Featured Application
CaMn2O4, a green biomimetic catalyst, has the potential to generate oxygen by splitting water for sustainability applications.
Abstract
Calcium manganese oxide catalysts are a new class of redox catalysts with significant importance because of their structural similarity to natural oxygen-evolving complex in plant cells and the earth-abundant elemental constituents. In the present study, the photo-electrocatalytic properties of CaMn2O4 in water-splitting were investigated. CaMn2O4 powders with irregular shapes and nanowire shapes were synthesised using mechanochemical processing and a hydrothermal method, respectively. The anode in a photo-electrochemical cell was fabricated by embedding CaMn2O4 powders within polypyrrole. The results showed that CaMn2O4 induced a higher dark and light current in comparison to the control sample (polypyrrole alone). CaMn2O4 nanowires exhibited higher dark and light current in comparison to irregular-shaped CaMn2O4 powders. The difference was attributable to the higher surface area of nanowires compared to the irregular-shaped particles, rather than the difference in exposed crystal facets.
1. Introduction
The photocatalytic water splitting into its constituents, hydrogen and oxygen, has attracted much attention as a clean and renewable source of fuel. In the past, various semiconductor materials have been investigated as water oxidation catalysts [1]. However, it is still challenging to develop effective catalysts that split water under sunlight. Recently, calcium manganese oxides have gained significant attention as water oxidation catalysts, due to their earth-abundant and environmentally friendly elemental constituents and also a structural similarity to the natural photocatalytic centre in plant cells. In natural photosynthesis, bio-electricity decomposes water into hydrogen and oxygen within a unique catalytic centre composed of a CaMn4O5 cluster, in the oxygen-evolving complex (OEC) enzyme in the Photosystem II protein [2,3,4]. This natural process is the most efficient known, for the photo-generation of H2 from water.
Among many calcium manganese oxides, CaMn2O4 contains structural sub-units of (Mn4CaOx), similar to the structure of natural CaMn4O5 cluster in OEC [5,6]. This has led to extensive investigation of catalytic water oxidation properties of CaMn2O4 [7,8,9]. Han et al. reported an oxygen-reduction reaction (ORR) catalytic activity of CaMn2O4 microspheres consisting of aggregated nanoparticles [7]. Du et al. demonstrated that one-dimensional CaMn2O4 nanostructures, synthesised using a solvothermal method, enabled quasi-four-electron transfer in the ORR [8]. These studies were electrochemical in nature and therefore were conducted without any light irradiation. It is very important to study the water splitting in the presence of light because the use of natural sunlight, as the source of energy, is the final goal in practical applications.
Najafpour et al. studied light-driven oxygen evolution by using an aqueous suspension of CaMn2O4 particles, however only in the presence of various sacrificial reagents [9]. Studies of photo-catalysts in the dispersed form are of significant interest, however, from a practical perspective, it is crucial to study the immobilisation of catalysts on conducting electrodes as an anode [10]. To the best of our knowledge, there is no report on the study of light-driven electrochemical activity of CaMn2O4 as a fixed anode material without sacrificial reagents.
In this study, CaMn2O4 ultrafine powders were studied as an anode material in photo-electrochemical cells for their catalytic and photocatalytic properties in the water oxidation reaction. It has been reported that photocatalytic properties are greatly affected by the morphology of catalysts [11]. Therefore, we also investigated the effects of particle shapes on the photo-electrocatalytic properties of CaMn2O4.
2. Materials and Methods
2.1. Synthesis of Irregular-Shaped CaMn2O4 Particles
All chemicals, except MnSO4.4H2O (99%, metals basis, Alfa Aesar, Heysham, Lancashire, England), were used without further purification. MnSO4.4H2O was dehydrated using the procedure reported elsewhere [12]. CaMn2O4 ultrafine particles were synthesised by first producing CaMn2(CO3)3 nanoparticles by mechanochemical processing and subsequently thermally decompose the CaMn2(CO3)3 nanoparticles into CaMn2O4. CaMn2(CO3)3 was produced via the following reaction:
CaSO4 + 2MnSO4 + 3K2CO3 +11K2SO4 → CaMn2(CO3)3 + 14K2SO4.
