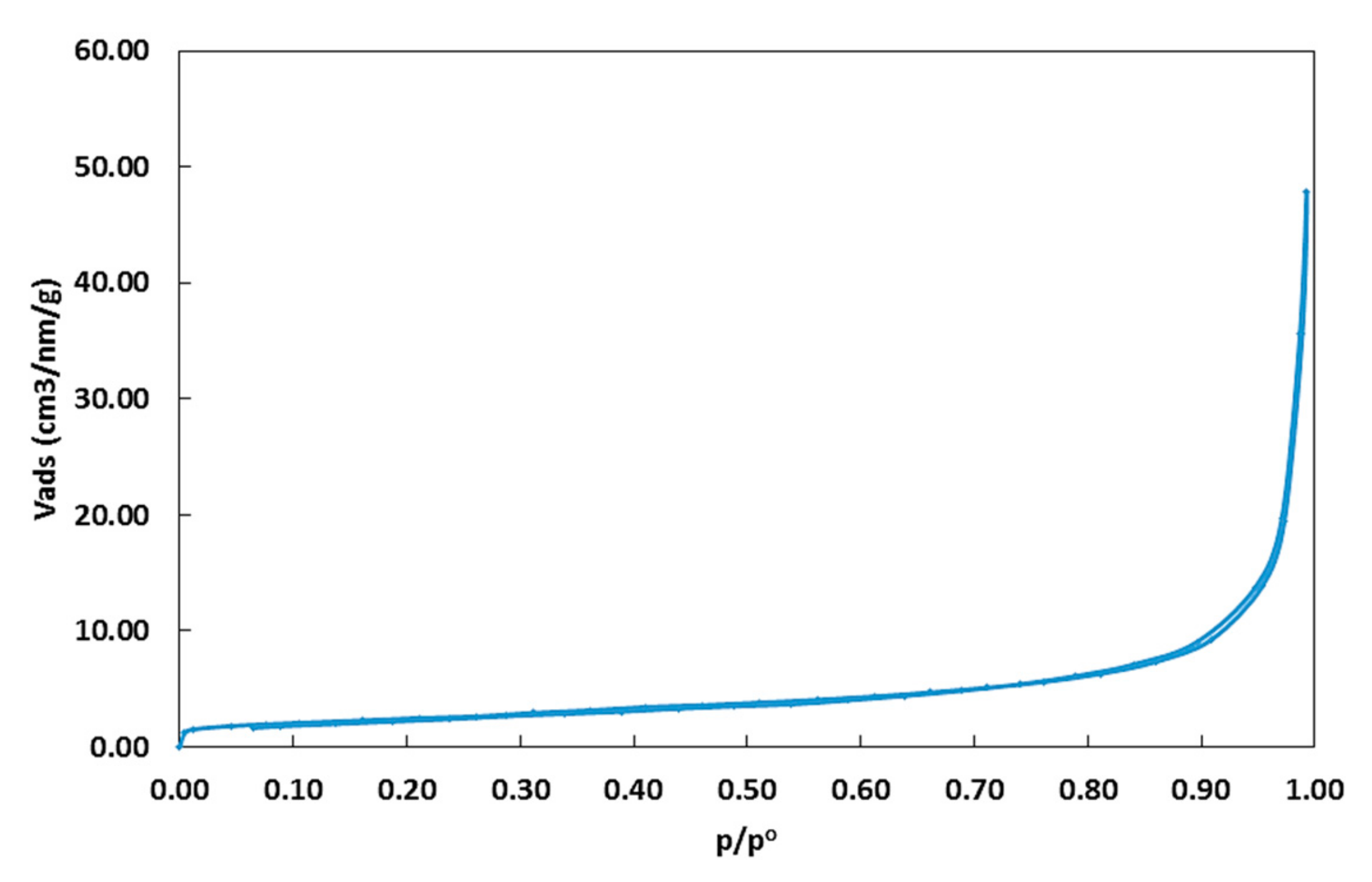
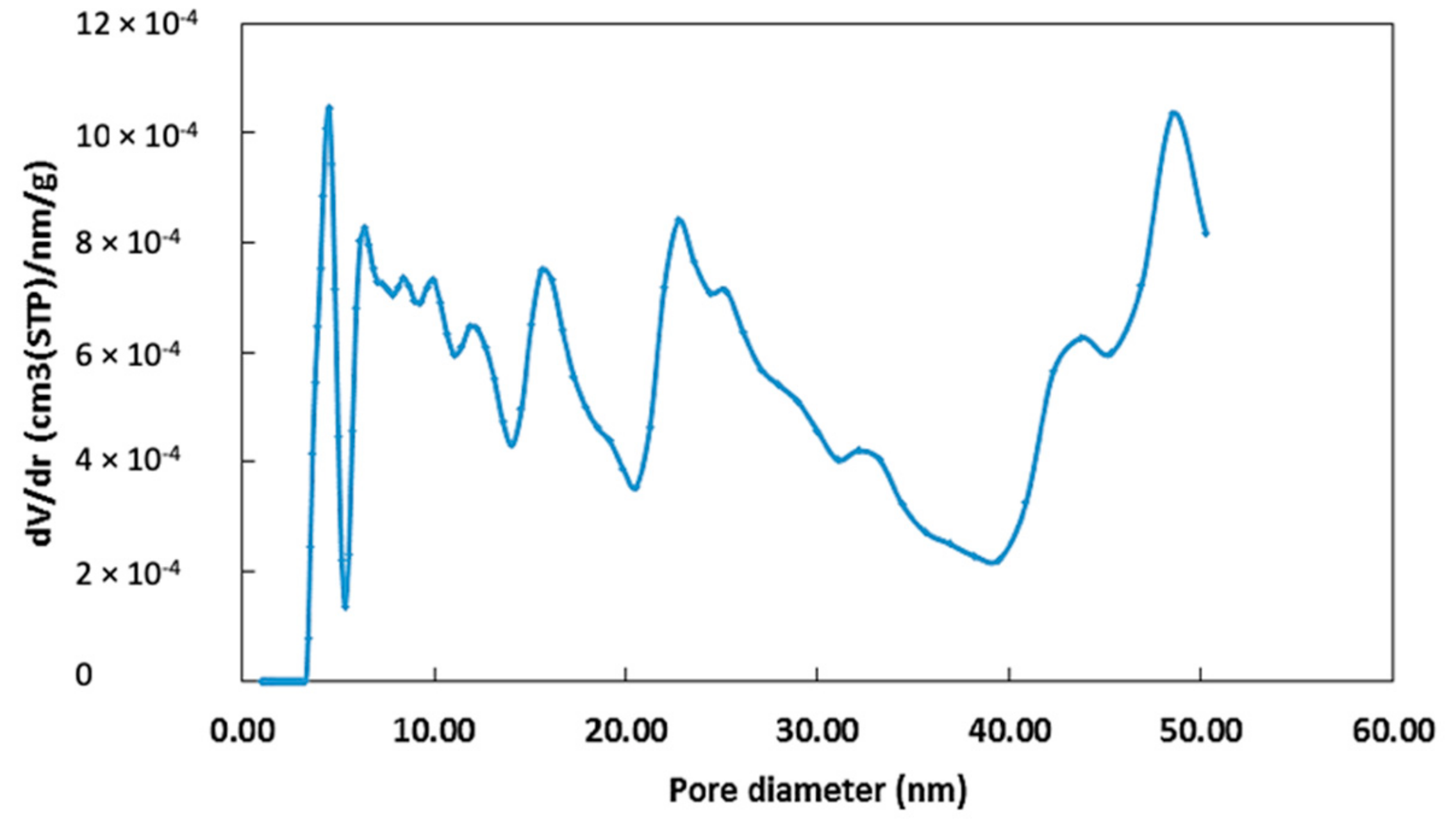
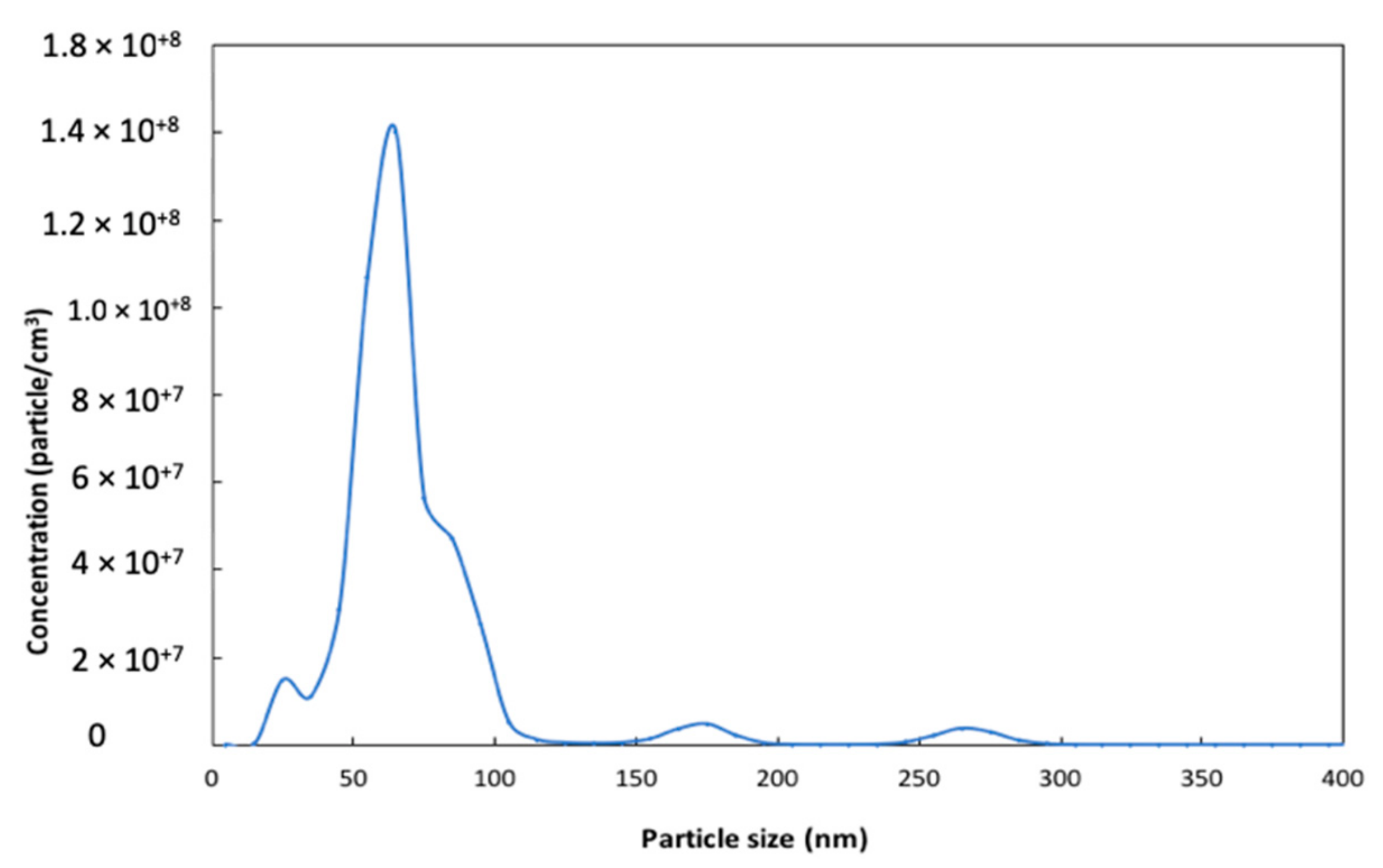
2.1.1. Surface Area and Particle Size
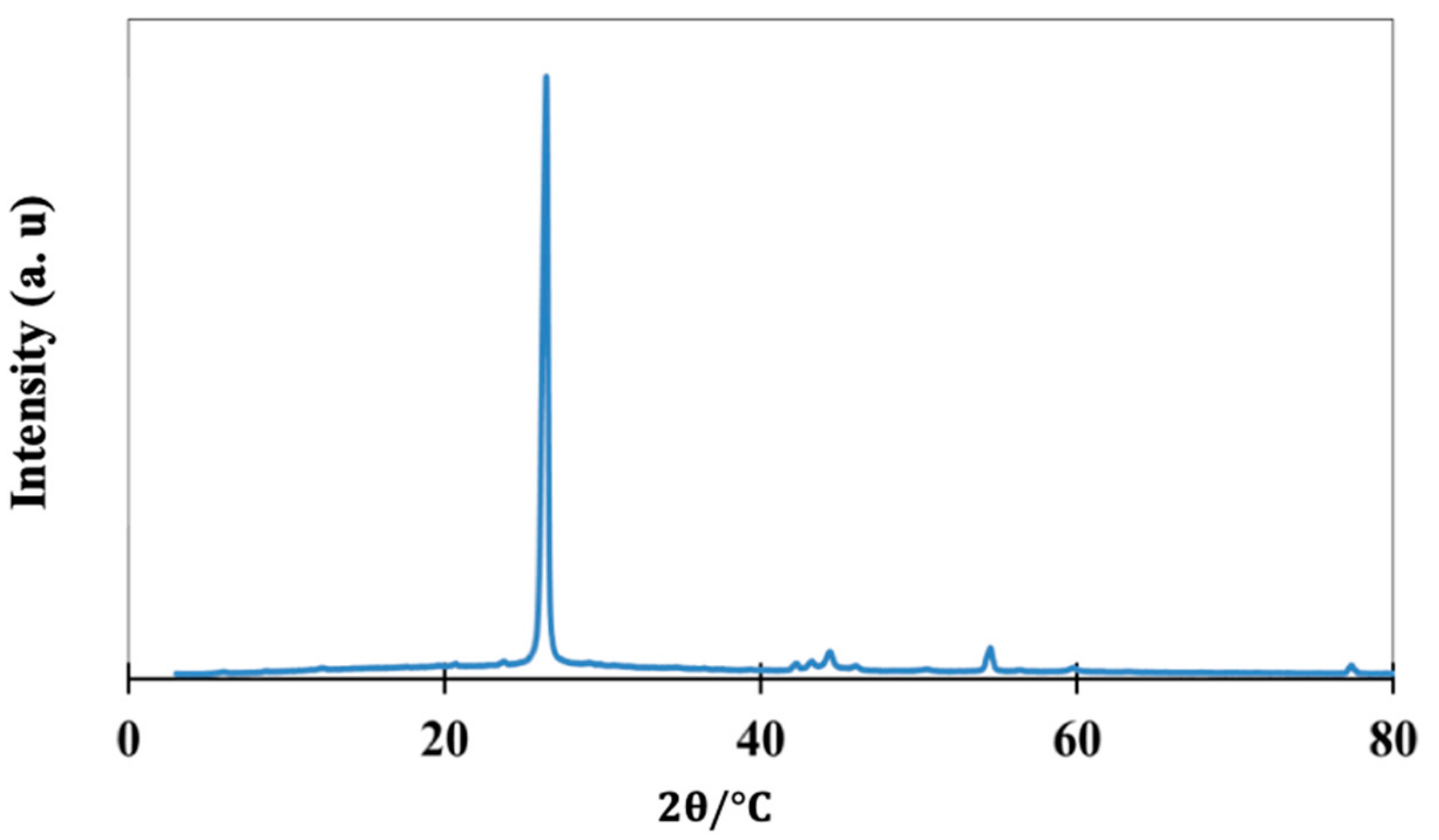
2.1.2. Structure and Composition
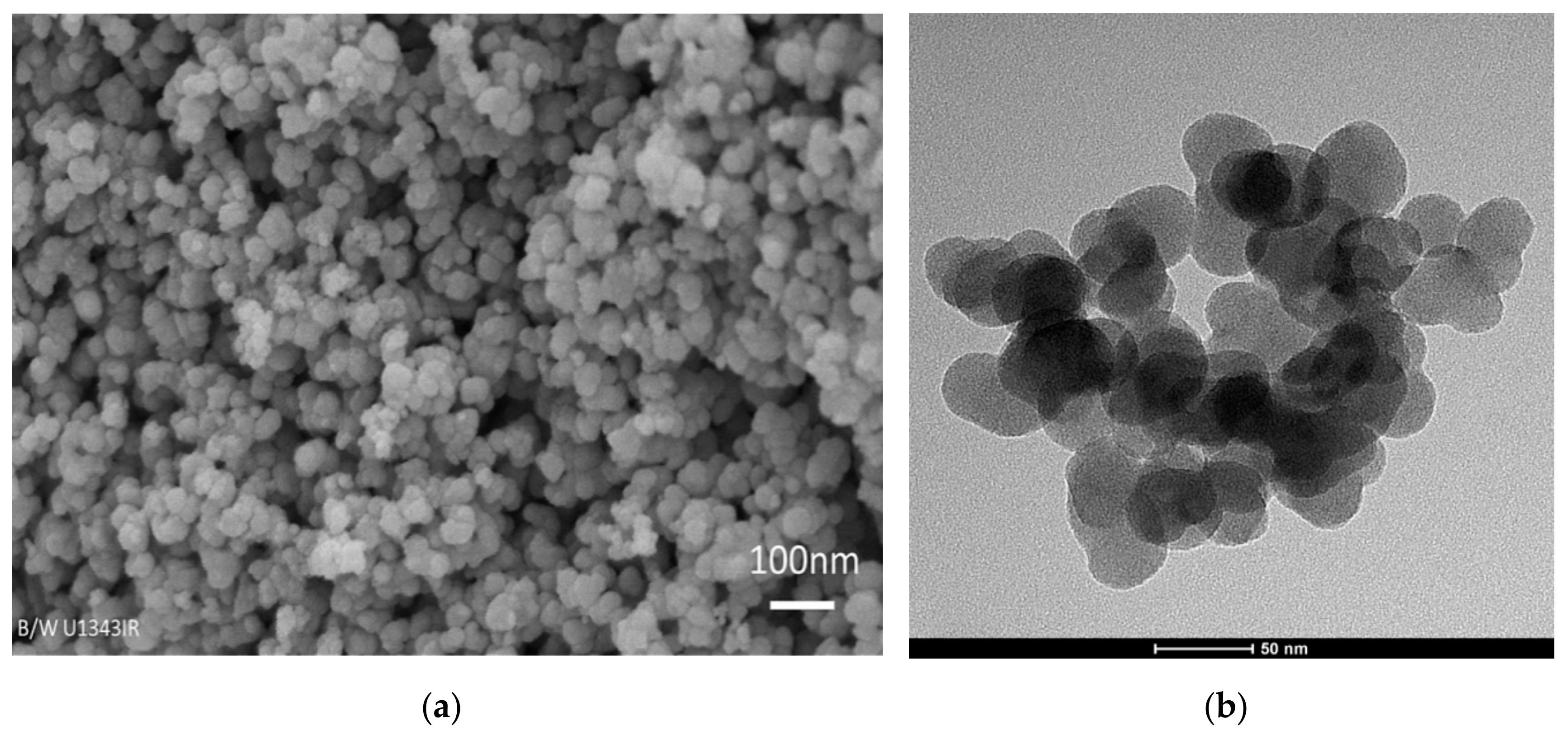
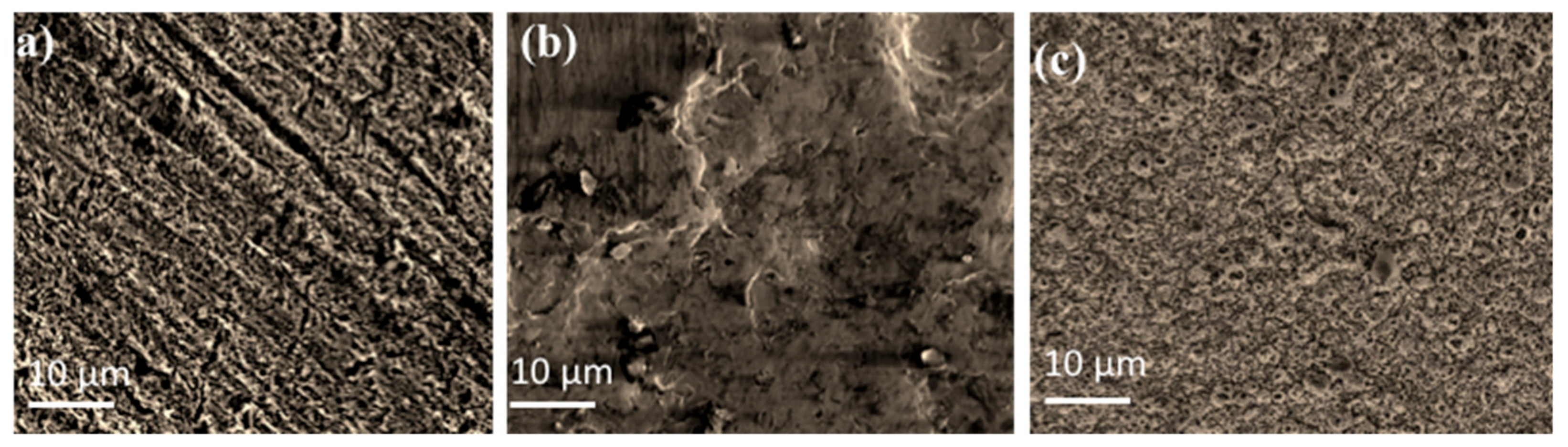
2.1.3. Morphology
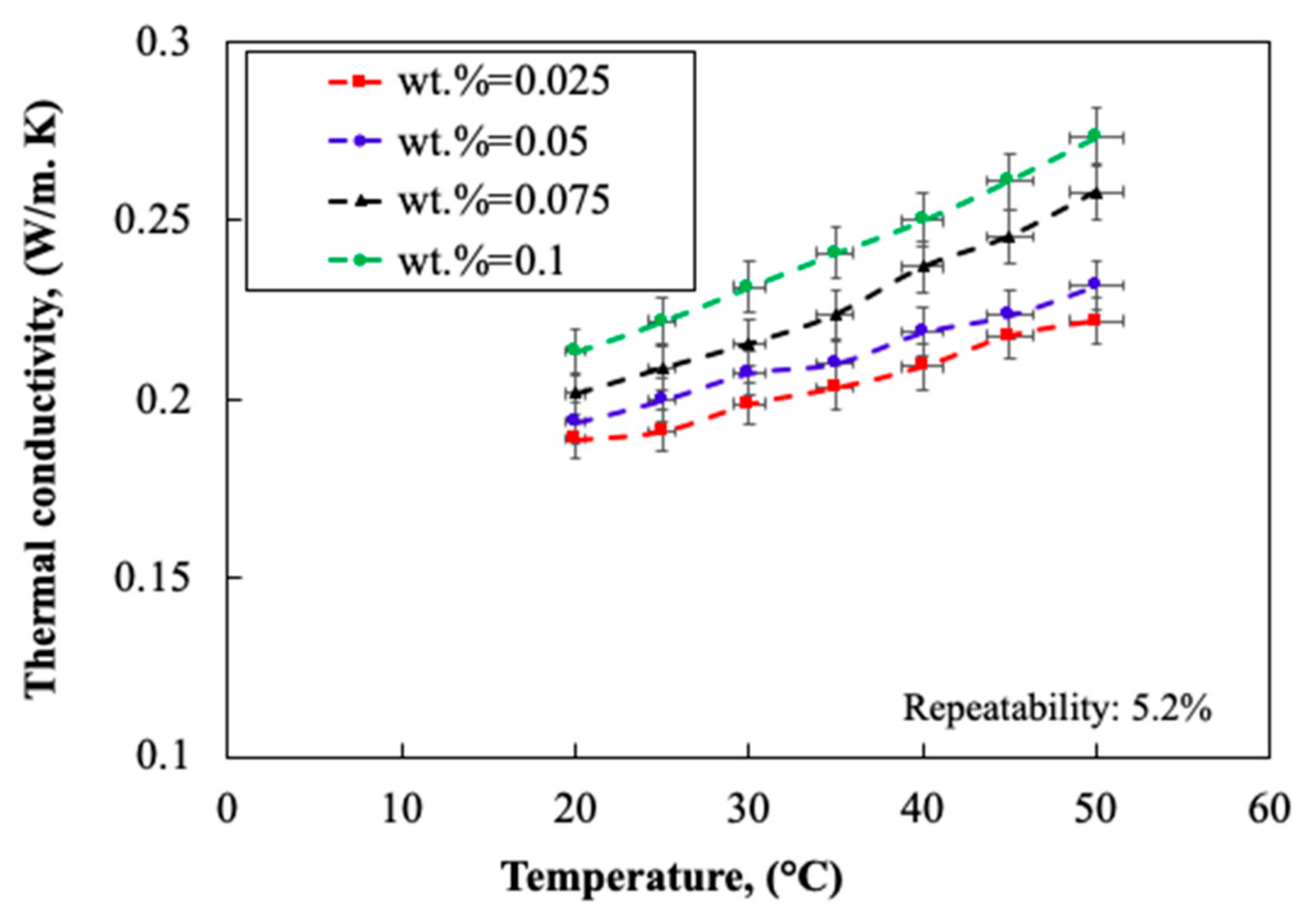
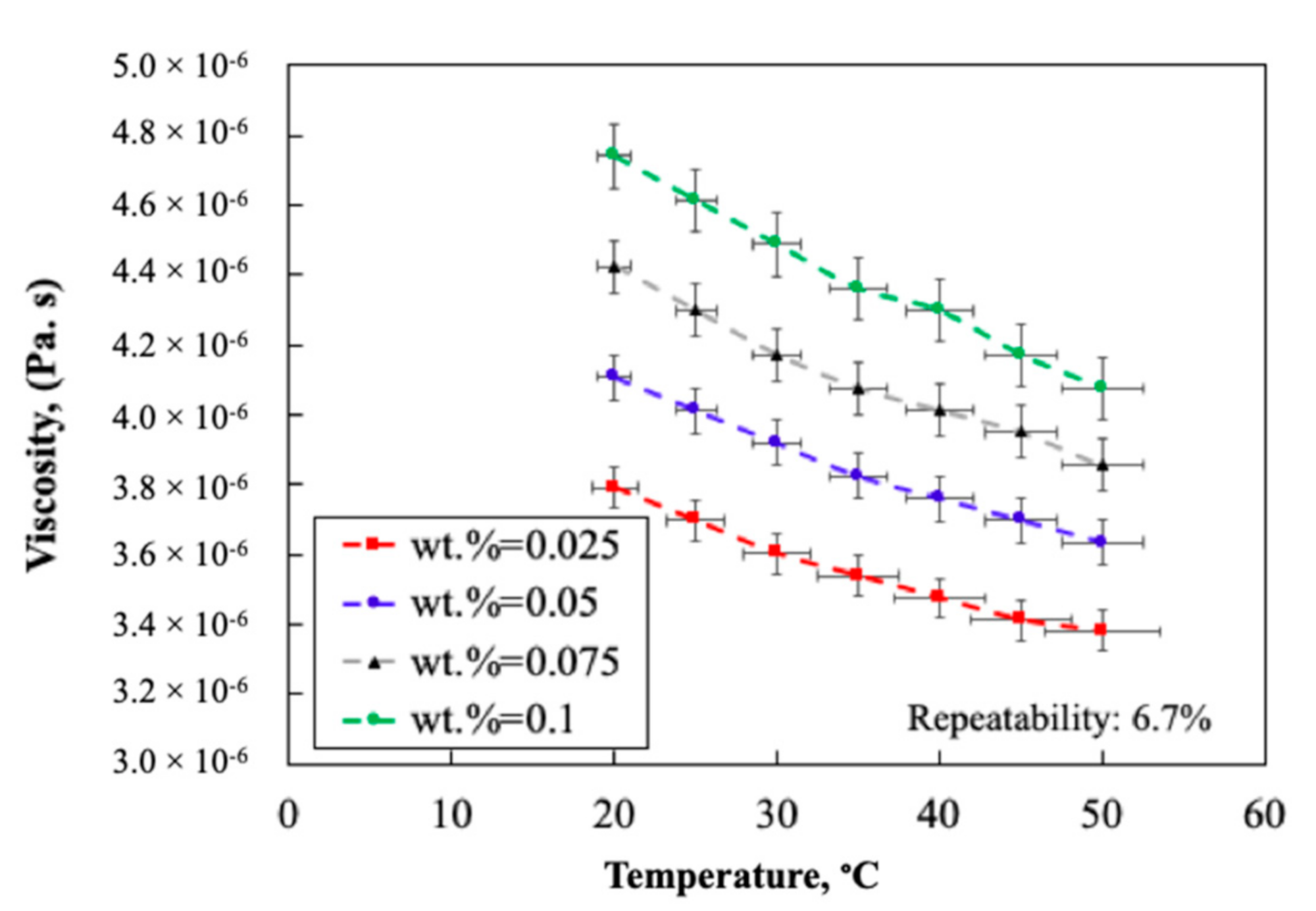
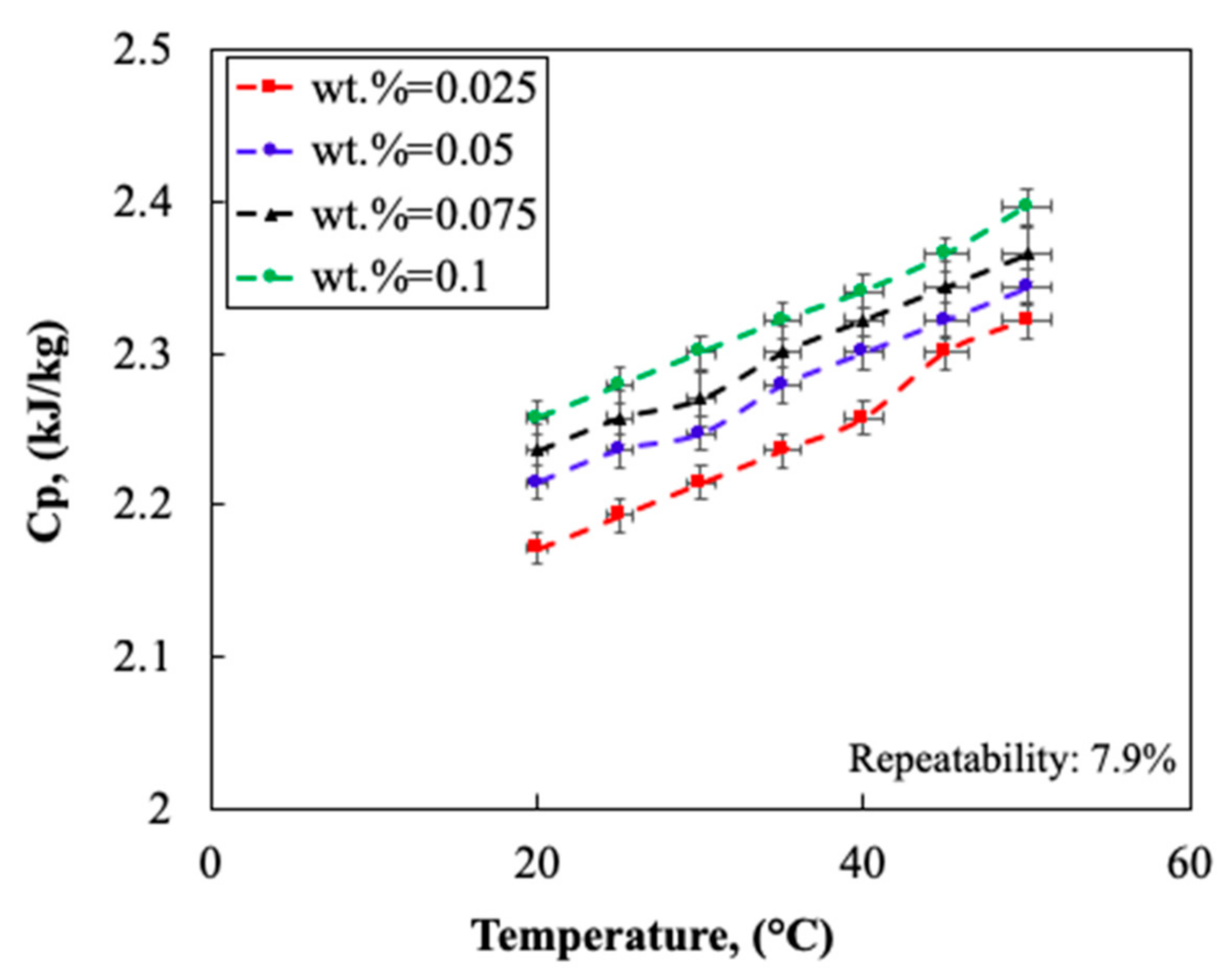
2.1.4. Thermo-Physical Properties
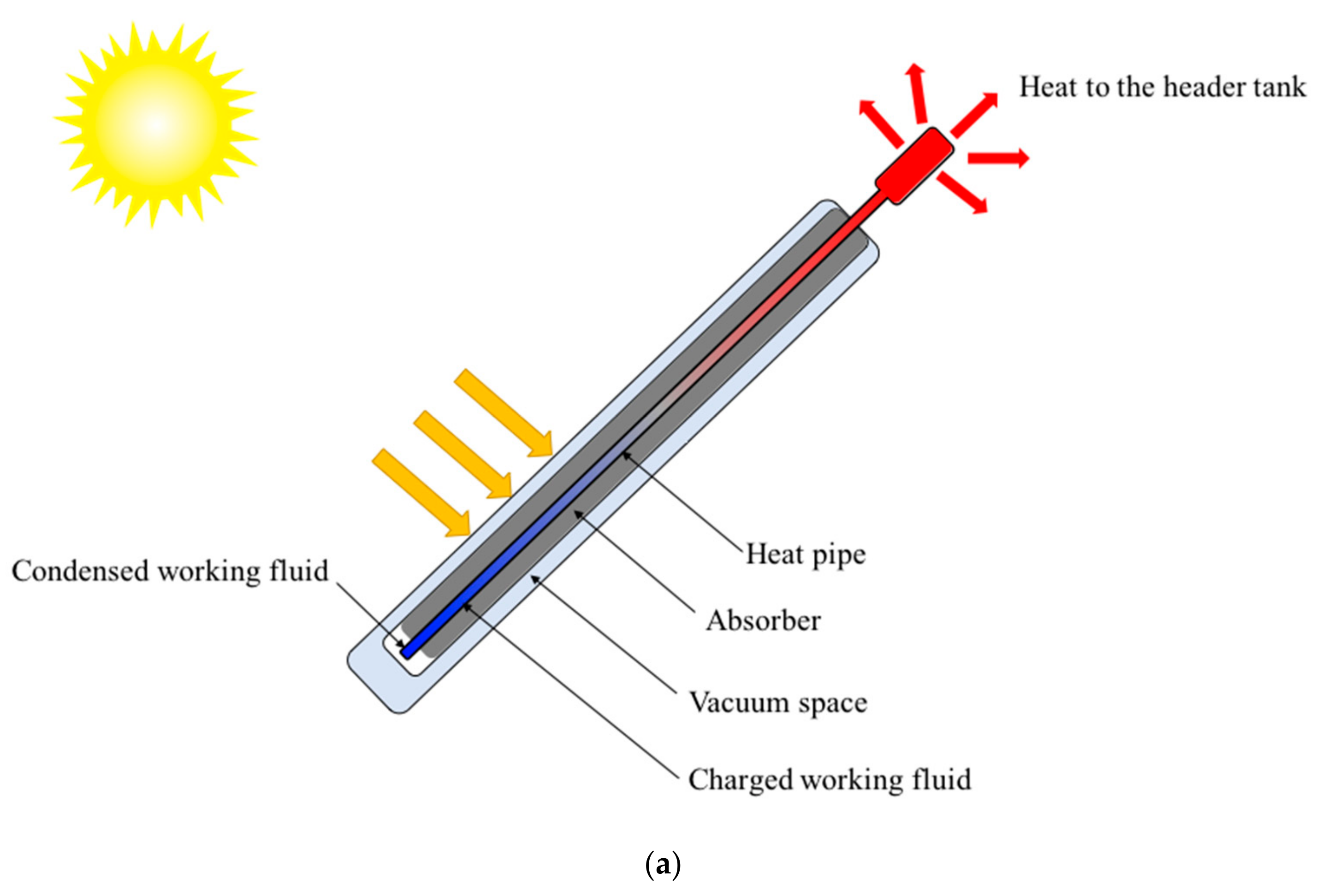
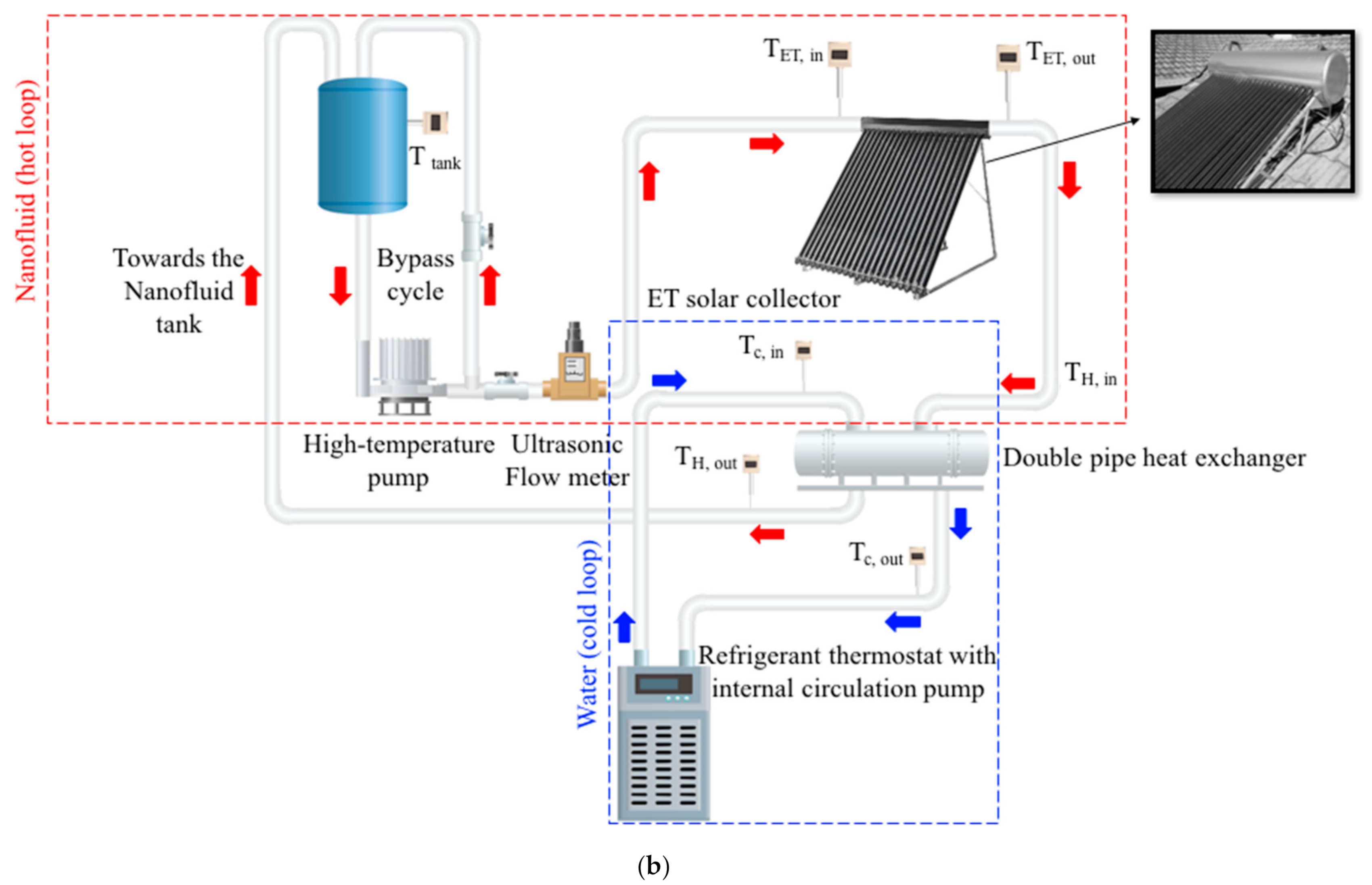
2.2. Experimental Setup
2.2.1. Experimental Setup
2.2.2. Data Reduction and Uncertainty Analysis
3. Results and Discussion
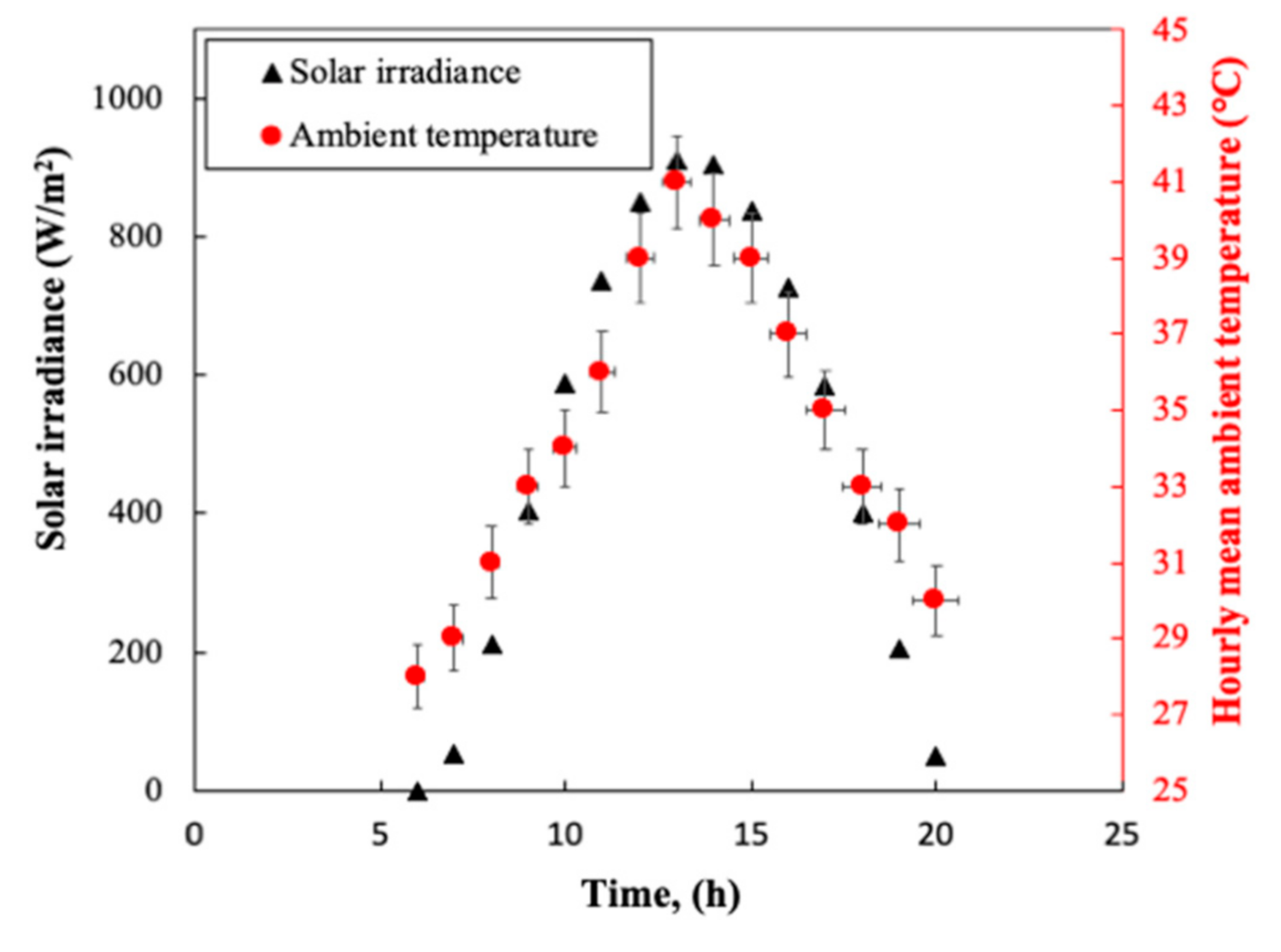
3.1. Solar Irradiance and Ambient Temperature
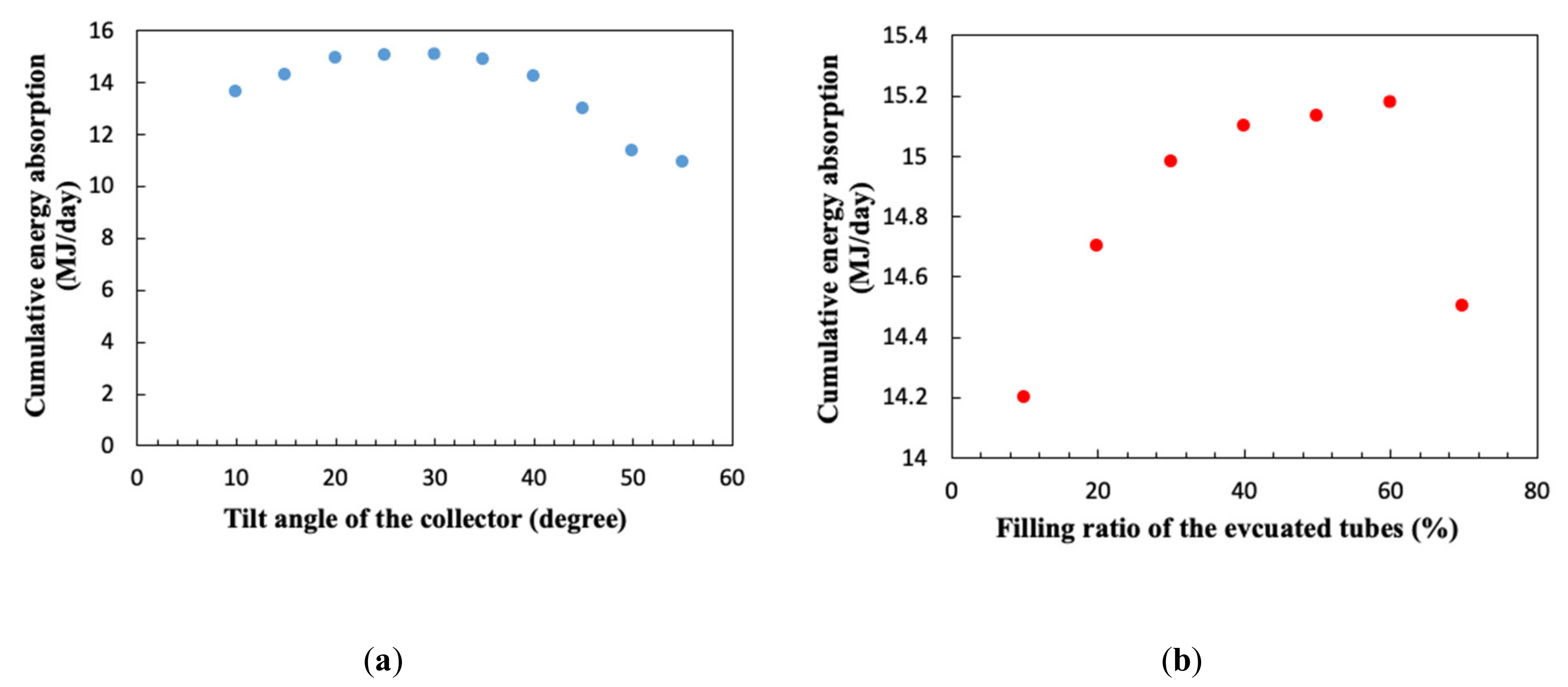
3.2. Tilt Angle and Filling Ratio
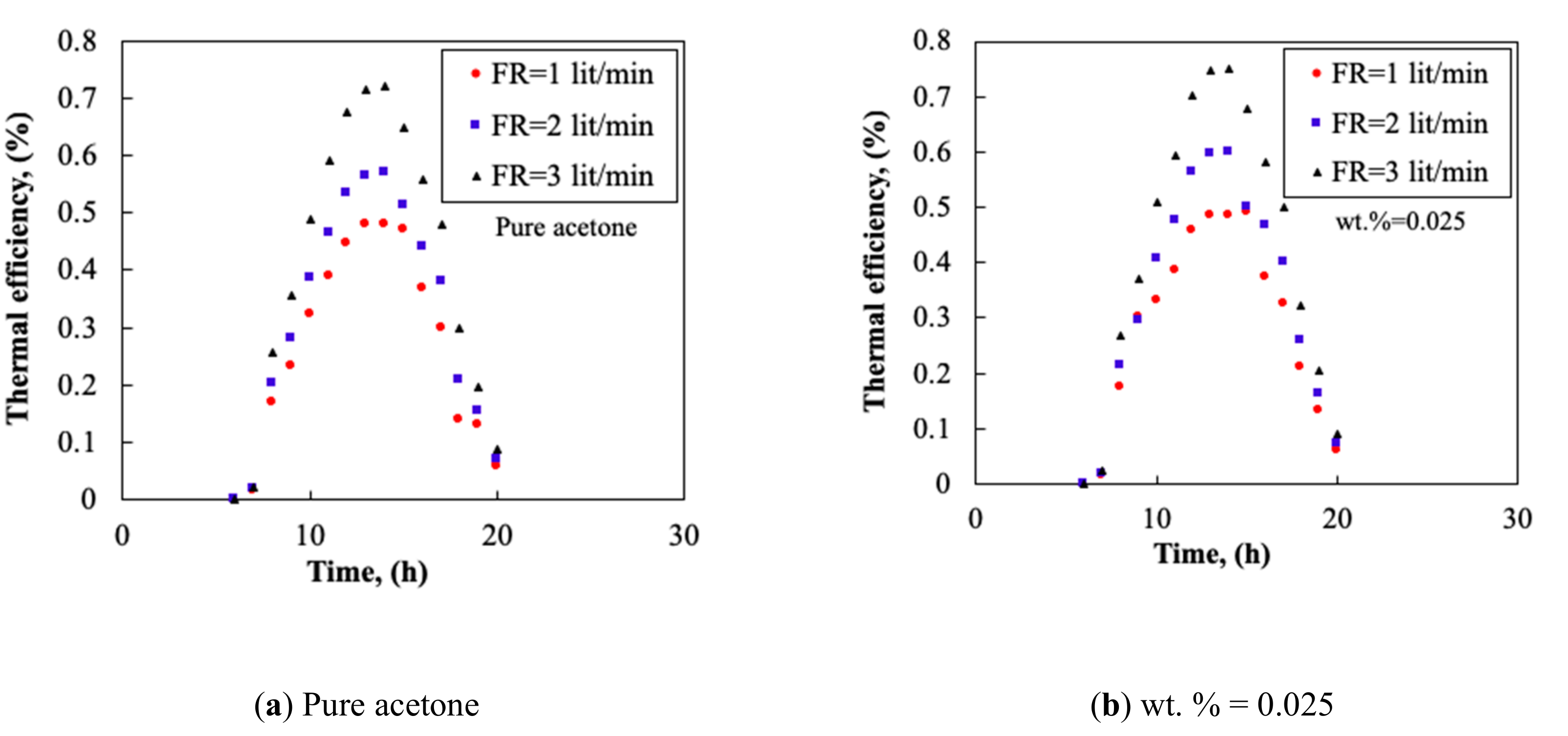
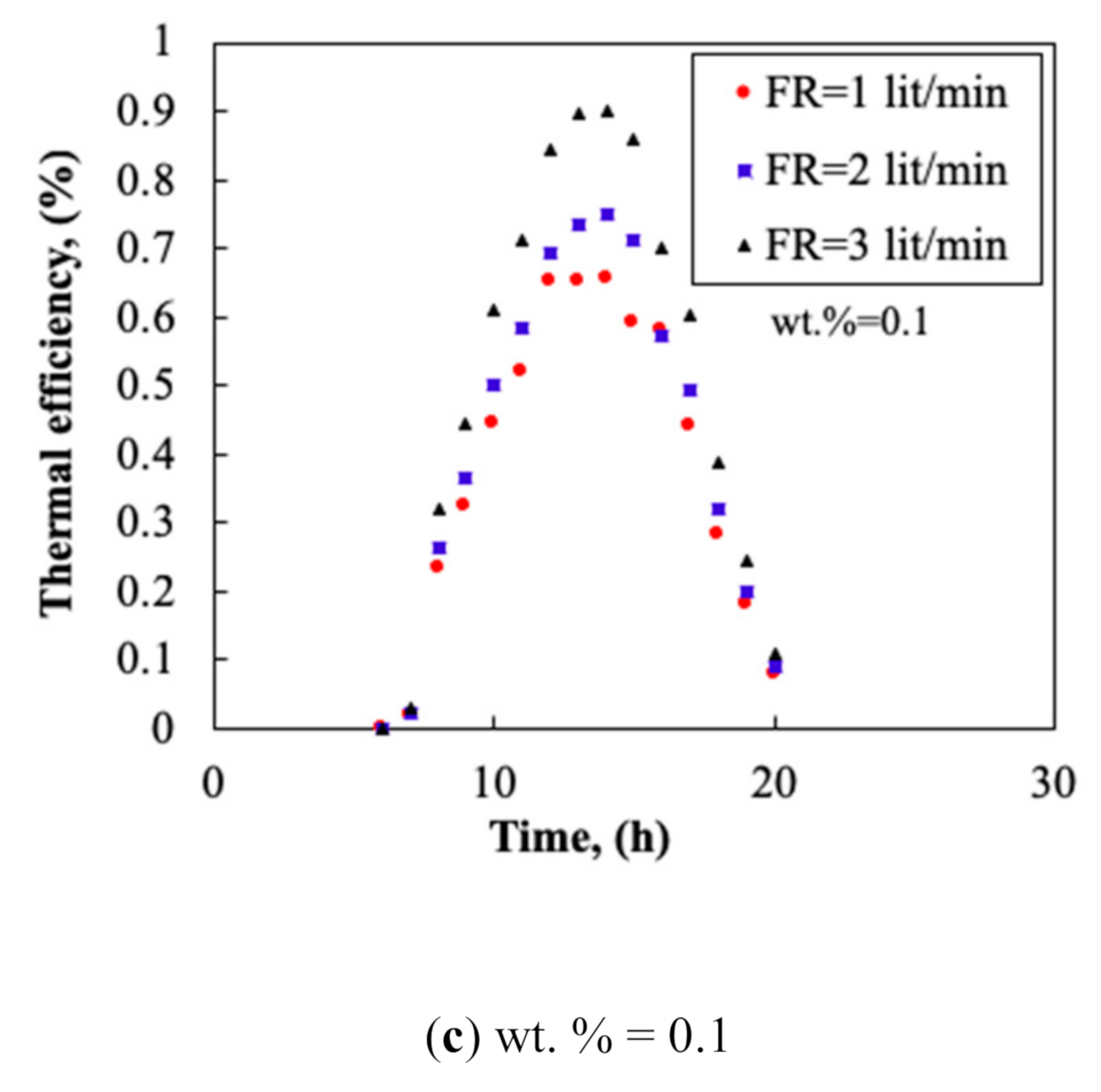
3.3. Collector’s Flow Rate
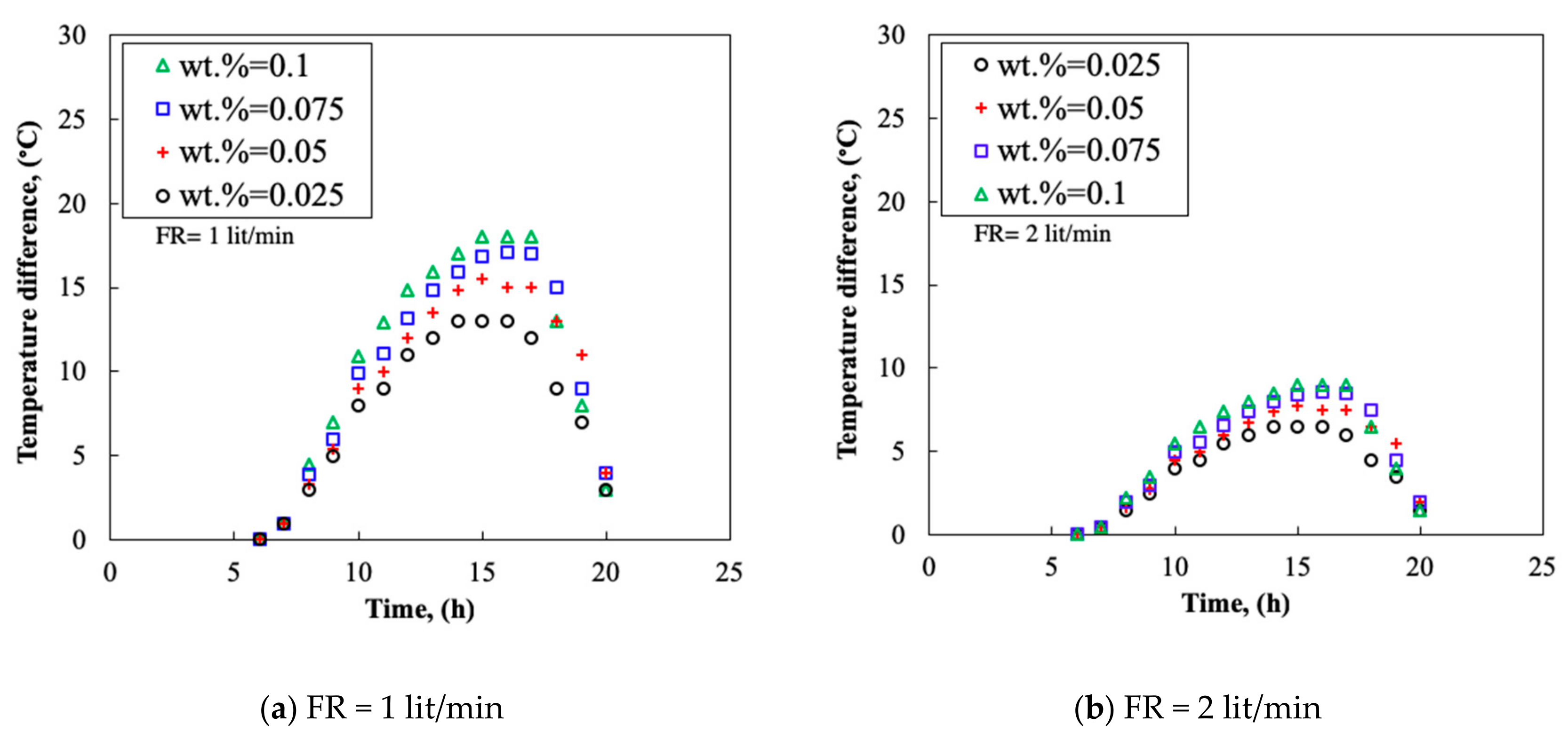
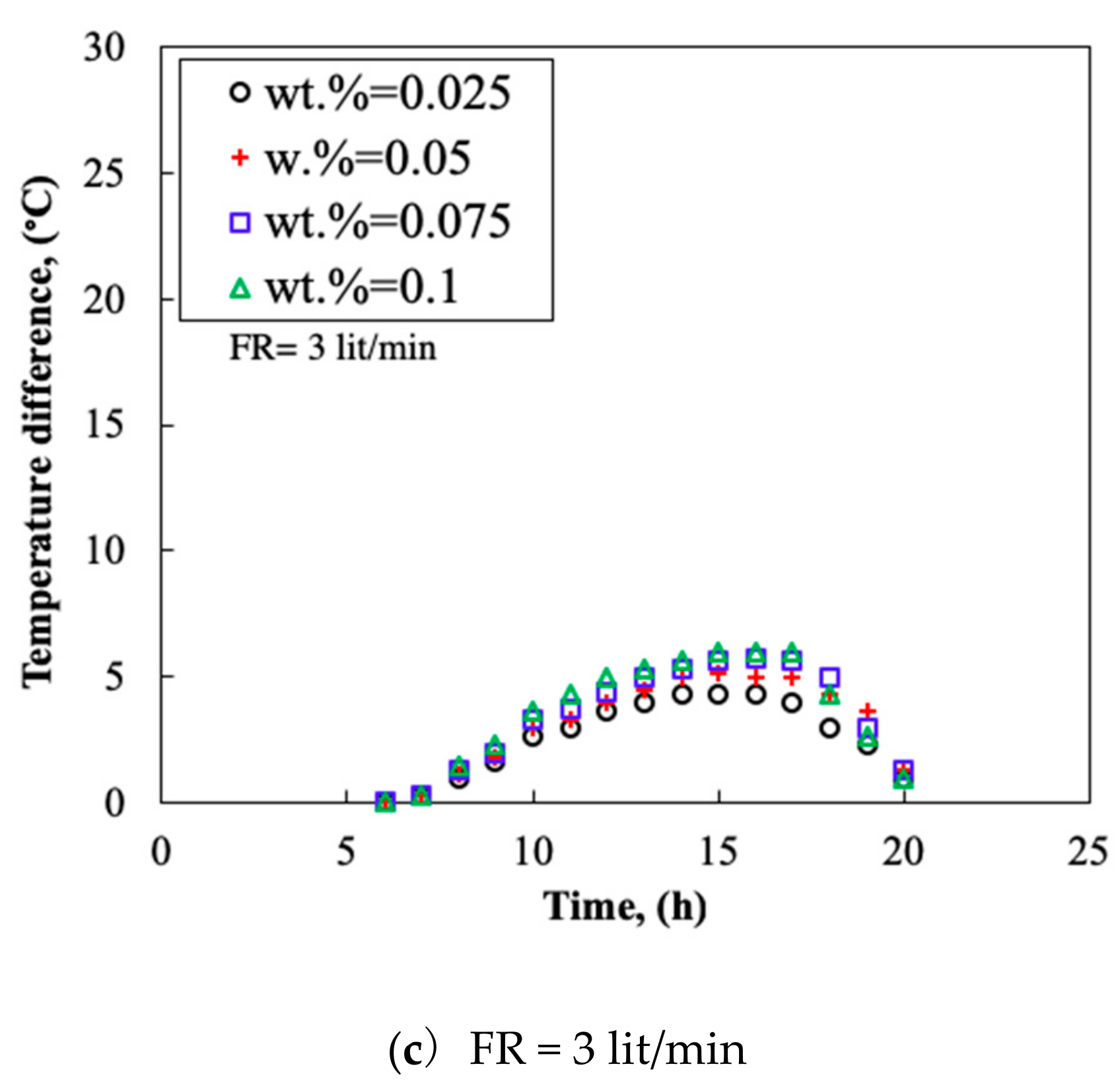
3.4. Temperature Difference
3.5. Evaporator’s Surface
4. Conclusions
- Carbon nanoparticles were stable up to 10 days within acetone and provided a significant change in the thermo-physical properties of the acetone. The thermal conductivity enhancement of the nanofluid improved the heat transfer mechanism in the HP and enhanced the TP of the collector. The maximum thermal efficiency of 91% was achieved which was well above the average thermal performance of the current collectors working with conventional working fluid (e.g., 72.6% for an acetone solar collector).
