Abstract
Cadmium (Cd) is a ubiquitous environmental pollutant with no nutritional value or physiological role in the body. It readily accumulates in various tissues as it is easily absorbed from the diet but only poorly excreted. Due to the widespread contamination of staple foods, exposure to this toxic metal is inevitable for most people. The health risk due to dietary Cd exposure has long been underappreciated. This is primarily due to the use of urinary excretion of β2-microglobulin (β2M) as an indicator of an adverse health effect. This study employed advanced benchmark dose (BMD) modeling in a Thai cohort (n = 799) to reassess health risks from dietary Cd exposure. The BMD limit for urinary Cd was identified as 0.17 μg/g creatinine when using a reduction in estimated glomerular filtration rate (eGFR) as the endpoint, whereas no reliable BMD could be established using β2-microglobulin excretion. Given that eGFR reduction is a more reliable indicator of chronic kidney disease, its use is recommended for deriving health-protective guidelines. The findings demonstrate that the current urinary Cd threshold of 5.24 μg/g creatinine is inadequate, supporting the adoption of a threshold below 0.20 μg/g creatinine in future exposure guidelines.
1. Introduction
Consumption of rice contaminated with the toxic metal cadmium (Cd) can cause itai-itai disease, marked by loss of kidney mass, osteoporosis, and osteomalacia [1,2,3,4]. Based on a lifetime intake of 2 g of Cd, and an excretion of β2-microglobulin (β2M) at a rate above 300 µg/g creatinine (cr), a tolerable Cd intake level was estimated to be 0.83 µg/kg b.w./d (58 µg/d for a 70 kg person), with a threshold of 5.24 µg/g cr [5]. By definition, a threshold level for an adverse effect of any food contaminant reflects the exposure level from a normal diet that can produce an adverse effect in 5% of the general populations [6,7,8,9]. However, rates of many Cd-related health effects, notably chronic kidney disease (CKD), are higher than the acceptable environmental-related disease prevalence of 5%.
The diagnosis of CKD is based on a fall of estimated glomerular filtration rate (eGFR) to one-third of a normal range (60 mL/min/1.73 m2) and/or the presence of albuminuria for at least 3 months [10,11,12]. Presently, CKD affects 8–13% of the adult population worldwide, and it has now reached epidemic proportions in many parts of the world [13,14,15]. A dose–response relationship between Cd exposure and CKD risk has been reported in a meta-analysis by Doccioli et al. [13]. The urinary Cd excretion levels associated with an increased risk of CKD were below a Cd excretion threshold of 5.24 µg/g cr. These human population data imply that current Cd exposure guidelines do not afford adequate health protection. Concerningly, CKD is predicted to be the fifth-leading cause of years of life lost by 2040 [14,15]. Developing strategies to prevent CKD and to reduce its progression to kidney failure is thus of great public health significance.
Dietary Cd exposure guidelines were also determined by the European Food Safety Agency (EFSA) and US Food and Drug Administration (US FDA) [6]. The EFSA guideline describes a reference dose (RfD), while the US FDA guideline describes a toxicological reference value (TRV). Notably, however, most countries adopted the JECFA guidelines [5].
The present work has three main objectives; firstly, to apply advanced benchmark dose (BMD) modeling to define the BMD limit (BMDL) value for urinary Cd excretion, which may have a negligible impact on the kidneys [16,17,18,19]. The BMDL figure for any health-hazardous substance in foodstuffs has now become a replacement for the no-observed-adverse-effect level (NOAEL) [18,19]. To the best of our knowledge, the present study is the first report of estimating a urinary Cd threshold based on a clinically relevant outcome.
The second objective it was to ascertain whether there is a significant impact of Cd exposure on CKD risk. To this end, urinary Cd excretion rate was used as an indicator of kidney burden, while changes in eGFR and β2M excretion reflected adverse effects on the kidneys. Data were from a Thai cohort of 799 individuals, 18–87 years of age, who did not have diabetes nor had workplace exposure to metals.
The third objective was to reiterate that the practice of adjusting urinary concentrations of Cd and β2M to creatinine excretion (Ecr) can obscure Cd’s effects on the kidneys. This phenomenon is described as reverse causality due to the imprecision in measuring Cd exposure dose levels and its effects [20]. These biases have led to erroneous conclusions that Cd did not diminish eGFR nor did it promote progressive eGFR reduction toward kidney failure [21,22]. Accordingly, results of Cd nephrotoxicity assessment obtained from adjusting excretion rates of Cd and β2M to Ecr as ECd/Ecr were compared with those adjusted by creatinine clearance (Ccr) as ECd/Ccr and Eβ2M/Ccr.
2. Materials and Methods
2.1. Data Sourcing
A reliable toxicological risk assessment requires a population exposed to a wide range of Cd doses to establish a clear dose–response relationship. The Mae Sot District in western Thailand, a geographic area with endemic environmental Cd pollution, provided a well-circumscribed population of people with the same level of exposure that would enable one to discern the health impact of excessive dietary Cd exposure [23]. More than 40% of residents 40 years of age and older were at risk of Cd nephrotoxicity [24].
The Cd concentration of the paddy soil samples from the Mae Sot District exceeded the standard of 0.15 mg/kg, and the rice samples collected from household storage contained four times the amount of the permissible Cd level of 0.1 mg/kg [25]. In a health survey of the Mae Sot residents (n = 5273), urinary Cd excretion levels correlated with the prevalence rates of hypertension and diabetes [26].
The present study selected individuals without diabetes from a pre-existing Thai population cohort (n = 1189) where residents of Bangkok, Nakhon Si Thammarat Province and the Mae Sot District, Tak Province were enrolled [27]. Following an exclusion of outliers and those with incomplete data, 799 individuals were analyzed in the present study. The Mae Sot residents represented the moderate-to-high Cd exposure group. The residents of Nakhon Si Thammarat Province were considered to represent low environmental Cd exposure conditions in non-urban areas, given that levels of arsenic, chromium, lead, and Cd in samples of soils and food crops in Nakhon Si Thammarat were within permissible ranges [28]. In addition, water arsenic concentrations were not significantly related to risk of diabetes in the region [29].