Excess K2SO4 (ACS, 99.0% min, Alfa Aesar, Ward Hill, MA, USA) salt was added to reduce the volume fraction of CaMn2(CO3)3 in the product phase so as to minimise particle agglomeration [13]. Before inducing the mechanochemical reaction, a stoichiometric amount of CaSO4 (anhydrous, 99%, Alfa Aesar, Ward hill, MA, USA) and MnSO4 were mixed in a hardened-steel milling vial using a SPEX 8000M mill (SPEX SamplePrep, Metuchen, NJ, USA). After 2 h of mixing, a stoichiometric amount of K2CO3 (AnalaR NORMAPUR, VWR, Leuven, Belgium) along with K2SO4 was introduced in the same milling vial along with hardened steel balls of 9.2 mm in diameter. The ball-to-powder mass ratio was 10:1. Then milling was performed for 4 h in a SPEX 8000M mill to obtain CaMn2(CO3)3 nanoparticles embedded in a K2SO4 salt matrix. The as-milled powder was calcined at 950 °C for 1 h in air. Upon cooling the powder, K2SO4 was removed by washing with deionised water (18 MΩ cm), using an ultrasonic bath and a centrifuge, until the total dissolved solids in the supernatant became less than 100 ppm. The washed powder was dried in an oven at 50 °C for several hours.
2.2. Synthesis of CaMn2O4 Nanowires
CaCl2 (>93%, 2 mmol, granular, anhydrous, Sigma Aldrich, Saint Louis, MO, USA) and 2.8 mmol of MnCl2 (99.9%, Sigma Aldrich, Saint Louis, MO, USA) were mixed in 10 mL of deionised water. In another beaker, 1.2 mmol of KMnO4 (analytical grade, Univar, Sydney, NSW, Australia) and 150 mmol of KOH (AnalaR NORMAPUR, VWR, Leuven, Belgium) were mixed in 10 mL of deionised water. Then, the KMnO4-KOH solution was added into the CaCl2-MnCl2 solution dropwise, while stirring. A dark-brown precipitate was formed. The suspension was centrifuged to separate the precipitate from the supernatant. The remaining wet-cake was re-dispersed into 12 mL of ethanol and subjected to hydrothermal treatment at 190 °C for 40 h. The resulting precipitate was collected and washed 5-times with ethanol using a centrifuge and an ultrasonic bath.
2.3. Characterization Techniques
The crystal structure of the powders was examined at room temperature using a Bruker X-ray diffraction instrument (XRD, D2 Phaser, Billerica, MA, USA) with Cu-Kα radiation of 1.54059 Å wavelength. The nanostructure and surface morphology of the powders were studied using a JEOL2100F field emission transmission electron microscope (TEM, JEOL, Tokyo, Japan) at 200 kV and also an Ultraplus field-emission scanning electron microscope (FESEM, Zeiss, Oberkochen, Germany) at 1 kV. The specific surface area was measured by the Brunauer-Emmett-Teller (BET) N2-gas adsorption method using a TriStar II 3020 system (Micromeritics, Norcross, GA, USA). Prior to BET measurements, the powders were degassed under vacuum at 150 °C for 24 h. The bandgap energy of samples was estimated from UV-Vis optical absorption spectra taken using a Cary 60 spectrophotometer (Agilent, Santa Clara, CA, USA). Optical transmittance of the samples coated on a quartz plate was measured and the absorption spectra were converted into the Tauc plot of (hvα)2 versus hv, where h is the Planck’s constant, ν is the photon’s frequency, and α is the optical absorption coefficient. The linear zone of the Tauc curve was extrapolated to the energy axis to estimate the direct bandgap. X-ray photoelectron spectroscopy (XPS) was conducted using a SPECS PHOIBOS 100 Analyser installed in a high-vacuum chamber with a base pressure below 10–8 mbar. An incident monochromated X-ray beam of Al-Kα radiation with a photon energy of 1486.6 eV was used at 12 kV and 144 W. XPS binding energy spectra were recorded at a pass energy of 20 eV and a step width of 0.05–0.1 eV in the fixed analyser transmission mode. To increase the signal/noise ratio, multi-scans were conducted and the signal intensity was accumulated. Broad survey scans were recorded at a pass energy of 60 eV and a step width of 0.5 eV. XPS data analysis was carried out using the commercial CasaXPS software package.
