Abstract
Antarctica, with its harsh environmental conditions, is home to a wide variety of microorganisms, including filamentous fungi. The survival of Antarctic mycoflora has led to increasing interest in their adaptation. Extreme low temperatures, low water availability, frequent freeze–thaw cycles, strong winds, ultraviolet radiation, etc., are all prerequisites for accelerated production of reactive oxygen species (ROS) and the occurrence of oxidative stress (OS). Antarctic fungi have effective mechanisms to cope with the damaging effects of ROS. While the distribution, morphology, physiology, and biotechnological potential of these fungi are well documented, the role of oxidative stress in their adaptation is poorly understood. This has been one of the main research topics of our team for a long time, and a number of papers on our results have been published. This review summarizes the existing literature on the induction of oxidative stress in Antarctic psychrophilic, psychrotolerant, and mesophilic fungi by extreme conditions. The most recent and relevant studies reporting on the relationship between cold stress and OS biomarkers and the damaging effects of ROS on key intracellular biomolecules are presented. The contribution of both enzymatic and non-enzymatic antioxidant defenses to the fungal cell response is discussed.
1. Introduction
Fungi are known for their ability to live in different ecological niches [1]. They can adapt to a wide range of extracellular stimuli such as low and high temperatures, UV radiation, low water availability, high salt concentrations, osmotic pressure, etc. In many cases, the fungi have to deal with a combination of these stimuli. Antarctica is one such habitat, characterized by extreme environmental conditions. Nevertheless, representatives of different phyla of the Kingdom Fungi have been isolated from this region [2,3]. Their survival is a complex process, and mechanisms have been identified at the morphological, physiological, biochemical, and genetic levels [1,4,5,6].
All the noted environmental conditions have a common mechanism of action. This is the phenomenon of oxidative stress (OS), which results from the exposure of fungal cells to biotic and non-biotic agents found in nature [7]. The term “oxidative stress” is widely used in many areas of science. It is one of the keywords of the 21st century, along with global warming and drug resistance. Oxidative stress is a complex process having negative consequences on aerobic cells, characterized as a state in which cells generate reactive oxygen species (ROS) at a higher rate than internal antioxidant defenses [8]. ROS are characterized by the presence of free radicals such as the superoxide anion (•O2−), and the hydroxyl radical (•OH), along with nonradical molecules like hydrogen peroxide (H2O2) and singlet oxygen (1O2), among additional species. In optimal growth environments, the synthesis of these substances occurs primarily at low levels within the organelles of fungal cells, such as mitochondria and peroxisomes [9]. However, under conditions of stress, there is a notable increase in the ROS generation rate.
A variety of environmental stresses, including temperature, drought, salinity, chilling conditions, metal toxicity, and UV-B radiation, as well as the impact of pathogens, have been demonstrated to contribute to an increased production of ROS in fungi [10]. Temperature is a critical factor influencing their growth and development. Exposure to extremely low temperatures induces oxidative stress, prompting a distinct response characterized by various stress tolerance strategies at the morphological, physiological, and molecular levels [3,6,11]. Despite the extensive research conducted on the cold stress responses of bacteria and plants, the adaptation strategies employed by fungi to cold conditions have not received adequate attention. Moreover, there is a notable lack of knowledge regarding the cellular response of Antarctic fungal isolates to low-temperature stress. Therefore, the present review was focused on the impact of low temperatures on the release of cold stress in the fungi isolated from Antarctica as well as on the cell response as a component of their adaptive strategies to survive under so harsh conditions.
2. Biodiversity of Filamentous Fungi in Antarctica
Despite the harsh conditions, Antarctica is home to a variety of microorganisms. The unique ecosystems here are dominated by fungi [12], which are the subject of growing interest in recent decades. Research on fungi in cold environments began in the late 19th century [13,14]. However, the initial research on the biodiversity of Antarctic fungi dates back to the 1960s [15]. The first evidence of the presence of fungi was obtained by studying mosses and lichens [16,17,18,19,20]. Microscopically viable fungi have been found on the surface and deep in permafrost sediments [21]. In the Antarctic, the most common fungal taxa to be found are Ascomycota, Basidiomycota, Mucoromycota, Chytridiomycota, and Glomeromycota. The reports described species belonging to the genera Alternaria, Botrytis, Cladosporium, Fusarium, Penicillium, Phaeosphaeria, and Phoma [22,23,24]. Fletcher et al. [17] reported that the major taxa identified in samples from Mac. Robertson Land and Enderby Land between December 1979 and October 1980 were Penicillium spp., especially P. brevicompactum and P. cyclopium, and Cladosporium herbarum. In this region, Sporothrix ramosissima was found for the first time in Antarctica. A large number of sterile forms were also isolated.
Since the beginning of the 21st century, research on the abundance and diversity of fungi in the Antarctic regions has made a great deal of progress (Table 1) [25,26,27]. The number of habitats under investigation is increasing, and the list of fungi isolated and identified is rapidly growing. Antarctic soils are characterized by great fungal diversity because of their exceptional capacity for cold adaptation. Spores in the dormant state make up a greater portion of fungal biomass [21,28]. In general, Antarctic soils contain mainly Ascomycota filamentous fungi and Basidiomycota yeasts [29], and Zygomycota and Chytridiomycota have been very rarely isolated [30,31]. Dos Santos et al. [32] presented information on 309 strains isolated from soils of Collins Glacier. Most of them belong to the phylum Ascomycota (more than 90%), followed by phylum Mortierellomycota, and phylum Basidiomycota. The strains were assigned to the genera Pseudogymnoacus, Pseudeurotium, Mortierella, Passalora, Pholiota, Russulales, Acremonium, Schizophyllum, Thelebolus, and Xylaria. Similar data for the phylum Ascomycota were reported by Frisvad [33], who mentioned 9 orders (Eurotiales, Hypocreales, Leotiales, Microascales, Onygenales, Pezizales, Saccharomycetales, Sordariales, and Trichosphaeriales) with 12 families. For phylum Basidiomycota, the classes Tremellomycetes and Cystobasidiomycetes were reported [34], and for Mucoromycota—the family Mortierellaceae [35]. Marfenina et al. [36] isolated 38 species from Antarctic soils belonging to the phylum Ascomycota (the genera Aspergillus, Penicillium, Trichoderma, Alternaria, Aureobasidium, Cladosporium, Doratomyces, Trichurus, and Emericella) and 1 species belonging to the phylum Zygomycota. A similar ratio between phyla was found in the fungal community identified in the soil of Syowa Station, Antarctica (11 genera of Ascomycota, 7 genera of Basidiomycota, and 2 genera of Zygomycota) [37]. A total of 145 fungal strains have been isolated from soil samples collected at Casey Station, Terra Nova Bay, and South Georgia [38]. They belong to the phyla Deuteromycota, Ascomycota, and Zygomycota and the genera Penicillium, Aspergillus, Mucor, Cladosporium, Alternaria, Verticillium, Botrytis, etc.

Table 1.
Fungal strains isolated from Antarctica.
From the lakes in the Antarctic Peninsula have been isolated species such as Geomyces pannorum, Thelebolus sp., Mortierella sp., Cadophora cf. luteo-olivacea, Cadophora malorum, Davidiella tassiana, G. pannorum, Mortierella cf. alpina, Thelebolus cf. microspores, etc. [45]. DNA metabarcoding indicates that the dominant phyla in lakes on the South Shetland Islands and James Ross Island are Ascomycota, Basidiomycota, Mortierellomycota, Mucoromycota, and Chytridiomycota. Species belonging to the phyla Aphelidiomycota, Basidiobolomycota, Blastocladiomycota, Monoblepharomycota, Rozellomycota, Zoopagomycota, and unknown fungal taxa were also detected [57].
Microbiological culture approaches or different molecular techniques have been used to demonstrate the presence of fungi in Antarctica [26,58,59,60,61,62]. Culturable soil fungi isolated from King George Island, Deception Island, and Robert Island were identified to 19 genera, namely, Penicillium, Pseudogymnoascus, Lambertella, Cadophora, Candida, Mortierella, Oxygenales, Geomyces, Vishniacozyma, Talaromyces, Rhizopus, Antarctomyces, Cosmospora, Tetracladium, Leptosphaeria, Lecanicillium, Thelebolus, Bjerkandera [63]. The culture-dependent method has been used successfully to assess fungal diversity in a number of Antarctic regions [28,41,64,65]. In addition, various methods such as direct or luminescence microscopy, scanning electron microscopy, and microarray analysis have been used to study Antarctic mycobiota [49,66,67,68].
On the other hand, molecular approaches allowed for the expansion of the number of identified fungi from different niches in Antarctica [26,57,69,70,71]. The simultaneous use of both methods increases the chances of obtaining more accurate information about fungal diversity [72]. The miscellany of black fungi in the Antarctic cryptoendolithic habitat was described by combining high-throughput sequencing and culture-dependent methods (genera Cyphellophora, Cryomyces, Catenelustroma, Coniospora, Elasticomyces, Friedmanniomyces, Knufia, Meristemomyces, Rachicladosporium, Cladophialophora, Exophiala, Extremus, Hortaea, Neodevriesia, Oleoguttula) [59]. Varrella et al. [60] reviewed the use of culture-dependent and molecular methods for the identification of fungi in the Antarctic marine environment. In coastal and offshore Antarctic waters, genera belonging to the orders Eurotiales, Hypocreales, Chaetothyriales, Kriegeriales, Penicillium, Metschnikowia, Rhodotorula, and Glaciozyma were found. In addition, the genera Pseudocercosporella, Toxicocladosporium, Trichoderma, Humicola, Paraconiothyrium, Phaeoacremonium, and Phenoliferia have been identified in Antarctic marine sediments. Molecular tools have allowed the identification of the orders Saccharomycetales and Eurotiales, the genera Wickerhamomyces, Geotrichum, Letendraea, and Bullera, and the families Saccharomycetaceae and Aspergillaceae.
Soil samples collected from the permanent Bulgarian Antarctic base “St. Kliment Ohridski” (62°38′29″ S, 60°21′53″ W) on Livingston Island have demonstrated the presence of 109 filamentous fungi [43,47]. Among them, the strains belonging to the genera Penicillium, Aspergillus, Cladosporium, Alternaria, Geomyces, Lecanicillium, Monodictys, Talaromyces, Epicoccum, etc.
3. Growing Temperature Requirements of Antarctic Fungi
The Antarctic mycoflora includes endemic and indigenous fungi that are able to grow and reproduce at low temperatures. They are well adapted, with the majority of them being psychrotolerant or rapid sporulating forms that can complete their lifecycles in an extremely short period. In addition, mesophilic cosmopolitan fungi may be found, transported as propagules from nearby areas, which are unable to grow in extreme conditions [24,25,73]. In most reports, the terminology used to characterize the temperature type of Antarctic fungi is that of Morita [6,34,74,75,76,77]. Fungi isolated from Antarctica can be classified as psychrophilic, psychrotolerant (psychrotrophic), and mesophilic–psychrotolerant [25,78]. Psychrophilic fungi can grow below 0 °C with optimum growth temperatures (OGT) around 15 °C or lower and maximum growth temperatures (MGT) around 20 °C, while psychrotolerant (psychrotrophic) fungi are able to grow at 5 °C or lower and have MGT between 20 and 25 °C [79]. Mesophilic–psychrotolerant fungi (mesophilic behavior and psychrotolerant adaptation) have been classified as strains that do not grow at 5 °C, showing optimal growth at 35 °C, and can grow at 45 °C [54,80,81,82]. In addition to the above-noted classification of microorganisms isolated from cold environments, Cavicchioli and Siddiqui [83] proposed the terms ‘eurypsychrophile’ (instead of ‘psychrotolerant’ or ‘psychrotroph’) and ‘stenopsychrophile’ (instead of ‘true psychrophile’). In the present review, we follow the definition of Morita [74].
The published data indicate a correlation between the temperature class of Antarctic fungi and their provenance [81]. In most cases, the Antarctic psychrophilic fungi are endemic species that can grow and reproduce in these harsh conditions [25,55,84,85]. From the soils of Schirmacher Oasis, East Antarctica, isolated 16 psychrophilic fungal and one yeast strains belonging to genera Acremonium, Aspergillus, Cladosporium, Fusarium, Trichoderma, and Torulopsis [56]. Endemic psychrophilic strains were classified as species Metschnikowia australis, Antarctomyces psychrotrophicus, Antarctomyces pellizariae, Cryomyces antarcticus, Friedmanniomyces simplex, Friedmanniomyces endolithicus, Mortierella antarctica, Penicillium antarcticum, Penicillium tardochrysogenum, Thelebolus globosus, Thelebolus ellipsoideus, Thelebolus balaustiformis, and Thelebolus spongiae [84]. Two endemic psychrophilic species of the genus Antarctomyces (A. psychrotrophicus and A. pellizariae) have been identified in the samples from the seasonal snow of the Antarctic Peninsula [44]. However, the Antarctic mycoflora consists mainly of cosmopolitan psychrotolerant taxa. For example, several cold-tolerant species belonging to the genera Penicillium, Aspergillus, Cladosporium, Colletotrichum, and Rhodotorula have been recognized as cosmopolitan taxa, adapted to the extreme low temperatures of Antarctica [84]. Out of the 51 fungal isolates from King George Island, 76% have been identified as psychrotolerant and 26% as psychrophilic [86]. The prevalence of psychrotolerant fungi in surface soils of cold environments has been documented in several studies. According to Robinson [6], this could be attributed to solar radiation causing seasonal and diurnal increases in soil temperature, which favors the development of cold-adapted microorganisms over strict psychrophiles. Furthermore, the Antarctic mycoflora includes species displaying mesophilic–psychrotolerant behavior as a cold-adaptive strategy [24]. Such mesophilic–psychrotolerant fungi have been isolated from soils and marine sediment samples collected from King George Island and Whalers Bay [79]. The authors found that two strains of Penicillium cf. oxalicum (LAMAI 2400 and LAMAI 2402) could grow at temperatures between 15 and 30 °C. Similar results have been reported for Antarctic isolates belonging to the genus Penicillium [38,54,87], as well as for Friedmanniomyces sp. CCFEE 507 [75], Aspergillus glaucus [88], Chaetomium thermophilum [89], and so forth.
Antarctic fungi are not always tolerant of the temperature of the cold environment from which they have been isolated. For example, psychrophilic fungi can grow at optimum and maximum temperatures much higher than the temperature of the original habitat [90]. It is worth mentioning that the isolation of Antarctic fungi belonging to different temperature classes depends on the isolation temperature, which can affect the isolation frequency [6]. The fungal strains obtained from forest soils in Rhode Island at an isolation temperature of 0 °C were mainly psychrotolerant, and some psychrophiles [91]. Isolations conducted at 25 °C yielded predominantly mesophilic organisms, exhibiting optimal growth between 5 and 10 °C and reaching a maximum at temperatures above 25 °C. Similar trends in the ratio between psychrophilic, psychrotolerant, and mesophilic isolates have been described for samples from King George Island [63]. Furthermore, the mitochondrial genomes of psychrophilic and psychrotolerant fungi demonstrated a higher fraction of protein-coding regions and a lower GC content compared to those of mesophilic fungi [82]. Tosi et al. [43] reported that among 109 fungal isolates, 16 were collected from agar plates incubated at 4 °C, 61 from those at 10 °C, and 32 from plates at 25 °C. The results revealed that the preferred growth temperature for most of the isolated strains was between 15 and 25 °C.
4. ROS and Oxidative Stress
4.1. ROS: Origin, Sources, Damages
The designation ROS denotes a collective term of oxygen-containing reactive species that are formed during the process of incomplete oxygen reduction. They include superoxide radicals (●O2−), hydrogen peroxide (H2O2), hydroxyl radicals (OH•), singlet oxygen (1O2), peroxyl radical (LOO●), alkoxyl radical (LO●), lipid hydroperoxide (LOOH), peroxynitrite (ONOO−), hypochlorous acid (HOCl), and ozone (O3), among others [92]. Some of ROS contain one or more unpaired electrons, which are called oxygen radicals or oxygen free radicals, for example ●O2−, OH•, LOO●, and LO●. The ROS that do not contain any unpaired electrons, such as H2O2, HOCl, and O3, are not free radicals [93,94]. ROS are inevitable by-products of the normal aerobic metabolism of cells. The mitochondrial respiratory chain is the major source of ROS. Under normal oxy conditions, only 1–2% of total oxygen is converted into free radicals [95]. The primary species ●O2−, H2O2, and HO• are the result of successive processes of monovalent O2 reduction [Figure 1].
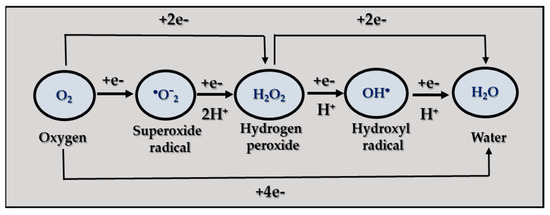
Figure 1.
Mechanisms of ROS generation.
The initial monovalent reduction results in the formation of ●O2−, which is subsequently followed by the reduction of a second electron, leading to the release of H2O2. ●O2− also reacts with H2O2 to generate HO• (Haber–Weiss reaction) [96]. The superoxide radical is most commonly produced in mitochondria. It has a very short lifetime and relatively low reactivity. ●O2− does not react with most biological molecules. Its chemical activity depends on where it is produced in the cell, its ability to be protonated to a more powerful oxidant (peroxyl radical), and its collision with a suitable substrate [97].
Hydrogen peroxide is an unstable and slowly decaying molecule containing an oxygen–oxygen bond. The main source of H2O2 is the superoxide radical dismutation reaction or the two-electron reduction of oxygen in reactions catalyzed by xanthine oxidase, glucose oxidase, and others. The H2O2 molecule is uncharged and can cross biological membranes. It is a powerful oxidant, but the kinetics of its reaction with biomolecules are quite slow, leading to its accumulation in cells at relatively high concentrations [98].
The hydroxyl radical is one of the most reactive radicals present in biological systems and has a relatively short half-life (10−8 to 10−9 s) [99]. It exhibits a direct effect near its point of origin, without making long transitions into the living cell, causing damage to the first molecule it encounters.
Singlet oxygen is a non-radical form of oxygen. It is an oxygen molecule in which one of the two free electrons is in an excited state. Thus, 1O2 can transfer energy to other molecules and act as a catalyst for the formation of free radicals.
