Highly Dispersed Mn–Ce Binary Metal Oxides Supported on Carbon Nanofibers for Hg0 Removal from Coal-Fired Flue Gas
Abstract
1. Introduction
2. Material and Methods
2.1. Sorbent Preparation and Characterization
2.2. Experimental
3. Results and Discussion
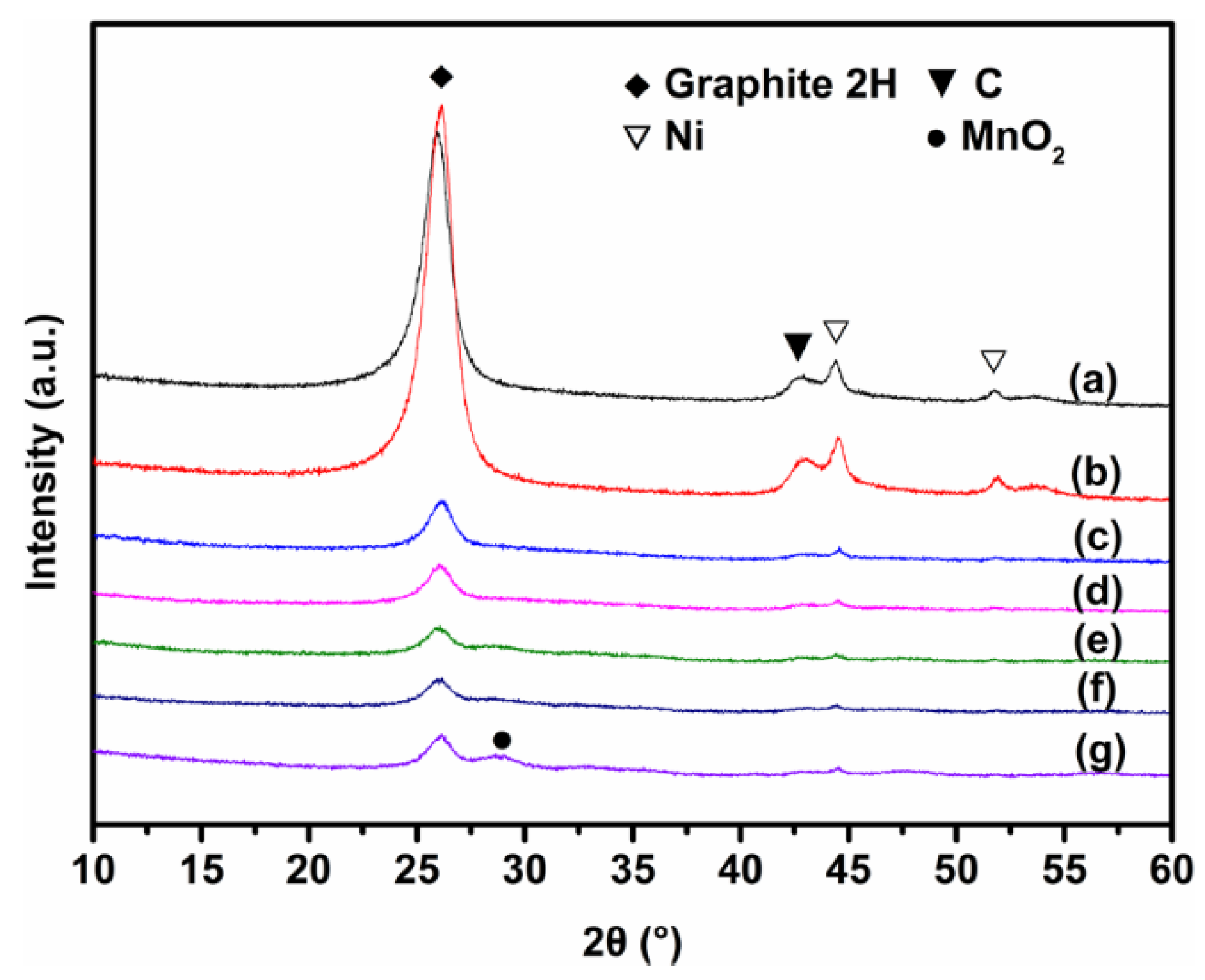
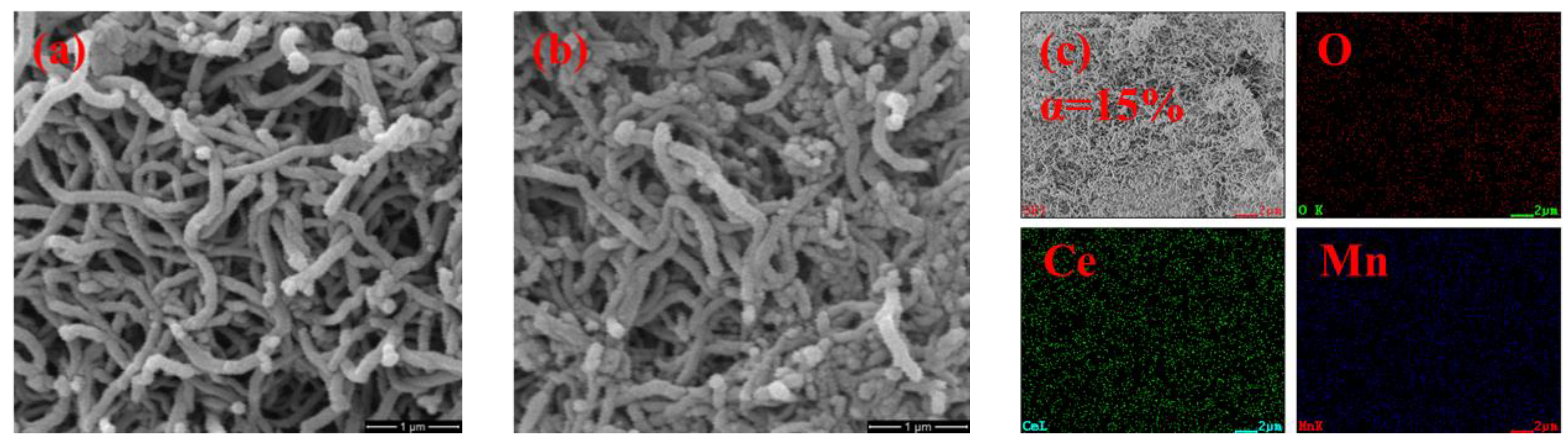
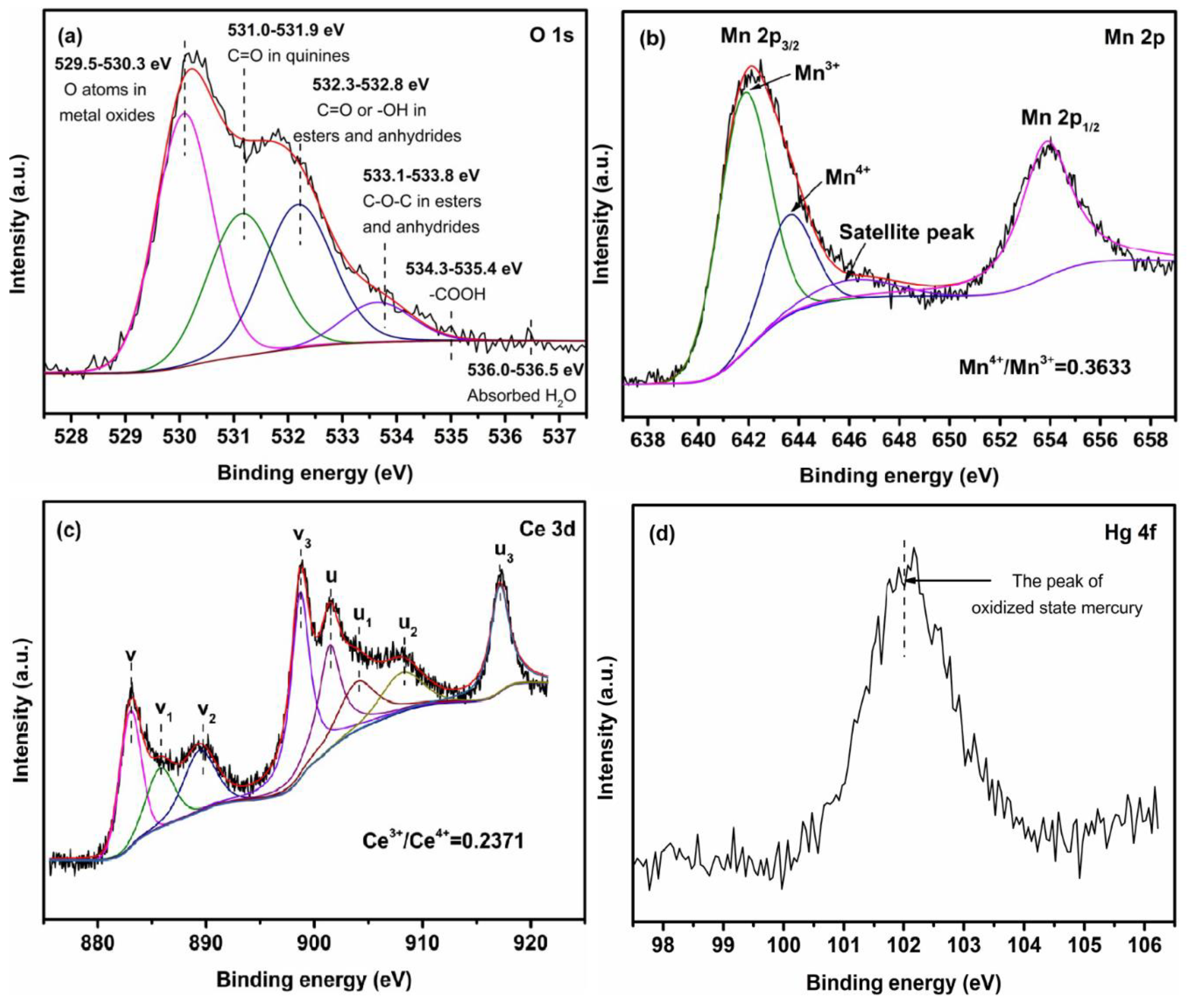
3.1. Sorbent Characterization
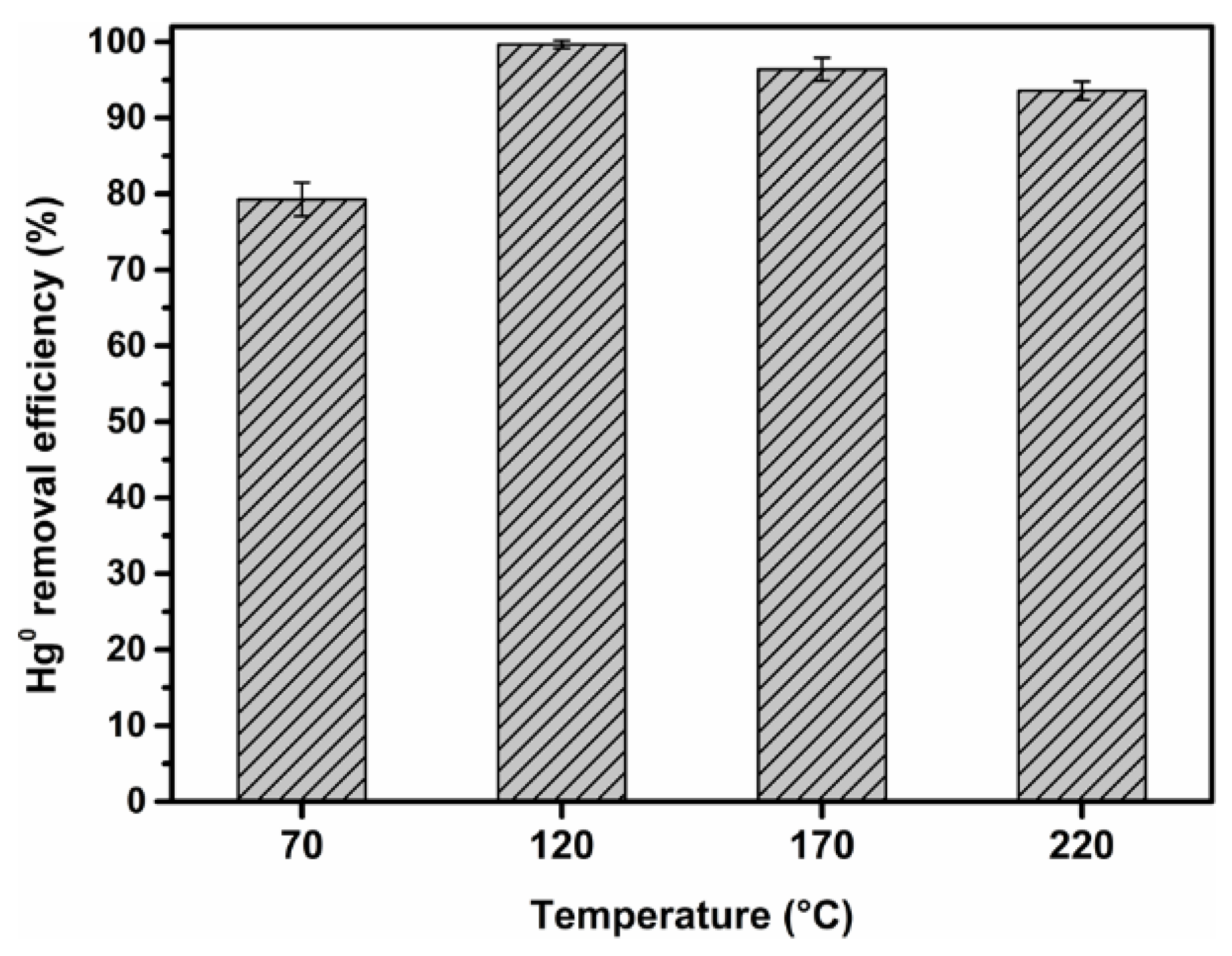
3.2. Influence of Loading Value and Reaction Temperature on Hg0 Removal Performance
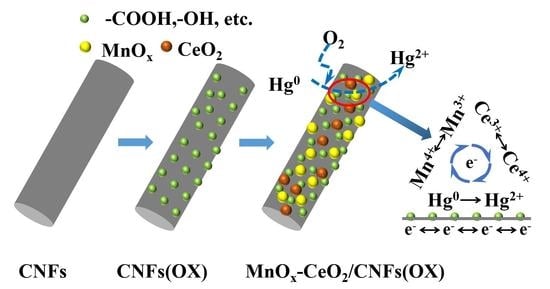
3.3. Hg0 Removal Mechanism Analysis
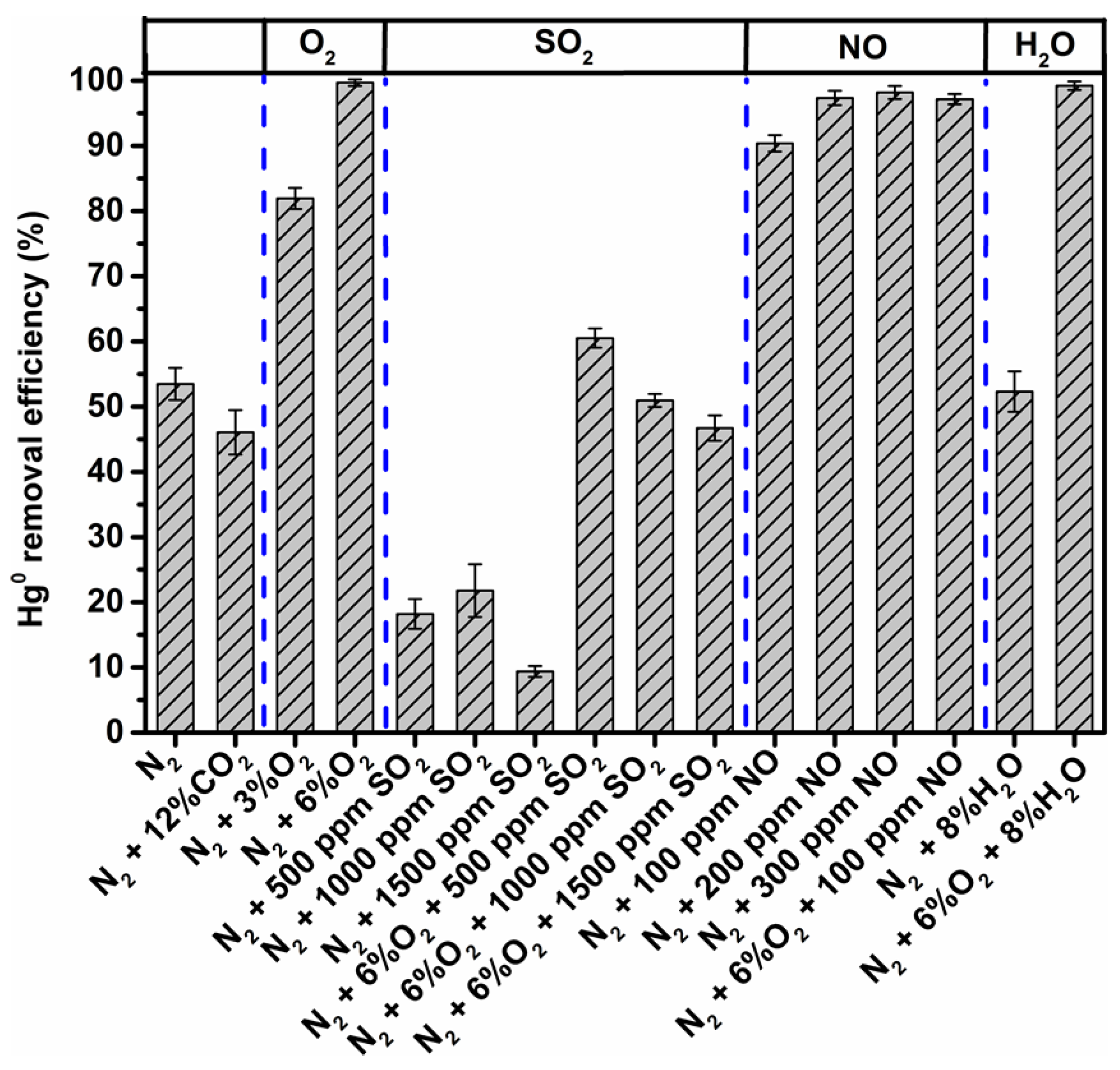
3.4. Influence of Individual Flue Gas Components on Hg0 Removal Performance
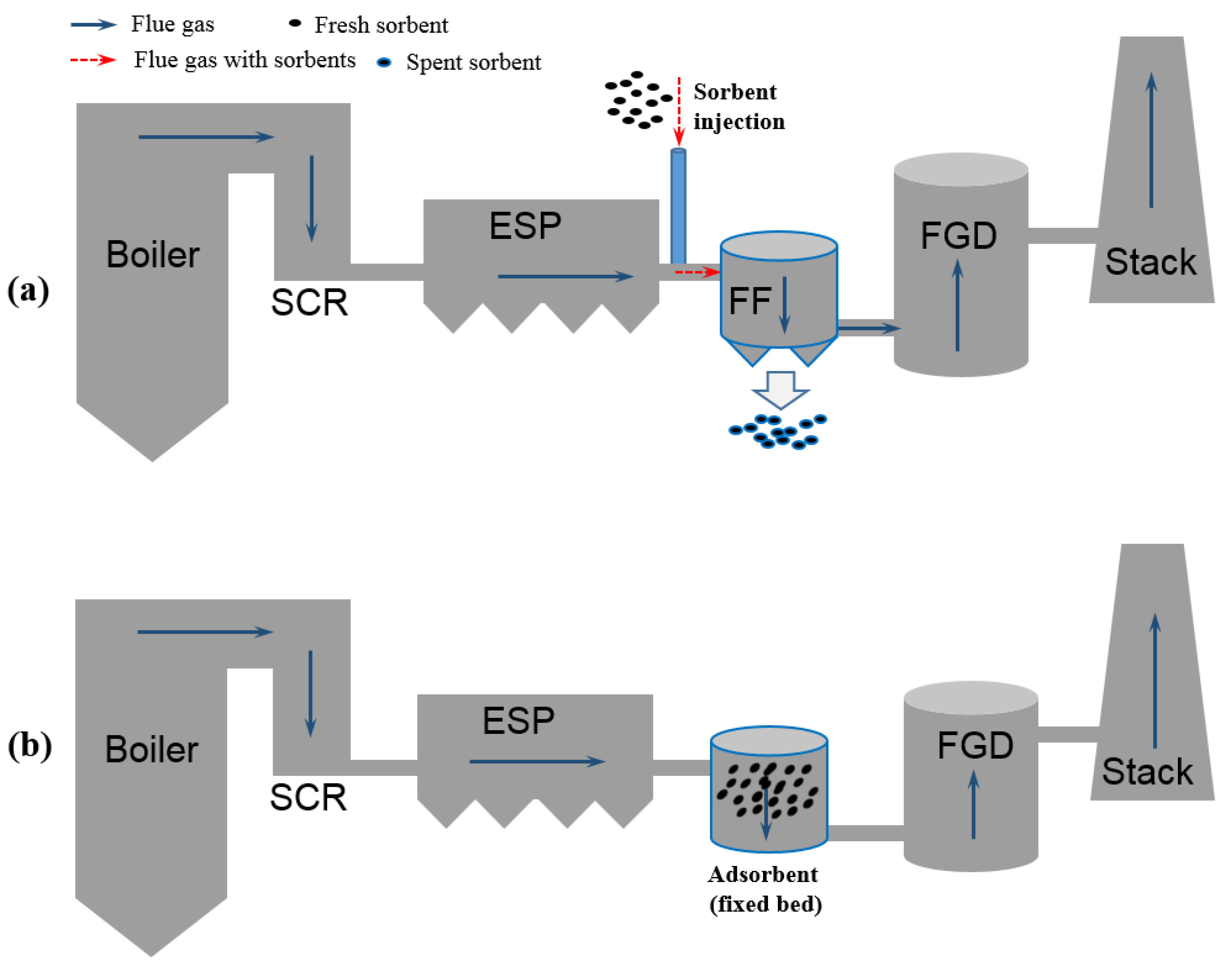
3.5. Possible Application Mode of the Sorbent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doney, S.C. The Growing Human Footprint on Coastal and Open-Ocean Biogeochemistry. Science 2010, 328, 1512–1516. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, J.; Rupp, E.; Ying, S.C.; Lim, D.-H.; Negreira, A.S.; Kirchofer, A.; Feng, F.; Lee, K. Mercury adsorption and oxidation in coal combustion and gasification processes. Int. J. Coal Geol. 2012, 90–91, 4–20. [Google Scholar] [CrossRef]
