Application of Lignin for Slope Bioengineering: Effect on Soil Improvement and Plant Growth
Abstract
1. Introduction
2. Literature Review
3. Materials and Methods
3.1. Materials Used
3.2. Experimental Program
3.2.1. Soil Specimen Preparations
3.2.2. Soil Testing
4. Results and Discussion
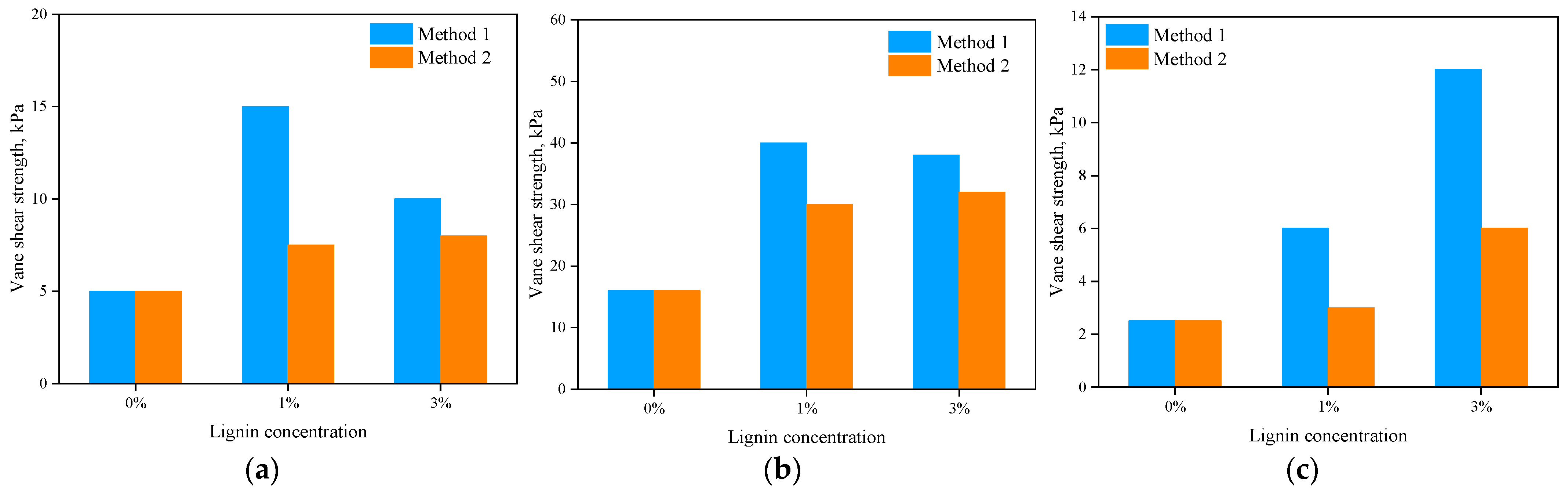
4.1. Compressive and Shear Strength Characteristics
- -
- For each measurement, at least five pocket penetrometer tests were conducted, and the average value was used to estimate the soil surface strength. The PP measurements were performed in different parts of the soil samples. As the soil mass was not homogenous (due to the soil nature and experimental preparations), different values were sometimes obtained, depending on the point selected for the measurement.
- -
- For each experiment, the soil surface was sprayed with 100 mL of water twice per week to stimulate seed germination and growth, and changes in moisture on the soil surface contributed to the soil inhomogeneity.
- -
- Over time, the soil samples had a natural tendency to dry on the surface, initiating surface cracks. This process contributed to the soil inhomogeneity as well, and impacted the measured soil strength.