ROS can originate from both external sources and intracellular processes. Their formation occurs across a variety of biochemical and physiological functions [94]. Besides the mitochondrial electron transport chain, various cellular organelles, including peroxisomes, lysosomes, the endoplasmic reticulum, etc., which depend on oxygen for their activities, also contribute to the intracellular reservoir of ROS. A range of enzymes such as NADPH oxidases, xanthine oxidase, cyclooxygenase, lipoxygenase, and other free ions (such as iron and copper) has been associated with the generation of ROS. The group of exogenous sources includes ultraviolet radiation, polycyclic aromatic hydrocarbons (PAHs), γ-irradiation, and environmental pollution. Exposure to different drugs like cisplatin, doxorubicin, paclitaxel, metformin, atorvastatin, and others can also cause an increase in the ROS level.
Under normal conditions, the level of radicals is adequate to the level of the cell’s defense system, and they manage to maintain an oxidation–reduction equilibrium; i.e., a balance is observed between the formation and elimination of ROS. However, the overproduction of ROS can exceed the protective mechanisms of cells, leading to detrimental impacts on cellular elements such as lipids, proteins, and nucleic acids [97] [Figure 2]. Carbohydrates, albeit to a lesser degree, are also affected by ROS [100].
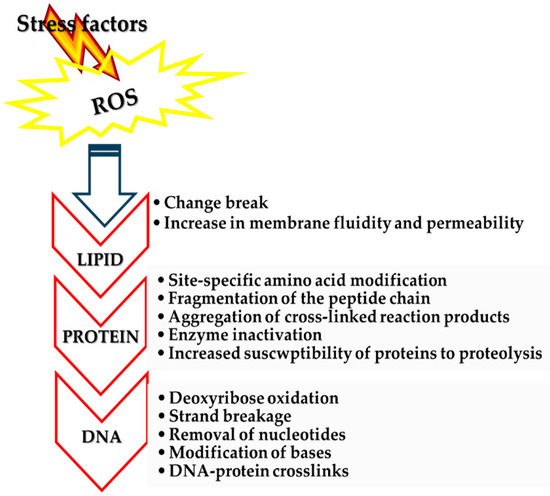
Figure 2.
Oxidative damage to intracellular molecules.
Although DNA is a stable, well-protected molecule, ROS can interact with it and cause various types of damage. In the human body, such processes have been shown to occur at a rate of 104 lesions per cell per day. ROS damages the DNA molecule by removing an electron from it and turning it into an unstable molecule. Mitochondrial and chloroplast DNA are more susceptible to oxidative damage than nuclear DNA due to the lack of protective proteins and histones and their proximity to ROS-producing systems. ROS has been demonstrated to induce a series of molecular events, including binding to DNA bases or deoxyribose, DNA chain breakage, removal of nucleotides, modifications in nucleotides, and subsequent mutations. Additionally, covalent binding to a protein molecule has been observed as a result of ROS action (Figure 3) [97,101,102].
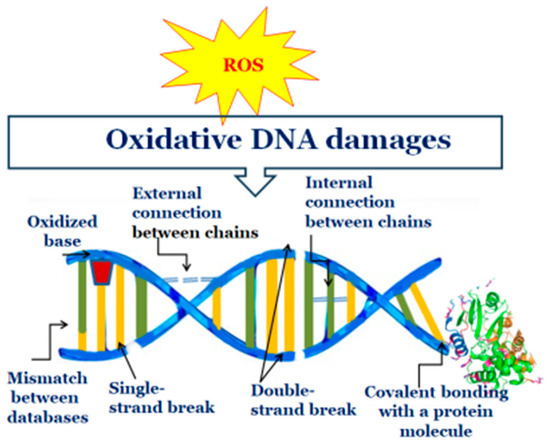
Figure 3.
Changes in DNA as a result of oxidative damage.
Proteins are also susceptible to biological oxidation due to their prevalence in cells and their high rate of constant interaction with numerous types of free radicals. Consequently, protein damage is a significant consequence of free radical formation within and outside the cell. ROS can cause hydrogen removal, electron transfer (oxidation or reduction), addition, fragmentation and rearrangement reactions, dimerization, disproportionation, and substitution in amino acids, peptides, and proteins, etc. (Figure 4) [100,103,104].
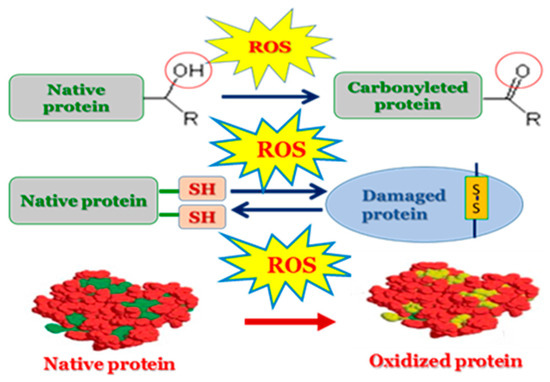
Figure 4.
Oxidative-induced damage of proteins.
Oxidative effects on lipids play a particularly important role in cell biology, human health, and the food industry. In cells, a process called lipid peroxidation takes place in which free radicals attack lipids (particularly polyunsaturated fatty acids, PUFAs), removing hydrogen while incorporating oxygen. Responsible for this process are hydroxyl radicals (OH•), which are mainly formed by the Fenton [Reaction 1] and Haber–Weiss [Reaction 2] reactions [10]. Theoretically, the Haber–Weiss reaction cannot play a major role in vivo because its rate constant is lower than that of the dismutation reaction. However, the Fenton reaction, which exploits the ability of iron ions to participate in redox–cyclic interactions and increases the rate of the process, is much more feasible.
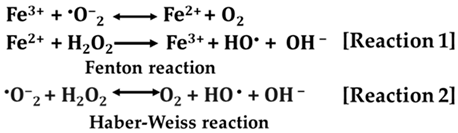
Lipid peroxidation is considered to be a major molecular mechanism involved in oxidative damage to all structures that can lead to cell death [105,106]. Cell membranes and membranes of subcellular organelles composed of polyunsaturated fatty acids are the first targets of radical attack. These include mitochondrial membranes, microsomes, peroxisomes, and cytoplasmic membranes. Glycolipids, phospholipids, and cholesterol are also damaged or fatally modified by lipid peroxidation (Figure 5).
Figure 5.
Lipid peroxidation in cell membranes.
4.2. The “Beneficial” Role of ROS
Is it possible that nature has involved ROS in beneficial activities? There is no doubt that the “helpful” effect of these radicals is very limited. From the data currently available, it is clear that ROS are important for the modeling of secondary signaling molecules, differentiation of cells, metabolism of xenobiotics, stimulation of the acrosome reaction in cell fertilization, etc. [107,108]. In addition, maintaining an optimum ROS level plays a vital role in molecular signaling in fungal growth, development, adaptation, and response to various abiotic and biotic stresses, as well as for the virulence of pathogenic fungi [109,110]. The beneficial role of ROS for fungi has been demonstrated in their resistance to antifungal compounds [111] or in the degradation of lignocellulose [112].
5. Oxidative Stress and Antioxidant Defence
5.1. Oxidative Stress and Its Physiological Role
A delicate balance between the rate of their formation and the rate of their removal controls the toxicity of ROS by the cell’s defense mechanisms. In cells with normal aerobic respiration, ROS formation proceeds at a relatively low rate. The antioxidant system present in normoxia is able to protect the organism against the action of radicals. However, under certain conditions, the concentration of toxic metabolites exceeds the physiological capacity of the cell, leading to an imbalance between pro- and antioxidants with a predominance of the former [96,113,114]. Excessive levels of pro-oxidants can cause OS. The concept of oxidative stress was introduced in greater detail for the first time in 1985 [115]. This concept caused extensive research on the relationship between oxidants and antioxidants, including their origins and metabolic features [116,117,118]. It is known that the OS plays a significant role in different physiological processes, in particular, cell signaling, differentiation, proliferation, tissue repair, aging, immune response, etc. [1,110,118]. OS is involved in the regulation of cell growth, differentiation, and apoptosis [119]. The detoxification of various compounds like drugs and xenobiotics can also be realized due to the OS [120].
5.2. Antioxidants and Antioxidant Defence
According to Halliwell [121], antioxidants are “any substance that delays prevents or removes oxidative damage to a target molecule”. They can also be defined as sufficiently stable molecules capable of accepting or donating an electron to any free radical and eliminating its unpaired state (Figure 6) [122]. Antioxidants cover a wide range of substances produced within cells or supplied by food substrates. Their function includes reducing the potential of ROS to cause damage, or interacting with ROS and preventing the chain reaction of their generation before damage occurs to molecules vital to the cell.
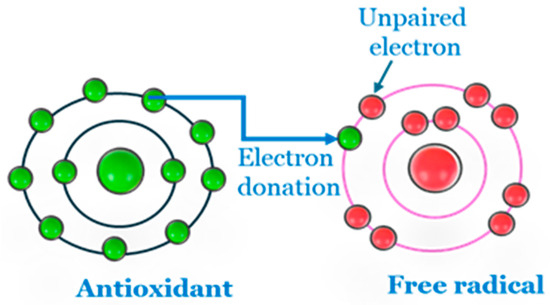
Figure 6.
Antioxidant action against free radicals.
Antioxidant activity can be found in the cellular defense system at different levels, depending on the strategy used: prevention, scavenging and destroying radicals, repairing damage, and adaptation. They can also be classified as natural and synthetic, enzymatic and non-enzymatic, low molecular and high molecular, intracellular and extracellular, water-soluble and lipid-soluble (Figure 7) [96,123].
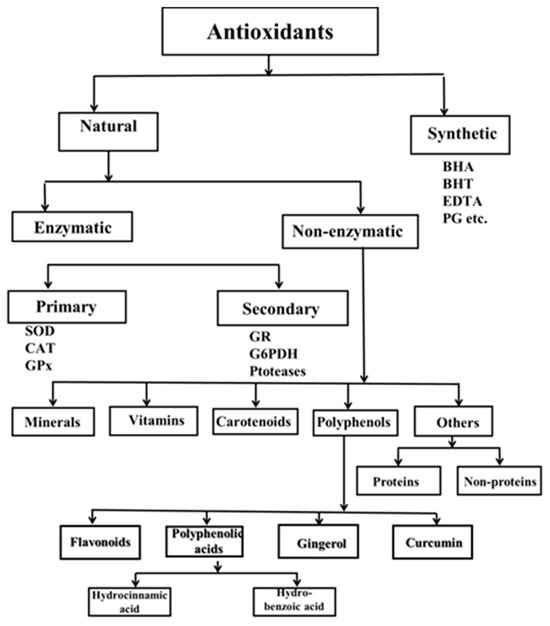
Figure 7.
Classification of antioxidants.
Enzymatic antioxidants are the first line of defense and are the most effective antioxidants in aerobic cells. They are classified as primary and secondary. Primary enzymes include superoxide dismutase (SOD) and catalase (CAT). Depending on the developmental stage of the organism, they are joined by glutathione peroxidase (GPx). SOD is responsible for the dismutation reaction in which ●O2− is converted to H2O2. In subsequent reactions catalyzed by CAT or GPx, H2O2 is converted to water and oxygen. Glutathione reductase and glucose-6-phosphate dehydrogenase are part of the secondary enzymatic defense. Glutathione reductase converts glutathione from its oxidized to its reduced state to recycle glutathione and scavenge more free radicals. Nicotinamide adenine dinucleotide phosphate, a coenzyme involved in anabolic processes, is renewed by glucose-6-phosphate (NADP), resulting in a reducing environment [124]. These two enzymes do not directly eliminate free radicals but rather provide support to the other endogenous antioxidants.
Third-line antioxidants recognize, degrade, and remove oxidized or damaged proteins, DNA, and lipids to prevent their accumulation, which can be toxic to cells. These include DNA repair enzyme systems (polymerases, glycosylases, and nucleases) and proteolytic enzymes (proteinases, proteases, and peptidases) found in both the cytosol and mitochondria of cells. The third lineage includes direct and indirect repair systems [125,126].
The fourth line of defense is the adaptive response to oxidative stress. This line uses the signals the cell receives about the formation or action of ROS and transports the appropriate antioxidants to the right place. The adaptive response depends on changes in gene expression or increased translation of a pre-existing mRNA to induce the synthesis of antioxidant enzymes. This leads to increased resistance to the stressors [126].
5.2.1. Enzymatic Antioxidants
The antioxidant enzymes SOD, CAT, and GPx play a crucial role in the ability of biological systems to fend off attacks from the free radicals. SOD (EC 1.15.1.1.) eliminates •O−2 radicals in a “ping-pong” mechanism. The enzyme catalyzes the dismutation of two molecules of •O−2 to hydrogen H2O2 and O2, which limits ●O2− availability [Reaction 3]. This situation led to the reduction of iron and a decrease in the amount of H2O2 formed by the Haber–Weiss/Fenton reaction [127,128].
The enzyme was discovered in 1969 by Joe McCord and Irwin Fridovich and has since been the subject of much research. Initially, three types of SOD were identified, and now there are a known four. The first and most obvious similarity between these enzymes is that they all contain redox-active metal ions in the active center: Fe2+/3+ in Fe-SOD, Mn2+/3+ in Mn-SOD, Cu1+/2+ in Cu/Zn-SOD, and Ni2+/3+ in Ni-SOD [128,129,130,131,132,133]. Manganese- and Fe-SOD are evolutionarily older than Cu/Zn-SOD and are derived from the same precursor enzyme [134]. In later stages of phylogenetic development, they diverged, and their amino acid sequences formed two distinct groups. The evolution of the Cu/Zn SOD family proceeded independently, resulting in a significant departure from the characteristics of the initial two enzymes.
The distribution of SOD isoenzymes across various organisms is indicative of both the developmental stage of the organism and the specific cellular organelles with which these enzymes are associated [135,136]. Cu/Zn-SOD is predominantly present in eukaryotic organisms, while Fe-SOD is typically found in prokaryotic organisms. Mn-SOD is observed in both prokaryotic and eukaryotic organisms, and Ni-SOD is specifically located in cyanobacteria and aquatic eukaryotes. Additionally, extracellular SOD (EC-SOD) is produced in the tissues of higher eukaryotes, playing a crucial role in the detoxification of superoxide radicals generated from external sources [137]. These isoenzymes exhibit distinct localization within cellular components [138]. Cu/Zn-SOD is found in the cell wall, cytosol, chloroplasts, and peroxisomes; Fe-SOD is present in the cytosol of prokaryotic organisms and within the chloroplasts of plants; Mn-SOD is located in mitochondria and peroxisomes.
Fe-SOD is the most ancient SOD metalloenzyme with a high degree of homology with Mn-SOD. There are two recognized categories of Fe-SOD: homodimeric protein structures with an approximate molecular weight of 20 kDa, which incorporate 1–2 g-atoms of iron in their active site, and tetrameric forms with a molecular weight ranging from 80 to 90 kDa, containing 2–4 g-atoms of iron in their active site.
In bacterial cells, manganese superoxide dismutase (MnSOD) is localized in the cytoplasm, whereas in eukaryotic cells, it is found within the mitochondria. MnSOD can exist as either a homotetramer or a homodimer, with molecular weights of approximately 96 kDa and 48 kDa, respectively. Each form comprises four or two identical subunits, with each subunit containing a single manganese atom.
Cu/Zn-SOD is predominantly found in the cytosolic compartment of eukaryotic cells [139]. However, exceptions exist, as Cu/Zn-SOD has been identified in certain prokaryotic organisms and within the intermembrane space of mitochondria, albeit in considerably lower concentrations than its iron- and manganese-containing counterparts. In prokaryotes, it is frequently observed as periplasmic SOD, indicating its potential function as a defensive mechanism for pathogenic bacteria against oxidative stress generated by mammalian macrophages. The molecular weight of Cu/Zn-SOD is approximately 32 kDa, consisting of two identical subunits (homodimer), with each subunit incorporating one atom of copper and one atom of zinc [137]. The coordination of these metal ions at the active site is facilitated by the histidine residue at position 61. The inclusion of zinc ions is likely crucial for maintaining the structural integrity of the enzyme. In periplasmic bacterial SOD, both monomeric and dimeric forms are present, with the dimeric forms exhibiting distinct characteristics compared to those found in eukaryotic systems.
The presence of Ni-SOD can be attributed to evolutionary adaptations that enable the utilization of nickel as an essential redox metal.
This adaptation arose in response to the diminished availability of iron in oceanic environments, a consequence of photosynthetic processes. Within the SOD family, this particular enzyme is distinctive, characterized by its unique amino acid sequence and specific spectroscopic properties. The enzyme is structured as a homohexamer, consisting of six identical subunits, each formed from monomers comprising 120 amino acids, resulting in a molecular mass of approximately 13 kDa, with each subunit containing one atom of nickel.
EC-SOD shares homology with intracellular Cu/Zn-SOD and is characterized as a tetrameric glycoprotein that incorporates a Cu/Zn prosthetic group [137]. This enzyme is released into the interstitial spaces of tissues as well as extracellular fluids, including plasma, lymph, and synovial fluid, in both humans and animals. It exhibits a binding affinity for the plasma membrane and heparin-associated components of the extracellular matrix. Notably, EC-SOD has been identified in certain instances within plants and prokaryotic organisms. Its primary role is to catalyze the dismutation of superoxide radicals that are generated extracellularly.
Catalase (EC 1.11.1.6) is an enzyme present in nearly all aerobic organisms and a variety of microaerophilic microorganisms, such as lactic acid bacteria, which thrive in low-oxygen environments [140,141]. This enzyme serves a crucial protective function for aerobic organisms by decomposing H2O2 generated within cells before it can escape into the surrounding environment. Although CAT may not be critical for every cell type, it significantly enhances the organism’s resilience against various oxidants. Historically, it was believed that CAT was primarily located in peroxisomes; however, recent findings indicate its presence in mitochondria, cytosol, and chloroplasts as well. CAT found in peroxisomes originates from the nucleus and requires transport to reach these organelles. Within the peroxisomes, the individual monomers assemble into tetramers, and the heme group is incorporated during this process.
CAT is an enzyme characterized by the presence of porphyrin and is composed of four identical subunits, forming a homotetrameric structure. Each subunit has a molecular weight of 60 kDa, resulting in a total molecular mass of 240 kDa for the complete enzyme, and comprises approximately 500 amino acids. This enzyme is recognized for its high catalytic efficiency. It facilitates the breakdown of H2O2 into water and oxygen, while also interacting with hydrogen donors such as methanol, ethanol, and phenol, utilizing one mole of peroxide in the process. The active site of the enzyme contains either manganese or iron ions.