- Xu, M.; Yan, R.; Zheng, C.; Qiao, Y.; Han, J.; Sheng, C. Status of trace element emission in a coal combustion process: A review. Fuel Process. Technol. 2004, 85, 215–237. [Google Scholar] [CrossRef]
- Hower, J.C.; Senior, C.L.; Suuberg, E.M.; Hurt, R.H.; Wilcox, J.L.; Olson, E.S. Mercury capture by native fly ash carbons in coal-fired power plants. Prog. Energy Combust. Sci. 2010, 36, 510–529. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qiao, Y.; Zheng, C.; Li, L.; Liu, J. Modeling of homogeneous mercury speciation using detailed chemical kinetics. Combust. Flame 2003, 132, 208–218. [Google Scholar] [CrossRef]
- Zhou, Z.-J.; Liu, X.-W.; Zhao, B.; Chen, Z.-G.; Shao, H.-Z.; Wang, L.-L.; Xu, M.-H. Effects of existing energy saving and air pollution control devices on mercury removal in coal-fired power plants. Fuel Process. Technol. 2015, 131, 99–108. [Google Scholar] [CrossRef]
- Lee, S.J.; Seo, Y.-C.; Jang, H.-N.; Park, K.-S.; Baek, J.-I.; An, H.-S.; Song, K.-C. Speciation and mass distribution of mercury in a bituminous coal-fired power plant. Atmos. Environ. 2006, 40, 2215–2224. [Google Scholar] [CrossRef]