- By increasing the mass fraction of the nanofluid, the TP and the TD of the collector increased. The thermal efficiency could reach 0.91 and the maximum TD of 20 °C was obtained at flow rate of 3.0 L/min.
- The deposition of the nanoparticles on the internal wall of the evaporator created an irregular coating on the surface, which was a suitable medium for the nucleation and the formation of the bubbles. Besides the augmentation in the thermal conductivity of acetone, this irregular surface can intensify the bubble formation and their interaction, which resulted in the improvement of the HTC in the evaporator section.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahendran, M.; Lee, G.; Sharma, K.; Shahrani, A.; Bakar, R. Performance of evacuated tube solar collector using water-based titanium oxide nanofluid. J. Mech. Eng. Sci. 2012, 3, 301–310. [Google Scholar]
- Sabiha, M.; Saidur, R.; Hassani, S.; Said, Z.; Mekhilef, S. Energy performance of an evacuated tube solar collector using single walled carbon nanotubes nanofluids. Energy Convers. Manag. 2015, 105, 1377–1388. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Hu, R.-L.; Lu, L.; Zhao, F.; Xiao, H.-s. Thermal performance of an open thermosyphon using nanofluid for evacuated tubular high temperature air solar collector. Energy Convers. Manag. 2013, 73, 135–143. [Google Scholar] [CrossRef]
- Tsai, C.; Chien, H.; Ding, P.; Chan, B.; Luh, T.; Chen, P. Effect of structural character of gold nanoparticles in nanofluid on heat pipe thermal performance. Mater. Lett. 2004, 58, 1461–1465. [Google Scholar] [CrossRef]