All participants enrolled in the parent cohort gave informed consent prior to participation. Inclusion criteria for the Mae Sot and Nakhon Si Thammarat groups were living at their current addresses for 30 years or longer. For the Bangkok group, living at their current addresses for at least 16 years was the inclusion criterion.
Exclusion criteria were pregnancy, breastfeeding, a history of exposure to metals in workplace settings, and a hospital record or physician’s diagnosis of an advanced chronic disease. The sociodemographic data, smoking status, educational attainment, occupation, health status, family history of diabetes, and use of food supplements were obtained by structured interview questionnaires.
2.2. Blood and Urine Sampling and Chemical Compositional Analysis
Samples of venous blood and morning voided urine were collected after an overnight fast. Aliquots of urine, whole blood, serum, and plasma were stored at −80 °C for later analysis. To prevent the degradation of β2M in acidic conditions, 1 M NaOH was added to adjust the pH of urine aliquots to >6.
The concentrations of creatinine in urine and plasma samples were determined by alkaline picrate Jaffe’s reaction [30]. The urine concentration of β2M was determined by the immunoagglutination method [31] or the human beta-2 microglobulin/β2M ELISA pair set (Sino Biological Inc., Wayne, PA, USA).
Urinary Cd concentration was determined by graphite furnace atomic absorption spectrometry [32]. To calibrate the instrument, multielement standards (Merck KGaA, Darmstadt, Germany) were used. Reference urine metal controls (Lyphocheck, Bio-Rad, Hercules, CA, USA) or urine standard reference material No. 2670 (National Institute of Standards, Washington, DC, USA) was used for quality control, accuracy, and precision assurance of Cd analysis.
The limit of detection (LOD) value for urinary Cd was defined as the standard deviation values of at least ten blank sample measurements multiplied by 3. For any urine sample containing Cd below the LOD, the Cd concentration assigned was the LOD value divided by the square root of 2 [33].
2.3. Calculation of eGFR and Normalization of Cd and β2M Excretion Rates
The eGFR was computed with equations of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [34]. CKD stages 1, 2, 3, 4, and 5 corresponded to eGFR of 90–119, 60–89, 30–59, 15–29, and <15 mL/min/1.73 m2, respectively [34].
Because urine samples were collected at a single time point (voided urine), a correction for interindividual differences in urine volume (dilution) was undertaken. Accordingly, the excretions of Cd (ECd) and β2M (Eβ2M) were adjusted to creatinine excretion (Ecr) and creatinine clearance (Ccr), using the below equations.
Ex/Ecr = [x]u/[cr]u, where x = Cd or β2M; [x]u = urine concentration of x (mass/volume) and [cr]u = urine creatinine concentration (mg/dL). Ex/Ecr was expressed as an amount of x excreted per g of creatinine.
Ex/Ccr = [x]u[cr]p/[cr]u, where x = Cd or β2M; [x]u = urine concentration of x (mass/volume); [cr]p = plasma creatinine concentration (mg/dL); and [cr]u = urine creatinine concentration (mg/dL). Ex/Ccr was expressed as an amount of x excreted per volume of the glomerular filtrate [35].
2.4. Benchmark Dose Modeling
We used the PROAST software version 71.1 (https://proastweb.rivm.nl, accessed on 28 May 2025) to estimate benchmark dose limit (BMDL) values for urinary Cd excretion rate associated with two toxic endpoints: eGFR reduction and abnormal β2M excretion rates. The BMD was estimated through fitting an entire exposure–effect dataset using multiple dose–response models with a pre-defined specific effect size, referred to as the benchmark response (BMR) [16,17,18,19].
For continuous endpoints (eGFR reduction and abnormal β2M excretion rates), a lower 95% confidence bound of BMD is referred to as the BMDL value, and the BMDL value derived when BMR is set at 5% can represent the NOAEL equivalent [18,19]. An upper 95% confidence bound of BMD is referred to as BMDU. The BMDU/BMDL ratio represents the degree of uncertainty in BMD estimates [18,19]. The mathematical equations applied to continuous endpoints were inverse exponential, natural logarithmic, exponential, and Hill dose–response models [36,37].
For quantal endpoints (% CKD and % abnormal β2M excretion rates), respective BMDL5 and BMDL10 values refer to the lower 95% confidence bound of BMD values derived when the prevalence of any outcome is set at 5% and 10% [18,19]. Similarly, respective BMDU5 and BMDU10 refer to the upper 95% confidence bound of BMD values derived when the prevalence of any outcome (% CKD and % abnormal β2M excretion rates) is set at 5% and 10%. The BMDL5 could be considered as a population exposure threshold level. The mathematical equations applied to quantal endpoints were two-stage, logarithmic logistic, Weibull, logarithmic probability, gamma, exponential, and Hill dose–response models [16,38,39].
For both continuous and quantal response endpoints, we evaluated model performance using the Akaike information criterion (AIC), which balances goodness of fit against model complexity to reduce the risk of overfitting and underfitting. Model weights, reflecting the relative information retained by each model, were higher for models with less information loss, indicating better quality.
2.5. Statistical Analysis
Data were analyzed with IBM SPSS Statistics 21 (IBM Inc., New York, NY, USA). The variability of any continuous variable and differences in percentages across the eGFR groups were assessed by the Kruskal–Wallis test and the Pearson chi-squared test, respectively. The one-sample Kolmogorov–Smirnov test was used to assess departure from a normal distribution of any continuous variable. Logarithmic transformation was applied to the excretion rates of Cd and β2M that showed rightward skewing before they were subjected to parametric statistics analyses.