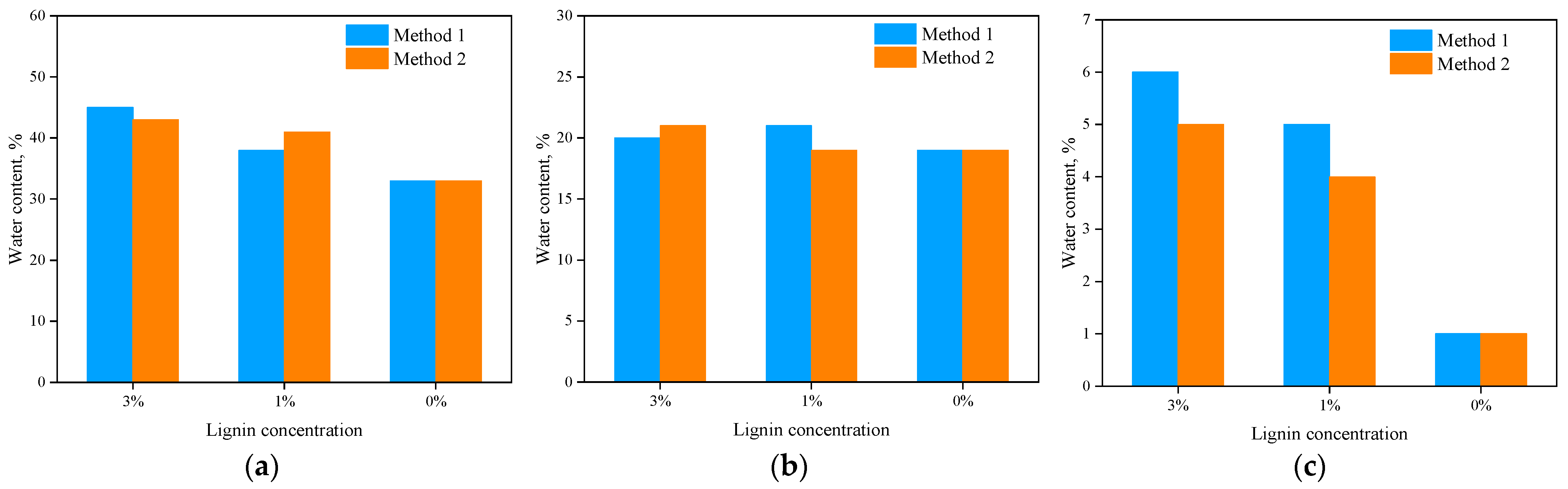
4.2. Visual Observations and Water Content
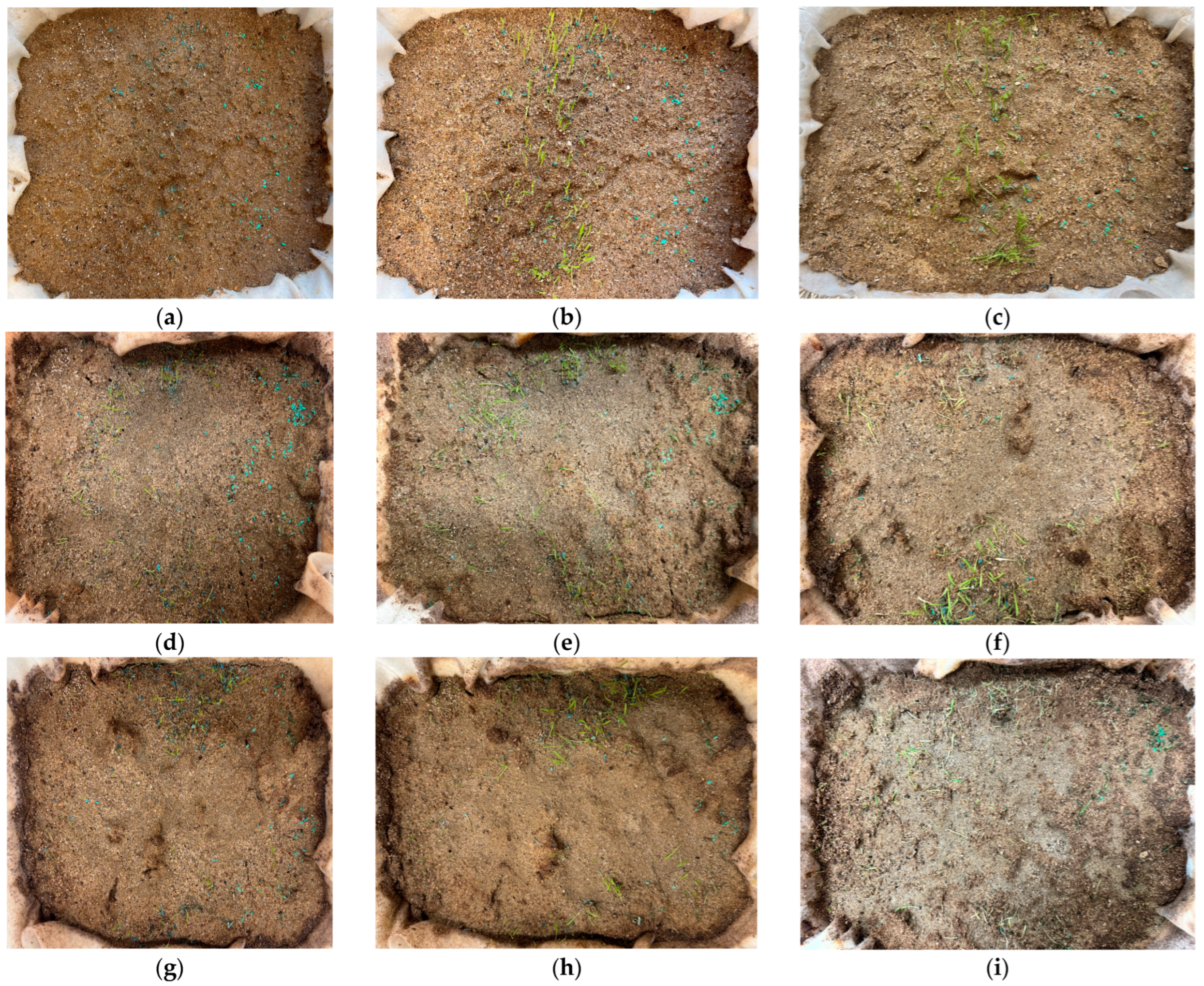
4.3. Seed Germination and Growth
5. Conclusions
- -
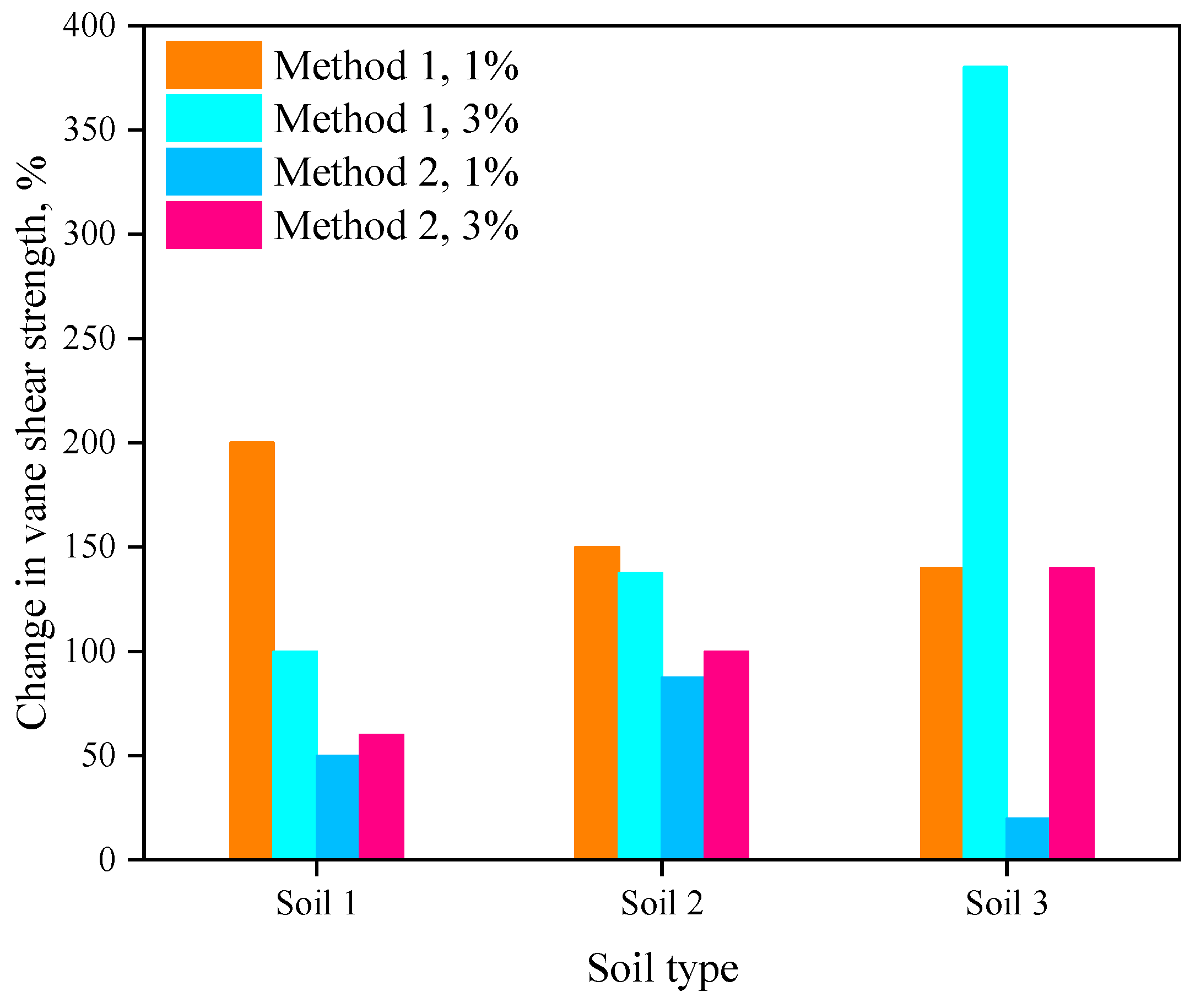
- The application of lignin biopolymer increased the strength of all three tested soil types. Both pocket penetrometer and vane shear test results indicated that the soil treated with lignin produced greater strength, especially when a 3% lignin solution was used. For high-plasticity Soil 1, the maximum increase in the strength, compared to the untreated control soil sample, occurred after the first 10 days, while for low-plasticity Soil 2, the maximum increase in the strength occurred later, within 20–30 days.