There are four types of CATs, categorized based on the characteristics of their active sites:
- The first type encompasses the typical or monofunctional catalases, which consist of four identical subunits. Each subunit contains either heme b or heme d, both of which are prosthetic groups that incorporate iron within their active sites. The molecular weight of each subunit ranges from 55 to 84 kDa. This category of CATs is the most prevalent in the natural world and demonstrates catalytic activity across a broad spectrum of pH levels. The heme group situated in the active site is positioned deep within the tetrameric structure. Monofunctional catalase enzymes operate through a two-step mechanism to facilitate the breakdown of H2O2. Initially, a single molecule of H2O2 is oxidized by the enzyme, resulting in the formation of ferryl porphyrin, referred to as compound I [Reaction 4]. Subsequently, in the second phase, compound I further oxidizes another molecule of H2O2, yielding molecular oxygen and water as products [Reaction 5].
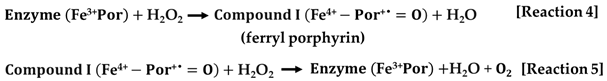
- The second category of enzymes, known as catalase-peroxidases, demonstrates a bifunctional nature, possessing both catalase and peroxidase activities. However, their activity levels are significantly lower, by two to three orders of magnitude, compared to conventional catalases. These enzymes typically exist as dimers or tetramers, with each subunit having an approximate molecular mass of 80 kDa, and they contain heme, which serves as a prosthetic group with iron. Catalase-peroxidases have been identified in a variety of organisms, including bacteria, archaea, fungi, and even more complex eukaryotes. The catalytic mechanism they employ mirrors that of conventional CATs, comprising the same two distinct stages.
- The third category encompasses manganese-containing CATs. In contrast to the previous two categories, these catalases lack heme as a prosthetic group and instead utilize manganese ions at their active sites. Mn-CATs are structured as hexamers, with each subunit exhibiting a molecular mass ranging from 28 to 35 kDa, and they contain two manganese ions within their active center. They operate through a two-step mechanism that is similar to that of heme-containing enzymes [Reactions 6 and 7]. During the first phase, H2O2 acts as the oxidizing agent, while in the subsequent phase, it serves as the reducing agent.
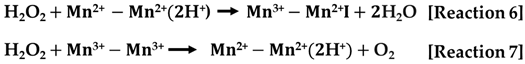
Glutathione peroxidase (GPx) is an enzyme that contains selenium and is classified under the peroxidase category (EC 1.11.1.19). This enzyme utilizes glutathione (GSH) to facilitate the conversion of hydrogen peroxide (H2O2) into water and to reduce organic peroxides to their corresponding alcohols in mammalian systems. The GPX family comprises eight distinct isoforms (GPX1 to GPX8), among which GPX1, GPX2, GPX3, GPX4, and GPX6 are recognized as selenoproteins with significant antioxidant properties. Notably, GPX6 is uniquely expressed in humans. Despite the importance of these enzymes, comprehensive investigations into the functions of the entire GPX family in the context of oxidative stress responses remain insufficient. To date, only a limited number of studies have systematically explored the involvement of GPX in oxidative stress [142].
Reductase enzymes, including glutathione reductase (GTR) and thioredoxin reductase (TRR), are integral components of the secondary antioxidant defense system. These enzymes facilitate the continuous production of NADPH, which is essential for counteracting reactive oxygen species (ROS) [143]. The presence of NADPH is crucial for mitigating the harmful effects of toxic substances that may enter aerobic cells. Additionally, NADPH can also be generated through the pentose phosphate pathway, aided by the enzymes glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase [144,145].
5.2.2. Non-Enzymatic Antioxidants
Natural compounds without enzymatic characteristic include vitamins, flavonoids, carotenoids, glutathione, plant polyphenols, melatonin, bilirubin, curcumin, and polyamines. Some of them are water-soluble, and in most cases, localized in the cytosol or cytoplasmic matrix, while others are fat-soluble and are present in cell membranes. Vitamins are complex organic compounds essential for the body in minimal quantities. Among these, antioxidant vitamins such as A, E, and C play a crucial role in neutralizing reactive oxygen species and exhibit a diverse array of biological functions. They contribute to cellular protection against genomic alterations by mitigating DNA damage caused by oxidative stress.
Vitamin A, also known as retinol, is a fat-soluble vitamin classified within the retinoid family. This family comprises 20 distinct compounds characterized by a methyl-substituted cyclohexenyl and a tetraene side chain, which contains four double bonds. Beta-carotene serves as the precursor to vitamin A, undergoing hydrolysis to form retinol in the gastrointestinal system. The mechanism through which vitamin A exerts its antioxidant effects involves the neutralization of peroxyl radicals (LOO•). This is achieved by the addition of these radicals to the β-ionone ring of retinol, resulting in the formation of a highly conjugated retinol-carbon radical. This intermediate compound is relatively stable and, under typical physiological conditions, does not possess sufficient reactivity to promote further lipid peroxidation [146].
Vitamin C, also known as ascorbic acid, is a water-soluble vitamin that was first isolated in 1928. It serves as an essential trace element necessary for the proper metabolism of aerobic cells [146]. Within the cytosol, vitamin C engages directly with reactive species such as ●O2− and HO•, thereby protecting cellular membranes against oxidative damage [125]. The antioxidant mechanism of vitamin C operates through its role as an electron donor, functioning as a reducing agent that can neutralize oxidized compounds. Upon interaction with a free radical, vitamin C donates an electron, resulting in the formation of the ascorbyl radical (AscR), which exhibits greater stability compared to other free radicals. The AscR can subsequently neutralize more reactive radicals, including OH•. In a further reaction, AscR can donate an additional electron to another reactive radical, leading to the production of dihydroascorbic acid. Notably, a single molecule of ascorbic acid has the capacity to neutralize two free radicals.
Vitamin E is a group of compounds that includes four tocopherols and four tocotrienols, with alpha- and gamma-tocopherols being the most abundant in human tissues. The antioxidant mechanism of vitamin E operates through its interaction with free radicals, resulting in the formation of a tocopheroxyl radical along with a non-radical by-product [146]. In the cellular context, vitamin E serves several critical functions: it quenches peroxyl radicals produced during lipid peroxidation, protects polyunsaturated fatty acids in cell membranes, assists in the neutralization of singlet oxygen, and participates in reactions with peroxynitrites. It is recognized as the most potent antioxidant in the lipid environment, and its efficacy is greatly enhanced when combined with other antioxidants such as vitamin C, selenium, and beta-carotene [147].
Carotenoids represent a diverse class of red-orange pigments found in plants. These pigments are characterized as fat-soluble chromophores that utilize a system of conjugated double bonds to absorb light within the wavelength range of 400 to 500 nm [148]. More than 600 different carotenoids have been identified, with six being particularly prominent: alpha-carotene, beta-carotene, lycopene, lutein, beta-cryptoxanthin, and zeaxanthin. The antioxidant properties of carotenoids are attributed to their polyene structure, which enables them to interact effectively with reactive oxygen species, including singlet oxygen [149]. Alterations to this polyene structure, such as modifications in the number of conjugated double bonds or the introduction of oxygen-containing functional groups, can significantly influence the reactivity of carotenoids.
Polyphenols represent a significant category of secondary metabolites derived from plants, encompassing approximately 10,000 distinct structures. The increasing interest in polyphenols is attributed to their potential in mitigating oxidative stress and associated diseases. This group is classified into two primary categories: flavonoids and non-flavonoids [150]. Non-flavonoid polyphenols consist of phenolic acids, such as hydroxybenzoic and hydroxycinnamic acids, as well as stilbenes and lignans.
Flavonoid polyphenols function as exogenous antioxidants and are characterized by a C6-C3-C6 structural framework, which consists of two aromatic rings linked by a three-carbon bridge. These metabolites play a crucial role in various cellular functions, influencing pigmentation, aroma, flavor, growth, and reproductive processes. The principal dietary flavonoids are categorized into six groups based on the configuration of the C ring: anthocyanidins, flavan-3-ols, flavanols, flavones, isoflavones, and flavanones [143]. The process by which these antioxidants exert their effects involves the transfer of a hydrogen atom from a flavonoid to a free radical, resulting in the formation of a phenoxy radical. This phenoxy radical is characterized by its stability, which effectively halts the progression of the chain reaction. Subsequently, the phenoxy radical may engage with another free radical, leading to the production of a quinone. Alternatively, rather than donating a hydrogen atom, the flavonoid can also provide an electron to the free radical, resulting in the generation of a stable cation radical.
Melatonin (N-acetyl-5-methoxytryptamine) is a hormone produced intracellularly by the pineal gland in mammals. Its antioxidant properties are realized through both direct and indirect mechanisms [151]. In the direct detoxification of reactive oxygen species, melatonin donates one or more electrons to free radicals (such as OH•), facilitating their transformation into stable, non-radical forms. This action is particularly effective in curtailing lipid peroxidation and neutralizing singlet oxygen. The indirect antioxidant effects involve the activation of key antioxidant enzymes (GPx, glutathione S-transferase, and CAT, as well as the stimulation of glutathione production.
Ubiquinones possess a 1,4-benzoquinone framework, with coenzyme Q10 being the most biologically active variant. This compound, resembling a vitamin, is fat-soluble and exhibits antioxidant properties while playing a crucial role in the electron transport chain involved in ATP synthesis. Its antioxidant mechanism is linked to the safeguarding of unsaturated fatty acids within cellular membranes from oxidative damage caused by peroxides, the neutralization of reactive carbonyl species, and its synergistic interactions with other antioxidants, such as vitamin E [152].
Glutathione is a tripeptide composed of three amino acids: cysteine, glutamine, and glycine. It functions primarily as an intracellular antioxidant, with approximately 90% of its presence in the reduced form and only 10% in the disulfide form. The antioxidant mechanism of glutathione involves its role as a substrate for various enzymes that facilitate the detoxification of reactive metabolites produced by the interaction of ROS with DNA, proteins, and other biomolecules. Additionally, certain enzymes utilize glutathione to neutralize potentially harmful substances, including estrogens and xenobiotics. Furthermore, glutathione serves as an endogenous antioxidant, effectively neutralizing free radicals. It also plays a crucial role in regenerating the oxidized forms of ascorbic acid, alpha-tocopherol, and ubiquinones.
6. Low Temperature-Induced Oxidative Stress in Antarctic Fungi
Among the mentioned harsh parameters of growth in Antarctica, the temperature plays an important role in the realization of oxidative stress in fungi [1,123]. The cold temperatures result in a decline in the metabolic activity of microorganisms, which leads to a reduction in the utilization of ATP and the accumulation of electrons at specific points within the respiratory chain (see Figure 1) [153]. The situation gives rise to a rapid increase in the production of ROS [Figure 8].
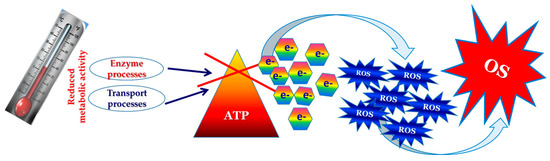
Figure 8.
Molecular mechanism of cold stress realization.
There are data that support the modification of metabolic pathways to ensure some species survive under cold stress conditions. The findings suggest the presence of a multifaceted interaction between physiological and biochemical mechanisms that contribute to the adaptation of these species to low-temperature environments [154]. Reduced metabolic activity in response to cold stress has been observed in the fungal strains P. chrysogenum [155], Mrakia psychrophila [156], and Mortierella isabellina [157]. Tsuji [50] demonstrated significant metabolite changes in the carbon-metabolite pathway (glycolysis, pentose-phosphate pathway, and TCA cycle) in the Antarctic basidiomycetous yeast Mrakia blollopis grown at 10 and 3 °C. Proteomic analysis of fungal strains belonging to the genus Pseudogymnoascus revealed a positive correlation between protein levels and transcription levels in certain pathways when subjected to low-temperature stress, indicating an up-regulation of chaperones and pathways associated with energy metabolism [11,154]. Moreover, the expression of cold-responsive genes, such as the FRO1 gene, as well as the accumulation of ROS, can be influenced by the disruption of mitochondrial function in plants [158]. Suzuki and Mittler [159] propose a model that elucidates the relationship between temperature stress and ROS production. This model is primarily based on the disruption of cellular homeostasis and the uncoupling of metabolic processes.
The scientific literature contains only a very small number of studies that make a connection between low temperatures and oxidative stress in fungi (Table 2). As shown by Zhang et al. [160], a rapid reduction in growth temperature from 30 to 10 °C increased H2O2 levels in S. cerevisiae, coinciding with increased transcripts of genes responsible for antioxidant defense. Similar results have been reported for the S. cerevisiae BY4743 strain growth at low temperatures (10–15 °C) [161]. The authors suggested that the suboptimal temperature caused an enhanced level of ROS and induction of the antioxidant response. Temperature changes induced ROS generation in plant pathogen A. flavus [162]. A transient temperature downshift from 30 °C to 10 °C or 4 °C resulted in a significant increase in ●O2− and H2O2 levels within both the intact cells and mitochondria of the Antarctic strains Penicillium sp. 161 and A. glaucus 363 [163]. The reduction in temperature to 4 °C resulted in a more pronounced elevation of ROS levels compared to the conditions at 10 °C. Furthermore, the mesophilic strain A. glaucus 363 exhibited a more vigorous ROS production process in contrast to the psychrotolerant Penicillium sp. 161. Similar results have been reported for the Antarctic strains P. griseofulvum P29 and P. chrysogenum P27 [164], as well as for P. waksmanii m12 and P. olsonii p14 [165]. An association between cold stress and ROS generation has been reported on two strains of the cold-adapted oleaginous yeast Rhodosporidium kratochvilovae [166]. In the psychrophilic fungus Pseudogymnoascus pannorum, increased levels of unsaturated fatty acids allow membrane function to be maintained under high ROS levels at low temperatures [76].

Table 2.
Biomarkers of Cold-Induced Oxidative Stress in Antarctic Fungi.
The oxidative burst of ROS formed under cold exposure causes significant injury in aerobic fungal cells. However, only a few studies have reported on the damage caused by cold stress to the main biological molecules of fungi, especially those found in Antarctica. It is well-known that cold stress leads to the process of lipid peroxidation in fungi and thus alters the fluidity of lipid-containing membranes and disrupts membrane-bound processes [3]. Schade et al. [173] reported the accumulation of the reserve carbohydrates, i.e., glycogen and trehalose. The experimental findings indicate that low temperatures result in an increase in unsaturated lipid content [168]. Specifically, a psychrotrophic isolate of Geomyces pannorum cultivated at 5 °C exhibited a modified lipid profile in comparison to the same isolate grown at 15 °C. Unsaturated fatty acids are very sensitive to oxidative degradation [174]. For example, the grey mold fungus Botrytis cinerea demonstrated oxidative lipid damage under low-temperature growth [175]. A similar response to suboptimal growth temperatures has been documented for the strains Humicola marvinii, Geomyces pannorum, and Mortierella elongata, isolated from Signy Island, Antarctica [168]. Short-term temperature downshift from 25 to 10 °C caused a significant increase in the malondialdehyde (MDA) level (a marker for lipid peroxidation degree) in Antarctic strain P. griseofulvum P29 [171,176].
Modification in lipid composition has been reported as a component of the survival strategy employed by cold-adapted fungi. In psychrophilic yeasts, such as species from the genera Candida, Leucosporidium, and Torulopsis, the fatty acids present exhibit a lower degree of saturation compared to those found in mesophilic yeasts. Furthermore, a reduction in incubation temperature serves to further enhance this unsaturation [177,178]. At lower temperatures, there is an increased production of long-chain polyunsaturated fatty acids (LCPUFAs), including eicosapentaenoic, arachidonic, and docosahexaenoic acids [179]. These compounds form hydrophobic boundaries between lipid bilayers, thereby preventing ROS damage and the intake of H2O2 into cells. Consequently, LCPUFAs have been identified as playing a crucial role in the protection of cells from oxidative stress under these lower temperature conditions. Moreover, the shortening of fatty acid chain lengths has been detected as a response to cold stress [180].
Changes in lipids are significantly associated with the stability of membrane structures in fungi and their integrity under low-temperature conditions [154]. The authors found a decreased phospholipid metabolism in the Antarctic isolate Pseudogymnoascus spp. (sp3) in response to cold stress. The Antarctic strain of Geomyces pannorum exhibited enhanced membrane fluidity due to alterations in the phospholipid composition of its membrane lipids when exposed to low temperatures [181]. Sterols serve an essential structural and regulatory function within eukaryotic cell membranes, affecting their fluidity. They intercalate within the fatty acid chains, generally contributing to the stabilization and fortification of the membrane lipid bilayer [180]. Using transcriptomic analysis, Su et al. [182] demonstrated an up-regulation of genes associated with the biosynthesis of unsaturated fatty acids in Mrakia psychrophile grown at 4 °C. This suggests that the cold adaptation mechanism of the obligatory psychrophilic fungus involves the synthesis of unsaturated fatty acids to preserve membrane fluidity. Data on the fungus Thermomyces dupontii revealed a lipid-mediated fungal response to cold stress [183].
In the context of low-temperature stress, cold-adapted fungi demonstrated significant damage in the tertiary structures of molecules such as enzymes and other functional proteins, which compromise their functionality and may lead to degradation. Similar data on the cold stress response of fungi isolated from Antarctica are described in previous publications of our group. Research demonstrated that protein carbonyls serve as reliable biomarkers for evaluating protein oxidation, which is a consequence of temperature stress. The long-term growth of Antarctic strains P. olsonii p14 and P. waksmanii m12, along with the temperate strain Penicillium sp. t35, under suboptimal temperature conditions, resulted in a significant increase in protein carbonyl concentrations [87]. A six-hour temperature downshift applied to exponentially growing cultures of these strains led to an observable increase in the number of carbonyl groups in cell proteins throughout the entire period. The mesophilic strains (P. waksmanii m12 and Penicillium sp. t35) exhibited a more substantial increase in the content of damaged proteins compared to the psychrotolerant strain (P. olsonii p14) [170]. Thus, the cold-adapted Antarctic psychrotolerant strain showed enhanced tolerance to cold stress in contrast to the mesophilic strains. The stationary-phase cultures also demonstrated a remarkable increase in carbonylated protein content, but to a greater extent [52]. It could be assumed that the exponential-phase cells exhibit higher resistance to cold stress.