2.4. Preparation of CaMn2O4 Electrodes on Conducting Glass Slide
Prior to the deposition of electrode materials, Fluorine doped tin oxide (FTO) coated glass slides were cleaned in the following manner. First, the glass slides, immersed in acetone within a TLC chamber, were sonicated for 90 min using a MTH ultrasonic bath (Branson, Danbury, CT, USA), washed with water and air dried. Subsequently, the remaining organic contaminants from the slides were removed using a DIG UV PSD PR SERIES digital ozone-UV cleaner (Novascan, Boone, IA, USA) for 20 min. The slides were further cleaned in a PLAMAFLO PDC-FMG plasma cleaner (Harrick Plasma, Ithaca, NY, USA), followed by drying using an IKA® RCT basic hotplate (IKA, Staufen, Germany) at 60 °C.
Polypyrrole/CaMn2O4 films were deposited on the cleaned FTO glass slides by spin-coating, using the following procedure. First, 85 mg of iron (III) p-toluenesulfonate (Fe(III)-pTS) (Sigma Aldrich, Saint Louis, MO, USA) was dissolved in 1 mL ethanol (Sigma Aldrich, Saint Louis, MO, USA) and sonicated for 10 min. Then, 8 mg of CaMn2O4 was added to the solution and sonicated for a further hour. The resulting solution (100 µL) was drop-cast onto the slide surface using a micropipette. The slides were spun at 1500 revolutions per min (rpm) for 120 s using a WS-400B-6NPP/LITE spin-coater (Laurell Technologies, North Wales, PA, USA). After spin-coating, the sample was quickly transferred to a preheated hot plate and dried at 60 °C for 15 min. Control samples were prepared without the addition of CaMn2O4.
Vapour phase polymerisation of polypyrrole was carried out in a separate conical flask (500 mL capacity), equipped with a rubber stopper containing a crocodile clip. In the flask, 0.500 mL pyrrole (Sigma Aldrich, Saint Louis, MO, USA) was placed and the spin-coated FTO glass substrates were held above the pyrrole solution by the crocodile clip. The flask was placed in an oven at 60 °C for 25 min to allow polymerization of pyrrole on the slide surface. The sample was washed thoroughly with ethanol and left to dry overnight. A copper wire was attached to the FTO surface with conductive silver paint and epoxy resin. Upon solidification of the silver paste, the contact area of the wire was covered with epoxy glue.
2.5. Studies of CaMn2O4 Electrode on FTO as OER Photocatalysts
Working electrodes were placed within a fully-enclosed quartz cell (5 × 5 × 5 cm) inside a closed cabinet that comprised a Faraday cage. A Pt mesh (1 × 2 cm) was used as the counter electrode. A BASi Ag/AgCl aqueous salt bridge (KCl, 3 M) was used as a reference electrode. The cell was filled with a 0.2 M Na2SO4 aqueous solution electrolyte, adjusted to pH 12 by adding NaOH. Prior to the experiment, nitrogen gas was bubbled in the electrolyte for 30 min. Linear sweep voltammetry (LSV) and chronoamperograms (CA) were performed using an EDAQ466 potentiostat (Biologic, Seyssinet-Pariset, ARA, France). Where applicable, a SoLux daylight MR16 halogen light bulb (12 V, 50 W, 24° ca. 0.25 sun intensity) with a stable output range of 275–750 nm was used to illuminate the sample. The distance between light and working electrode was 10 cm. A Thorlabs visible-light bandpass filter (315–710 nm) was placed 1.5 cm in front of the light source to remove infra-red wavelengths.
3. Result and Discussion
3.1. Characterisation of CaMn2O4 Powder
Figure 1 shows the X-ray diffraction pattern of the synthesised CaMn2O4 powders, where the diffraction peaks can be indexed to the orthorhombic phase of CaMn2O4 (JCPDS card No 74-2293) [14].