- Padak, B.; Wilcox, J. Understanding mercury binding on activated carbon. Carbon 2009, 47, 2855–2864. [Google Scholar] [CrossRef]
- Rupp, E.C.; Wilcox, J. Mercury chemistry of brominated activated carbons-Packed-bed breakthrough experiments. Fuel 2014, 117, 351–353. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Wu, C.-Y.; Zhang, J. Oxidation and capture of elemental mercury over SiO2–TiO2–V2O5 catalysts in simulated low-rank coal combustion flue gas. Chem. Eng. J. 2011, 169, 186–193. [Google Scholar] [CrossRef]
- Kamata, H.; Ueno, S.-I.; Naito, T.; Yukimura, A. Mercury Oxidation over the V2O5(WO3)/TiO2 Commercial SCR Catalyst. Ind. Eng. Chem. Res. 2008, 47, 8136–8141. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Zhang, J.; Zheng, C. Regenerable Cobalt Oxide Loaded Magnetosphere Catalyst from Fly Ash for Mercury Removal in Coal Combustion Flue Gas. Environ. Sci. Technol. 2014, 48, 14837–14843. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, L.; Wu, S.; Liu, Y.; Shih, K. Synergy of CuO and CeO2 combination for mercury oxidation under low-temperature selective catalytic reduction atmosphere. Int. J. Coal Geol. 2017, 170, 69–76. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Wang, Z.; Liu, F. Heterogeneous reaction kinetics of mercury oxidation by HCl over Fe2O3 surface. Fuel Process. Technol. 