A remarkable increase in carbonylated protein content has also been evaluated in two Antarctic strains, particularly the psychrotolerant Penicillium sp. 161 and mesophilic A. glaucus 363, when subjected to temperatures of 4 °C or 10 °C [163]. The same typical response to oxidative stress regarding protein damage by low-temperature exposure has been reported for P. griseofulvum [171,176].
The modification of DNA is also significant in understanding the interplay between low temperatures and oxidative stress. Cells may either accumulate DNA molecules that have been damaged by reactive oxygen species (ROS) or successfully repair them to their original state [184]. The psychrophilic strain Cryomyces antarcticus exhibited an increase in the GC content of its genes, as well as in the bases located at the third positions of codons, when subjected to extremely low temperatures during growth [172].
7. Cold Stress Response of Antarctic Fungi and Adaptation
We have summarized a compendium of research reports on the antioxidant cell response data for fungi with a geographical origin of Antarctica (Table 3).

Table 3.
Antioxidant cell response of Antarctic fungi.
7.1. Morphological Modification Caused by Cold Stress
The morphological adaptations of cold-adapted fungi are well-suited for surviving in challenging environmental conditions. The phenomenon of meristematic growth, which involves a shift toward isodiametric expansion, serves as a specific adaptation to stress, establishing a stable characteristic among fungi that thrive in extreme environments. These fungi demonstrate a high degree of adaptability to significant environmental stressors, particularly low temperatures, through mechanisms such as melanization and the promotion of meristematic development [185]. Such growth helps maintain an optimal volume-to-surface ratio, effectively limiting their interaction with external stressors [76,204]. Meristematic development of hyphae has been reported for black yeast-like fungus Exophiala dermatitidis [204,205], Friedmanniomyces endolithicus [185,206], rock-inhibiting Antarctic fungi [207], etc. Fourteen fungal species isolated from Livingston Island, Antarctica, demonstrated morphological peculiarities growing at low temperatures [47]. The prevalence of melanized species indicates the advantageous function of melanin in their survival in extreme conditions. It has been observed that certain colonies exhibit multicellular meristematic clusters, in addition to formations characterized by concentric rings that display variations in density and thickness. Furthermore, some colonies present rings composed of sterile mycelium. In certain instances, the colony surface is marked by irregularities, featuring convoluted wrinkles and pronounced radial furrows. The mycelium of Penicillium sp. 1-6-4 isolated at a temperature of 4 °C exhibited a significant presence of intercalary, swollen, and thick-walled cells that develop into chlamydospores, known as long-term survival structures. The significance of chlamydospores for survival at low temperatures has been evidenced in the case of the plant pathogen Zymoseptoria tritici [208]. In the microbial communities of soils found in the Antarctic, Arctic, and Alpine regions, fungi exhibiting dark septate hyphae are the most prevalent [6].
Considerable ultrastructural changes have been observed in the cells of P. griseofulvum following a short-term temperature downshift to 6 °C [188]. Analysis via transmission electron microscopy (TEM) indicated an increase in vacuolar volume, focal damage to the cell wall, and structural changes in the mitochondria. Moreover, ultrastructural findings revealed evidence of plasma membrane turnover, characterized by frequent deep infoldings of the plasmalemma that penetrate the cytoplasm, extending toward the center of the hyphae. The study of two Antarctic fungal strains under extreme cold conditions revealed that the mesophilic A. glaucus 363 exhibited more substantial ultrastructural changes in both its cells and conidia compared to the psychrotolerant P. commune 161 [187]. Scanning electron microscopy (SEM) results suggest that the psychrotolerant strain has a significantly enhanced ability to adapt and thrive in such harsh environments. This assertion is further substantiated by the findings of Sato et al. [209], who observed ultrastructural changes in the mitochondrial matrix and rupture of nuclear membranes in a temperature-sensitive mutant of Schizosaccharomyces pombe following a temperature decrease from 20 °C to 4 °C. A related trend has been identified in various studies. Psychrophilic yeasts of the genera Mrakia and Mrakiella, sourced from glacial regions in Antarctica and the Alps, reveal marked differences in their ultrastructure compared to mesophilic yeasts [186]. Onofri et al. [35] also observed changes in the morphological characteristics of Antarctic fungi subsequent to several freeze–thaw cycles.
7.2. Physiological Modification Caused by Cold Stress
It is evident that to overcome any type of stress, including cold stress, changes in cell physiology are required, which subsequently result in positive selection [7]. Adaptation at the physiological level may involve individual strategies or a combination of them. One of the initial responses to a reduction in the growth temperature of microorganisms is a cessation of growth. This is followed by an acclimation phase, which is a period of latency preceding the restoration of cell growth [210]. During this phase, there is a transient inhibition of the synthesis of the majority of cellular proteins, most likely at the level of translation.
A drastic effect of low temperatures on the growth of Antarctic fungal cultures was found in Pseudogymnoascus roseus [190], Mrakia blollopis SK-4 and TKG1-2 [50], and P. griseofulvum [171]. In addition, a decrease in biomass content has been documented in fungal strains isolated from soil samples in three areas of Antarctica: Casey Station, Terra Nova Bay, and South Georgia, due to both long-term and short-term exposure to low temperatures [38,87,170]. A comparative analysis of the cell response of Antarctic fungi to a reduction in temperature, focusing on two thermal classifications, psychrotolerant and mesophilic, indicated a complete cessation of growth or a marked reduction in biomass [163]. This effect was notably more significant in the mesophilic fungus than in the psychrophilic ones. A similar trend has been observed in the Antarctic strains P. olsonii p14 and P. waksmanii m12, as well as in the temperate strain P. rugulosum t35, when exposed to the temperature downshift during the stationary growth phase [52]. The reduction in biomass, known as cold-shock-induced autolysis, is linked to the activation of genes that encode peptidoglycan hydrolases, which facilitate the process of autolysis [210]. Similar findings have been reported for various microorganisms exposed to cold stress, such as Pichia pastoris [189], Volvariella volvacea [191], etc. The phenomenon of low-temperature autolysis coincided with reduced glucose consumption [37,52,163,171].
The findings presented by Chang et al. [211] indicated that the low-temperature autolysis in V. volvacea resulted from both intracellular and extracellular interactions. The authors proposed that the variations in metabolites and genes associated with malate metabolism played a role in supplying electrons to the electron transport chain. However, prolonged exposure to low temperatures led to an inadequate intracellular energy supply, ultimately resulting in cold-induced damage.
The accumulation of trehalose and glycogen in microbial cells under adverse growth conditions can serve as indicators of stress [11,212,213,214]. Trehalose is a vital storage compound found in both the vegetative cells and spores of fungi, and it is recognized as the most commonly occurring disaccharide among fungal species [6]. Its role as a universal stress protectant in the cytosol is significant, particularly in its ability to stabilize membranes during episodes of dehydration [214]. The stabilizing properties of trehalose can be attributed to the formation of a strong hydrogen bonding network that links biomolecules to the sugar–water matrix [215]. This connection inhibits the unfolding of biomolecules, as it involves a reconfiguration of the surrounding medium.
Glycogen serves as the common carbohydrate storage and energy reservoir in fungi, especially during periods of stress. This polysaccharide consists of glucose units connected by α, 1–4 glycosidic bonds, with additional α, 1–6 branching points. The metabolism of glycogen is governed by a complex network of interactions involving numerous genes and biochemical pathways. Key to this metabolic process are five critical enzymes: ADP-glucose pyrophosphorylase (GlgC), glycogen synthase (GlgA), glycogen branching enzyme (GlgB), glycogen phosphorylase (GlgP), and glycogen debranching enzyme (GlgX) [216,217]. Studies have shown that the activation of genes responsible for glycogen synthesis and degradation can occur simultaneously, which may lead to no net increase in glycogen within cells exposed to diverse forms of stress, such as heat shock [213,216]. Although this transcription pattern is also observed when subjected to low temperatures, the results demonstrate a substantial accumulation of glycogen in yeast and fungal cells.
Cold stress led to the accumulation of trehalose and glycogen and triggered the pathways involved in their biosynthesis [11]. The evidence for the increased content of these carbohydrates in response to low-temperature treatment of many fungal species was observed [218]. As an illustration, the Antarctic fungi P. commune 161 and A. glaucus 363 [88,163], as well as P. griseofulvum [171], demonstrated enhanced glycogen and trehalose content when exposed to both short-term and long-term cold stress. In addition, the Antarctic fungal strains P. olsonii p14 and P. waksmanii m12 demonstrated a significant increase in intracellular trehalose and glycogen levels after being subjected to low temperatures. It is noteworthy that exponentially growing cells experienced a more pronounced enhancement in reserve carbohydrate levels than their counterparts in the stationary growth phase [52]. In contrast, the late cold response in S. cerevisiae triggered the accumulation of the carbohydrate reserves trehalose and glycogen [173].
Furthermore, the intracellular content of trehalose was significantly greater in the temperature-tolerant strain V. volvacea (VH3) relative to the temperature-sensitive strain V. volvacea (V23) when both were grown at optimal temperatures [192]. During exposure to low-temperature stress, the expression levels of the trehalose-6-phosphate phosphatase (TPP) gene and the trehalose phosphorylase (TP) gene in VH3 were found to be significantly higher than those in V23. The authors indicated that the TP gene might be a pivotal component of trehalose metabolism, potentially promoting trehalose synthesis in response to low-temperature stress.
7.3. Metabolic Adaptation Response
In recent years, there has been a significant rise in the volume of research focused on the effect of low temperature on protein modifications through the application of proteomic methodologies [11]. Research conducted by Tesei et al. [155] on the proteomic profiles of fungi that inhabit black rocks under conditions of low-temperature stress demonstrated an increase in the total number of protein spots. The findings revealed significant changes in high molecular mass protein spots, particularly those ranging from 70 to 170 kDa, in the species Friedmanniomyces endolithicus and Coniosporium perforans. Conversely, the analysis of Exophiala jeanselmei exhibited alterations in protein patterns at a lower molecular mass range of 25 to 100 kDa. Exposure of fungus E. dermatitidis to low-temperature stress resulted in a decline in proteins associated with metabolic activity, particularly those involved in general carbon metabolism. A comprehensive array of proteins engaged in energy metabolism pathways underwent down-regulation, encompassing malate synthase, malate dehydrogenase, acetyl-coenzyme A synthetase, and glyceraldehyde-3-phosphate dehydrogenase [219]. The proteomic study on the low-temperature stress response indicated a positive correlation between protein levels and transcription levels in certain pathways due to an increase in chaperones and energy metabolism pathways. As an illustration, a comparable modification in protein profiles was observed in response to both short-term and long-term low-temperature exposure to Flammulina velutipes [156]. Furthermore, Abu Bakar et al. [154] demonstrated a substantial modification in the levels of various metabolic enzymes and ribosomal proteins in Antarctic isolates of Pseudogymnoascus spp. Through pathway enrichment analysis, the authors further demonstrate diversity in the pathways associated with cold stress responses, particularly those related to metabolism and translation processes. Of interest are the studies conducted by Chen et al. [183], which reported for the first time the involvement of tryptophan-based metabolic reprogramming in the response of T. dupontii to cold stress. The response mechanisms of the psychrophilic strain Mrakia psychrophile [182], Flammulina velutipes [156], and Mortierella isabellina [157] to low-temperature stress encompass several physiological changes. These include a desaturation of fatty acids, an accumulation of glycerol, an increase in the expression of glutamine synthetase and MFS transport proteins, and a decrease in the expression levels of citrate synthase, succinyl Co-A ligase, pyruvate decarboxylase, and ribosomal proteins.
The impact of a significant reduction in growth temperature on carbon metabolism has been investigated in two Antarctic strains, one psychrotolerant and one mesophilic, alongside a temperate mesophilic strain [165]. Findings indicated a pronounced elevation in the activity of hexokinase (HK), the enzyme that initiates glycolysis. A similar increase was noted for glucose-6-phosphate dehydrogenase (GPDH), which catalyzes the initial reaction in the pentose phosphate pathway (PPP), as well as glyceraldehyde 3-phosphate dehydrogenase (GAPDH), which plays a crucial role in energy production. A key conclusion drawn from this study is that low-temperature stress prompts a shift in carbohydrate metabolism from glycolysis toward the PPP, contributing to the survival mechanisms at suboptimal temperatures. Additionally, it is noteworthy that the enhancement in the activity of these three enzymes has been markedly more pronounced in cells during the exponential growth phase compared to those in the stationary phase.
It should be noted that the short-term cold stress (4 °C) applied to Antarctic fungi Penicillium sp. 161 and A. glaucus 363 resulted in the re-routing of carbon metabolism away from glycolysis into the PPP, serving as a stress-resistance mechanism [220]. Additionally, the stress response encompasses changes in the enzymatic activity of the TCA cycle, which acts as a major supplier of precursors, thereby facilitating adaptation to cold stress [165,220].
The ability of fungi to withstand cold temperatures is accelerated by the production of various extracellular hydrolases. Notably, chitinolytic enzymes play a crucial role in the development of the cell wall [48]. Additionally, lipases contribute to the metabolism of fatty acids [221], while proteases facilitate the breakdown of damaged proteins [193]. The response to low-temperature stress in two Antarctic strains is characterized by a multifaceted activation of both biosynthetic and trehalose-degrading pathways, albeit to varying degrees [187]. Notably, the increase in trehalose-6-phosphate synthetase (TPS) during stress is significantly greater than that of neutral trehalase (NT). This disparity results in a substantial accumulation of trehalose during the stress period. Following the alleviation of stress, there is a marked decline in TPS activity, while NT levels remain stable. Consequently, the intracellular concentration of trehalose is determined by a meticulously regulated equilibrium between the expression of synthetic and degradative enzymes [222].
7.4. Cold-Active Enzymes
The survival of fungal strains in conditions of extremely low temperatures is also promoted by so-called cold-active enzymes, which favor their metabolic processes, substrate uptake, development, and reproduction [6]. These enzymes are known to increase the affinity for the substrates by decreasing the activation energy required for the enzyme–substrate complex formation. This is facilitated by a greater flexibility in the enzyme’s structure, either in part or as a whole, which enhances the rate of enzymatic reactions at lower temperatures, thereby contributing to reduced energy usage [194].
The International Commission of Enzyme Nomenclature classified the cold-active enzymes that have been discovered thus far into six principal categories: hydrolases, oxidoreductases, transferases, lyases, isomerases, and synthetases. Duarte et al. [9] reviewed the ability of fungi and yeasts from terrestrial and marine Antarctic settings to synthesize such enzymes as part of their survival strategy. The authors presented a wide variety of fungal genera belonging to the phyla Ascomycota and Basidiomycota, producing enzymes with a low temperature optimum. The analysis encompassed a variety of hydrolases, such as α-amylase, cellulase, chitinase, glucosidase, invertase, lipase, pectinase, phytase, protease, subtilase, tannase, and xylanase, in addition to oxidoreductases, specifically laccase and superoxide dismutase.
Fungi from Antarctic soil and moss samples obtained from various sites in Victoria Land (continental Antarctica) were evaluated for their ability to produce cold-active enzymes, such as lipases, polygalacturonase, pectin lyase, amylase, cellulase, chitinase, phosphatase, glucose oxidase, urease, protease, and RNase [46]. Among these, the psychrotolerant strain of Lecanicillium muscarium (CCFEE 5003) emerged as a notable candidate for the production of cold-tolerant extracellular enzymes, particularly those that hydrolyze chitin.
A total of 27 filamentous fungi were isolated from marine sponges obtained from King George Island, Antarctica, and subsequently evaluated as potential sources of cold-active pectinases [223]. Of these, eight demonstrated pectinolytic activity at a temperature of 15 °C. The most effective producer identified was Geomyces sp. F09-T3-2, which exhibited optimal enzymatic activity at a temperature that is 10 °C lower than that of commercially available mesophilic enzymes. Singh et al. [224] identified the psychrophilic fungal strain Truncatella angustata BPF5 as a promising candidate for the production of pectinase, which holds potential applications in the wine industry. The presence of cold-active enzymes, including SOD [225], catalase [51,53], sialidase [226], lipases [227], invertase [228], chitinolytic enzymes [48], etc., has been detected in fungal strains isolated from Antarctica. The application of metagenomic techniques facilitates the discovery of a greater number of cold-active enzymes [229].
8. Antioxidant Defence in Antarctic Fungi
8.1. Enzymatic Antioxidant Defense
Accelerated ROS production in Antarctic fungi induced by cold stress activates their antioxidant enzyme defense mechanisms. Their ability to survive in extremely cold ecosystems is associated with a cellular response that includes increased levels of SOD, CAT, and GPx. In particular, psychrophilic and psychrotolerant fungal strains isolated from Antarctica show higher enzyme activities compared to mesophilic strains from the same region [52,163]. In contrast, the situation of a drastic decrease in growth temperature led to a more significant increase in enzyme activity in mesophilic Antarctic strains than in their psychrophilic counterparts. Enhanced enzymatic antioxidant defense has also been observed in Antarctic strains P. olsonii [170], P. griseofulvum [171], and A. fumigatus I-9 [167]. Of interest are transcriptomic studies of a psychrophilic strain of M. psychrophila, which showed a lack of up-regulation of genes responsible for SOD synthesis at temperatures of 4 °C and 20 °C. Thirty microbial strains isolated from the Bulgarian Antarctic base “St. Kliment Ohridski” on Livingston Island were screened for the synthesis of antioxidant enzymes, specifically SOD and CAT [43]. Species belonging to the genera Cladosporium, Penicillium, Monodictys, Geomyces, Aspergillus, Alternaria, Lecanicillium, Epicoccum, and Rhizopus have been included in the study. The analysis revealed that six of these strains exhibited significantly higher enzyme activity at 15 °C than at 30 °C, with A. glaucus strain 363 being recognized as the most proficient producer of cold-active SOD.
An up-regulation of the CAT gene has been observed in the psychrophilic yeast strain Rhodotorula sp. (USM-PSY62) when subjected to a temperature downshift to 0 °C. This phenomenon coincides with a rise in ROS concentration and variations in the antioxidant defense mechanisms [196]. Cold-adapted fungi belonging to the species Chrysosporium pannosum, Cylindrocarpon magnusianum, Mortierella minutissima, P. chrysogenum, P. cyclopium, and P. islandicum exhibited increased production of extra- and intracellular CAT at 15 °C compared to 20 °C [195]. Arbuscular mycorrhizal fungi (AMF) have been observed to respond to cold stress by increasing the levels of several antioxidant enzymes, including SOD, CAT, ascorbate peroxidase (APX), and glutathione reductase (GR) [230]. The gpx gene, essential for GPx synthesis in V. volvacea, showed expression levels critical for fruiting body formation under low-temperature conditions [197]. Conversely, overexpression of this gene has been shown to enhance fruiting body regeneration in degenerated strains of V. volvacea T19. Transcriptomic analysis of A. alternata revealed that exposure to cold stress significantly altered its transcriptional profile, particularly affecting the glutathione and thioredoxin systems [231].