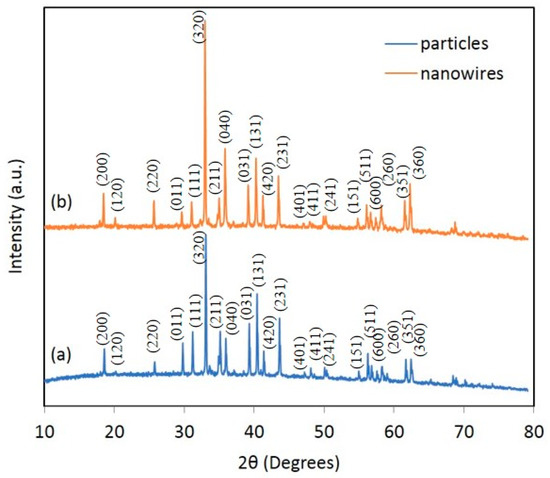
Figure 1.
X-ray diffraction instrument (XRD) patterns of (a) irregular-shaped CaMn2O4 particles, and (b) CaMn2O4 nanowires.
Figure 2 shows a typical SEM and TEM images of CaMn2O4 powders. The CaMn2O4 powder made by mechanochemical processing were irregular in shape with sizes from 200 nm to 2 µm (Figure 2a,b). The large particle sizes can be attributed to the higher temperature required to decompose the carbonate precursors. As evidenced in Figure 2c, irregular-shaped CaMn2O4 particles showed high crystallinity with clearly distinguished lattice fringes. The CaMn2O4 powder made using the hydrothermal method had wire shapes with diameters of ~500 nm and varying lengths (Figure 2d,e). The inset in Figure 2e shows the electron diffraction pattern of the wires, from which the growth direction of nanowires was identified as [001] (Figure 2f). The BET surface areas of the irregular-shaped powder and nanowire powder were 4.7 m2 g−1 and 5.4 m2 g−1, respectively.
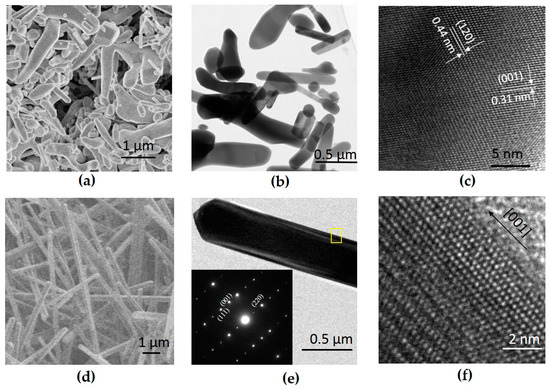
Figure 2.
Electron micrographs of CaMn2O4 powder made by mechanochemical processing and subsequent heat treatment, (a) SEM, (b,c) TEM; CaMn2O4 powder made using a hydrothermal method, (d) SEM and (e,f) TEM. The inset in Figure (e) is an electron diffraction pattern taken in the area indicated with a yellow square.
Figure 3 shows the XPS spectra of CaMn2O4 nanowires and irregular-shaped particles. As shown in Figure 3a, two strong peaks corresponding to Mn2p3/2 and Mn2p1/2 were observed at approximately 641 and 653 eV, respectively. Figure 3b shows the spectra near Mn3s peaks. Average oxidation state (AOS) was calculated using the linear relationship with splitting energy between two Mn3s peaks [15]. AOS for irregular-shaped particles and nanowires was found to be 3.07 and 2.96, respectively, which is in good agreement with the theoretical value of 3.

Figure 3.
X-ray photoelectron spectroscopy (XPS) spectra of irregular-shaped CaMn2O4 particles and CaMn2O4 nanowires; (a) Mn 2p and (b) Mn 3s regions.
Figure 4b shows transmittance spectra of irregular-shaped CaMn2O4 particles and CaMn2O4 nanowires, respectively. The Tauc plots in Figure 4c,d suggest an optical band gap of 1.5 eV and 1.6 eV for irregular-shaped particles and nanowires, respectively.
