Effect of Spirulina platensis Biomass with High Polysaccharides Content on Quality Attributes of Common Carp (Cyprinus carpio) and Common Barbel (Barbus barbus) Fish Burgers
Abstract
1. Introduction
2. Material and Methods
2.1. Collection of Fish
2.2. Fish Burger Manufacture
2.3. Physicochemical Properties of Fish Burger
2.4. Functional Properties of Fish Burger
2.5. Sensory Evaluation
2.6. Color Analysis
2.7. Textural Analysis
2.8. Antioxidant Properties Evaluation Using DPPH and FRAP Methods
2.9. Microbiological Analyses
2.10. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Results
3.2. Textural Parameters
3.3. Functional Properties of Fish Burgers
3.4. Microbiological Quality of Fish Burgers
3.5. Color Measurement of Burger Samples
3.6. Antioxidant Properties of Fish Burgers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gökoglu, N.; Yerlikaya, P. Seafood Chilling, Refrigeration and Freezing: Science and Technology, 1st ed.; John Wiley and Sons, Ltd.: Chichester, UK, 2015. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Fisheries and Aquaculture Department, 2015. Available online: http://www.fao.org/fishery/en (accessed on 27 July 2015).
- Pascoe, S. The sunken billions: The economic justification for fisheries reform. Mar. Resour. Econ. 2012, 27, 193–194. [Google Scholar] [CrossRef]
- Mancini, I.; Defant, A.; Mesarič, T.; Potočnik, F.; Batista, U.; Guella, G.; Turk, T.; Sepčić, K. Fatty acid composition of common barbel (Barbus barbus) roe and evaluation of its haemolytic and cytotoxic activities. Toxicon 2011, 57, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Maiditsch, I.P.; Ladich, F. Effects of temperature on auditory sensitivity in eurythermal fishes: Common Carp Cyprinus carpio (Family Cyprinidae) versus Wels Catfish Silurus glanis (Family Siluridae). PLoS ONE 2014, 9, e108583. [Google Scholar] [CrossRef][Green Version]
- Ljubojevic, D.; Trbovic, D.; Lujic, J.; Bjelic-Cabrilo, O.; Kostic, D.; Novakov, N.; Cirkovic, M. Fatty acid composition of fishes from inland waters. Bulg. J. Agric. Sci. 2013, 19, 62–74. [Google Scholar]
- Cedola, A.; Cardinali, A.; Nobile, M.A.D.; Conte, A. Fish burger enriched by olive oil industrial by-Product. Int. J. Food Sci. Nutr. 2017, 5, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Ben Amor, F.; Barkallah, M.; Elleuch, F.; Karkouch, N.; Dammak, M.; Baréa, B.; Villeneuve, P.; Abdelkafi, S.; Fendri, I. Cyanobacteria as source of marine bioactive compounds: Molecular specific detection based on Δ9 desaturase gene. Int. J. Biol. Macromol. 2017, 105, 1440–1445. [Google Scholar] [CrossRef]