8.2. Non-Enzymatic Antioxidants
In addition to the production of antioxidant enzymes, fungi have been shown to synthesize a wide variety of molecules with antioxidant and scavenging properties. For example, marine fungi produce phenolic compounds, anthraquinones, xanthones, carotenoids, indole derivatives, and alkaloids that participate in their cold stress response [200,232]. Magi et al. [181] reported that the content of phenolic compounds increased under low-temperature conditions, thereby facilitating their ROS scavenging activity. Furthermore, the melanin present in the cells of Antarctic fungi demonstrated remarkable stability as well as significant antioxidant properties, which included a robust ability to scavenge DPPH free radicals [6]. It plays a critical role in protecting injured cells by reducing the presence of ROS. The function of melanin in protecting against cold stress has also been established in Cryptococcus neoformans [233] and Cryomyces antarcticus [201]. In addition, the elevated carotenoid biosynthesis exhibited a concomitant correlation with the adaptation to low temperatures in the Antarctic yeast Rhodosporidium kratochvilovae [166] and rock-inhabiting fungus Knufia petricola A95 [202]. The cold adaptation in Antarctic yeasts, isolated from soils in King George Island, correlated with the production of freeze proteins and ergosterols [169].
8.3. The Role of Cold Shock Proteins (CSPs)
Upon exposure to cold temperatures, fungal cells quickly trigger the production of a substantial number of cold shock proteins (CSPs). These proteins play a crucial role in transcription and translation mechanisms [173]. In eukaryotes, CSPs are incorporated as cold shock domains in multidomain proteins, distinguished by their variable sequences at the N- and C-terminal regions. While the function of CSPs in the adaptation of bacteria to low-temperature environments is well understood, the function of cold shock domains in eukaryotes remains to be fully elucidated [234]. Furthermore, there is a paucity of information regarding the contributions of these proteins to fungal growth and survival in low-temperature environments. Louet et al. [234] found that the up-regulation of CSP genes in the rust fungus Melampsora larici-populina significantly bolstered its cold stress tolerance. CSPs have been identified in A. clavatus and Metarhizium anisopliae growing under conditions of extremely low temperatures [235]. The study of Keto-Timonen et al. [236] provides a comprehensive overview of the functions of CSP-family proteins, emphasizing their significance in the stress response mechanisms of enteropathogenic Yersinia. In the context of budding yeast cultures, the identification of two distinct groups of genes encoding CSPs has been made. These groups are designated as the “early response to low temperatures” group and the “late response” group. The “early response to low temperatures” group is associated with transcription, transport, and the metabolism of lipids and fatty acids. The second group of genes encodes proteins that support carbohydrate metabolism and the metabolism of trehalose and glycogen. The results of this study demonstrate that yeast cells employ two distinct programs in their response to low temperatures, thereby enhancing their capacity to adapt [173].
9. Genetic Basis of Cell Response to Cold Stress
Research on mycoflora in Antarctica has identified a limited number of endemic psychrophilic species, while the predominant findings indicate the presence of cosmopolitan psychrotolerant taxa [84]. The endemic species have been described as genuine psychrophilic fungi, which can only thrive and reproduce in particular Antarctic conditions [25]. On the other hand, cosmopolitan psychrotolerant fungi are ecotypes that exhibit mesophilic–psychrotolerant behavior, which has developed as an adaptation to the cold Antarctic climate [24]. Various species of the genera Penicillium, Aspergillus, Cladosporium, Colletotrichum, Fusarium, and Rhodotorula, among others, isolated from Antarctica, are characteristic of temperate environments. Robinson [6] suggested that fungi in Arctic and Antarctic regions might employ a cold avoidance strategy through recolonization in the spring, utilizing spores or hyphal fragments that have been transported from warmer areas. In addition to the morphological, physiological, and metabolic mechanisms of response against oxidative stress noted above, researchers are looking for molecular genetic evidence for the cold tolerance of such species. Zakharova et al. [237] reported that the Antarctic rock-inhabiting fungus Cryomyces antarcticus, one of the most stress-resistant eukaryotic microorganisms, transcribes a limited part of its genome as a strategy for cold adaptation. Probably, a significant portion of its genome has been silenced due to an ‘energy conservation mode’. The identified proteins are predominantly derived from highly conserved protein families that are indispensable for the maintenance of central metabolism, which is critical for growth and recovery after dormancy [238]. A set of novel proteins, characterized by their remarkable resistance to stress, has been evaluated [239]. Additionally, the first assessment of the draft genome did not uncover any substantial variations in the C. antarcticus genome relative to comparative species and mesophilic hyphomycetes [240].
Tesei et al. [156] discovered that a significant decrease in temperature (up to 1 °C) resulted in an increase in the number of proteins in three extremotolerant black fungi: Coniosporium perforans, Exophiala jeanselmei, and Friedmanniomyces endolithicus. This increase was not observed in the mesophilic hyphomycete P. chrysogenum. These variations in the cell response to temperature stress could indicate a distinct strategy to survive suboptimal temperatures.
The data on six Pseudogymnoascus spp., collected from different biogeographical regions, presented novel findings regarding the reactions to temperature changes in their habitats [241]. The proteomic study revealed no definitive correlation between protein alterations and the geographical backgrounds of the strains. At the same time, gene ontology analysis indicated a significant presence of proteins associated with various protective strategies, such as the maintenance of protein homeostasis, the regulation of energy metabolism, and the initiation of DNA damage response and repair mechanisms.
The research of Gomez-Gutiérrez et al. [173] extended the genetic basis of fungal cell response to oxidative stress. Stajich [242] emphasized the relationship between the ecological niche adaptation and the evolution of the fungal genome. The expansion or contraction of orthologous protein families has been described as one of the most straightforward mechanisms by which organisms adapt to harsh environments. Similar gene modifications contributing to the evolution of fungal genomes have been published for the microcolonial black fungus Saxispiralis lemnorum MUM 23.14 [243], Cryptococcus neoformans [244], Mrakia psychrophila [183], etc. The transcriptomic analysis of M. psychrophila grown at 4 °C demonstrated up-regulation of the genes responsible for energy metabolism [183]. At the same time, other genes such as those involved in protein binding, proteasome, spliceosome, and mRNA surveillance were up-regulated at 20 °C. However, further investigation is required to establish the role of fungal gene evolution in the capacity of fungi to thrive under extreme conditions.
10. Concluding Remarks
In recent years, survival in extreme cold temperatures has sparked growing interest in the biodiversity, ecology, and biotechnological potential of Antarctic microorganisms. Furthermore, they are the subject of exobiology as a unique model for evolutionary studies. Several scientists point out the similarity between the conditions of existence of microorganisms in some regions of Antarctica (e.g., deep ice, rock crevices, frozen soil, etc.) and those on the planet Mars. The Antarctic cold, arid climate, and remoteness from the rest of the Earth’s surface are responsible for the preservation of many life forms untouched by human civilization. In addition, low temperatures, short summers, high concentrations of salts and UV radiation, and many other factors have a significant impact on the type of microorganisms that survive in this place.
Despite the extremely negative effect of cold temperatures on biochemical reactions, Antarctic fungi grow at rates similar to those achieved by related species living in temperate climates. This tolerance in conditions of extremely low temperatures is related to the cellular response to oxidative stress induced by the available abiotic factors. Antarctic fungi demonstrate the formation of ROS even at an optimal growth temperature, which is adequate for their survival mechanisms. Lowering the temperature, however, causes accelerated generation of ROS, which leads to the realization of oxidative stress. Data on the induction of oxidative stress in Antarctic fungi after exposure to low temperatures and their response are very scarce. Even less studied are the fungi isolated from the area of the Bulgarian base “St. Kliment Ohridski” on Livingston Island. This review presents data on the biodiversity of filamentous fungi in the Antarctic region and their tolerance to low temperatures.
As is clear from this review, cold stress causes significant oxidative damage to all cellular molecules in Antarctic fungi, such as lipids, proteins, and DNA. This results in substantial changes in the structure and fluidity of cell membranes, modifications to the structures of enzymes and other functional proteins, changes in the profile of bases in the DNA molecule, breakage of DNA chains, and numerous additional effects.
In the course of their evolution, Antarctic fungi have developed efficient survival mechanisms in harsh conditions, specifically at extremely low temperatures. Their survival strategies encompass modifications in cell and colony morphology, such as melanization, and the promotion of meristematic development. The literature documents significant ultrastructural changes, including an increase in vacuolar volume, focal damage to the cell wall, rupture of nuclear membranes, and structural changes in the mitochondria. The physiological adaptation of these organisms may encompass a variety of individual strategies or a synthesis of multiple approaches. Growth retardation, including a decrease in the amount of biomass, has been observed to occur as a result of the cold-shock-induced autolysis process. Furthermore, under cold stress conditions, it has been observed that the metabolic pathways of reserve carbohydrate synthesis and stress protectants trehalose and glycogen are triggered, resulting in their accumulation within the cells. Cold tolerance is facilitated by the presence of cold-active enzymes, which enhance metabolic activities, substrate assimilation, growth, and reproduction of these organisms.
In this review, we have also focused on the antioxidant defense mechanisms employed by Antarctic fungi. They promptly identify and respond to the changes in the level of ROS by inducing protective enzymes that counteract oxidative damage (SOD, CAT, GPx) as well as non-enzyme antioxidants. A significant research direction pertains to the comparison of the cellular response to oxidative stress in strains isolated from Antarctica and those from temperate habitats. The acquisition of such data may contribute to the elucidation of the cold-adapted mechanism.
The knowledge about the cold-stress response of Antarctic fungi is increasing rapidly, and, in the future, studies should be focused on the significance of combined stress exposure.
Author Contributions
Conceptualization, M.A.; methodology, E.K., R.A., J.M.-S., V.D., and B.S.; software, J.M.-S. and G.S; validation, R.A. and Y.G.; formal analysis, M.A.; investigation, E.K. and R.A.; resources, E.K., R.A., J.M.-S., Y.G., G.S., V.D., and B.S.; data curation, R.A.; writing—original draft preparation, M.A.; writing—review and editing, M.A.; visualization, R.A.; supervision, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Bulgarian Ministry of Education and Science through the National Centre for Polar Studies, and Sofia University “St. Kliment Ohridski” in the framework of the National Program for Polar Studies 2022–2025.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yaakoub, H.; Mina, S.; Calenda, A.; Bouchara, J.P.; Papon, N. Oxidative stress response pathways in fungi. Cell. Mol. Life Sci. 2022, 79, 333. [Google Scholar] [CrossRef]
- Kochkina, G.; Ivanushkina, N.; Ozerskaya, S.; Chigineva, N.; Vasilenko, O.; Firsov, S.; Spirina, E.; Gilichinsky, D. Ancient fungi in Antarctic permafrost environments FEMS Microb. Ecol. 2012, 82, 501–509. [Google Scholar] [CrossRef]
- Gostinčar, C.; Gunde-Cimerman, N. Understanding fungi in glacial and hypersaline environments. Annu. Rev. Microbiol. 2023, 77, 89–109. [Google Scholar] [CrossRef]
- Son, Y.; Min, J.; Shin, Y.; Park, W. Morphological and physiological adaptations of psychrophilic Pseudarthrobacter psychrotolerans YJ56 under temperature stress. Sci. Rep. 2023, 13, 14970. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, I.; Valdés-Muñoz, G.; Moreno-Perlin, T.; Mouriño-Pérez, R.R.; del Rayo Sánchez-Carbente, M.; Folch-Mallol, J.L.; Pérez-Llano, Y.; Gunde-Cimerman, N.; del C. Sánchez, N.; Batista-García, R.A. Haloadaptative Responses of Aspergillus sydowii to Extreme Water Deprivation: Morphology, Compatible Solutes, and Oxidative Stress at NaCl Saturation. J. Fungi 2020, 6, 316. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.H. Cold adaptation in Arctic and Antarctic fungi. New Phytol. 2001, 151, 341–353. Available online: http://www.jstor.org/stable/1353786 (accessed on 30 April 2025). [CrossRef]
- Branco, S.; Schauster, A.; Liao, H.L.; Ruytinx, J. Mechanisms of stress tolerance and their effects on the ecology and evolution of mycorrhizal fungi. New Phytol. 2022, 235, 2158–2175. [Google Scholar] [CrossRef]
- McCord, J.M. The Evolution of Free Radicals and Oxidative Stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Duarte, A.W.F.; dos Santos, J.A.; Vianna, M.V.; Vieira, J.M.F.; Mallagutti, V.H.; Inforsato, F.J.; Wentzel, L.C.P.; Lario, L.D.; Rodrigues, A.; Pagnocca, F.C.; et al. Cold-adapted enzymes produced by fungi from terrestrial and marine Antarctic environments. Crit. Rev. Biotechnol. 2018, 38, 600–619. [Google Scholar] [CrossRef]
- Gessler, N.N.; Averyanov, A.A.; Belozerskaya, T.A. Reactive Oxygen Species in Regulation of Fungal Development. Biochemistry 2007, 72, 1091–1109. [Google Scholar] [CrossRef]
- Abu Bakar, N.; Karsani, S.A.; Alias, S.A. Fungal survival under temperature stress: A proteomic perspective. PeerJ 2020, 15, e10423. [Google Scholar] [CrossRef] [PubMed]
- Bridge, P.D.; Spooner, B.M. Non-lichenized Antarctic fungi: Transient visitors or members of a cryptic ecosystem? Fungal Ecol. 2012, 5, 381–394. [Google Scholar] [CrossRef]
- Bommer, E.; Rousseau, M. Champignons in resultats du voyage du SY Belgica, expedition Antarctique Beige 1897–1899. Rapp. Sci. Botan. 1905, 4, 1–15. [Google Scholar]
- Brown, R.N.R. Antarctic botany: Its present state and future problems. Scottish Geogr. Mag. 1906, 22, 473–484. [Google Scholar] [CrossRef]
- Nikitin, D.A. Ecological Characteristics of Antarctic Fungi. Dokl. Biol. Sci. 2023, 508, 32–54. [Google Scholar] [CrossRef]
- Pugh, G.J.F.; Allosopp, D. Microfungi on Signy Island, South Orkney Islands. Br. Antarct. Surv. Bull. 1982, 57, 55–67. [Google Scholar] [CrossRef]
- Fletcher, L.D.; Kerry, E.J.; Weste, G.M. Microfungi of Mac Robertson and Enderby Lands, Antarctica. Polar. Biol. 1985, 4, 81–88. [Google Scholar] [CrossRef]
- Onofri, S.; Tosi, S. II contributo della micologia alla IV spedizione italiana in Antartide. Micol. Veget. Medit. 1989, 4, 57–62. [Google Scholar]
- Onofri, S.; Tosi, S. Arthrobotrys ferox sp. nov. a springtail-capturing hyphomycete from continental Antarctica. Mycotaxon 1992, 44, 445–451. Available online: http://hdl.handle.net/2067/30394 (accessed on 1 January 1992).