2017, 159, 266–271. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Hu, Y.; Liao, Z.; Cheng, S.; Xu, M. An efficient sorbent based on CuCl2 loaded CeO2-ZrO2 for elemental mercury removal from chlorine-free flue gas. Fuel 2018, 216, 356–363. [Google Scholar] [CrossRef]
- He, C.; Shen, B.; Chen, J.; Cai, J. Adsorption and Oxidation of Elemental Mercury over Ce-MnOx/Ti-PILCs. Environ. Environ. Sci. Technol. 2014, 48, 7891–7898. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Zhao, B.; Shao, H.; Xu, Y.; Xu, M. Elemental mercury oxidation over manganese-based perovskite-type catalyst at low temperature. Chem. Eng. J. 2016, 288, 701–710. [Google Scholar] [CrossRef]
- Li, H.; Wu, C.-Y.; Li, Y.; Zhang, J. CeO2–TiO2 catalysts for catalytic oxidation of elemental mercury in low-rank coal combustion flue gas. Environ. Sci. Technol. 2011, 45, 7394–7400. [Google Scholar] [CrossRef]
- Li, H.; Wu, C.-Y.; Li, Y.; Zhang, J. Superior activity of MnOx-CeO2/TiO2 catalyst for catalytic oxidation of elemental mercury at low flue gas temperatures. Appl. Catal. B Environ. 2012, 111–112, 381–388. [Google Scholar] [CrossRef]