- Kang, S.-W.; Wei, W.-C.; Tsai, S.-H.; Yang, S.-Y. Experimental investigation of silver nano-fluid on heat pipe thermal performance. Appl. Therm. Eng. 2006, 26, 2377–2382. [Google Scholar] [CrossRef]
- Barber, J.; Brutin, D.; Tadrist, L. A review on boiling heat transfer enhancement with nanofluids. Nanoscale Res. Lett. 2011, 6, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.A.; Phelan, P.E. Pool boiling of nanofluids: Comprehensive review of existing data and limited new data. Int. J. Heat Mass Transf. 2009, 52, 5339–5347. [Google Scholar] [CrossRef]
- Kwark, S.M.; Kumar, R.; Moreno, G.; Yoo, J.; You, S.M. Pool boiling characteristics of low concentration nanofluids. Int. J. Heat Mass Transf. 2010, 53, 972–981. [Google Scholar] [CrossRef]
- Das, S.K.; Choi, S.U.; Patel, H.E. Heat transfer in nanofluids—A review. Heat Transf. Eng. 2006, 27, 3–19. [Google Scholar] [CrossRef]
- Verma, S.K.; Tiwari, A.K. Progress of nanofluid application in solar collectors: A review. Energy Convers. Manag. 2015, 100, 324–346. [Google Scholar] [CrossRef]
- Xuan, Y.; Roetzel, W. Conceptions for heat transfer correlation of nanofluids. Int. J. Heat Mass Transf. 2000, 43, 3701–3707. [Google Scholar] [CrossRef]
- Nakhjavani, M.; Nikkhah, V.; Sarafraz, M.; Shoja, S.; Sarafraz, M. Green synthesis of silver nanoparticles using green tea leaves: Experimental study on the morphological, rheological and antibacterial behaviour. Heat Mass Transf. 2017, 53, 3201–3209. [Google Scholar] [CrossRef]