- Park, W.S.; Kim, H.J.; Li, M.; Lim, D.H.; Kim, J.; Kwak, S.S.; Kang, C.M.; Ferruzzi, M.G.; Ahn, M.J. Two classes of pigments, carotenoids and C-phycocyanin, in Spirulina powder and their antioxidant activities. Molecules 2018, 23, 2065. [Google Scholar] [CrossRef] [PubMed]
- Kurd, F.; Samavati, V. Water soluble polysaccharides from Spirulina platensis: Extraction and in vitro anti-cancer activity. Int. J. Biol. Macromol. 2015, 74, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Ghaeni, M.; Roomiani, L. Review for application and medicine effects of Spirulina, Spirulina platensis microalgae. JOAAT 2016, 3, 114–117. [Google Scholar] [CrossRef]
- Suliburska, J.; Szulińska, M.; Tinkov, A.A.; Bogdański, P. Effect of Spirulina maxima supplementation on calcium, magnesium, iron, and zinc status in obese patients with treated hypertension. Biol. Trace Elem. Res. 2016, 173, 1–6. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X. Inhibitory effects of small molecular peptides from Spirulina (Arthrospira) platensis on cancer cell growth. Food Funct. 2016, 7, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Reichert, M.; Bergmann, S.M.; Hwang, J.; Buchholz, R.; Lindenberger, C. Antiviral activity of exopolysaccharides from Arthrospira platensis against koi herpesvirus. J. Fish Dis. 2017, 40, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Baky, H.H.; El-Baroty, G.S. Characterization and bioactivity of phycocyanin isolated from Spirulina maxima grown under salt stress. Food Funct. 2012, 3, 381–388. [Google Scholar] [CrossRef]
- Ben Atitallah, A.; Hentati, F.; Dammak, M.; Hadrich, B.; Fendri, I.; Ayadi, M.-A.; Michaud, P.; Abdelkafi, S.; Barkallah, M. Effect of microalgae incorporation on quality characteristics, functional and antioxidant capacities of ready-to-eat fish burgers made from common carp (Cyprinus carpio). Appl. Sci. 2019, 9, 1830. [Google Scholar] [CrossRef]
- Latimer, G.W. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–9170. [Google Scholar] [CrossRef]
- Fendri, I.; Chaari, A.; Dhouib, H.; Jlassi, B.; Abousalham, A.; Carrière, F.; Sayadi, S.; Abdelkafi, S. Isolation, identification and characterization of a new lipolytic Pseudomonas sp., strain AHD-1, from Tunisian soil. Environ. Technol. 2010, 31, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.-A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT Food Sci. Technol. 2017, 84, 323–330. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophylls A and B or leaf in dissolved solvents. Biochem. Soc. Trans. 1985, 11, 591–592. [Google Scholar] [CrossRef]