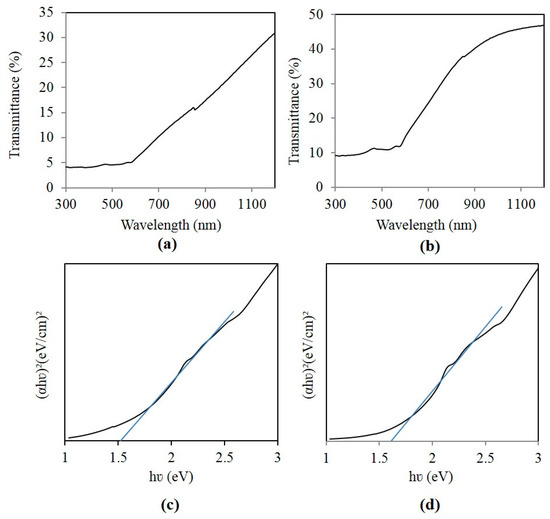
Figure 4.
Transmittance spectra of (a) irregular-shaped CaMn2O4 particles and (b) CaMn2O4 nanowires; Tauc plots of (c) irregular-shaped CaMn2O4 particles and (d) CaMn2O4 nanowires.
3.2. Studies on Current Density
Figure 5 shows the electrocatalytic performance of the samples, tested in 0.2 M Na2SO4 aqueous solution, with the pH adjusted to 12, at 1.00 V (vs Ag/AgCl) bias, with and without light illumination.
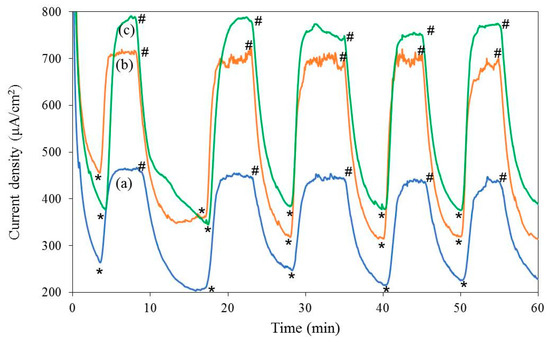
Figure 5.
Chrono-amperograms at 1.00 V (vs Ag/AgCl) in 0.2 M Na2SO4 (pH 12) over 1 h of operation, with and without light illumination (0.25 sun), of Fluorine doped tin oxide (FTO) glass slides coated with thin films of: (a) polypyrrole only (control), (b) polypyrrole containing irregular-shaped CaMn2O4 particles, and (c) polypyrrole containing CaMn2O4 nanowires. (* = ‘light on’, # = ‘light off’).
As shown in Figure 5, a significant increase in both dark- and photo-current was observed for the PPy/CaMn2O4 films in comparison to the control PPy film alone. A significant increase in photocurrent was also observed when a small amount of CaMn2O4 powder was added to the PPy polymer. The control PPy film exhibited light and dark current densities of 447 µA cm−2 and 230 µA cm−2, respectively, whereas the films prepared from the mixture of CaMn2O4 and PPy produced 701 µA cm−2 and 326 µA cm−2 for irregular-shaped particles, and 769 µA cm−2 and 380 µA cm−2 for nanowires in light and dark, respectively. It is also clear that the CaMn2O4 nanowires/PPy film produced higher dark and photocurrents than the irregular-shaped CaMn2O4/PPy film. Evolved gas was traced using a customised apparatus, as shown in Figure S1. The analysis confirmed the production of oxygen at the anode as shown in Figure S2.
Catalytic and photocatalytic activities occur on the surface of catalysts. Hence, in order to examine the intrinsic catalytic properties of materials, it is often convenient to normalise the indicators of photocatalytic activity with the specific surface area of materials. Table 1 compares the BET specific surface areas and increases in dark- and photo-current with regard to the control electrode, between irregular-shaped CaMn2O4 powder and CaMn2O4 nanowires. Since the optical bandgap energies of irregular-shaped CaMn2O4 powder and CaMn2O4 nanowires are nearly the same, the differences in the surface-area-normalised increase in photo-current, ΔIL/S, is expected to arise from the difference in particle shapes. Table 1 shows that ΔIL/S values of irregular-shaped CaMn2O4 powder and CaMn2O4 nanowires are similar to each other. The results suggest that the influence of particle shapes on the light-driven catalysis of CaMn2O4 is relatively weak. It is speculated that the exposed facets along the growth-direction of nanowires do not have significantly different catalytic activities compared to the other crystal faces. On the other hand, the surface-area-normalised increase in photo-current, ΔID/S, was higher for the electrode with nanowires than the electrode with irregular-shaped particles. The reason may be attributed to the difference in electric conductivity within the CaMn2O4/PPy composite electrode. Rod- or wire- shaped particles have a lower percolation threshold [16] and, given the semiconducting nature of CaMn2O4, wire-shaped particles will result in higher electrical conductivity than irregular-shaped particles in polymer composites.