- Devaiah, D.; Reddy, L.H.; Park, S.-E.; Reddy, B.M. Ceria–zirconia mixed oxides: Synthetic methods and applications. Catal. Rev. 2018, 60, 177–277. [Google Scholar] [CrossRef]
- He, J.; Reddy, G.K.; Thiel, S.W.; Smirniotis, P.G.; Pinto, N.G. Simultaneous Removal of Elemental Mercury and NO from Flue Gas Using CeO2 Modified MnOx/TiO2 Materials. Energy Fuels 2013, 27, 4832–4839. [Google Scholar] [CrossRef]
- Xie, Y.; Li, C.; Zhao, L.; Zhang, J.; Zeng, G.; Zhang, X.; Zhang, W.; Tao, S. Experimental study on Hg0 removal from flue gas over columnar MnOx-CeO2/activated coke. Appl. Surf. Sci. 2015, 333, 59–67. [Google Scholar] [CrossRef]
- Wang, P.; Su, S.; Xiang, J.; You, H.; Cao, F.; Sun, L.; Hu, S.; Zhang, Y. Catalytic oxidation of Hg0 by MnOx–CeO2/γ-Al2O3 catalyst at low temperatures. Chemosphere 2014, 101, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-H.; Sui, Z.-J.; Zhu, J.; Li, P.; Chen, D.; Dai, Y.-C.; Yuan, W.-K. Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS and FT-IR. Carbon 2007, 45, 785–796. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Z.; Zong, C.; Huang, W.; Quan, F.; Yan, N. MnOx/Graphene for the Catalytic Oxidation and Adsorption of Elemental Mercury. Environ. Sci. Technol. 