- -
- The increase in soil strength was greater when lignin solutions were mixed with soil (Method 1) compared to the other method (Method 2) when lignin solutions were sprayed on the surface of the soil mass.
- -
- The lignin-treated soil could retain more water over the long term compared to the untreated soil. This was more pronounced for very plastic soil (Soil 1) and non-plastic sand (Soil 3) than for Soil 2. This extra moisture could contribute to better vegetation growth, leading to greater resistance to soil erosion.
- -
- Both lignin-treated and untreated soils produced similar results on seed germination and growth, suggesting that lignin does not have a negative effect on vegetation. The outcomes of this study indicate that the addition of lignin to soil can improve soil strength without negative effects on the environment and vegetation growth, which is important for alternative slope bioengineering methods.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bagheri, P.; Gratchev, I.; Rybachuk, M. Effects of Xanthan Gum Biopolymer on Soil Mechanical Properties. Appl. Sci. 2023, 13, 887. [Google Scholar] [CrossRef]
- Dehghan, H.; Tabarsa, A.; Latifi, N.; Bagheri, Y. Use of xanthan and guar gums in soil strengthening. Clean Technol. Environ. Policy 2019, 21, 155–165. [Google Scholar] [CrossRef]
- Bagheri, P.; Gratchev, I.; Son, S.; Rybachuk, M. Durability, Strength, and Erosion Resistance Assessment of Lignin Biopolymer Treated Soil. Polymers 2023, 15, 1556. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Prasidhi, A.K.; Cho, G.-C. Effects of Xanthan gum biopolymer on soil strengthening. Constr. Build. Mater. 2015, 74, 65–72. [Google Scholar] [CrossRef]
- Jang, C.; Yang, B.; Hong, W.-T.; Ahn, J.; Jung, J. Soil improvement using agar gum polymer for seismic liquefaction mitigation. Soil Dyn. Earthq. Eng. 2024, 177, 108405. [Google Scholar] [CrossRef]
- Chang, I.; Cho, G.-C. Strengthening of Korean residual soil with β-1, 3/1, 6-glucan biopolymer. Constr. Build. Mater. 2012, 30, 30–35. [Google Scholar] [CrossRef]
- Soldo, A.; Miletic, M.; Auad, M.L. Biopolymers as a sustainable solution for the enhancement of soil mechanical properties. Sci. Rep. 2020, 10, 267. [Google Scholar] [CrossRef]
- Bagheri, P.; Gratchev, I.; Zolghadr, M.; Son, S.; Kim, J.M. Mitigation of Soil Erosion and Enhancement of Slope Stability Through the Utilization of Lignin Biopolymer. Polymers 2024, 16, 1300. [Google Scholar] [CrossRef]
- Tingle, J.S.; Santoni, R.L. Stabilization of clay soils with nontraditional additives. Transp. Res. Rec. 2003, 1819, 72–84. [Google Scholar] [CrossRef]
- Al Mahamud, M.A.; Indraratna, B. Elastic modules of soils treated with lignosulfonate. In Proceedings of the 11th Australia New Zealand Conference on Geomechanics, Melbourne, Australia, 15–18 July 2012. [Google Scholar]
- Bai, Z.; Li, D.; Zhao, D.; Lu, W.; Liu, J. Experimental research on collapsibility of Xi’an Loess improved by calcium lignosulfonate. Coatings 2023, 13, 157. [Google Scholar] [CrossRef]
- Loranger, B.; Barbieri, D.M.; Rieksts, K.; Økern, J.; Stolpestad, S.S.; Hoff, I.; Scibilia, E. Mechanical and freezing behavior of quarry waste sands stabilized with two nontraditional additives. Cold Reg. Sci. Technol. 2024, 221, 104168. [Google Scholar] [CrossRef]
- Vaiana, R.; Oliviero Rossi, C.; Perri, G. An eco-sustainable stabilization of clayey road subgrades by lignin treatment: An overview and a comparative experimental investigation. Appl. Sci. 2021, 11, 11720. [Google Scholar] [CrossRef]
- Bolander, P. Laboratory testing of nontraditional additives for stabilization of roads and trail surfaces. Transp. Res. Rec. 1999, 1652, 24–31. [Google Scholar] [CrossRef]
- Indraratna, B.; Mahamud, M.A.A.; Vinod, J.S. Chemical and mineralogical behaviour of lignosulfonate treated soils. In Proceedings of the GeoCongress 2012: State of the Art and Practice in Geotechnical Engineering, Oakland, CA, USA, 25–29 March 2012; pp. 1146–1155. [Google Scholar]
- Chen, Q.; Indraratna, B. Shear behaviour of sandy silt treated with lignosulfonate. Can. Geotech. J. 2015, 52, 1180–1185. [Google Scholar] [CrossRef]