Table 1.
Comparison between irregular-shaped CaMn2O4 powder and CaMn2O4 nanowires for Brunauer-Emmett-Teller (BET) specific surface area, increase in dark- and photo-current with regard to control electrode; BET specific surface area (S), dark current (ID), photo-current (IL), increase in dark current with respect to control (ΔID), increase in photo-current with respect to control (ΔIL).
3.3. EIS and Tafel Plot Studies
In order to investigate the nature of the catalytic process, electrochemical impedance spectroscopy (EIS) and Tafel plot studies were undertaken. EIS was conducted potentiostatically at 1.00 V (vs. Ag/AgCl) under the same conditions as the amperometric studies, with the same light source. Figure 6a,b shows the Nyqist and Bode plots, respectively, of the irregular-shaped particles/PPy film, the nanowires/PPy, and the control PPy electrodes without CaMn2O4, in the dark and under light illumination.

Figure 6.
(a) Nyquist and (b) Bode plots showing measured data (individual data points) and modelled data (solid lines) using the equivalent circuit depicted in (c): (i) CaMn2O4 nanowires/PPy (with light illumination),(ii) CaMn2O4 nanowires/PPy (without light illumination), (iii) irregular-shaped CaMn2O4 particles/PPy (with light illumination), (iv) irregular-shaped CaMn2O4 particles/PPy (without light illumination) (dark), (v) PPy only (with light illumination) and (vi) PPy only (without light illumination). (Figure 6 (c) is reprinted with permission from ref. [17]. Copyright (2018) American Chemical Society).
Following previous work in our laboratory [17], the EIS data were modelled with the equivalent circuit shown in Figure 6c. The modelled results are depicted as the solid lines in Figure 6a. As can be seen, the modelled data yielded an excellent match with the measured data.
Table 2 shows the results derived from the modelling. It is evident from Table 2 that Cdl was the highest for the CaMn2O4 nanowires/PPy sample. The capacitance Cdl is a comparative measure of the active area of the samples tested. Polypyrrole films exhibited the lowest values for Cdl. The higher catalytic performance of the CaMn2O4 nanowires/PPy sample could, therefore, be attributed, at least in part, to a larger catalytically active area of nanowires.

Table 2.
Data from electrochemical impedance spectroscopy (ohmic resistance (Rel)), adsorption resistance (Rad), diffuse layer capacitance (Cdl), catalytic charge transfer resistance (RCT), and capacitance expressed in terms of a constant phase element (QCPE, nCPE, and CCPE). Data from Tafel plot studies (slope (A), exchange current density (io)). ‘dark’ = without light illumination; ‘light’ = with light illumination.
In oxygen evolution (photo)electrocatalysis at pH 12, the catalytic performance may be controlled by reactant adsorption to the catalyst (with an associated resistance due to adsorption, Rad) or by the fundamental catalytic turnover process (with an associated charge transfer resistance, RCT) [17]. In the present study, operating at 1.00 V vs Ag/AgCl (compared to 0.8 V in previous studies [17]) appears to have made adsorption much less of an impediment than the catalytic turnover process, with the RCT being, in all cases greater than the Rad. Both the RCT and the Rad were low in comparison with previous conducting polymer films prepared by us [17]; this is to be expected from the higher applied bias.
Comparing the nanowires/PPy with the irregular-shaped particle/PPy, the resistances were marginally lower for the former than the latter, under both dark and light conditions, but of the same order as the control PPy film. The CaMn2O4 nanowires/PPy was, therefore, a slightly better catalyst than the irregular-shaped CaMn2O4 particles/PPy, particularly under light illumination. The ohmic resistance, Rel, was of the same order for all samples (17.83–17.91 Ω cm2) (Table 2). Higher Cdl values were observed for both samples under light illumination. Hence the major effect of the light illumination appears to be the increase in the electrochemically active area of the films.