2015, 49, 6823–6830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, P.; Qiu, Y.; Yu, Q.; Ma, J.; Wu, H.; Luo, G.; Xu, M.; Yao, H. Increasing oxygen functional groups of activated carbon with non-thermal plasma to enhance mercury removal efficiency for flue gases. Chem. Eng. J. 2015, 263, 1–8. [Google Scholar] [CrossRef]
- Mizokawa, T.; Nambu, T.; Fujimori, A.; Fukumura, T.; Kawasaki, M. Electronic structure of the oxide-diluted magnetic semiconductor Zn1−xMnxO. Phys. Rev. B 2002, 65, 085209. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Liao, Z.; Shao, H.; Lv, C.; Hu, Y.; Xu, M. Manganese doped CeO2-ZrO2 catalyst for elemental mercury oxidation at low temperature. Fuel Process. Technol. 2016, 152, 285–293. [Google Scholar] [CrossRef]
- Li, H.; Wu, S.; Li, L.; Wang, J.; Ma, W.; Shih, K. CuO–CeO2/TiO2 catalyst for simultaneous NO reduction and Hg0 oxidation at low temperatures. Catal. Sci. Technol. 2015, 5, 5129–5138. [Google Scholar] [CrossRef]
- Endo, M.; Kim, Y.A.; Hayashi, T.; Yanagisawa, T.; Muramatsu, H.; Ezaka, M.; Terrones, H.; Terrones, M.; Dresselhaus, M.S. Microstructural changes induced in “stacked cup” carbon nanofibers by heat treatment. Carbon 2003, 41, 1941–1947. [Google Scholar] [CrossRef]