- Rosa, L.; Vaz, A.; Caligiorne, R.; Campolina, S.; Rosa, C. Endophytic fungi associated with the Antarctic grass Deschampsia antarctica Desv. (Poaceae). Polar Biol. 2009, 32, 161–167. [Google Scholar] [CrossRef]
- Kochkina, G.A.; Ozerskaya, S.M.; Ivanushkina, N.E.; Chigineva, N.I.; Vasilenko, O.V.; Spirina, E.V.; Gilichinskii, D.A. Fungal diversity in the Antarctic active layer. Microbiology 2014, 83, 94–101. [Google Scholar] [CrossRef]
- Wynn-Williams, D.D. Aerobiology and colonization in Antarctica—The BIOTAS Programme. Grana 1991, 30, 380–393. [Google Scholar] [CrossRef]
- Möller, C.; Dreyfuss, M.M. Microfungi from antarctic lichens, mosses and vascular plants. Mycologia 1996, 88, 922–933. [Google Scholar] [CrossRef]
- Zucconi, L.; Pagano, S.; Fenice, M.; Selbmann, L.; Tosi, S.; Onofri, S. Growth temperature preferences of fungal strains from Victoria Land, Antarctica. Polar Biol. 1996, 16, 53–61. [Google Scholar] [CrossRef]
- Ruisi, S.; Barreca, D.; Selbmann, L.; Zucconi, L.; Onofri, S. Fungi in Antarctica. Rev. Environ. Sci. Biotechnol. 2007, 6, 127–141. [Google Scholar] [CrossRef]
- Selbmann, L.; Onofri, S.; Coleine, C.; Buzzini, P.; Canini, F.; Zucconi, L. Effect of environmental parameters on biodiversity of the fungal component in lithic Antarctic communities. Extremophiles 2017, 21, 1069–1080. [Google Scholar] [CrossRef]
- Gomes, E.C.Q.; Figueredo, H.M.; de Oliveira, F.S.; Schaefer, C.E.G.R.; Michel, R.F.; Rosa, C.A.; Rosa, L.H. Fungi present in soils of Antarctica. In Fungi of Antarctica; Rosa, L.H., Ed.; Springer: Cham, Switzerland, 2019; pp. 43–67. ISBN 978-3-030-18366-0. [Google Scholar]
- Nikitin, D.A.; Marfenina, O.E.; Kudinova, A.G.; Lysak, L.V.; Mergelov, N.S.; Dolgikh, A.V.; Lupachev, A.V. Microbial Biomass and Biological Activity of Soils and Soil-Like Bodies in Coastal Oases of Antarctica. Eur. Soil Sci. 2017, 50, 1086–1097. [Google Scholar] [CrossRef]
- Connell, L.B.; Redman, R.; Craig, S.D.; Scorzetti, G.; Iszard, M.; Rodriguez, R. Diversity of soil yeasts isolated from South Victoria Land, Antarctica. Microb. Ecol. 2008, 56, 448–459. [Google Scholar] [CrossRef]
- Paterson, R.A. The occurrence and distribution of some aquatic phycomycetes on Ross Island and the dry valleys of Victoria Land, Antarctica. Mycologia 1973, 65, 373–387. [Google Scholar] [CrossRef]
- Lawley, B.; Ripley, S.; Bridge, P.; Convey, P. Molecular analysis of geographic patterns of eukaryotic diversity in Antarctic soils. Appl. Environ. Microbiol. 2004, 70, 5963–5972. [Google Scholar] [CrossRef]
- dos Santos, J.A.; Meyer, E.; Sette, L.D. Fungal Community in Antarctic Soil Along the Retreating Collins Glacier (Fildes Peninsula, King George Island). Microorganisms 2020, 8, 1145. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C. Fungi in cold ecosystems. In Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Schinner, F., Marx, J.C., Gerday, C., Eds.; Springer: Berlin, Germany, 2008; pp. 137–156. [Google Scholar]
- Hassan, N.; Rafiq, M.; Hayat, M.; Shah, A.A.; Hasan, F. Psychrophilic and psychrotrophic fungi: A comprehensive review. Rev. Environ. Sci. Bio/Tech. 2016, 15, 147–172. [Google Scholar] [CrossRef]
- Onofri, S.; Selbmann, L.; de Hoog, G.S.; Grube, M.; Barreca, D.; Ruisi, S.; Zucconi, L. Evolution and adaptation of fungi at boundaries of life. Adv. Space Res. 2007, 40, 1657–1664. [Google Scholar] [CrossRef]
- Marfenina, O.E.; Nikitin, D.A.; Ivanova, A.E. The Structure of Fungal Biomass and Diversity of Cultivated Micromycetes in Antarctic Soils (Progress and Russkaya Stations). Eurasian Soil Sci. 2016, 49, 934–941. [Google Scholar] [CrossRef]
- Tsuji, M. Survey on Fungi in Antarctica and High Arctic Regions, and Their Impact on Climate Change. Climate 2023, 11, 195. [Google Scholar] [CrossRef]
- Gocheva, Y.G.; Krumova, E.; Slokoska, L.S.; Gesheva, V.; Angelova, M. Isolation of filamentous fungi from Antarctica. Compt. Rend. Acad bulg. Sci. 2005, 58, 403–408. [Google Scholar]
- Kochkina, G.; Ivanushkina, N.; Lupachev, A.; Starodumov, I.; Vasilenko, O.; Ozerskaya, S. Diversity of mycelial fungi in natural and human-affected Antarctic soils. Polar Biol. 2019, 42. [Google Scholar] [CrossRef]
- Abrashev, R.; Krumova, E.; Petrova, P.; Eneva, R.; Kostadinova, N.; Miteva-Staleva, J.; Engibarov, S.; Stoyancheva, G.; Gocheva, Y.; Kolyovska, V.; et al. Distribution of a novel enzyme of sialidase family among native filamentous fungi. Fungal Biol. 2021, 125, 412–425. [Google Scholar] [CrossRef]
- Devoto, B.T.; Toscanini, M.A.; Hermida Alava, K.; Etchecopaz, A.N.; Pola, S.J.; Martorell, M.M.; Ansaldo, M.; Negrete, J.; Ruberto, L.; Mac Cormack, W.; et al. Exploring fungal diversity in Antarctic wildlife: Isolation and molecular identification of culturable fungi from penguins and pinnipeds. N. Z. Vet. J. 2022, 70, 263–272. [Google Scholar] [CrossRef]
- Zucconi, L.; Selbmann, L.; Buzzini, P.; Turchetti, B.; Guglielmin, M.; Frisvad, J.C.; Onofri, S. Searching for eukaryotic life preserved in Antarctic permafrost. Polar Biol. 2012, 35, 749–757. [Google Scholar] [CrossRef]
- Tosi, S.; Kostadinova, N.; Krumova, E.; Pashova, S.; Dishliiska, V.; Spassova, B.; Vassilev, S.; Angelova, M. Antioxidant enzyme activity of filamentous fungi isolated from Livingston Island, Maritime Antarctica. Polar Biol. 2010, 33, 1227–1237. [Google Scholar] [CrossRef]
- de Menezes, G.C.A.; Godinho, V.M.; Porto, B.A.; Goncalves, V.N.; Rosa, L.H. Antarctomyces pellizariae sp. nov., a new, endemic, blue, snow resident psychrophilic ascomycete fungus from Antarctica. Extremophiles 2017, 21, 259–269. [Google Scholar] [CrossRef]
- Gonçalves, V.N.; Vaz, A.B.; Rosa, C.A.; Rosa, L.H. Diversity and distribution of fungal communities in lakes of Antarctica, FEMS Microb. Ecol. 2012, 82, 459–471. [Google Scholar] [CrossRef]
- Fenice, M.; Selbmann, L.; Zucconi, L.; Onofri, S. Production of extracellular enzymes by Antarctic fungal strains. Polar Biol. 1997, 17, 275–280. [Google Scholar] [CrossRef]
- Kostadinova, N.; Krumova, E.; Tosi, S.; Pashova, S.; Angelova, M. Isolation and identification of filamentous fungi from Livingston Island, Antarctica. Biotechnol. Biotechnol. Equip. 2009, 23, 10. [Google Scholar] [CrossRef]
- Fenice, M. The psychrotolerant antarctic fungus Lecanicillium muscarium CCFEE 5003: A powerful producer of cold-tolerant chitinolytic enzymes. Mollecules 2016, 21, 447. [Google Scholar] [CrossRef]
- Figueredo, H.M.; Gonçalves, V.N.; Godinho, V.M.; Lopes, D.V.; Oliveira, F.S.; Rosa, L.H. Diversity and ecology of cultivable fungi isolated from the thermal soil gradients in Deception Island, Antarctica. Extremophiles 2020, 24, 219–225. [Google Scholar] [CrossRef]
- Tsuji, M. Cold-stress responses in the Antarctic basidiomycetous yeast Mrakia blollopis. R. Soc. Open Sci. 2016, 3, 160106. [Google Scholar] [CrossRef]
- Krumova, E.; Abrashev, R.; Dishliyska, V.; Stoyancheva, G.; Kostadinova, N.; Miteva-Staleva, J.; Spasova, B.; Angelova, M. Cold-active catalase from the psychrotolerant fungus Penicillium griseofulvum. J. Basic Microbiol. 2021, 61, 782–794. [Google Scholar] [CrossRef]
- Miteva-Staleva, J.G.; Krumova, E.T.; Vassilev, S.V.; Angelova, M.B. Cold-stress response during the stationary-growth phase of Antarctic and temperate-climate Penicillium strains. Microbiology 2017, 163, 1042–1051. [Google Scholar] [CrossRef]
- Koleva, Z.; Abrashev, R.; Angelova, M.; Stoyancheva, G.; Spassova, B.; Yovchevska, L.; Dishliyska, V.; Miteva-Staleva, J.; Krumova, E. A novel extracellular catalase produced by the Antarctic filamentous fungus Penicillium rubens III11-2. Fermentation 2024, 10, 58. [Google Scholar] [CrossRef]
- Gonçalves, V.N.; Campos, L.S.; Melo, I.S.; Pellizari, V.H.; Rosa, C.A.; Rosa, L.H. Penicillium solitum: A mesophilic, psychrotolerant fungus present in marine sediments from Antarctica. Polar Biol. 2013, 36, 1823–1831. [Google Scholar] [CrossRef]
- Gomes, E.C.Q.; Gonçalves, V.N.; da Costa, M.C.; de Freitas, G.J.C.; Santos, D.A.; Johann, S.; Oliveira, J.B.S.; da Paixão, T.A.; Convey, P.; Rosa, L.H. Pathogenicity of psychrotolerant strains of Antarctic Pseudogmynoascus fungi reveals potential opportunistic profiles. Microbe 2024, 5, 100186. [Google Scholar] [CrossRef]
- Singh, S.M.; Puja, G.; Bhat, D.J. Psychrophilic fungi from Schirmacher Oasis, East Antarctica. Curr. Sci. 2006, 90, 1388–1392. Available online: https://www.jstor.org/stable/24091989 (accessed on 25 May 2006).
- de Souza, L.M.D.; Lirio, J.M.; Coria, S.H.; Lopes, F.A.C.; Convey, P.; Carvalho-Silva, M.; de Oliveira, F.S.; Rosa, C.A.; Câmara, P.E.A.S.; Rosa, L.H. Diversity, distribution and ecology of fungal communities present in Antarctic Lake sediments uncovered by DNA metabarcoding. Sci. Rep. 2022, 12, 8407. [Google Scholar] [CrossRef] [PubMed]
- Halıcı, M.G.; Güllü, M.; Kahraman Yiğit, M.; Barták, M. Three new records of lichenised fungi for Antarctica. Polar Record. 2022, 58, e22. [Google Scholar] [CrossRef]
- Selbmann, L.; Stoppiello, G.A.; Onofri, S.; Stajich, J.E.; Coleine, C. Culture-Dependent and Amplicon Sequencing Approaches Reveal Diversity and Distribution of Black Fungi in Antarctic Cryptoendolithic Communities. J. Fungi 2021, 16, 213. [Google Scholar] [CrossRef]
- Varrella, S.; Barone, G.; Tangherlini, M.; Rastelli, E.; Dell’Anno, A.; Corinaldesi, C. Diversity, Ecological Role and Biotechnological Potential of Antarctic Marine Fungi. J. Fungi 2021, 7, 391. [Google Scholar] [CrossRef]
- Doytchinov, V.V.; Dimov, S.G. Microbial Community Composition of the Antarctic Ecosystems: Review of the Bacteria, Fungi, and Archaea Identified through an NGS-Based Metagenomics Approach. Life 2022, 12, 916. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Liu, F.; Liu, L.R.; Li, M.; Cai, L.; Liu, S.; Mao, J. Culturing the uncultured marine fungi in the omics age: Opportunities and challenges. Fungal Biol. Rev. 2024, 48, 100353. [Google Scholar] [CrossRef]
- Durán, P.; Barra, P.J.; Jorquera, M.A.; Viscardi, S.; Fernandez, C.; Paz, C.; Mora, M.L.; Bol, R. Occurrence of Soil Fungi in Antarctic Pristine Environments. Front. Bioeng. Biotechnol. 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Li, L.; Che, Q.; Li, D.; Gu, Q.; Zhu, T. Richness and bioactivity of culturable soil fungi from the Fildes Peninsula, Antarctica. Extremophiles 2016, 20, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Enireb, E.; Cucalón, R.V.; Cárdenas, D.; Ordóñez, N.; Coello, S.; Elizalde, P.; Cárdenas, W.B. Antarctic fungi with antibiotic potential isolated from Fort William Point, Antarctica. Sci. Rep. 2022, 12, 21477. [Google Scholar] [CrossRef]
- Wierzchos, J.; De Los Ríos, A.; Sancho, L.G.; Ascaso, C. Viability of endolithic micro-organisms in rocks from the McMurdo Dry Valleys of Antarctica established by confocal and fluorescence microscopy. J. Microsc. 2004, 216, 57–61. [Google Scholar] [CrossRef]
- De los Ríos, A.; Sancho, L.G.; Grube, M.; Wierzchos, J.; Ascaso, C. Endolithic growth of two Lecidea lichens in granite from continental Antarctica detected by molecular and microscopy techniques. New Phytol. 2005, 165, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ortega, S.; Garrido-Benavent, I.; De Los Ríos, A. Austrostigmidium, a new austral genus of lichenicolous fungi close to rock-inhabiting meristematic fungi in Teratosphaeriaceae. Lichenologist 2015, 47, 143–156. [Google Scholar] [CrossRef]
- Taylor, D.L.; Hollingsworth, T.N.; McFarland, J.W.; Lennon, N.J.; Nusbaum, C.; Ruess, R.W. A first comprehensive census of fungi in soil reveals both hyper diversity and fine-scale niche partitioning. Ecol. Monogr. 2014, 84, 3–20. [Google Scholar] [CrossRef]
- Rosa, L.H.; da Silva, T.H.; Ogaki, M.B.; Pinto, O.H.B.; Stech, M.; Convey, P.; Carvalho-Silva, M.; Rosam, C.A.; Câmara, P.E.A.S. DNA metabarcoding uncovers fungal diversity in soils of protected and non-protected areas on Deception Island, Antarctica. Sci. Rep. 2020, 10, 21986. [Google Scholar] [CrossRef]
- Rabelo, N.G.; Gonçalves, V.N.; Carvalho, M.A.; Scheffler, S.M.; Santiago, G.; Sucerquia, P.A.; Oliveira, F.S.; Campos, L.P.; Lopes, F.A.C.; Santos, K.C.R.; et al. Endolithic Fungal Diversity in Antarctic Oligocene Rock Samples Explored Using DNA Metabarcoding. Biology 2024, 13, 414. [Google Scholar] [CrossRef]
- Dziurzynski, M.; Gorecki, A.; Pawlowska, J.; Istel, L.; Decewicz, P.; Golec, P.; Styczynski, M.; Poszytek, K.; Rokowska, A.; Gorniak, D.; et al. Revealing the diversity of bacteria and fungi in the active layer of permafrost at Spitsbergen Island (Arctic)—Combining classical microbiology and metabarcoding for ecological and bioprospecting exploration. Sci. Total Environ. 2023, 856, 159072. [Google Scholar] [CrossRef]
- Tosi, S.; Begonã, C.; Gerdol, R.; Caretta, G. Fungi isolated from Antarctic mosses. Polar Biol. 2002, 25, 262–268. [Google Scholar] [CrossRef]
- Morita, R.Y. Psychrophilic bacteria. Bacteriol. Rev. 1975, 39, 144–167. [Google Scholar] [CrossRef]
- Selbmann, L.; de Hoog, G.S.; Mazzaglia, A.; Friedmann, E.I.; Onofri, S. Fungi at the edge of life: Cryptoendolithic black fungi from Antarctic desert. Stud. Mycol. 2005, 51, 1–32. [Google Scholar]
- Wang, M.; Tian, J.; Xiang, M.; Liu, X. Living strategy of cold-adapted fungi with reference to several representative species. Mycology 2017, 8, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Moyer, C.L.; Collins, R.E.; Morita, R.Y. Psychrophiles and psychrotrophs. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017; MS code 02282. [Google Scholar]
- Onofri, S.; Pagano, S.; Zucconi, L.; Tosi, S. Friedmanniomyces endolithicus (Fungi, Hyphomycetes), anam.-gen. and sp. nov., from continental Antartica. Nova Hedwigia 1999, 68, 175–181. Available online: http://hdl.handle.net/11571/137768 (accessed on 26 May 1999). [CrossRef]
- Kita, D.M.; Giovanella, P.; Yoshinaga, T.T.; Pellizzer, E.P.; Sette, L.D. Antarctic fungi applied to textile dye bioremediation. An. Acad. Bras. Cienc. 2022, 94, e20210234. [Google Scholar] [CrossRef]
- Onofri, S.; Selbmann, L.; Zucconi, L.; Pagano, S. Antarctic microfungi as models for exobiology. Planet Space Sci. 2004, 52, 229–237. [Google Scholar] [CrossRef]
- Godinho, V.; Furbino, L.; Santiago, I.; Pellizzari, F.M.; Yokoya, N.S.; Pupo, D.; Alves, T.M.A.; Junior, P.A.S.; Romanha, A.J.; Zani, C.L.; et al. Diversity and bioprospecting of fungal communities associated with endemic and cold-adapted macroalgae in Antarctica. ISME J. 2013, 7, 1434–1451. [Google Scholar] [CrossRef]
- Mi, Z.; Su, J.; Yu, L.; Zhang, T. Comparative mitochondrial genomics of Thelebolaceae in Antarctica: Insights into their extremophilic adaptations and evolutionary dynamics. IMA Fungus 2024, 15, 33. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Siddiqui, K.S. Cold adapted enzymes. In Enzyme Technology; Pandey, A., Webb, C., Soccol, C.R., Larroche, C., Eds.; Asiatech Publishers, Inc.: New Delhi, India, 2004; pp. 615–638. [Google Scholar]
- Rosa, L.H.; Zani, C.L.; Cantrell, C.L.; Duke, S.O.; Dijck, P.V.; Desideri, A.; Rosa, C.A. Fungi in Antarctica: Diversity, ecology, effects of climate change and bioprospection for bioactive compounds. In Fungi of Antarctica: Diversity, Ecology and Biotechnological Applications; Rosa, L.H., Ed.; Springer: Berlin, Germany, 2019; pp. 1–17. [Google Scholar]
- Batista, T.M.; Hilario, H.O.; Mendes de Brito, G.A.; Moreira, R.G.; Furtado, C.; de Menezes, G.C.A.; Rosa, C.A.; Rosa, L.H.; Franco, G.R. Whole-genome sequencing of the endemic Antarctic fungus Antarctomyces pellizariae reveals an ice-binding protein, a scarce set of secondary metabolites gene clusters and provides insights on Thelebolales phylogeny. Genomics 2020, 112, 2915–2921. [Google Scholar] [CrossRef]
- Martorell, M.M.; Ruberto, L.A.M.; Fernández, P.M.; De Figueroa, L.I.C.; Mac Cormack, W.P. Biodiversity and enzymes bioprospection of Antarctic filamentous fungi. Antarct. Sci. 2018, 31, 3–12. [Google Scholar] [CrossRef]
- Gocheva, Y.G.; Krumova, E.T.; Slokoska, L.S.; Miteva, J.G.; Vassilev, S.V.; Angelova, M.B. Cell response of Antarctic and temperate strains of Penicillium spp. to different growth temperatures. Mycol. Res. 2006, 110, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Kostadinova, N.; Tosi, S.; Spassova, B.; Angelova, M. Comparison of the oxidative stress response of two Antarctic fungi to different growth temperatures. Pol. Polar Res. 2017, 38, 393–408. [Google Scholar] [CrossRef]
- Wang, X.-W.; Zheng, R.-Y. Chaetomium acropullum sp. nov. (Chaetomiaceae, Ascomycota), a new psychrotolerant mesophilic species from China. Nova Hedwig. 2005, 80, 413–417. [Google Scholar] [CrossRef]
- Cavicchioli, R. Cold-adapted archaea. Nat. Rev. Microbiol. 2006, 4, 331–343. [Google Scholar] [CrossRef]
- Carreiro, M.M.; Koske, R.E. Room temperature isolations can bias against selection of low temperature microfungi in temperate forest soils. Mycologia 1992, 84, 886–900. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef]
- Li, R.; Jia, Z.; Trush, M.A. Defining ROS in Biology and Medicine. React. Oxyg. Species 2016, 1, 9–21. [Google Scholar] [CrossRef]
- Rauf, A.; Khalil, A.A.; Awadallah, S.; Khan, S.A.; Abu-Izneid, T.; Kamran, M.; Hemeg, H.A.; Mubarak, M.S.; Khalid, A.; Wilairatana, P. Reactive oxygen species in biological systems: Pathways, associated diseases, and potential inhibitors—A review. Food Sci. Nutr. 2024, 12, 675–693. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species (ROS), oxygen radicals and antioxidants: Where are we now, where is the field going and where should we go? Biochem. Biophys. Res. Commun. 2022, 633, 17–19. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.J.P.O.; de Oliveira, J.C.P.L.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxid. Med. Cell Longev. 2022, 19, 1225578. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 28, 4642. [Google Scholar] [CrossRef]
- Andres, C.M.C.; Perez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Perez-Lebena, E. Chemistry of hydrogen peroxide formation and eliminationin mammalian cells, and its role in various pathologies. Stresses 2022, 2, 256–274. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, U.; Das, D.; Banerjee, R.K. Reactive oxygen species: Oxidative damage and pathogenesis. Curr. Sci. 1999, 77, 658–666. Available online: https://www.jstor.org/stable/24102839 (accessed on 10 September 1999).