Figure 7 shows Tafel plots of the catalysts at much higher overpotentials than previously examined [17], with resulting data tabulated in the last two columns of Table 2. The exchange current density, io, provides a measure of the rate of catalysis at the reversible potential when the overpotential is zero. The catalytic performance as a function of increasing bias is given by the Tafel slope. As shown in Table 2, the higher applied potential also appears to have had a beneficial effect on the Tafel data, which were improved relative to previous studies [17]. The exchange current density and the Tafel slope of the prepared samples varied in the order nanowires/PPy < irregular-shaped particles/PPy. However, the difference between the nanowires/PPy and irregular-shaped particles/PPy was insignificant, which explains nearly the same photocurrent produced by the samples. The slightly higher dark- and photo-current for the nanowires/PPy in comparison to irregular-shaped particles/PPy could be attributed to the higher electrochemically active area of the former relative to the latter.

Figure 7.
Tafel plots of (i) CaMn2O4 nanowires/PPy (with light illumination), (ii) CaMn2O4 nanowires/PPy (without light illumination, (iii) irregular-shaped CaMn2O4 particles/PPy (with light illumination), (iv) irregular-shaped CaMn2O4 particles/PPy (without light illumination) (dark), (v) PPy only (with light illumination) and (vi) PPy only (without light illumination).
4. Conclusions
In the present study, CaMn2O4 ultrafine powders with different morphologies were synthesised and their catalytic performance in photo-electrochemical water splitting was investigated. CaMn2O4 powders with irregular-shapes and nanowire shapes were synthesised using a dry mechanochemical method and a hydrothermal method, respectively.
To study their photo-electrocatalytic properties, CaMn2O4 powders were embedded within polypyrrole to form an anode in a photo-electrochemical cell. It was found that irrespective of the morphology of CaMn2O4, the presence of CaMn2O4 in the electrode resulted in a higher dark and light current, in comparison to the control without CaMn2O4. The evolution of O2 gas from the electrode surface was also observed. Thus, CaMn2O4 showed a catalytic property in the water oxidation reaction. Upon light illumination, the current density was increased and the evolution of O2 gas also increased, demonstrating the photocatalytic nature of CaMn2O4.
The electrode with CaMn2O4 nanowires exhibited higher dark- and light- current in comparison to the electrode with irregular-shaped CaMn2O4 particles. Electrochemical impedance spectroscopy studies revealed that the electrochemically active area increased in the order: PPy alone < irregular-shaped CaMn2O4 particles/PPy < CaMn2O4 nanowires/PPy, which was identified as a reason for the higher dark and photocurrent of CaMn2O4 nanowires/PPy electrode. However, surface-area-normalised data suggest that the influence of particle shapes on the light-driven catalysis of CaMn2O4 is relatively weak. The result suggests that the difference in catalytic activities among the crystal facets of CaMn2O4 is small.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/9/11/2196/s1, Figures S1 and S2.
Author Contributions
Conceptualization, A.G., T.T. and G.F.S.; methodology, A.G., M.A.; writing—original draft preparation, A.G.; writing—review and editing, G.F.S., T.T., A.G.
Funding
This research received no external funding.