- Kumar, P.; Ramakritinan, C.M.; Kumaraguru, A.K. Solvent extraction and spectrophotometric determination of pigments of some algal species from the shore of puthumadam, southeast coast of India. Int. J. Oceans Oceanogr. 2010, 4, 29–34. [Google Scholar]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Kuniak, L.; Marchessault, R.H. Study of the crosslinking reaction between epichlorohydrin and starch. Starch Starke 1972, 24, 110–116. [Google Scholar] [CrossRef]
- Okezie, B.O.; Bello, A.B. Physicochemical and functional properties of winged bean flour and isolate compared with soy isolate. J. Food Sci. 1988, 53, 450–454. [Google Scholar] [CrossRef]
- Wong, K.H.; Cheung, P.C.K. Nutritional evaluation of some subtropical red and green seaweeds. Part I—Proximate composition, amino acid profiles and some physicochemical properties. Food Chem. 2000, 71, 475–482. [Google Scholar] [CrossRef]
- Bourne, M.C. Texture profile analysis. Food Technol. 1978, 32, 62–66. [Google Scholar]
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from a heated histidine glucose model system I: Investigation of the antioxidant role of histidine and isolation of antioxidants by high performance liquid chromatography. J. Am. Oil Chem. Soc. 1998, 75, 181–187. [Google Scholar] [CrossRef]
- Yildirim, A.; Mavi, A.; Kara, A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. Extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Compendium of Methods for the Microbiological Examination of Foods, 4th ed.; Downes, F.P., Ito, K., Eds.; American Public Health Association (APHA): Washington, DC, USA, 2001. [Google Scholar]
- Abdelkafi, S.; Labat, M.; Gam, Z.B.A.; Lorquin, J.; Sayadi, S. Optimized conditions for the synthesis of vanillic acid under hypersaline conditions by Halomonas elongata DSM 2581Tresting cells. World J. Microbiol. Biotechnol. 2008, 24, 675–680. [Google Scholar] [CrossRef]
- Shimamatsu, H. Mass production of Spirulina, an edible microalga. Hydrobiologia 2004, 512, 39–44. [Google Scholar] [CrossRef]
- Dammak, M.; Hadrich, B.; Miladi, R.; Barkallah, M.; Hentati, F.; Hachicha, R.; Laroche, C.; Michaud, P.; Fendri, I.; Abdelkafi, S. Effects of nutritional conditions on growth and biochemical composition of Tetraselmis sp. Lipids Health Dis. 2017, 16, 41. [Google Scholar] [CrossRef]
- Selani, M.M.; Shirado, G.A.N.; Margiotta, G.B.; Saldaña, E.; Spada, F.P.; Piedade, S.M.S.; Contreras-Castillo, C.J.; Canniatti-Brazaca, S.G. Effects of pineapple byproduct and canola oil as fat replacers on physicochemical and sensory qualities of low-fat beef burger. Meat Sci. 2016, 112, 69–76. [Google Scholar] [CrossRef]