- Nikkhah, V.; Sarafraz, M.; Hormozi, F. Application of spherical copper oxide (ii) water nano-fluid as a potential coolant in a boiling annular heat exchanger. Chem. Biochem. Eng. Q. 2015, 29, 405–415. [Google Scholar] [CrossRef]
- Salari, E.; Peyghambarzadeh, S.; Sarafraz, M.; Hormozi, F.; Nikkhah, V. Thermal behavior of aqueous iron oxide nano-fluid as a coolant on a flat disc heater under the pool boiling condition. Heat Mass Transf. 2017, 53, 265–275. [Google Scholar] [CrossRef]
- Sarafraz, M. Experimental investigation on pool boiling heat transfer to formic acid, propanol and 2-butanol pure liquids under the atmospheric pressure. J. Appl. Fluid Mech. 2013, 6, 73–79. [Google Scholar]
- Sarafraz, M.; Hormozi, F.; Peyghambarzadeh, S.; Vaeli, N. Upward flow boiling to di-water and cuo nanofluids inside the concentric annuli. J. Appl. Fluid Mech. 2015, 8, 651–659. [Google Scholar]
- Sarafraz, M.; Nikkhah, V.; Madani, S.; Jafarian, M.; Hormozi, F. Low-frequency vibration for fouling mitigation and intensification of thermal performance of a plate heat exchanger working with cuo/water nanofluid. Appl. Therm. Eng. 2017, 121, 388–399. [Google Scholar] [CrossRef]
- Sarafraz, M.; Nikkhah, V.; Nakhjavani, M.; Arya, A. Thermal performance of a heat sink microchannel working with biologically produced silver-water nanofluid: Experimental assessment. Exp. Therm. Fluid Sci. 2018, 91, 509–519. [Google Scholar] [CrossRef]
- Sarafraz, M.; Peyghambarzadeh, S.; Alavi Fazel, S.; Vaeli, N. Nucleate pool boiling heat transfer of binary nano mixtures under atmospheric pressure around a smooth horizontal cylinder. Period. Polytech. Chem. Eng. 2013, 57, 71–77. [Google Scholar] [CrossRef]