- Xu, H.; Ma, Y.; Huang, W.; Mei, J.; Zhao, S.; Qu, Z.; Yan, N. Stabilization of mercury over Mn-based oxides, Speciation and reactivity by temperature programmed desorption analysis. J. Hazard. Mater. 2017, 321, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, N.; Qu, Z.; Qiao, S.; Yang, S.; Guo, Y.; Liu, P.; Jia, J. Catalytic Oxidation of Elemental Mercury over the Modified Catalyst Mn/α-Al2O3 at Lower Temperatures. Environ. Sci. Technol. 2010, 44, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, X.; Hu, Y.; Xu, J.; Cao, X.E.; Liao, Z.; Xu, M. Investigation on synergistic oxidation behavior of NO and Hg0 during the newly designed fast SCR process. Fuel 2018, 225, 134–139. [Google Scholar] [CrossRef]



| α | Specific Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| 0 | 96.532 | 0.461 |
| 6% | 97.020 | 0.350 |
| 9% | 93.853 | 0.299 |
| 12% | 88.948 | 0.307 |
| 15% | 87.158 | 0.293 |
| 18% | 86.729 | 0.286 |
| α | Surface Atomic Concentration (%) | |||||
|---|---|---|---|---|---|---|
| C 1s | O 1s | Mn 2p | Mn4+/Mn3+ | Ce 3d | Ce3+/Ce4+ | |
| 0 | 96.76 | 3.24 | ||||
| 6% | 96.31 | 2.98 | 0.48 | 0.4277 | 0.23 | 0.5523 |
| 9% | 91.28 | 6.28 | 1.61 | 0.4212 | 0.83 | 0.3174 |
| 12% | 90.24 | 6.94 | 1.88 | 0.4171 | 0.94 | 0.2900 |