The prevalence odds ratio (POR) values for CKD and abnormal β2M excretion levels 1, 2, and 3 were determined by multivariable logistic regression modeling with adjustment for covariates, namely age, body mass index (BMI), gender, smoking, and hypertension.
3. Results
3.1. Cd, eGFR, and β2M Excretion Levels in Study Subjects
Subjects were grouped according to their eGFR values, where 426, 303, and 70 persons had eGFR ≥ 90, 61–89, and ≤60 mL/min/1.73 m2, respectively (Table 1).

Table 1.
Characteristics of study subjects grouped by eGFR.
The overall mean age was 49.2 years (range: 18−87 years), and the percentages (%) of women, smokers, and those with hypertension were 62.5, 32.5, and 42.7, respectively. The low-eGFR group was the oldest. It had the highest % of hypertension (42.9) and abnormal excretion of β2M; Eβ2M/Ecr ≥ 300 µg/g cr (65.7) and (Eβ2M/Ccr) × 100 ≥ 300 µg/L filtrate (70).
Respective arithmetic (geometric) means for ECd/Ecr and (ECd/Ccr) × 100 were 5.24 (2.15) µg/g creatinine and 5.05 (1.82) µg/L filtrate. Mean Eβ2M/Ecr and mean (Eβ2M/Ccr) × 100 values were the highest, moderate, and lowest in the groups with eGFR values ≤ 60, 61–89, and ≥90 mL/min/1.73 m2, respectively. The BMI variability in the three eGFR groups did not differ statistically.
3.2. Determinants of the Prevalence Odds for CKD (eGFR ≤ 60 mL/min/1.73 m2)
Logistic regression modeling was employed to assess effects of Cd exposure on the prevalence odds ratio (POR) for CKD (eGFR criterion) with adjustment for covariates: age, BMI, gender, smoking, and hypertension. Two models were constructed; ECd was incorporated in models A and B as ECd/Ecr and ECd/Ccr, respectively. All other independent variables in both models A and B were identical. Results are presented in Table 2.

Table 2.
Determinants of the prevalence odds ratios for CKD.
As shown in model A, POR for CKD was affected by age, BMI ≥ 24 kg/m2, and ECd/Ecr, while an effect of hypertension did not reach statistically significant levels (p = 0.068). The POR for CKD rose 1.98-fold and 4-fold by a 2-fold increase in ECd/Ecr and BMI ≥ 24 kg/m2. It rose 7.3% for every one-year increase in age.
As shown in model B, POR for CKD was affected by hypertension, age, BMI ≥ 24 kg/m2, and ECd/Ccr. POR for CKD rose 4.78-fold, 3.13-fold, and 2.66-fold by BMI ≥ 24 kg/m2, doubling ECd/Ccr, and hypertension, respectively. It rose 6.8% for every one-year increase in age.
3.3. Incremental β2M Excretion Rates in Relation to Cd Excretion Levels
Additional logistic regression models were conducted to examine whether the incremental levels of β2M excretion could be related to Cd excretion levels in a dose-dependent manner (Table 3).

Table 3.
Determinants of the prevalence odds ratios for abnormal β2M excretion rates.
As shown in Table 3, Cd excretion levels were incorporated in the regression model as categorial independent variables together with CKD (eGFR ≤ 60 mL/min/1.73 m2), age, BMI, gender, hypertension, and smoking. In both models A and B, the POR values for the β2M excretion levels 1, 2, and 3 were more prevalent in those with CKD. As expected, β2M excretion level 3 appeared to occur in the highest frequency in those with CKD in both models.
Notably, however, in model A, a dose–effect relationship could not be established between Cd excretion levels and the graded incremental β2M excretion levels. POR values for β2M excretion levels 1, 2, and 3 in the subjects with the highest Cd exposure category did not differ. Thus, β2M excretion levels 1, 2, and 3 occurred in the same high frequencies as ECd/Ecr rose above 2.50 µg/g creatinine.
In comparison, a dose–effect relationship between of Cd excretion and β2M excretion levels was apparent in model B. POR values for β2M excretion levels 1, 2, and 3 rose 5.8-fold, 6.4-fold, and 15.4-fold in the highest Cd exposure category. Thus, β2M excretion level 3 occurred in the highest frequency in the group with the highest Cd exposure [(ECd/Ccr) × 100 ≥ 5 µg/L filtrate] as did CKD.