- Cofrades, S.; López-López, I.; Solas, M.; Bravo, L.; Jiménez-Colmenero, F. Influence of different types and proportions of added edible seaweeds on characteristics of different types and proportions of added edible seaweeds on characteristics of low-salt gel/emulsion meat systems. Meat Sci. 2008, 79, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Martin, F.; Lopez-Lopez, I.; Cofrades, S.; Colmenero, F.J. Influence of adding Sea Spaghetti seaweed and replacing the animal fat with olive oil or a konjac gel on pork meat batter gelation. Potential protein/alginate association. Meat Sci. 2009, 83, 209–217. [Google Scholar] [CrossRef]
- Dolea, D.; Rizo, A.; Fuentes, A.; Barat, J.M.; Fernandez-Segovia, I. Effect of thyme and oregano essential oils on the shelf life of salmon and seaweed burgers. Food Sci. Technol. Int. 2018, 24, 394–403. [Google Scholar] [CrossRef]
- López-Vargas, J.H.; Fernández-López, J.; Pérez-Álvarez, J.Á.; Viuda-Martos, M. Quality characteristics of pork burger added with albedo-fiber powder obtained from yellow passion fruit (Passiflora edulis var. flavicarpa) co-products. Meat Sci. 2014, 97, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Huber, E.; Francio, D.L.; Biasi, V.; Mezzomo, N.; Ferreira, S.R.S. Characterization of vegetable fiber and its use in chicken burger formulation. J. Food Sci. Technol. 2016, 53, 3043–3052. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, L.M.J.; Díaz, I.S.; Rohner, C.S.; Ramirez, G.C.; Ambrosio, A.F. Effectiveness of high power ultrasound for surimi-based preparation of lionfish (Pterois volitans) patties by textural, sensory and shape preference. J. Culin. Sci. Technol. 2017, 17, 89–102. [Google Scholar] [CrossRef]
- Kumarathunge, N.C.; Jayasinghe, J.M.P.; Abeyrathne, E.D.N.S. Development of sea lettuce (Ulva lactuca) and Catla (Catla catla) incorporated protein and fiber rich fish burger. Int. J. Res. Agric. Sci. 2016, 4, 2348–3997. [Google Scholar]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2016, 99, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- López-López, I.; Cofrades, S.; Cañeque, V.; Díaz, M.T.; López, O.; Jiménez-Colmenero, F. Effect of cooking on the chemical composition of low-salt, low-fat Wakame/olive oil added beef patties with special reference to fatty acid content. Meat Sci. 2011, 89, 27–34. [Google Scholar] [CrossRef]
- Cox, S.; Abu-Ghannam, N. Enhancement of the phytochemical and fibre content of beef patties with Himanthalia elongata seaweed. Int. J. Food Sci. Technol. 2013, 48, 2239–2249. [Google Scholar]
- Luo, A.; Feng, J.; Hu, B.; Lv, J.; Chen, C.O.; Xie, S. Polysaccharides in Spirulina platensis improve antioxidant capacity of chinese-style sausage. J. Food Sci. 2017, 82, 2591–2597. [Google Scholar] [CrossRef] [PubMed]