- Zhang, T.; Cai, G.; Liu, S. Assessment of mechanical properties in recycled lignin-stabilized silty soil as base fill material. J. Clean. Prod. 2018, 172, 1788–1799. [Google Scholar] [CrossRef]
- Zhang, T.; Cai, G.; Liu, S. Application of lignin-stabilized silty soil in highway subgrade: A macroscale laboratory study. J. Mater. Civ. Eng. 2018, 30, 04018034. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, S.; Zhan, H.; Ma, C.; Cai, G. Durability of silty soil stabilized with recycled lignin for sustainable engineering materials. J. Clean. Prod. 2020, 248, 119293. [Google Scholar] [CrossRef]
- Kong, X.; Wang, G.; Liang, Y.; Zhang, Z.; Cui, S. The engineering properties and microscopic characteristics of high-liquid-limit soil improved with lignin. Coatings 2022, 12, 268. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, M.; Wang, Q.; Wang, Y.; Liu, J.; Cao, C.; Zheng, W.; Bao, Y.; Rocchi, I. Use of Sulfur-Free Lignin as a novel soil additive: A multi-scale experimental investigation. Eng. Geol. 2020, 269, 105551. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, W.; Wang, Q.; Cao, C.; Chang, M.; Rocchi, I. Evaluating sulfur-free lignin as a sustainable additive for soil improvement against frost resistance. J. Clean. Prod. 2020, 251, 119504. [Google Scholar] [CrossRef]
- Singh, S.P.; Palsule, P.S.; Anand, G. Strength properties of expansive soil treated with sodium lignosulfonate. In Problematic Soils and Geoenvironmental Concerns: Proceedings of IGC 2018; Springer: Singapore, 2020; pp. 665–679. [Google Scholar]
- Ji, S.G.; Wang, B.Z.; Yang, X.J.; Fan, H.H. Experimental study of dispersive clay modified by calcium lignosulfonate. Rock Soil Mech. 2021, 42, 3. [Google Scholar]
- Amulya, G.; Moghal, A.A.B.; Basha, B.M.; Almajed, A. Coupled Effect of Granite Sand and Calcium Lignosulphonate on the Strength Behavior of Cohesive Soil. Buildings 2022, 12, 1687. [Google Scholar] [CrossRef]
- Boobalan, S.C.; Devi, M.S. Investigational study on the influence of lime and coir fiber in the stabilisation of expansive soil. Mater. Today Proc. 2022, 60, 311–314. [Google Scholar] [CrossRef]
- Du, X.; Wu, Q.; Ma, Q.; Tian, Y.; Zhang, J. Study on Strengthening and Waterproofing Mechanism of Calcium Lignosulfonate in Silty Soil Sites. Coatings 2023, 13, 1402. [Google Scholar] [CrossRef]
- Varsha, B.; Moghal, A.A.B.; Rehman, A.U.; Chittoori, B.C.S. Shear, Consolidation Characteristics and Carbon Footprint Analysis of Clayey Soil Blended with Calcium Lignosulphonate and Granite Sand for Earthen Dam Application. Sustainability 2023, 15, 6117. [Google Scholar] [CrossRef]
- Vakili, A.H.; Awam, A.; Keskin, İ. Innovative application of recycled waste biopolymers to enhance the efficiency of traditional compacted clay liners of landfill systems: Mitigating leachate impact. Mater. Lett. 2024, 365, 136487. [Google Scholar] [CrossRef]
- Kavazanjian, E., Jr.; Iglesias, E.; Karatas, I. Biopolymer soil stabilisation for wind erosion control. In Proceedings of the 17th International Conference on Soil Mechanics and Geotechnical Engineering, Alexandria, Egypt, 5–9 October 2009; Volume 1–4, pp. 881–884. [Google Scholar]
- Luiz, C.; Schauffler, G.P.; Lemos-Blainski, J.M.; Rosa, D.J.; Di Piero, R.M. Mechanisms of action of aloe polysaccharides and xanthan gum for control of black rot in cauliflower. Sci. Hortic. 2016, 200, 170–177. [Google Scholar] [CrossRef]
- Josifovski, J.; Susinov, B.; Atanasovska, A.N. Experimental and Numerical Modeling of Soil-Vegetation-Atmospheric Interaction on Slopes and Erosion Control Using Biopolymers and Vegetation. In Proceedings of the 4th European Regional Conference of IAEG, EUROENGEO, Dubrovnik, Croatia, 8–12 October 2024. [Google Scholar]
- Tran, A.T.P.; Chang, I.; Cho, G.C. Soil water retention and vegetation survivability improvement using microbial biopolymers in drylands. Geomech. Eng. 2019, 17, 475–483. [Google Scholar]
- Wang, H.; De Vries Frits, P.; Jin, Y. A win-win technique of stabilizing sand dune and purifying paper mill black-liquor. J. Environ. Sci. 2009, 21, 488–493. [Google Scholar] [CrossRef]