- Sadhukhan, P.; Sil, P.C. The regulation of intracellular redox homeostasis in cancer progression and its therapy. In Pathology; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 105–114. ISBN 9780128159729. [Google Scholar]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2015; p. 696. [Google Scholar]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Patel, R.; Rinker, L.; Peng, J.; Chilian, W.M. Reactive Oxygen Species: The Good and the Bad. In Reactive Oxygen Species (ROS) in Living Cells; Filip, C., Albu, E., Eds.; Intech Open: Rijeka, Croatia, 2017. [Google Scholar]
- Breitenbach, M.; Weber, M.; Rinnerthaler, M.; Karl, T.; Breitenbach-Koller, L. Oxidative stress in fungi: Its function in signal transduction, interaction with plant hosts, and lignocellulose degradation. Biomolecules 2015, 3, 318–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, D.; Chen, T.; Li, B.; Zhang, Z.; Qin, G.; Tian, S. Production, Signaling, and scavenging mechanisms of reactive oxygen species in fruit–pathogen interactions. Int. J. Mol. Sci. 2019, 20, 2994. [Google Scholar] [CrossRef]
- Oiki, S.; Nasuno, R.; Urayama, S.I.; Takagi, H.; Hagiwara, D. Intracellular production of reactive oxygen species and a DAF-FM-related compound in Aspergillus fumigatus in response to antifungal agent exposure. Sci. Rep. 2022, 12, 13516. [Google Scholar] [CrossRef]
- Bugg, T.D.; Ahmad, M.; Hardiman, E.M.; Rahmanpour, R. Pathways for degradation of lignin in bacteria and fungi. Nat. Prod. Rep. 2011, 28, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Li, B.; Chen, T.; Tian, S. Reactive oxygen species: A generalist in regulating development and pathogenicity of phytopathogenic fungi. CSBJ 2020, 18, 3344–3349. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Cadenas, E. Oxidative stress: Damage to intact cells and organs. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1985, 311, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Gomez-Contreras, P.C.; Kluz, P.N.; Hines, M.R.; Coleman, M.C. Intersections between mitochondrial metabolism and redox biology mediate posttraumatic osteoarthritis. Curr. Rheumatol. Rep. 2021, 23, 32. [Google Scholar] [CrossRef]
- Abdelazim, A.M.; Abomughaid, M.M. Oxidative stress: An overview of past research and future insights. All Life 2024, 17, 2316092. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Banerjee, S.; Ghosh, J.; Sil, P.C. Drug Metabolism and Oxidative Stress: Cellular mechanism and new therapeutic insights. Biochem. Anal. Biochem. 2016, 5, 255. [Google Scholar] [CrossRef]
- Halliwell, B. The definition and measurement of antioxidants in biological systems. Free Rad. Biol. Med. 1995, 18, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.; Abdulkareem, S.D.; Abeer, A.M. Antioxidant Categories and Mode of Action. In Antioxidants; Shalaby, E., Ed.; IntechOpen: Rijeka, Croatia, 2019; pp. 3–18. [Google Scholar] [CrossRef]
- Zadrąg-Tęcza, R.; Maślanka, R.; Bednarska, S.; Kwolek-Mirek, M. Response Mechanisms to Oxidative Stress in Yeast and Filamentous Fungi. In Stress Response Mechanisms in Fungi; Skoneczny, M., Ed.; Springer Nature: Cham, Switzerland, 2018; pp. 1–34. [Google Scholar]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef]
- Rosa, A.C.; Corsi, D.; Cavi, N.; Bruni, N.; Dosio, F. Superoxide Dismutase Administration: A Review of Proposed Human Uses. Molecules 2021, 26, 1844. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. Available online: https://www.researchgate.net/publication/17780338 (accessed on 30 April 2025). [CrossRef]
- Yost, F.J.; Fridovich, I. An Iron-containing superoxide dismutase from Escherichia coli. J. Biol. Chem. 1973, 248, 4905–4908. [Google Scholar] [CrossRef]
- Fréalle, E.; Noël, C.; Viscogliosi, E.; Camus, D.; Dei-Cas, E.; Delhaes, L. Manganese superoxide dismutase in pathogenic fungi: An issue with pathophysiological and phylogenetic involvements. FEMS Immunol. Med. Microbiol. 2005, 45, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.C.; Maroney, M.J. Nickel Superoxide Dismutase. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 1505–1515. [Google Scholar]
- Nakane, D.; Akiyama, Y.; Suzuki, S.; Miyazaki, R.; Akitsu, T. Improvement of the SOD activity of the Cu2+ complexes by hybridization with lysozyme and its hydrogen bond effect on the activity enhancement. Front. Chem. 2024, 11, 1330833. [Google Scholar] [CrossRef] [PubMed]
- Valenti, R.; Jabłońska, J.; Tawfik, D.S. Characterization of ancestral Fe/Mn superoxide dismutases indicates their cambialistic origin. Protein Sci. 2022, 31, 4423. [Google Scholar] [CrossRef] [PubMed]
- Belozerskaya, T.A.; Gessler, N.N. Reactive oxygen species and the strategy of antioxidant defense in fungi: A review. Appl. Biochem. Microbiol. 2007, 43, 506–515. Available online: https://api.semanticscholar.org/CorpusID:30908199 (accessed on 30 April 2025). [CrossRef]
- Jiménez, A.; Correa, S.; Sevilla, F. Identification of Superoxide Dismutase (SOD) Isozymes in Plant Tissues. Methods Mol. Biol. 2024, 2798, 205–212. [Google Scholar] [CrossRef]
- Okado-Matsumoto, A.; Fridovich, I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria. J. Biol. Chem. 2001, 276, 38388–38393. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant. Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Islam, M.N.; Rauf, A.; Fahad, F.I.; Emran, T.B.; Mitra, S.; Olatunde, A.; Shariati, M.A.; Rebezov, M.; Rengasamy, K.R.R.; Mubarak, M.S. Superoxide dismutase: An updated review on its health benefits and industrial applications. Crit. Rev. Food Sci. Nutr. 2021, 62, 7282–7300. [Google Scholar] [CrossRef]
- Zámocký, M.; Koller, F. Understanding the structure and function of catalases: Clues from molecular evolution and in vitro mutagenesis. Prog. Biophys. Mol. Biol. 1999, 72, 19–66. [Google Scholar] [CrossRef]
- Karakus, Y.Y. Typical catalases: Function and structure. In Glutathione System and Oxidative Stress in Health and Disease; Bagatini, M.D., Ed.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Tumilaar, S.G.; Hardianto, A.; Dohi, H.; Kurnia, D. A comprehensive review of free radicals, oxidative stress, and antioxidants: Overview, clinical applications, global perspectives, future directions, and mechanisms of antioxidant activity of flavonoid compounds. J. Chem. 2024, 2024, 5594386. [Google Scholar] [CrossRef]
- Ho, H.Y.; Cheng, M.L.; Chiu, D. Glucose-6-phosphate dehydrogenase—From oxidative stress to cellular functions and degenerative diseases. Redox Rep. 2007, 12, 109–118. [Google Scholar] [CrossRef]
- Nóbrega-Pereira, S.; Fernandez-Marcos, P.J.; Brioche, T.; Gomez-Cabrera, M.C.; Salvodar-Pascual, A.; Flores, J.M.; Vina, J.; Serrano, M. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016, 7, 10894. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Razis, A.A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Atkinson, J. Vitamin E: Antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. β-Carotene: An unusual type of lipid antioxidant. Science 1984, 224, 569–573. [Google Scholar] [CrossRef]
- Bohn, T. Carotenoids and Markers of Oxidative Stress in Human Observational Studies and Intervention Trials: Implications for Chronic Diseases. Antioxidants 2019, 8, 179. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, metabolism and bioavailability of flavonoids: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef]
- Boutin, J.; Liberelle, M.; Yous, S.; Ferry, G.; Nepveu, F. Melatonin facts: Lack of evidence that melatonin is aradical scavenger in living systems. J. Pineal Res. 2024, 76, e12926. [Google Scholar] [CrossRef]
- Lee, S.Q.E.; Tan, T.S.; Kawamukai, M.; Chen, E.S. Cellular factories for coenzyme Q10 production. Microb. Cell Fact. 2017, 16, 39. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K. Low temperature and oxidative stress. Curr. Sci. 2002, 83, 109. [Google Scholar]
- Abu Bakar, N.; Lau, C.B.Y.; ·Gonzalez-Aravena, M.; Smykla, J.; ·Krzewicka, B.; Karsani, S.A.; Alias, S.A. Geographical Diversity of Proteomic Responses to Cold Stress in the Fungal Genus Pseudogymnoascus. Microb. Ecol. 2024, 87, 11. [Google Scholar] [CrossRef] [PubMed]
- Tesei, D.; Marzban, G.; Zakharova, K.; Isola, D.; Selbmann, L.; Sterflinger, K. Alteration of protein patterns in black rock inhabiting fungi as a response to different temperatures. Fungal Biol. 2012, 116, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Men, J.L.; Chang, M.C.; Feng, C.P.; Yuan, L.G. iTRAQ-based quantitative proteome revealed metabolic changes of Flammulina velutipes mycelia in response to cold stress. J. Proteom. 2017, 156, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Luo, M.; Ji, X.; Lin, L.; Wei, Y.; Zhang, Q. Proteomic analysis of Mortierella isabellina M6-22 during cold stress. Arch. Microbiol. 2016, 198, 869–876. [Google Scholar] [CrossRef]
- Lee, B.H.; Lee, H.; Xiong, L.; Zhu, J.K. A mitochondrial complex I defect impairs cold-regulated nuclear gene expression. Plant Cell 2002, 14, 1235–1251. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Zhang, L.; Onda, K.; Imai, R.; Fukuda, R.; Horiuchi, H.; Ohta, A. Growth temperature downshift induces antioxidant response in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2003, 307, 308–314. [Google Scholar] [CrossRef]
- García-Ríos, E.; Ramos-Alonso, L.; Guillamón, J.M. Correlation between low temperature adaptation and oxidative stress in Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 1199. [Google Scholar] [CrossRef]
- Tian, F.; Lee, S.Y.; Woo, S.Y.; Choi, H.Y.; Heo, S.; Nah, G.; Chun, H.S. Transcriptomic responses of Aspergillus flavus to temperature and oxidative stresses during aflatoxin production. Sci. Rep. 2021, 11, 2803. [Google Scholar] [CrossRef]
- Kostadinova, N.; Krumova, E.; Stefanova, T.; Dishliyska, V.; Angelova, M. Transient cold shock induces oxidative stress events in Antarctic fungi. In Oxidative Stress/BWe Confirmook 3; Lushchak, V.I., Stoliar, O., Eds.; InTechOpen: Rijeka, Croatia, 2012; pp. 75–99. [Google Scholar]
- Dishliyska, V. Catalase from Antarctic Fungi: Role in Antioxidant Defense, Regulation and Properties. PhD Thesis, The Stephan Angeloff Institute of Microbiology, Bulgarian Academy of Sciences, Sofia, Bulgaria, 2024. [Google Scholar]
- Miteva-Staleva, J.G. Low Temperature Stress and Cell Aging in Antarctic Fungi. PhD Thesis, The Stephan Angeloff Institute of Microbiology, Bulgarian Academy of Sciences, Sofia, Bulgaria, 2017. [Google Scholar]
- Guo, R.; Liu, T.; Guo, C.; Chen, G.; Fan, J.; Zhang, Q. Carotenoid biosynthesis is associated with low-temperature adaptation in Rhodosporidium kratochvilovae. BMC Microbiol. 2022, 22, 319. [Google Scholar] [CrossRef]
- Dishliyska, V.; Stoyancheva, G.; Abrashev, R.; Miteva-Staleva, J.; Spasova, B.; Angelova, M.; Krumova, E. Catalase from the Antarctic Fungus Aspergillus fumigatus I-9–Biosynthesis and Gene Characterization. Indian J. Microbiol. 2023, 63, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.N.; Montiel, P.O.; Johnstone, K. Influence of growth temperature on lipid and soluble carbohydrate synthesis by fungi isolated from fellfield soil in the Maritime Antarctic. Mycologia 2000, 92, 222–229. [Google Scholar] [CrossRef]
- Villarreal, P.; Carrasco, M.; Barahona, S.; Alcaíno, J.; Cifuentes, V.; Baeza, M. Antarctic yeasts: Analysis of their freeze-thaw tolerance and production of antifreeze proteins, fatty acids and ergosterol. BMC Microbiol. 2018, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Gocheva, Y.G.; Krumova, E.T.; Slokoska, L.S.; Miteva, J.G.; Vassilev, S.V.; Angelova, M.B. Temperature downshift induces antioxidant response in fungi isolated from Antarctica. Extremophiles 2009, 13, 273–281. [Google Scholar] [CrossRef]
- Krumova, E.T.; Koeva, E.K.; Stoitsova, S.R.; Paunova-Krasteva, T.S.; Stoyancheva, G.D.; Angelova, M.B. Cell response of Antarctic strain Penicillium griseofulvum against low temperature stress. Pol. Polar Res. 2022, 43, 125–143. [Google Scholar] [CrossRef]
- Gomez-Gutiérrez, S.V.; Sic-Hernandez, W.R.; Haridas, S.; LaButti, K.; Eichenberger, J.; Kaur, N.; Lipzen, A.; Barry, K.; Goodwin, S.B.; Gribskov, M.; et al. Comparative genomics of the extremophile Cryomyces antarcticus and other psychrophilic Dothideomycetes. Front. Fungal Biol. 2024, 5, 1418145. [Google Scholar] [CrossRef]
- Schade, B.; Jansen, G.; Whiteway, M.; Entian, K.D.; Thomas, D.Y. Cold adaptation in budding yeast. Mol. Biol. Cell 2004, 15, 5492–5502. [Google Scholar] [CrossRef]
- Suárez-Medina, M.D.; Sáez-Casado, M.I.; Martínez-Moya, T.; Rincón-Cervera, M.Á. The effect of low temperature storage on the lipid quality of fish, either alone or combined with alternative preservation technologies. Foods 2024, 13, 1097. [Google Scholar] [CrossRef]
- Yan, W.; Su, Z.; Zhou, G.; Lin, Y.; Ren, W.; Liao, J.; Zhu, C.; Wang, C.; Ren, D.; Wang, Y.; et al. Cold tolerance of postharvest fungal pathogens is regulated by the conserved high osmolarity glycerol (HOG) pathway. Postharvest Biol. Technol. 2025, 219, 113206. [Google Scholar] [CrossRef]
- Abrashev, R.; Krumova, E.; Petrova, P.; Dolashka, P.; Eneva, R.; Fenice, M.; Engibarov, S.; Stoyancheva, G.; Gocheva, Y.; Velkova, L.; et al. New Enzymes from the Sialidase Family in Filamentous Fungi. Monograph; Prees Product Lain Ltd.: Sofia, Bulgaria, 2024; p. 102. ISBN 978-619-7177-12-1. (In Bulgarian) [Google Scholar]
- Fabri, J.H.T.M.; de Sa, N.P.; Malavazi, I.; Del Poeta, M. The dynamics and role of sphingolipids in eukaryotic organisms upon thermal adaptation. Prog. Lipid Res. 2020, 80, 101063. [Google Scholar] [CrossRef]
- Shi, T.A.O.; Yu, Y.Y.; Dai, J.J.; Zhang, Y.T.; Hu, W.P.; Zheng, L.; Shi, D.Y. New polyketides from the Antarctic fungus Pseudogymnoascus sp. HSX2#-11. Mar. Drugs 2021, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Tendulkar, S.; Hattiholi, A.; Chavadar, M.; Dodamani, S. Psychrophiles: A journey of hope. J. Biosci. 2021, 46, 64. [Google Scholar] [CrossRef]
- Jodłowska, I.; Białkowska, A.M. Cold-Adapted Fungi: Goldmine of Biomolecules Applicable in Industry. Appl. Sci. 2024, 14, 11950. [Google Scholar] [CrossRef]
- Maggi, O.; Tosi, S.; Angelova, M.; Lagostina, E.; Fabbri, A.; Pecoraro, L.; Altobelli, E.; Picco, A.; Savino, E.; Branda, E.; et al. Adaptation of fungi, including yeasts, to cold environments. Plant Biosyst. 2013, 147, 247–258. [Google Scholar] [CrossRef]
- Su, Y.; Jiang, X.Z.; Wu, W.P.; Wang, M.M.; Hamid, M.I.; Xiang, M.C.; Liu, X.Z. Genomic, transcriptomic, and proteomic analysis provide insights into the cold adaptation mechanism of the obligate psychrophilic fungus Mrakia psychrophila. G3 2016, 6, 3603–3613. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Zhang, L.; Wu, Q.; Li, S.; Gou, J.; He, J.; Zhang, K.; Li, S.; Niu, X. Tryptophan-centered metabolic alterations coincides with lipid-mediated fungal response to cold stress. Heliyon 2023, 9, e13066. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radical oxidation of proteins and its relationship with the functional state of organisms. Biochemistry 2007, 72, 809–827. [Google Scholar] [CrossRef]
- Coleine, C.; Masonjones, S.; Sterflinger, K.; Onofri, S.; Selbmann, L.; Stajich, J.E. Peculiar genomic traits in the stress-adapted cryptoendolithic Antarctic fungus Friedmanniomyces endolithicus. Fungal Biol. 2020, 124, 458–467. [Google Scholar] [CrossRef]