- Senthil, M.A.; Mamatha, B.; Mahadevaswamy, M. Effect of using seaweed (Eucheuma) powder on the quality of fish cutlet. Int. J. Food Sci. Nutr. 2005, 56, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Bezerra, P.Q.M.; Matos, M.F.R.; Ramos, I.G.; Magalhães-Guedes, K.T.; Druzian, J.I.; Costa, J.A.V.; Nunes, I.L. Innovative functional nanodispersion: Combination of carotenoid from Spirulina and yellow passion fruit albedo. Food Chem. 2019, 258, 397–405. [Google Scholar] [CrossRef]
- Ismaiel, M.M.; El-Ayouty, Y.M.; Piercey-Normore, M. Role of pH on antioxidants production by Spirulina (Arthrospira) platensis. Braz. J. Microbiol. 2016, 47, 298–304. [Google Scholar] [CrossRef]
- Anbarasan, V.; Kumar, V.K.; Kumar, P.S.; Venkatachalam, T. In vitro evaluation of antioxidant activity of blue green algae S. platensis. Int. J. Pharm. Sci. Res. 2011, 2, 2616–2618. [Google Scholar]
- Ben Atitallah, A.; Barkallah, M.; Hentati, F.; Dammak, M.; Hlima, H.B.; Fendri, I.; Attia, H.; Michaud, P.; Abdelkafi, S. Physicochemical, textural, antioxidant and sensory characteristics of microalgae-fortified canned fish burgers prepared from minced flesh of common barbel (Barbus barbus). Food Biosci. 2019, 30, 100417. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Triratana, P.; Loha, V.; Tia, S.; Bunnag, B. Polysaccharide extraction from Spirulina sp. and its antioxidant capacity. Int. J. Biol. Macromol. 2013, 58, 73–78. [Google Scholar] [CrossRef]
- Pugh, N.; Ross, S.A.; ElSohly, H.N.; Elsohly, M.A.; Pasco, D.S. Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, Aphanizomenon flos-aquae and Chlorella pyrenoidosa. Planta Med. 2001, 67, 737–742. [Google Scholar] [CrossRef]
- Jónsdóttir, R.; Geirsdóttir, M.; Hamaguchi, P.Y.; Jamnik, P.; Kristinsson, H.G.; Undeland, I. The ability of in vitro antioxidant assays to predict the efficiency of a cod protein hydrolysate and brown seaweed extract to prevent oxidation in marine food model systems. J. Sci. Food Agric. 2015, 96, 2125–2135. [Google Scholar] [CrossRef] [PubMed]
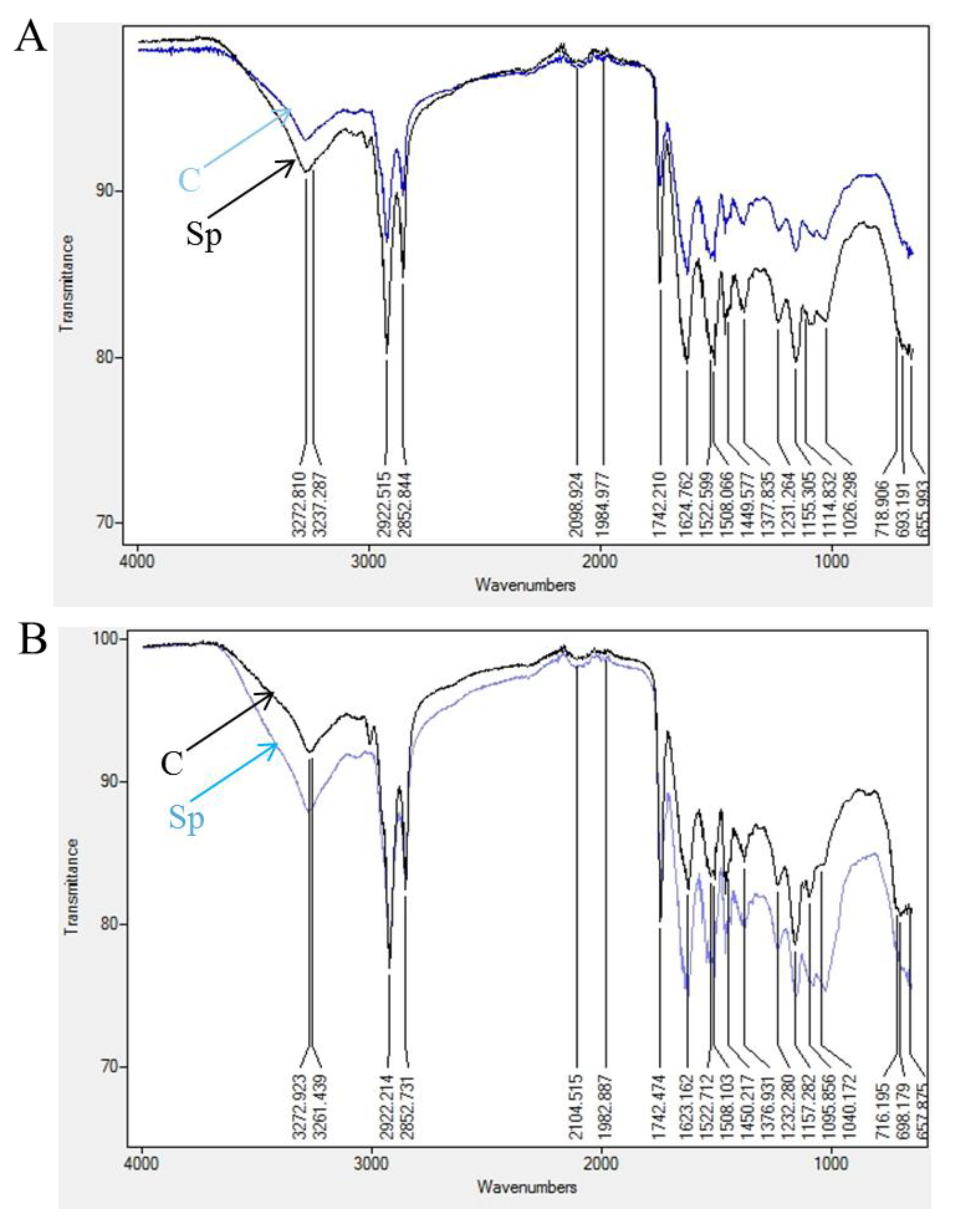
| Sensory Properties | Fish Burger Types | Formulations | |||
|---|---|---|---|---|---|
| Control burger | 0.5% Spirulina fish burger | 1% Spirulina fish burger | 1.5% Spirulina fish burger | ||
| Taste | CB | 4.42 ± 0.05 | 4.48 ± 0.145 | 4.26 ± 0.179 | 3.98 ± 0.034 b f g |
| BB | 4.12 ± 0.53 | 4.03 ± 0.42 | 3.98 ± 0.296 | 3.02 ± 0.203 c e h | |
| After-taste | CB | 4.35 ± 0.296 | 4.13 ± 0.014 | 4.11 ± 0.015 | 3.99 ± 0.020 a |
| BB | 4.08 ± 0.251 | 4.10 ± 0.428 | 4.07 ± 0.381 | 3.39 ± 0.307 a d g | |