- Sarafraz, M.; Peyghambarzadeh, S. Nucleate pool boiling heat transfer to al2o3-water and tio2-water nanofluids on horizontal smooth tubes with dissimilar homogeneous materials. Chem. Biochem. Eng. Q. 2012, 26, 199–206. [Google Scholar]
- Sarafraz, M.; Arjomandi, M. Demonstration of plausible application of gallium nano-suspension in microchannel solar thermal receiver: Experimental assessment of thermo-hydraulic performance of microchannel. Int. Commun. Heat Mass Transf. 2018, 94, 39–46. [Google Scholar] [CrossRef]
- Sarafraz, M.; Arjomandi, M. Thermal performance analysis of a microchannel heat sink cooling with copper oxide-indium (cuo/in) nano-suspensions at high-temperatures. Appl. Therm. Eng. 2018, 137, 700–709. [Google Scholar] [CrossRef]
- Sarafraz, M.; Arya, H.; Arjomandi, M. Thermal and hydraulic analysis of a rectangular microchannel with gallium-copper oxide nano-suspension. J. Mol. Liq. 2018, 263, 382–389. [Google Scholar] [CrossRef]
- Sarafraz, M.; Arya, H.; Saeedi, M.; Ahmadi, D. Flow boiling heat transfer to mgo-therminol 66 heat transfer fluid: Experimental assessment and correlation development. Appl. Therm. Eng. 2018, 138, 552–562. [Google Scholar] [CrossRef]
- Sarafraz, M.; Hart, J.; Shrestha, E.; Arya, H.; Arjomandi, M. Experimental thermal energy assessment of a liquid metal eutectic in a microchannel heat exchanger equipped with a (10 hz/50 hz) resonator. Appl. Therm. Eng. 2019, 148, 578–590. [Google Scholar] [CrossRef]