3.4. Cd Excretion Benchmarks Derive from Continuous eGFR and β2M Endpoints
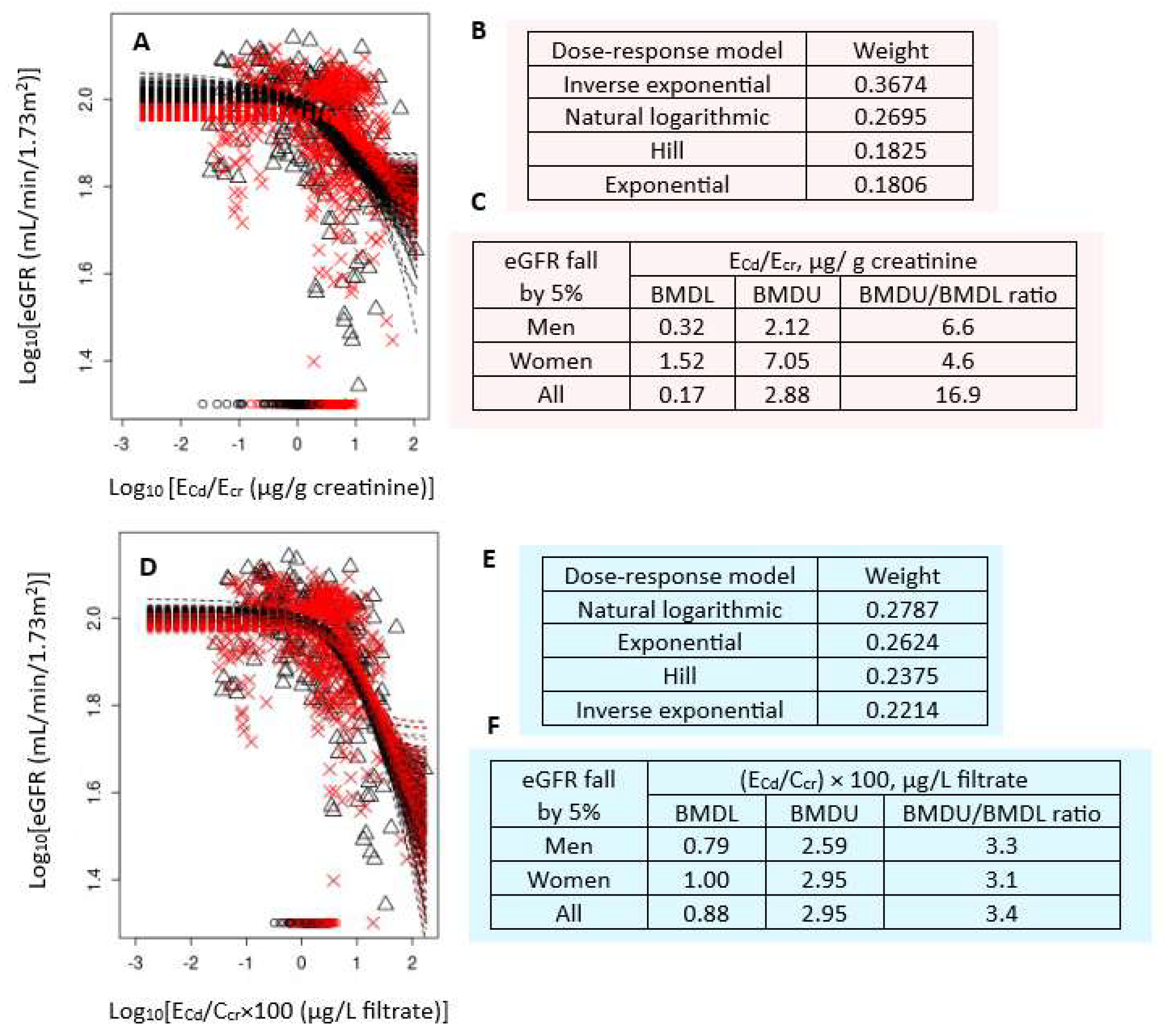
Figure 1 presents the BMD modeling of two eGFR/ECd datasets, including bootstrap model averaging curves (Figure 1A,D), model weights (Figure 1B,E), and BMDU/BMDL values of ECd (Figure 1C,F).
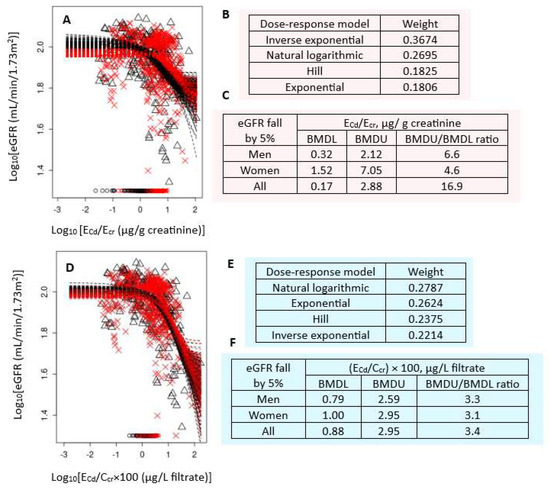
Figure 1.
Cadmium excretion benchmarks for a 5% reduction in eGFR. Bootstrap dose–response model averaging for eGFR-ECd/Ecr (A) and eGFR-ECd/Ccr (D); Model weights for ECd/Ecr (B) and ECd/Ccr (E); BMDL/BMDU values for ECd/Ecr (C) and ECd/Ccr (F). × and Δ represent male and female participants, respectively. eGFR: Estimated glomerular filtration rate; Cd: cadmium; Ecr: creatinine excretion; BMDL: benchmark dose limit; ECd: urinary Cd; BMDU: upper 95% confidence bound of BMD.
For Ecr-adjusted datasets, the inverse exponential model had the highest weight (0.3674), followed by the natural logarithmic model (0.2695) (Figure 1B). In all subjects, ECd/Ecr of 0.17 µg/g cr produced a 5% reduction in eGFR (Figure 1C). The BMDL value of ECd/Ecr producing the same BMR in women was 4.8-fold higher than in men (1.52 vs. 0.32 µg/g cr). The BMDU/BMDL ratio for the benchmark ECd/Ecr of 0.17 µg/g cr in all subjects was 16.9.
For Ccr-adjusted datasets, the weights for the natural logarithmic model (0.2787) and the exponential model (0.2624) were close (Figure 1E). In all subjects, (ECd/Ccr) × 100 of 0.88 µg/L filtrate (Figure 1F) produced a 5% reduction in eGFR. BMDL value of (ECd/Ccr) × 100 for the same BMR was only 21% higher in women compared to men (0.79 vs. 1 µg/L filtrate) (Figure 1F). The BMDU/BMDL ratio for the Ccr-based benchmark was 3.4. This indicated a higher degree of certainty, compared to the Ecr-based BMD estimates.