- Thomas-Hall, S.R.; Turchetti, B.; Buzzini, P.; Branda, E.; Boekhout, T.; Theelen, B.; Watson, K. Cold-adapted yeasts from Antarctica and the Italian Alps—Description of three novel species: Mrakia robertii sp. nov., Mrakia blollopis sp. nov. and Mrakiella niccombsii sp. nov. Extremophiles 2010, 14, 47–59. [Google Scholar] [CrossRef]
- Kostadinova, N. Cell Response of Antarctic Fungi to Temperature Stress. PhD Thesis, The Stephan Angeloff Institute of Microbiology, Bulgarian Academy of Sciences, Academic Publishing House “Prof. Marin Drinov”, Sofia, Bulgaria, 2013. [Google Scholar]
- Cassaro, A.; Pacelli, C.; Onofri, S. Survival, metabolic activity, and ultrastructural damage of Antarctic black fungus in perchlorates media. Front. Microbiol. 2022, 13, 992077. [Google Scholar] [CrossRef] [PubMed]
- Bartolo-Aguilar, Y.; Dendooven, L.; Chávez-Cabrera, C.; Flores-Cotera, L.B.; Hidalgo-Lara, M.E.; Villa-Tanaca, L.; Marsch, R. Autolysis of Pichia pastoris induced by cold. AMB 2017, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Misiak, M.; Goodall-Copestake, W.P.; Sparks, T.H.; Worland, M.R.; Boddy, L.; Magan, N.; Convey, P.; Hopkins, D.W.; Newsham, K.K. Inhibitory effects of climate change on the growth and extracellular enzyme activities of a widespread Antarctic soil fungus. Glob. Chang. Biol. 2020, 27, 1111–1125. [Google Scholar] [CrossRef]
- Gong, M.; Wang, Y.; Zhang, J.; Zhao, Y.; Wan, J.; Shang, J.; Yang, R.; Wu, Y.; Li, Y.; Tan, Q.; et al. Chilling stress triggers VvAgo1-mediated miRNA-like RNA biogenesis in Volvariella volvacea. Front. Microbiol. 2020, 11, 523593. [Google Scholar] [CrossRef]
- Zhao, X.; Song, X.; Li, Y.; Yu, C.; Zhao, Y.; Gong, M.; Shen, X.; Chen, M. Gene expression related to trehalose metabolism and its effect on Volvariella volvacea under low-temperature stress. Sci. Rep. 2018, 8, 11011. [Google Scholar] [CrossRef]
- Miteva-Staleva, J.; Krumova, E.; Spasova, B.; Angelova, M. Age-related changes in protease activity as a cold stress response by Penicillium strains from different temperature classes. Pol. Polar Res. 2023, 45, 67–79. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, N.; Ma, J.; Zhou, Y.; Wei, Q.; Tian, C.; Fang, Y.; Zhong, R.; Chen, G.; Zhang, S. Advances in cold-adapted enzymes derived from microorganisms. Front. Microbiol. 2023, 14, 1152847. [Google Scholar] [CrossRef] [PubMed]
- Fiedurek, J.; Gromada, A.; Słomka, A.; Korniłowicz-Kowalska, T.; Kurek, E.; Melke, J. Catalase activity in arctic microfungi grown at different temperatures. Acta Biol. Hung. 2003, 54, 105–112. [Google Scholar] [CrossRef]
- Chai, C.N.; Yam, H.C.; Rosli, N.; Azlan, A.; Azzam, G.; Halim, M.A.; Najimudin, N. Transcriptomic response of an Antarctic yeast Rhodotorula sp. USM-PSY62 to temperature changes. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wang, W.; Tan, Q.; Wang, Q.; Wang, J.; Zhang, F.; Zheng, X.; Yun, J.; Zhang, W.; Zhao, F. Glutathione peroxidase gene regulates substrate development and prevents strain aging in Volvariella volvacea. Int. J. Biol. Macromol. 2025, 289, 138835. [Google Scholar] [CrossRef]
- Govarthanan, M.; Fuzisawa, S.; Hosogai, T.; Chang, Y.C. Biodegradation of aliphatic and aromatic hydrocarbons using the filamentous fungus Penicillium sp. CHY-2 and characterization of its manganese peroxidase activity. RSC Adv. 2017, 7, 20716–20723. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Liu, D.; Proksch, P.; Yu, S.; Lin, W. Antioxidative phenolic compounds from a marine-derived fungus Aspergillus versicolor. Tetrahedron 2016, 72, 50–57. [Google Scholar] [CrossRef]
- Huang, J.N.; Zou, Q.; Chen, J.; Xu, S.; Luo, D.; Zhang, F.; Lu, Y. Phenols and diketopiperazines isolated from Antarctic-derived fungi, Penicillium citreonigrum SP-6. Phytochem. Lett. 2018, 27, 114–118. [Google Scholar] [CrossRef]
- Pacelli, C.; Cassaro, A.; Maturilli, A.; Timperio, A.M.; Gevi, F.; Cavalazzi, B.; Stefan, M.; Ghica, D.; Onofri, S. Multidisciplinary characterization of melanin pigments from the black fungus Cryomyces antarcticus. Appl. Microbiol. Biotechnol. 2020, 104, 6385–6395. [Google Scholar] [CrossRef]
- Flieger, K.; Knabe, N.; Toepel, J. Development of an Improved Carotenoid Extraction Method to Characterize the Carotenoid Composition under Oxidative Stress and Cold Temperature in the Rock Inhabiting Fungus Knufia petricola A95. J. Fungi 2018, 4, 124. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, S.B.; Biscaino, C.D.S.; Kreusch, M.G.; da Silva, A.F.; Duarte, R.T.D.; Robl, D. The hidden rainbow: The extensive biotechnological potential of Antarctic fungi pigments. Braz. J. Microbiol. 2023, 54, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Gostinčar, C.; Zalar, P.; Gunde-Cimerman, N. No need for speed: Slow development of fungi in extreme environments. Fungal Biol. Rev. 2022, 39, 1–14. [Google Scholar] [CrossRef]
- Novak Babič, M.; Zupančič, J.; Gunde-Cimerman, N.; de Hoog, S.; Zalar, P. Ecology of the human opportunistic black yeast Exophiala dermatitidis indicates preference for human-made habitats. Mycopathologia 2018, 183, 201–212. [Google Scholar] [CrossRef]
- Selbmann, L.; Onofri, S.; Zucconi, L.; Isola, D.; Rottigni, M.; Ghiglione, C.; Piazza, P.; Alvaro, M.C.; Schiaparelli, S. Distributional records of Antarctic fungi based on strains preserved in the Culture Collection of Fungi from Extreme Environments (CCFEE) Mycological Section associated with the Italian National Antarctic Museum (MNA). MycoKeys 2015, 10, 57–71. [Google Scholar] [CrossRef]
- Selbmann, L.; Zucconi, L.; Onofri, S.; Cecchini, C.; Isola, D.; Turchetti, B.; Buzzini, P. Taxonomic and phenotypic characterization of yeasts isolated from worldwide cold rock-associated habitats. Fungal Biol. 2014, 118, 61–71. [Google Scholar] [CrossRef]
- Francisco, C.S.; Ma, X.; Zwyssig, M.M.; McDonald, B.A.; Palma-Guerrero, J. Morphological changes in response to environmental stresses in the fungal plant pathogen Zymoseptoria tritici. Sci. Rep. 2019, 9, 9642. [Google Scholar] [CrossRef]
- Sato, M.; Hasegawa, K.; Shimada, S.; Osumi, M. Effects of pressure stress on the fission yeast Schizosaccharomyces pombe cold-sensitive mutant nda3. FEMS Microbiol. Lett. 1999, 176, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Araki, J.; Takano, M.; Sekiguchi, J. Characterization of Bacillus subtilis mutants resistant to cold shock-induced autolysis FEMS Microbiol. Lett. 1997, 150, 269–275. [Google Scholar] [CrossRef]
- Chang, T.; Zha, L.; Yu, C.; Chen, M.; Zhou, S.; Dong, Q.; Wu, Y.; Fan, S.; Zhao, Y. Expanding the understanding of Volvariella volvacea autolysis at 4 °C with transcriptomics and metabolomics. Sci. Hortic. 2024, 336, 113386. [Google Scholar] [CrossRef]
- Gonçalves, R.D.; Cupertino, F.B.; Freitas, F.Z.; Luchessi, A.D.; Bertolini, M.C. A genome-wide screen for Neurospora crassa transcription factors regulating glycogen metabolism. Mol. Cell Proteom. 2011, 10, M111.007963. [Google Scholar] [CrossRef] [PubMed]
- Parrou, J.L.; Teste, M.A.; François, J. Effects of various types of stress on the metabolism of reserve carbohydrates in Saccharomyces cerevisiae: Genetic evidence for a stress-induced recycling of glycogen and trehalose. Microbiology 1997, 143, 1891–1900. [Google Scholar] [CrossRef]
- Ribeiro, G.D.; de Holanda Paranhos, L.; Eleutherio, E.C.A. Trehalose promotes biological fitness of fungi. Fungal Biol. 2024, 128, 2381–2389. [Google Scholar] [CrossRef]
- França, M.B.; Panek, A.D.; Eleutherio, E.C.A. Oxidative stress and its effects during dehydration. CBPA 2007, 146, 621–631. [Google Scholar] [CrossRef]
- Aguilera, J.; Randez-Gil, F.; Prieto, J.A. Cold response in Saccharomyces cerevisiae: New functions for old mechanisms. FEMS Microbiol. Rev. 2007, 31, 327–341. [Google Scholar] [CrossRef]
- Xiong, L.; Li, Y.; Yu, H.; Wei, Y.; Li, H.; Ji, X. Whole genome analysis and cold adaptation strategies of Pseudomonas sivasensis W-6 isolated from the Napahai plateau wetland. Sci. Rep. 2023, 13, 14190. [Google Scholar] [CrossRef]
- Gancedo, C.; Flores, C.L. The importance of a functional trehalose biosynthetic pathway for the life of yeasts and fungi. FEMS Yeast Res. 2004, 4, 351–359. [Google Scholar] [CrossRef]
- Tesei, D.; Marzban, G.; Marchetti-Deschmann, M.; Tafer, H.; Arcalis, E.; Sterflinger, K. Proteome of tolerance fine-tuning in the human pathogen black yeast Exophiala dermatitidis. J. Proteom. 2015, 128, 39–57. [Google Scholar] [CrossRef]
- Kostadinova, N.; Vassilev, S.; Spasova, B.; Angelova, M. Cold stress in Antarctic fungi targets enzymes of the glycolytic pathway and tricarboxylic acid cycle. Biotechnol. Biotechnol. Equip. 2011, 25, 50–57. [Google Scholar] [CrossRef]
- Broberg, M.; Dubey, M.; Sun, M.H.; Ihrmark, K.; Schroers, H.J.; Li, S.D.; Jensen, D.F.; Brandström, D.M.; Karlsson, M. Out in the cold: Identification of genomic regions associated with cold tolerance in the biocontrol fungus Clonostachys rosea through genome-wide association mapping. Front. Microbiol 2018, 9, 2844. [Google Scholar] [CrossRef] [PubMed]
- Jules, M.; Beltran, G.; Francois, J.; Parrou, J.L. New insights into trehalose metabolism by Saccharomyces cerevisiae: NTH2 encodes a functional cytosolic trehalase, and deletion of TPS1 reveals Ath1p-dependent trehalose mobilization. Appl. Environ. Microbiol. 2008, 74, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Poveda, G.; Gil-Durán, C.; Vaca, I.; Levican, G.; Chavez, R. Cold-active pectinolytic activity produced by filamentous fungi associated with Antarctic marine sponges. Biol. Res. 2018, 51, 28. [Google Scholar] [CrossRef]
- Singh, P.; Hamid, B.; Ahmad Lone, M.; Ranjan, K.; Khan, A.; Chaurse, V. Evaluation of pectinase activity from the psychrophilic fungal strain Truncatella angustata-BPF5 for use in the wine industry. Endocyt. Cell Res. 2012, 22, 57–61. [Google Scholar]
- Abrashev, R.; Feller, G.; Kostadinova, N.; Krumova, E.; Alexieva, Z.; Gerginova, M.; Spasova, B.; Miteva-Staleva, J.; Vassilev, S.; Angelova, M. Production, purification, and characterization of a novel cold-active superoxide dismutase from the Antarctic strain Aspergillus glaucus 363. Fungal Biol. 2016, 120, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Dolashki, A.; Abrashev, R.; Kaynarov, D.; Krumova, E.; Velkova, L.; Eneva, R.; Engibarov, S.; Gocheva, Y.; Miteva-Staleva, J.; Dishliyska, V.; et al. Purification and characterization of a novel sialidase from Antarctic fungus Penicillium griseofulvum P29. Biochem. Biophis. Rep. 2024, 37, 101610. [Google Scholar] [CrossRef]
- Baeza, M.; Alcaíno, J.; Cifuentes, V.; Turchetti, B.; Buzzini, P. Cold-Active Enzymes from Cold-Adapted Yeasts. In Biotechnology of Yeasts and Filamentous Fungi; Sibirny, A., Ed.; Springer: Cham, Switzerland, 2017; pp. 297–334. [Google Scholar] [CrossRef]
- Taskin, M.; Ortucu, S.; Unver, Y.; Tasar, O.C.; Ozdemir, M.; Kaymak, H.C. Invertase production and molasses decolourization by cold-adapted filamentous fungus Cladosporium herbarum ER-25 in non-sterile molasses medium. Process Saf. Environ. Prot. 2016, 103, 136–143. [Google Scholar] [CrossRef]
- Santiago, M.; Ramírez-Sarmiento, C.A.; Zamora, R.A.; Parra, L.P. Discovery, molecular mechanisms, and industrial applications of cold-active enzymes. Front. Microbiol. 2016, 7, 1408. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.T.; Fu, J.J.; Sun, Y.F.; Xu, Y.M.; Miao, Y.J.; Xu, Y.F.; Hu, T.M. Effect of arbuscular mycorrhizal fungi inoculation on cold stress-induced oxidative damage in leaves of Elymus nutans Griseb. S. Afr. J. Bot. 2016, 104, 21–29. [Google Scholar] [CrossRef]
- Liang, X.; Niu, Q.; Kong, J.; Zhao, X.; Zhang, B.; Li, L.; Jiao, C.; Gai, Y. Role of antioxidant molecules in the oxidative stress response networks in the tangerine pathotype of Alternaria alternata. Agronomy 2023, 13, 2735. [Google Scholar] [CrossRef]
- Vitale, G.A.; Coppola, D.; Palma Esposito, F.; Buonocore, C.; Ausuri, J.; Tortorella, E.; de Pascale, D. Antioxidant molecules from marine fungi: Methodologies and perspectives. Antioxidants 2020, 9, 1183. [Google Scholar] [CrossRef]
- Rosas, A.L.; Casadevall, A. Melanization affects susceptibility of Cryptococcus neoformans to heat and cold. FEMS Microbiol. Lett. 1997, 153, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Louet, C.; Blot, C.; Shelest, E.; Guerillot, P.; Salamov, A.; Zannini, F.; Pétrowski, J.; Grigoriev, I.V.; Frey, P.; Duplessis, S. Annotation survey and life cycle transcriptomics of transcription factors in rust fungi (Pucciniales) identify a possible role for cold shock proteins in dormancy exit. Fungal Genet. Biol. 2022, 161, 103698. [Google Scholar] [CrossRef]
- Fang, W.; St Leger, R.J. RNA binding proteins mediate the ability of a fungus to adapt to the cold. Environ. Microbiol. 2010, 12, 810–820. [Google Scholar] [CrossRef]
- Keto-Timonen, R.; Hietala, N.; Palonen, E.; Hakakorpi, A.; Lindström, M.; Korkeala, H. Cold shock proteins: A minireview with special emphasis on Csp-family of enteropathogenic Yersinia. Front. Microbiol. 2016, 7, 1151. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, K.; Sterflinger, K.; Razzazi-Fazeli, E.; Nöbauer, K.; Marzban, G. Global proteomics of the extremophile black fungus Cryomyces antarcticus using 2D-electrophoresis. Nat. Sci. 2014, 6, 978–995. [Google Scholar] [CrossRef]
- Zakharova, K.; Tesei, D.; Marzban, G.; Dijksterhuis, J.; Wyatt, T.; Sterflinger, K. Microcolonial fungi on rocks: A life in constant drought? Mycopathologia 2012, 175, 537–547. [Google Scholar] [CrossRef]
- Sterflinger, K.; Lopandic, K.; Pandey, R.V.; Blasi, B.; Kriegner, A. Nothing special in the specialist? Draft genome sequence of Cryomyces antarcticus, the most extremophilic fungus from Antarctica. PLoS ONE 2014, 9, e109908. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, N.; Lau, B.Y.C.; Smykla, J.; Karsani, S.A.; Alias, S.A. Protein homeostasis, regulation of energy production and activation of DNA damage-repair pathways are involved in the heat stress response of Pseudogymnoascus spp. Environ. Microbiol. 2022, 24, 1849–1864. [Google Scholar] [CrossRef]
- Stajich, J.E. Fungal genomes and insights into the evolution of the kingdom. Microbiol. Spectr. 2017, 5, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Paiva, D.S.; Fernandes, L.; Portugal, A.; Trovão, J. First genome sequence of the microcolonial black fungus Saxispiralis lemnorum MUM 23.14: Insights into the unique genomic traits of the Aeminiaceae family. Microorganisms 2024, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Loftus, B.J.; Fung, E.; Roncaglia, P.; Rowley, D.; Amedeo, P.; Bruno, D.; Vamathevan, J.; Miranda, M.; Anderson, I.J.; Fraser, J.A.; et al. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 2005, 307, 1321–1324. [Google Scholar] [CrossRef]
- Janbon, G.; Ormerod, K.L.; Paulet, D.; Byrnes, E.J., 3rd; Yadav, V.; Chatterjee, G.; Mullapudi, N.; Hon, C.C.; Billmyre, R.B.; Brunel, F.; et al. Analysis of the genome and transcriptome of Cryptococcus neoformans var. grubii reveals complex RNA expression and microevolution leading to virulence attenuation. PLoS Genet. 2014, 10, e1004261. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).