| 15% | 85.98 | 9.74 | 2.85 | 0.4140 | 1.43 | 0.2859 |
| 18% | 79.95 | 13.12 | 3.64 | 0.3218 | 1.85 | 0.2734 |
| α | Capacity (μg·g−1) |
|---|---|
| 12% | 74.8 ± 1.7 |
| 15% | 118.4 ± 2.3 |
| 18% | 54.1 ± 1.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Liao, Z.; Zheng, Y.; Zhou, Z. Highly Dispersed Mn–Ce Binary Metal Oxides Supported on Carbon Nanofibers for Hg0 Removal from Coal-Fired Flue Gas. Appl. Sci. 2018, 8, 2501. https://doi.org/10.3390/app8122501
Xia Y, Liao Z, Zheng Y, Zhou Z. Highly Dispersed Mn–Ce Binary Metal Oxides Supported on Carbon Nanofibers for Hg0 Removal from Coal-Fired Flue Gas. Applied Sciences. 2018; 8(12):2501. https://doi.org/10.3390/app8122501
Chicago/Turabian StyleXia, Yongjun, Zhiqiang Liao, Yan Zheng, and Zijian Zhou. 2018. "Highly Dispersed Mn–Ce Binary Metal Oxides Supported on Carbon Nanofibers for Hg0 Removal from Coal-Fired Flue Gas" Applied Sciences 8, no. 12: 2501. https://doi.org/10.3390/app8122501
APA StyleXia, Y., Liao, Z., Zheng, Y., & Zhou, Z. (2018). Highly Dispersed Mn–Ce Binary Metal Oxides Supported on Carbon Nanofibers for Hg0 Removal from Coal-Fired Flue Gas. Applied Sciences, 8(12), 2501. https://doi.org/10.3390/app8122501