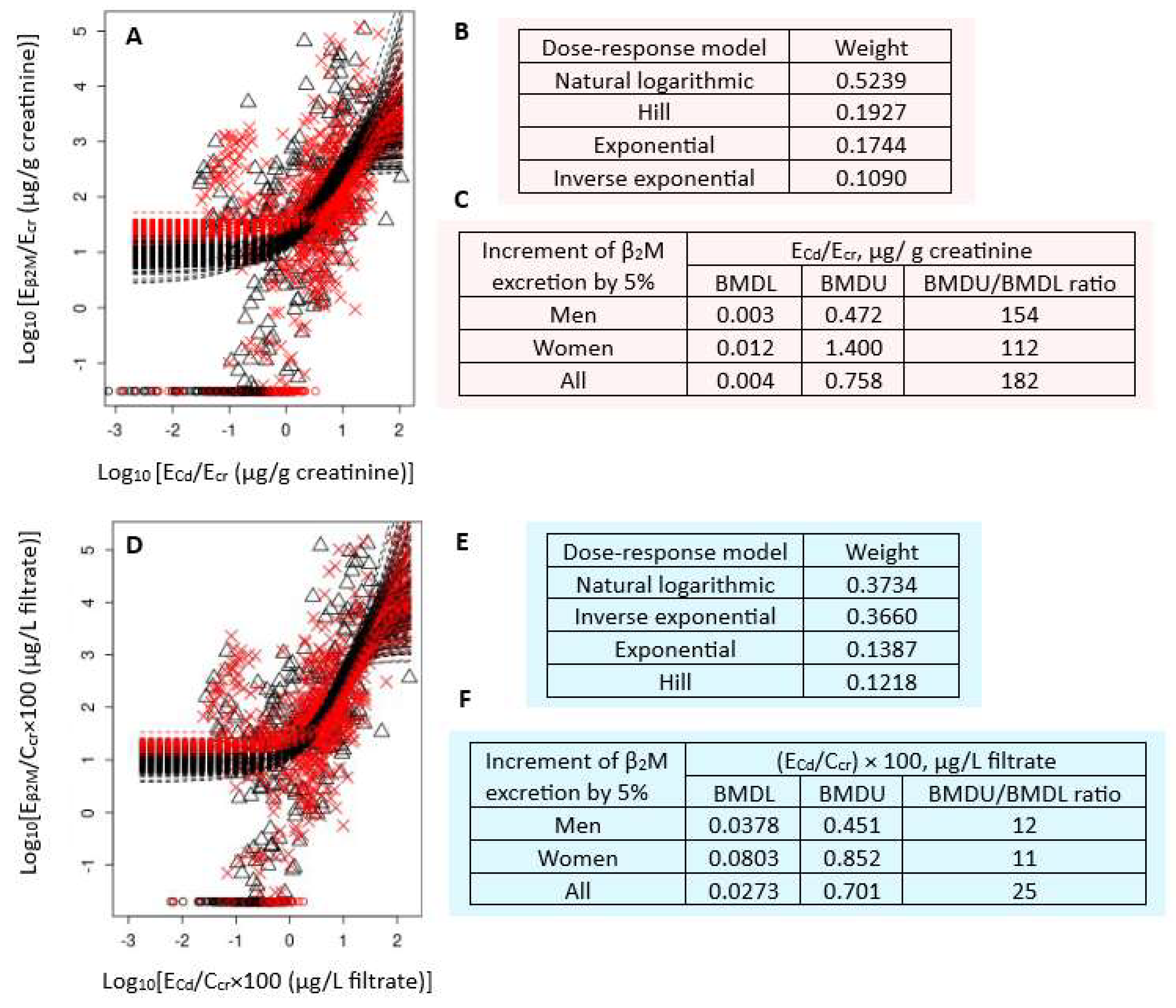
Figure 2 presents the BMD modeling of two Eβ2M/ECd datasets, including bootstrap model averaging curves (Figure 2A,D), model weights (Figure 2B,E), and BMDU/BMDL values of ECd (Figure 2C,F).
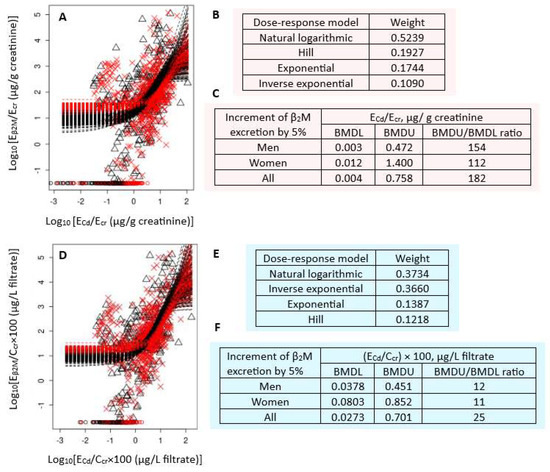
Figure 2.
Cadmium excretion benchmarks for a 5% increase in β2M excretion levels. Bootstrap dose–response model averaging for Eβ2M/Ecr-ECd/Ecr (A) and Eβ2M/Ccr-ECd/Ccr (D); Model weights for Eβ2M/Ecr (B) and Eβ2M/Ccr (E); BMDL/BMDU values ECd/Ecr (C) and ECd/Ccr (F). × and △ represent male and female participants, respectively. β2M: β2-microglobulin; Eβ2M: urinary β2M; Cd: cadmium; Ecr: creatinine excretion; BMDL: benchmark dose limit; ECd: urinary Cd; BMDU: upper 95% confidence bound of BMD.
For Ecr-adjusted datasets, the weights of the natural logarithmic model (0.3734) and inverse exponential model (0.3660) were nearly the same (Figure 2B). The BMDL value of ECd/Ecr producing a 5% increment in Eβ2M/Ecr were unreliable because the BMDU/BMDL ratios (an uncertainty index) were higher than 100 (Figure 2C). The uncertainty indices for men, women, and all subjects were 154, 112, and 182, respectively.
For Ccr-adjusted datasets, the natural logarithmic model had the highest weight (0.5239), followed by the Hill model (0.1927) (Figure 2B). The BMDL values of (ECd/Ccr) × 100 producing a 5% Eβ2M/Ccr increment endpoint were 0.04, 0.08, and 0.03 µg/L filtrate in men, women, and all subjects, respectively (Figure 2F). These ECd benchmarks were of high certainty, reflected by BMDU/BMDL ratios of 12, 11, and 15 for men, women, and all subjects, respectively.