- Zezin, A.B.; Mikheikin, S.V.; Rogacheva, V.B.; Zansokhova, M.F.; Sybachin, A.V.; Yaroslavov, A.A. Polymeric stabilizers for protection of soil and ground against wind and water erosion. Adv. Colloid Interface Sci. 2015, 226, 17–23. [Google Scholar] [CrossRef]
- Naeimi, M.; Chu, J.; Khosroshahi, M.; Zenouzi, L.K. Soil stabilisation for dunes fixation using microbially induced calcium carbonate precipitation. Geoderma 2023, 429, 116183. [Google Scholar] [CrossRef]
- Nikolovska, A.; Josifovski, J.O.; Susinov, B. Stabilization of surface erosion on slopes using polymers and vegetation. In Proceedings of the 16th International Symposium on Water Management and Hydraulic Engineering, Skopje, North Macedonia, 5–7 September 2019; pp. 488–499. [Google Scholar]
- Yang, Q.W.; Pei, X.J.; Huang, R.Q. Impact of polymer mixtures on the stabilisation and erosion control of silty sand slope. J. Mt. Sci. 2019, 16, 470–485. [Google Scholar] [CrossRef]
- Chang, I.; Prasidhi, A.K.; Im, J.; Shin, H.D.; Cho, G.C. Soil treatment using microbial biopolymers for anti-desertification purposes. Geoderma 2015, 253, 39–47. [Google Scholar] [CrossRef]
- Ni, J.; Wang, Z.T.; Geng, X.Y. Vegetation growth promotion and overall strength improvement using biopolymers in vegetated soils. Can. Geotech. J. 2023, 61, 1294–1310. [Google Scholar] [CrossRef]
- Cho, G.C.; Chang, I. Cementless soil stabilizer–biopolymer. In Proceedings of the 2018 World Congress on Advances in Civil, Environmental, & Materials Research (ACEM18), Incheon, Republic of Korea, 27–31 August 2018; pp. 27–31. [Google Scholar]
- Cho, G.C.; Kwon, Y.M. Biopolymer-based soil treatment (BPST). In Proceedings of the 2022 World Congress on Advances in Civil, Environmental, & Materials Research (ACEM22), Seoul, Republic of Korea, 16–19 August 2022. [Google Scholar]
- Ko, D.; Kang, J. Biopolymer-Reinforced Levee for Breach Development Retardation and Enhanced Erosion Control. Water 2020, 12, 1070. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Zhang, J.; Jiang, T.; Wang, S.; Zhao, J.; Meng, Z. Water retention characteristics and vegetation growth of biopolymer-treated silt soils. Soil Tillage Res. 2023, 225, 105544. [Google Scholar] [CrossRef]
- Savy, D.; Cozzolino, V. Novel fertilising products from lignin and its derivatives to enhance plant development and increase the sustainability of crop production. J. Clean. Prod. 2022, 366, 132832. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Kanungo, D.P.; Song, Z.; Bai, Y.; Wang, Y.; Li, D.; Qian, W. Topsoil reinforcement of sandy slope for preventing erosion using water-based polyurethane soil stabilizer. Eng. Geol. 2019, 252, 125–135. [Google Scholar] [CrossRef]
- Seo, S.; Lee, M.; Im, J.; Kwon, Y.M.; Chung, M.K.; Cho, G.C.; Chang, I. Site application of biopolymer-based soil treatment (BPST) for slope surface protection: In-situ wet-spraying method and strengthening effect verification. Constr. Build. Mater. 2021, 307, 124983. [Google Scholar] [CrossRef]
- Che, W.; Liu, J.; Hao, S.; Ren, J.; Song, Z.; Bu, F. Application of colloid-sand coating treated by a hydrophilic polysaccharide biopolymer material for topsoil stability control. Geoderma 2022, 424, 115994. [Google Scholar] [CrossRef]
- Ni, J.; Chen, J.; Liu, S.; Hao, G.; Geng, X. Experimental Study of the Usage of Combined Biopolymer and Plants in Reinforcing the Clayey Soil Exposed to Acidic and Alkaline Contaminations. Appl. Sci. 2022, 12, 5808. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, R.J.; Chen, J.Q.; Geng, X.Y. Mechanical and hydraulic characteristics of unvegetated or vegetated loess soils amended with xanthan gum. Transp. Geotech. 2024, 48, 101350. [Google Scholar] [CrossRef]
- Wan, J.; Tang, Z.; Liu, Y.; Xiao, H.; Wang, H. Study on the improvement of clay properties by xanthan gum and its application on ecological slope protection engineering. Environ. Technol. 2024, 45, 2762–2775. [Google Scholar] [CrossRef] [PubMed]
- ASTM D4318-17; Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D698-12; Standard Test Methods for Laboratory Compaction Characteristics of Soil Using Standard Effort (12,400 ft-lbf/ft3 (600 kN-m/m3)). ASTM International: West Conshohocken, PA, USA, 2012.