| Odor | CB | 3.99 ± 0.021 | 4.03 ± 0.024 | 4.04 ± 0.050 | 4.02 ± 0.017 |
| BB | 4.01 ± 0.283 | 4.00 ± 0.261 | 4.01 ± 0.312 | 4.05 ± 0.311 | |
| Color | CB | 4.26 ± 0.08 | 4.29 ± 0.06 | 4.15 ± 0.07 | 3.91 ± 0.08 c f h |
| BB | 4.01 ± 0.432 | 4.02 ± 0.419 | 4.02 ± 0.521 | 3.88 ± 0.489 | |
| Mouth-texture | CB | 4.03 ± 0.090 | 4.09 ± 0.060 | 4.24 ± 0.050 b d | 4.17 ± 0.022 a |
| BB | 3.98 ± 0.123 | 4.09 ± 0.327 | 4.02 ± 0.222 | 4.01 ± 0.284 | |
| Overall-acceptability | CB | 4.12 ± 0.032 | 4.12 ± 0.020 | 4.13 ± 0.034 | 4.03 ± 0.023 b e h |
| BB | 4.12 ± 0.156 | 4.11 ± 0.238 | 4.08 ± 0.145 | 3.80 ± 0.05 a d g | |
| Physicochemical Characteristics | Fish Burger Types | Burger Formulations | ||
|---|---|---|---|---|
| Control burger | 0.5% Spirulina fish burger | 1% Spirulina fish burger | ||
| pH | CB | 7.21 ± 0.07 | 7.16 ± 0.01 | 7.17 ± 0.01 |
| BB | 7.14 ± 0.02 | 7.1 ± 0.015 | 7.07 ± 0.025 | |
| Water activity (aw) | CB | 0.982 ± 0.000 | 0.979 ± 0.000 a | 0.978 ± 0.000 c |
| BB | 0.982 ± 0.001 | 0.974 ± 0.000 c | 0.969 ± 0.001 c f | |
| Moisture (% FW) | CB | 77.03 ± 0.055 | 76.55 ± 0.053 | 76.36 ± 0.078 |
| BB | 77.57 ± 0.32 | 77.17 ± 0.072 a | 77.00 ± 0.046 b | |
| Total solids (% FW) | CB | 22.96 ± 0.055 | 23.44 ± 0.053 c | 23.63 ± 0.078 c e |
| BB | 22.43 ± 0.32 | 22.82 ± 0.072 | 23 ± 0.046 a | |
| Protein (% DW) | CB | 79.66 ± 0.119 | 79.50 ± 0.019 | 79.20 ± 0.096 |
| BB | 78.24 ± 0.125 | 78.21 ± 0.070 | 78.02 ± 0.012 a | |
| Fat (% DW) | CB | 7.79 ± 0.15 | 7.67 ± 0.04 | 7.46 ± 0.068 |
| BB | 8.33 ± 0.085 | 8.25 ± 0.095 | 8.24 ± 0.010 | |
| Ash (% DW) | CB | 11.12 ± 0.105 | 11.18 ± 0.013 | 11.69 ± 0.046 c f |
| BB | 11.53 ± 0.03 | 11.56 ± 0.315 | 11.84 ± 0.155 c f | |
| Total dietary fiber (g/100 g DW) * | CB | 0.32 ± 0.024 | 0.5 ± 0.075 c | 0.76 ± 0.024 c f |
| BB | 0.203 ± 0.000 | 0.308 ± 0.016 a | 0.817 ± 0.158 c f | |
| Textural Parameters | Fish Burger Types | Burger Formulations | |
|---|---|---|---|
| Control burger | 1% Spirulina fish burger | ||
| Hardness (N) | CB | 8.35 | 13.53 c |
| BB | 6.69 | 7.39 a | |
| Elasticity (cm) | CB | 0.36 | 0.38 |
| BB | 0.56 | 0.85 c | |
| Chewiness (N.cm) | CB | 1.11 | 2.21 c |
| BB | 0.88 | 2.40 c | |
| Gumminess (N) | CB | 3.09 | 5.84 c |
| BB | 1.58 | 2.83 c | |
| Cohesion | CB | 0.371 | 0.432 a |
| BB | 0.239 | 0.383 c | |
| Functional Characteristics | Fish Burger Types | Burger Formulations | |
|---|---|---|---|
| Control burger | 1% Spirulina fish burger | ||
| SWC (mL/g DW) * | CB | 3.13 ± 0.115 | 3.56 ± 0.110 a |
| BB | 2.99 ± 0.03 | 3.43 ± 0.02 a | |
| OHC (g/g DW) * | CB | 0.85 ± 0.07 | 0.90 ± 0.017 a |