3.5. Cd Excretion Benchmarks Derived from CKD and Abnormal β2M Excretion Prevalences
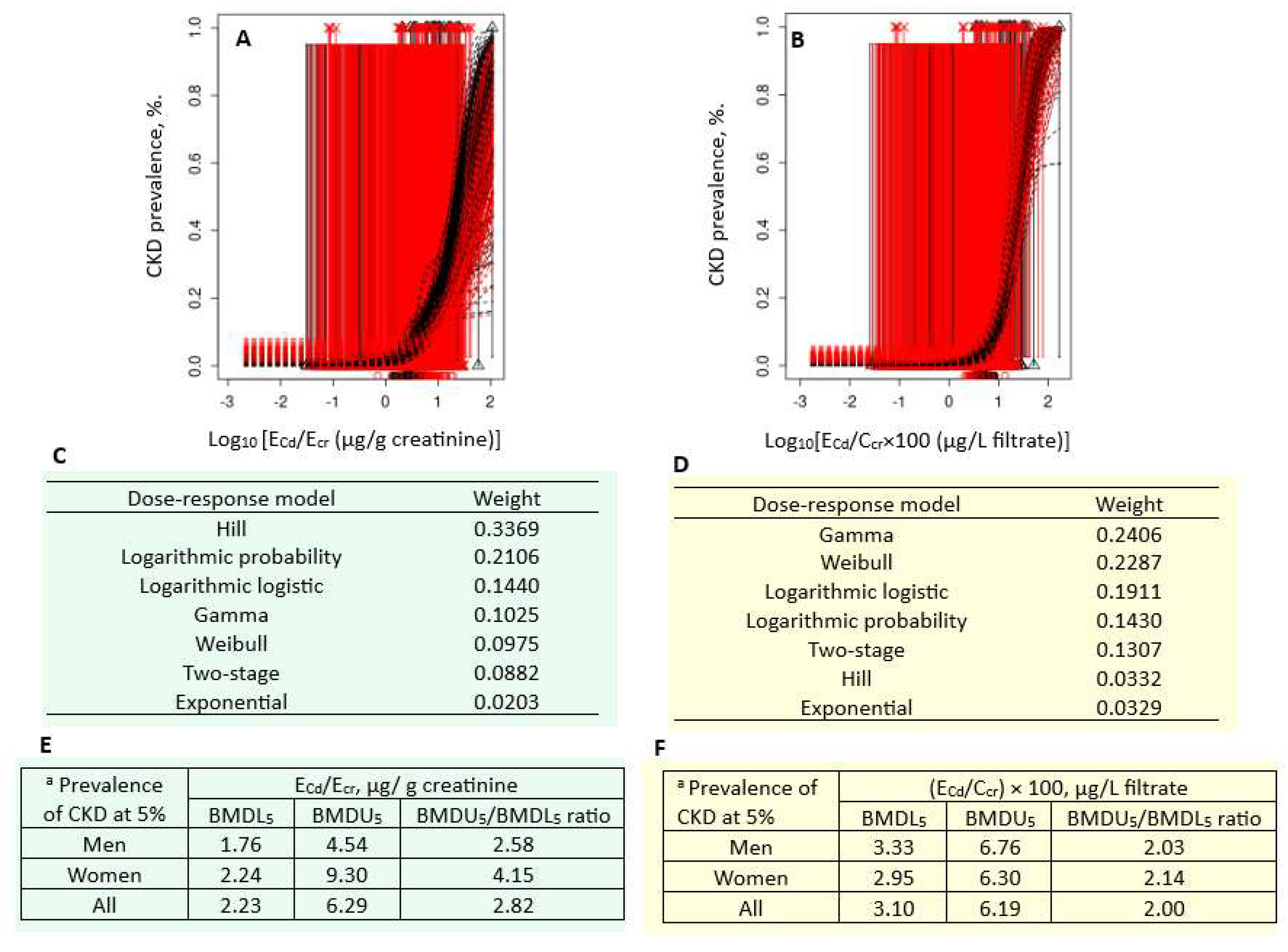
Figure 3 presents the quantal BMD modeling of two CKD prevalence/ECd datasets, including bootstrap model averaging curves (Figure 3A,B), model weights (Figure 3C,D), and BMDU/BMDL values of ECd (Figure 3E,F).
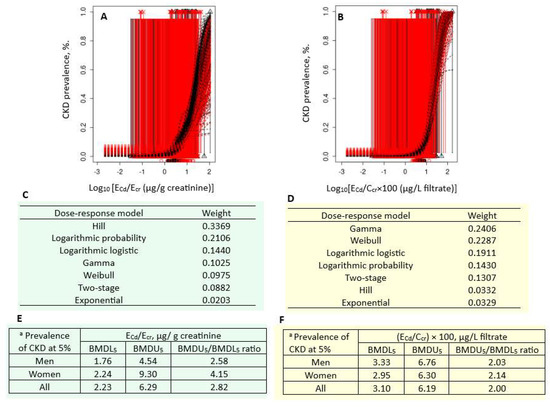
Figure 3.
Cadmium excretion levels associated with 5% prevalences of CKD. Bootstrap dose–effect model averaging for CKD prevalence/ECd/Ecr (A) and CKD prevalence/ECd/Ccr (B). Model weights for ECd/Ecr (C) and ECd/Ccr (D). BMDL5/BMDU5 values of ECd/Ecr (E) and ECd/Ccr (F). a CKD was defined as eGFR ≤ 60 mL/min/1.73 m2. CKD: chronic kidney disease; Cd: cadmium; ECd: urinary Cd; Ecr: creatinine excretion; BMDL: benchmark dose limit; BMDU: upper 95% confidence bound of BMD.