- ASTM D854-23; Standard Test Methods for Specific Gravity of Soil Solids by the Water Displacement Method. ASTM International: West Conshohocken, PA, USA, 2023.
- NSW Government. Rhodes Grass. Available online: https://www.dpi.nsw.gov.au/agriculture/pastures-and-rangelands/species-varieties/pf/factsheets/rhodes-grass (accessed on 8 April 2025).
- ASTM D2573-08; Standard Test Method for Field Vane Shear Test in Cohesive Soil. ASTM International: West Conshohocken, PA, USA, 2008. [CrossRef]
- Gratchev, I.; Bagheri, P.; Sugawara, J. Application of biopolymer for soil improvement: Laboratory and field experience. In Proceedings of the Thirteenth International Conference on Geotechnique, Construction Materials and Environment, GEOMATE, Tsu, Japan, 14–16 November 2023; The GEOMATE International Society: Kyoto, Japan, 2023. [Google Scholar]
- Burt, L.G. Handbook of Geotechnical Investigation and Design Tables; Taylor & Francis: Oxfordshire, UK, 2007. [Google Scholar]
- Ravindran, S.; Gratchev, I. Effect of Water Content on Apparent Cohesion of Soils from Landslide Sites. Geotechnics 2022, 2, 385–394. [Google Scholar] [CrossRef]
- Narjary, B.; Aggarwal, P.; Singh, A.; Chakraborty, D.; Singh, R. Water availability in different soils in relation to hydrogel application. Geoderma 2012, 187, 94–101. [Google Scholar] [CrossRef]
- Sharma, K.; Kaith, B.S.; Kumar, V.; Kalia, S.; Kumar, V.; Swart, H.C. Water retention and dye adsorption behavior of Gg-cl-poly (acrylic acid-aniline) based conductive hydrogels. Geoderma 2014, 232, 45–55. [Google Scholar] [CrossRef]






| Soil Type | Lignin Type | Additive Dosage (%) | Curing Period | Test Method | Key Findings | Reference |
|---|---|---|---|---|---|---|
| Aggregate mixed | Lignosulfonate | - | 7–28 days | UCS, durability |
| Bolander [14] |
| Clays | Lignosulfonate | 0.5, 0.6, 1, 1.5 | 0–28 days | UCT, EC |
| Indraratna et al. [15] |
| Sandy silt | Lignosulfonate | 0.5, 1, 2, 3, 4 | 7–28 days | TT |
| Chen et al. [16] |
| Silty soil | Lignin, quick lime | 2, 5, 8, 12, 15 | 0–60 days | UCT, CBR, Vs |
| Zhang et al. [17,18,19] |
| Silt soil | Lignin, lime, cement, fly ash | 7, 10, 12, 14, 16 | 1–28 days | UCT |
| Kong et al. [20] |
| Silty soil | Sulfur-free lignin | 3, 7, 10, 12, 15 | 1–60 days | UCT |
| Liu et al. [21,22] |
| Black cotton soil | Sodium lignosulfonate | 1, 3, 6, 9, 12 | 3, 7, 28 days | UCT, DST |
| Singh et al. [23] |
| Dispersive soil | Calcium-lignosulfonate | 0.5, 1, 2, 3, 4 | 28 days | UCT |
| Ji et al. [24] |
| Clay mixed granite sand | Calcium-lignosulfonate | 0.5, 1, 1.5, 2 | 0, 7, 14, 28, 90 days | UCT, HT |
| Amulya et al. [25] |
| Clay | Lignin-coir fibers, lime | 0.5, 1, 1.5 | – | UCT, CBR, VST |
| Boobalan and Sivakami, [26] |
| Silty soil | Calcium lignosulfonate | 0.5, 1.0, 1.5 | 28 days | UCT, DST |
| Du et al. [27] |
| Clay soil | Calcium lignosulfonate, granite sand | 0.25, 0.5, 1, 1.5; 30, 40, 50 | 7, 14 days | DST |
| Varsha et al. [28] |
| Silty soil | Lignosulfonate | 1, 3 | 1–35 days | TT, UCT |
| Bagheri et al. [8] |
| Clay soil | Sodium lignosulfonate | 0.5, 1.0, 1.5 | 7, 28, 90 | UCT |
| Vakili et al. [29] |
| Soil Type | Biopolymers | Content (%) | Method | Vegetation Type | Curing Days | Outcomes | Reference |
|---|---|---|---|---|---|---|---|
| Sand | Lignin-(guaiac, syringyl, p-hydroxyl phenyl) | 2 | Mixing—spraying | Agriophyllum squarrosum, Artemisia desertorum Spreng, etc. | 120–150 | The lignin biopolymer and the species used could form a community within 2 to 3 years and stabilize the desert dune significantly. | Hanjie et al. [34] |