Acknowledgments
Ankita Gagrani would like to recognise the PhD research fellowship from the Australian National University. Access to the facilities of the Centre for Advanced Microscopy (CAM) with funding through the Australian Microscopy and Microanalysis Research Facility (AMMRF) is gratefully acknowledged. MA thanks the Government of Iraq for a PhD scholarship. The authors thank A/Prof Attila Mozer of the University of Wollongong for use of a GC gas analyser. The authors acknowledge the Australian National Fabrication Facility (ANFF) Materials Node for equipment use and Adam Taylor for the design and printing of the custom-built apparatus. Support from the Australian Research Council Centre of Excellence Scheme (Project Number CE140100012) is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gan, J.; Lu, X.; Tong, Y. Towards highly efficient photoanodes: boosting sunlight-driven semiconductor nanomaterials for water oxidation. Nanoscale 2014, 6, 7142–7164. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.; Bogdanoff, P.; Friedrich, D.; Fiechter, S. Synthesis of Ca2Mn3O8 films and their electrochemical studies for the oxygen evolution reaction (OER) of water. Nano Energy 2012, 1, 282–289. [Google Scholar] [CrossRef]
- Najafpour, M.M.; Pashaei, B.; Nayeri, S. Calcium manganese (IV) oxides: biomimetic and efficient catalysts for water oxidation. Dalton Trans. 2012, 41, 4799–4805. [Google Scholar] [CrossRef] [PubMed]
- Najafpour, M.M. Calcium-manganese oxides as structural and functional models for active site in oxygen evolving complex in photosystem II: Lessons from simple models. J. Photochem. Photobiol. B Biol. 2011, 104, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Najafpour, M.M. Amorphous Manganese-Calcium Oxides as a Possible Evolutionary Origin for the CaMn4 Cluster in Photosystem II. Orig. Life Evol. Biosph. 2011, 41, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Gagrani, A.; Tsuzuki, T. Calcium manganese oxides as biomimetic catalysts in energy applications: A short review. Chem. Eng. Sci. 2018, 194, 116–126. [Google Scholar] [CrossRef]
- Han, X.; Zhang, T.; Du, J.; Cheng, F.; Chen, J. Porous calcium-manganese oxide microspheres for electrocatalytic oxygen reduction with high activity. Chem. Sci. 2013, 4, 368–376. [Google Scholar] [CrossRef]
- Du, J.; Pan, Y.; Zhang, T.; Han, X.; Cheng, F.; Chen, J. Facile solvothermal synthesis of CaMn2O4 nanorods for electrochemical oxygen reduction. J. Mater. Chem. 2012, 22, 15812–15818. [Google Scholar] [CrossRef]
- Najafpour, M.M.; Ehrenberg, T.; Wiechen, M.; Kurz, P. Calcium Manganese (III) Oxides (CaMn2O4⋅x H2O) as Biomimetic Oxygen-Evolving Catalysts. Angew. Chem. Int. Ed. 2010, 49, 2233–2237. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, Z.; Li, X. Synergetic photoelectrocatalytic reactors for environmental remediation: A review. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 83–101. [Google Scholar] [CrossRef]
- Peter, I.J.; Praveen, E.; Vignesh, G.; Nithiananthi, P. ZnO nanostructures with different morphology for enhanced photocatalytic activity. Mater. Res. Express 2017, 4, 124003. [Google Scholar] [CrossRef]
- Sinha, S.G.; Deshpande, N.D.; Deshpande, D.A. Dehydration of crystalline MnSO4·4H2O. Thermochim. Acta 1987, 113, 95–104. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Pethick, K.; McCormick, P.G. Synthesis of CaCO3 nanoparticles by mechanochemical processing. J. Nanoparticle Res. 2000, 2, 375–380. [Google Scholar] [CrossRef]
- Lepicard, G.; Protas, J. Étude structurale de l’oxyde double de manganése et de calcium orthorhombique CaMn2O4 (marokite). Bulletin de Minéralogie 1966, 89, 318–324. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, B.; Liu, H.; He, B.; Ye, F.; Yu, L.; Sun, C.; Wen, H. The effect of acid/alkali treatment on the catalytic combustion activity of manganese oxideoctahedral molecular sieves. RCS Adv. 2017, 7, 3958–3965. [Google Scholar]
- Schilling, T.; Miller, M.A.; Van der Schoot, P. Percolation in suspensions of hard nanoparticles: From spheres to needles. EPL (Europhys. Lett.) 2015, 111, 56004. [Google Scholar] [CrossRef]
- Alsultan, M.; Balakrishnan, S.; Choi, J.; Jalili, R.; Tiwari, P.; Wagner, P.; Swiegers, G.F. Synergistic Amplification of Water Oxidation Catalysis on Pt by a Thin-Film Conducting Polymer Composite. ACS Appl. Energy Mater. 2018, 1, 4235–4246. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).