For the Ecr-adjusted datasets, the weight of the Hill model (0.3369) was the highest, followed by the logarithmic probability (0.2106) and the logarithmic logistic (0.1440) models (Figure 3C). Respective ECd/Ecr benchmarks for a 5% CKD prevalence in men, women, and all subjects were 1.76, 2.24, and 2.23 µg/g cr (Figure 3E). The uncertainty indices for the ECd/Ecr benchmark estimates for men, women, and all subjects were 2.58, 4.15, and 2.82.
For the Ccr-adjusted datasets, the weight of the gamma model (0.2406) was close to that of the Weibull model (0.2287) (Figure 3D). Respective ECd/Ecr benchmarks for a 5% CKD prevalence in men, women, and all subjects were 3.33, 2.95, and 3.10 µg/g cr (Figure 3F). The uncertainty indices for the ECd/Ecr benchmark estimates for men, women, and all subjects were 2.03, 2.14, and 2.00.
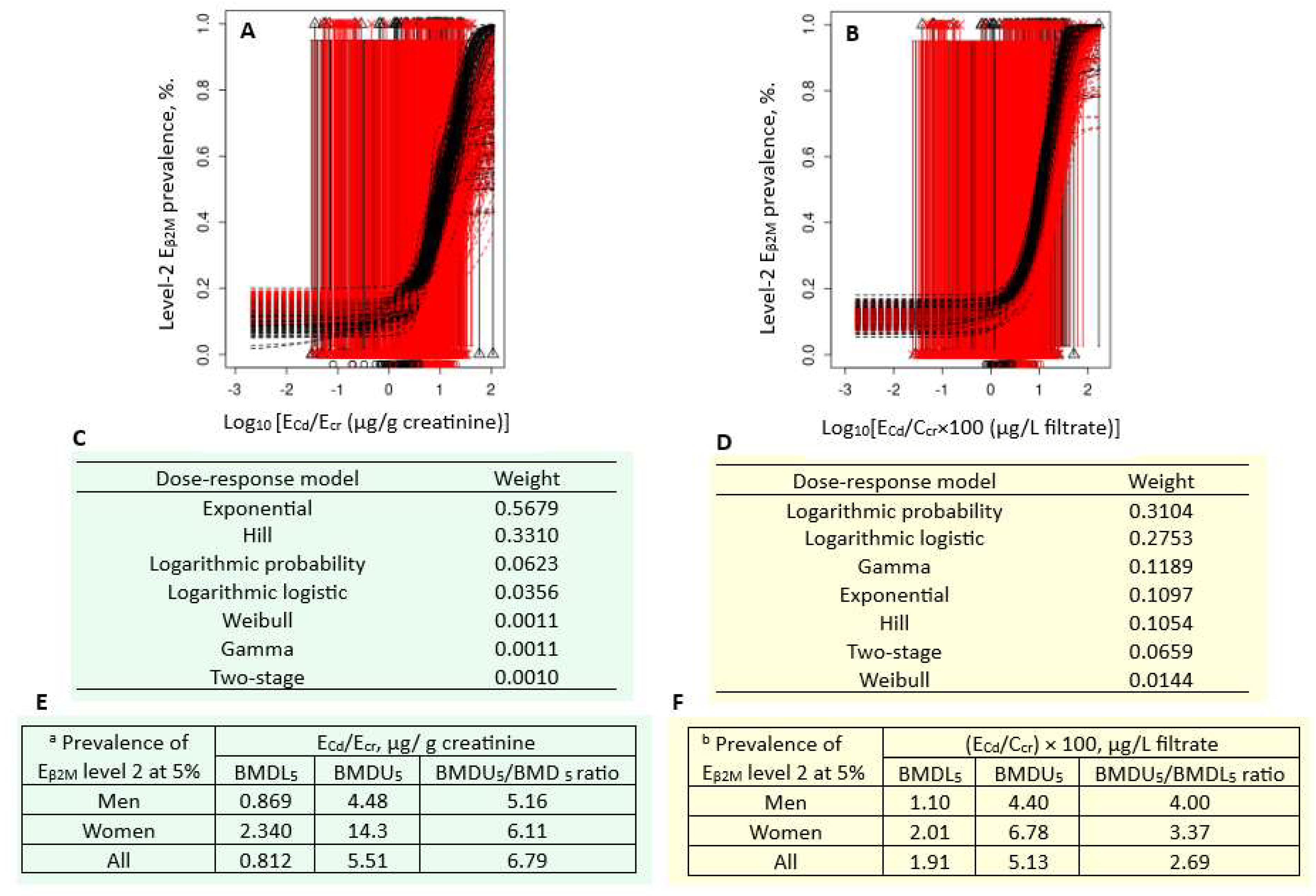
Figure 4 presents the quantal BMD modeling of two abnormal β2M excretion prevalence/ECd datasets, including bootstrap model averaging curves (Figure 4A,B), model weights (Figure 4C,D), and BMDU/BMDL values of ECd (Figure 4E,F).
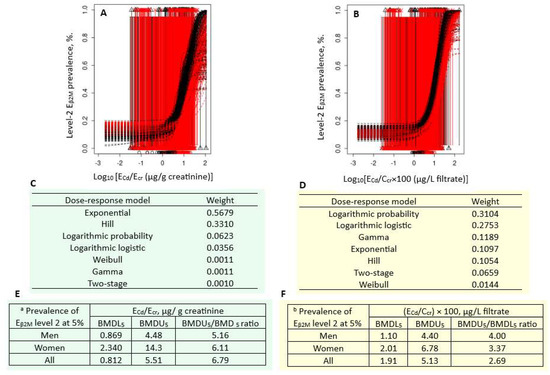
Figure 4.