- Dehaj, M.S.; Mohiabadi, M.Z. Experimental investigation of heat pipe solar collector using mgo nanofluids. Sol. Energy Mater. Sol. Cells 2019, 191, 91–99. [Google Scholar] [CrossRef]
- Ziyadanogullari, N.B.; Yucel, H.; Yildiz, C. Thermal performance enhancement of flat-plate solar collectors by means of three different nanofluids. Therm. Sci. Eng. Prog. 2018, 8, 55–65. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Gróf, G. Efficiency of evacuated tube solar collector using wo3/water nanofluid. Renew. Energy 2019, 134, 453–460. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Gróf, G. Evacuated tube solar collector performance using ceo 2/water nanofluid. J. Clean. Prod. 2018, 185, 347–356. [Google Scholar] [CrossRef]
- Ozsoy, A.; Corumlu, V. Thermal performance of a thermosyphon heat pipe evacuated tube solar collector using silver-water nanofluid for commercial applications. Renew. Energy 2018, 122, 26–34. [Google Scholar] [CrossRef]
- Nazari, M.A.; Ghasempour, R.; Ahmadi, M.H.; Heydarian, G.; Shafii, M.B. Experimental investigation of graphene oxide nanofluid on heat transfer enhancement of pulsating heat pipe. Int. Commun. Heat Mass Transf. 2018, 91, 90–94. [Google Scholar] [CrossRef]
- Arya, A.; Sarafraz, M.; Shahmiri, S.; Madani, S.; Nikkhah, V.; Nakhjavani, S. Thermal performance analysis of a flat heat pipe working with carbon nanotube-water nanofluid for cooling of a high heat flux heater. Heat Mass Transf. 2018, 54, 985–997. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Navaneethakrishnan, P.; Kumaresan, G. Thermal characteristics studies on sintered wick heat pipe using cuo and al2o3 nanofluids. Exp. Therm. Fluid Sci. 2016, 79, 25–35. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Gor, G.Y.; Thommes, M.; Cychosz, K.A.; Neimark, A.V. Quenched solid density functional theory method for characterization of mesoporous carbons by nitrogen adsorption. Carbon 2012, 50, 1583–1590. [Google Scholar] [CrossRef]
- Sarafraz, M.; Hormozi, F. Experimental study on the thermal performance and efficiency of a copper made thermosyphon heat pipe charged with alumina–glycol based nanofluids. Powder Technol. 2014, 266, 378–387. [Google Scholar] [CrossRef]
- Sarafraz, M.; Hormozi, F. Heat transfer, pressure drop and fouling studies of multi-walled carbon nanotube nano-fluids inside a plate heat exchanger. Exp. Therm. Fluid Sci. 2016, 72, 1–11. [Google Scholar] [CrossRef]
- Sarafraz, M.; Hormozi, F.; Peyghambarzadeh, S. Role of nanofluid fouling on thermal performance of a thermosyphon: Are nanofluids reliable working fluid? Appl. Therm. Eng. 2015, 82, 212–224. [Google Scholar] [CrossRef]
- Sarafraz, M.; Hormozi, F.; Peyghambarzadeh, S. Thermal performance and efficiency of a thermosyphon heat pipe working with a biologically ecofriendly nanofluid. Int. Commun. Heat Mass Transf. 2014, 57, 297–303. [Google Scholar] [CrossRef]
- Sarafraz, M.; Safaei, M.R. Diurnal thermal evaluation of an evacuated tube solar collector (ETSC) charged with graphene nanoplatelets-methanol nano-suspension. Renew. Energy 2019. In press, accepted manuscript. [Google Scholar] [CrossRef]
- Moffat, R.J. Describing the uncertainties in experimental results. Exp. Therm. Fluid Sci. 1988, 1, 3–17. [Google Scholar] [CrossRef] [Green Version]