| BB | 0.86 ± 0.014 | 1.06 ± 0.056 a | |
| WHC (g/g DW) * | CB | 2.31 ± 0.135 | 2.78 ± 0.162 a |
| BB | 2.12 ± 0.042 | 2.52 ± 0.042 a | |
| Color Parameters | Fish Burger Types | Burger Formulations | |
|---|---|---|---|
| Control burger | 1% Spirulina fish burger | ||
| L* | CB | 46.75 ± 0.335 | 40.23 ± 0.961 a |
| BB | 40.6 ± 0.07 | 33.27 ± 0.530 a | |
| a* | CB | 10.65 ± 0.22 | 6.23 ± 0.084 a |
| BB | 8.18 ± 0.084 | 5.2 ± 0.098 a | |
| b* | CB | 11.39 ± 0.715 | 7.77 ± 0.692 a |
| BB | 18.11 ± 0.664 | 5.73 ± 0.001 a | |
| Color Parameters | Fish Burger Types | Burger Formulations | |
|---|---|---|---|
| Control burger | 1% Spirulina fish burger | ||
| Chlorophylls (mg/100g DW) | CB | 0 | 23.93 ± 1.675 a |
| BB | 0 | 20.05 ± 1.97 a | |
| Phycocyanin (mg/100g DW) | CB | 0 | 0.431 ± 0.033 a |
| BB | 0 | 0.446 ± 0.199 a | |
| Carotenoids (mg/100g DW) | CB | 0 | 12.39 ± 0.657 a |
| BB | 0 | 14.06 ± 0.848 a | |
| Scavenging activity (%) * | CB | 56.28 | 86.76 a |
| BB | 40.09 | 94.09 a | |
| Reducing power * | CB | 0.315 | 0.615 a |
| BB | 0.410 | 0.584 a | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkallah, M.; Ben Atitallah, A.; Hentati, F.; Dammak, M.; Hadrich, B.; Fendri, I.; Ayadi, M.A.; Michaud, P.; Abdelkafi, S. Effect of Spirulina platensis Biomass with High Polysaccharides Content on Quality Attributes of Common Carp (Cyprinus carpio) and Common Barbel (Barbus barbus) Fish Burgers. Appl. Sci. 2019, 9, 2197. https://doi.org/10.3390/app9112197
Barkallah M, Ben Atitallah A, Hentati F, Dammak M, Hadrich B, Fendri I, Ayadi MA, Michaud P, Abdelkafi S. Effect of Spirulina platensis Biomass with High Polysaccharides Content on Quality Attributes of Common Carp (Cyprinus carpio) and Common Barbel (Barbus barbus) Fish Burgers. Applied Sciences. 2019; 9(11):2197. https://doi.org/10.3390/app9112197
Chicago/Turabian StyleBarkallah, Mohamed, Ali Ben Atitallah, Faiez Hentati, Mouna Dammak, Bilel Hadrich, Imen Fendri, Mohamed Ali Ayadi, Philippe Michaud, and Slim Abdelkafi. 2019. "Effect of Spirulina platensis Biomass with High Polysaccharides Content on Quality Attributes of Common Carp (Cyprinus carpio) and Common Barbel (Barbus barbus) Fish Burgers" Applied Sciences 9, no. 11: 2197. https://doi.org/10.3390/app9112197
APA StyleBarkallah, M., Ben Atitallah, A., Hentati, F., Dammak, M., Hadrich, B., Fendri, I., Ayadi, M. A., Michaud, P., & Abdelkafi, S. (2019). Effect of Spirulina platensis Biomass with High Polysaccharides Content on Quality Attributes of Common Carp (Cyprinus carpio) and Common Barbel (Barbus barbus) Fish Burgers. Applied Sciences, 9(11), 2197. https://doi.org/10.3390/app9112197