| Red–yellow sand | Xanthan gum, β-glucan | 0.5 | Mixing | Oats (600 seeds) | 7–21 | Xanthan gum and β-glucan biopolymers stimulated seed germination and growth in natural and cultured soil. | Chang et al. [39] |
| Granular soil | IPCs, HPAN, PDADMAC, KNO3 | 1–2 wt.% | Mixing | Sudan grass | 1095–1460 | IPCs offer a considerable soil binder and enhance grass growth compared to the other polymers. | Zezin et al. [35] |
| Clayey soil | Xanthan gum, guar gum, agar gum, beta-glucan | 0.25, 0.5, 0.75, 1 | Mixing | Oats (160 seeds) | 2–14 | Xanthan gum efficiently promotes vegetation growth and increases the seed germination ratio by about 300% at 0.5% compared to other biopolymers. | Ni et al. [40] |
| Silt | Xanthan gum, guar gum, agar gum | 0.5 | Mixing | Ryegrass | 7, 14, 21, 28 | Xanthan gum-treated silt has the highest growth and germination rate compared to guar- and agar-treated silt. | Wang et al. [44] |
| Sand | Xanthan gum-starch | 0.5 | Spraying | Turfgrasses | 16 | Xanthan gum enhanced water retention, soil cohesion, and vegetation growth to improve erosion resistance. | Tran et al. [33] |
| Sand | Polyurethane | 0–20 | Mixing | Ryegrasses (60 g of seeds) | 10–60 | Polyurethane polymer did not show effective promotion of vegetation growth compared to untreated sand, especially at concentrations above 10%. | Liu et al. [46] |
| Sand | Xanthan gum, cationamyl, potassium nitrate, carboxy methyl cellulose | 0.5–1 | Mixing | Festuca, Poa pratensis, Lolium perenne and Trifolium repens seeds | 1–27 | Biopolymers are capable of improving the overall vegetation growth. | Nikolovska et al. [37] |
| Weathered granite soil | Xanthan gum, starch, β-glucan | 0.45–0.5 | Mixing—spray | Seeds | 30 | Xanthan gum, starch, and β-glucan compounds were implemented using the wet-spraying method to strengthen the structure and promote vegetation growth on levee slopes. | Seo et al. [47] |
| Sand | Hydrophilic polysaccharide biopolymer (HPB) | 0.05–1 | Spraying | Ryegrass and Bermuda grass | 12 | HPB concentration of less than 5% promotes vegetation growth. | Che et al. [48] |
| Clay soil | Xanthan gum | 0.25–1 | Mixing | Oats (160 seeds) | 1–14 | Xanthan gum promotes vegetation growth at 0.25% to 0.5% dosage; above 0.5% may impede vegetation growth. | Ni et al. [49] |
| Desert sand | MICP | 0.1–0.5 M | Spraying | Heracleum persicum | 7–30 | MICP biopolymer promotes the germination of Heracleum persicum. | Naeimi et al. [36] |
| Loess | Xanthan gum | 0–1 | Mixing | Oats (160 seeds) | 14 | Xanthan gum yields higher germination from 0.25% to 1% dosage and high root content at 0.25% to 0.75%. | Ni et al. [50] |
| Clay | Xanthan gum | 0–4 | Mixing | LP seeds | 60 | Xanthan gum promotes LP growth, and increasing Xanthan dosage increases the growth rate. | Wan et al. [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gratchev, I.; Tang, Q.; Akosah, S.; Sugawara, J. Application of Lignin for Slope Bioengineering: Effect on Soil Improvement and Plant Growth. Appl. Sci. 2025, 15, 4173. https://doi.org/10.3390/app15084173
Gratchev I, Tang Q, Akosah S, Sugawara J. Application of Lignin for Slope Bioengineering: Effect on Soil Improvement and Plant Growth. Applied Sciences. 2025; 15(8):4173. https://doi.org/10.3390/app15084173
Chicago/Turabian StyleGratchev, Ivan, Qianhao Tang, Stephen Akosah, and Jun Sugawara. 2025. "Application of Lignin for Slope Bioengineering: Effect on Soil Improvement and Plant Growth" Applied Sciences 15, no. 8: 4173. https://doi.org/10.3390/app15084173
APA StyleGratchev, I., Tang, Q., Akosah, S., & Sugawara, J. (2025). Application of Lignin for Slope Bioengineering: Effect on Soil Improvement and Plant Growth. Applied Sciences, 15(8), 4173. https://doi.org/10.3390/app15084173