| Parameter | Instrument | Uncertainty |
|---|---|---|
| G | Pyranometer, EKO | ±1% of reading value (W/m2) |
| Temperature | Omega k type thermocouple | ±1 (°C) |
| Ambient temperature | RTD, PT-100 Omega | ±0.5 (°C) |
| Flow rate | FLownetix ultrasonic sensor | 1% of reading value (L/min) |
| Tilt angle | EuropacTM Inclinometer | ±0.1 (degree) |
| Weight | A&D balancer | ±1% of reading value (g) |
| Parameter | Value |
|---|---|
| Tube’s gross area | 1.75 m2 |
| Tube’s aperture area | 0.77 m2 |
| Total liquid capacity of the tube | 0.4 lit |
| Absorbance | 0.91 |
| Emission parameter | 0.04 |
| Collector’s dimension (L × H × W) | 2 × 0.15 × 0.9 m |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarafraz, M.M.; Tlili, I.; Abdul Baseer, M.; Safaei, M.R. Potential of Solar Collectors for Clean Thermal Energy Production in Smart Cities using Nanofluids: Experimental Assessment and Efficiency Improvement. Appl. Sci. 2019, 9, 1877. https://doi.org/10.3390/app9091877
Sarafraz MM, Tlili I, Abdul Baseer M, Safaei MR. Potential of Solar Collectors for Clean Thermal Energy Production in Smart Cities using Nanofluids: Experimental Assessment and Efficiency Improvement. Applied Sciences. 2019; 9(9):1877. https://doi.org/10.3390/app9091877
Chicago/Turabian StyleSarafraz, M. M., Iskander Tlili, Mohammad Abdul Baseer, and Mohammad Reza Safaei. 2019. "Potential of Solar Collectors for Clean Thermal Energy Production in Smart Cities using Nanofluids: Experimental Assessment and Efficiency Improvement" Applied Sciences 9, no. 9: 1877. https://doi.org/10.3390/app9091877