Cadmium excretion rates associated with 5% prevalence β2M excretion level 2. Bootstrap dose–effect model averaging for Eβ2M/Ecr (A) and Eβ2M/Ccr (B). Model weights for Eβ2M/Ecr (C) and Eβ2M/Ccr (D). BMDL5/BMDU5 values of ECd/Ecr (E) and ECd/Ccr (F). a Eβ2M/Ecr level 2 was defined as Eβ2M/Ecr ≥ 300 µg/g creatinine. b Eβ2M/Ccr level 2 was defined as (Eβ2M/Ccr) × 100 ≥ 300 µg/L filtrate. β2M: β2-microglobulin; Eβ2M, urinary β2M; Cd, cadmium; ECd, urinary Cd; Ecr, creatinine excretion; BMDL, benchmark dose limit; BMDU, upper 95% confidence bound of BMD.
For the Ecr-adjusted datasets, the weight of the exponential model (0.5679) was the highest, followed by the Hill model (0.3310) (Figure 4C). Respective ECd/Ecr benchmarks for a 5% prevalence of Eβ2M/Ecr values ≥ 300 µg/g cr in men, women, and all subjects were 0.869, 2.340, and 0.812 µg/g cr (Figure 4E). The uncertainty indices for the ECd/Ecr benchmark estimates were 4, 14.3, and 5.51 for men, women, and all subjects, respectively.
For the Ccr-adjusted datasets, the weights of the logarithmic probability model (0.3104) and the logarithmic logistic model (0.2753) were close (Figure 4D). Respective ECd/Ecr benchmarks for a 5% prevalence of (Eβ2M/Ccr) × 100 values ≥ 300 µg/L filtrate in men, women, and all subjects were 1.10, 2.01, and 1.91 µg/L filtrate (Figure 4F). All estimated benchmarks were of the high statistical certainty, given that the uncertainty indices were as little as 1.10, 2.01, and 1.91 for men, women, and all subjects, respectively.
Equivalent quantal BMD modeling for prevalences of abnormal Eβ2M excretion values were conducted using two additional cut-off values: ≥100 and ≥1000 µg/g cr for Ecr-adjusted datasets and ≥100 and ≥1000 µg/L filtrate for Ccr-adjusted datasets. Full results are provided in Figures S1 and S2. Urinary Cd excretion benchmarks estimated from these cut-off values are displayed in Table 4 together with those reported in Figure 3E,F and Figure 4E,F.

Table 4.
Quantal Cd excretion benchmarks derived from abnormal eGFR values and abnormal β2M excretion rate.
For the Ecr-normalized dataset, benchmark Cd excretion rates producing 5% prevalences of low eGFR with Eβ2M/Ecr values ≥ 100, ≥ 300, and 1000 µg/g cr were 0.640, 0.812, and 2.35 µg/g cr, respectively. Notably, the benchmark Cd excretion for 5% prevalence of Eβ2M/Ecr levels ≥ 1000 µg/g cr was higher than the figure for low eGFR.
For the Ccr-normalized dataset, benchmark Cd excretion rates producing 5% prevalences of low eGFR with (Eβ2M/Ccr) × 100 values ≥ 100, ≥300, and 1000 µg/L filtrate were 0.93, 1.91, and 3.62 µg/L filtrate, respectively. Similarly, the benchmark Cd excretion for 5% prevalence of (Eβ2M/Ccr) × 100 values ≥ 1000 µg/L filtrate was higher than the figure for low eGFR.
3.6. Validity Analysis of a Proposed Urinary Cd Threshold
In a 4 × 4 cross-tabulation analysis of 799 subjects, the % of low eGFR (CKD) in those with ECd/Ecr below a new threshold figure was 5.1%. The CKD prevalence rate rose to 9.2% in those with ECd/Ecr ≥ 0.2 µg/g cr. The chi-square test of the prevalence rate ratio (9.2/5.1) did not reach statistical significance levels (p = 0.232). However, in a logistic regression analysis adjusting for age, BMI, smoking, gender, and hypertension, ECd/Ecr at 0.2 µg/g cr still showed an association with an increased risk of CKD (p = 0.020) (Table S1).
4. Discussion
4.1. Cd Exposure as a Risk Factor for CKD and Understated Toxicity by Ecr Adjustment
Consistent with a large body of literature, and a recent meta-analysis evaluating an effect of Cd exposure on CKD risk [10], age, BMI, blood pressure, and chronic exposure to Cd independently influenced risk for CKD, defined as eGFR falling to 60 mL/min/1.73 m2 or below. For every one-year increase in age, CKD risk rose 6.8%, while BMI ≥ 24 kg/m2, doubling ECd/Ccr, and hypertension raised risk of CKD 4.78-fold, 3.13-fold, and 2.66-fold, respectively (model B, Table 2).
As revealed by model A (Table 2), an independent effect of hypertension on CKD risk was not apparent because of adjusting urinary Cd excretion to Ecr. For the same reason, the effect size of Cd on CKD dropped 68%; the POR for CKD rose 1.98 vs. 3.13 by two-fold increments of ECd/Ecr vs. ECd/Ccr.
Another notable result is that adjusting ECd and Eβ2M to Ecr nullified the dose–response relationship between Cd exposure and the severity of Cd toxicity, reflected by β2M excretion (Table 3). The POR for β2M excretion levels 1, 2, and 3 in the highest exposure group (ECd/Ecr ≥ 2.50 µg/g cr) increased 5.3-fold, 3.5-fold, and 4.5-fold. These data were in line with a study from Japan, where differences in the prevalence of abnormal β2M excretion rates were unrelated to Cd exposure but were related to age [40]
In comparison, however, a dose–response relationship was observed in Ccr-normalized datasets; the POR for β2M excretion levels 1, 2, and 3 rose 5.8-fold, 6.4-fold, and 15.4-fold in the highest exposure group ((ECd/Ccr) × 100 ≥ 5 µg/L filtrate). Thus, for accurate measurements of Cd exposure levels and kidney effects, urinary concentrations of Cd and biomarkers of kidney effects, e.g., β2M, should be normalized to creatinine clearance (Ccr).
4.2. Reduction in eGFR Due to Cd-Induced Nephron Destruction
Notably, β2M excretion level 3 was most prevalent in those with low eGFR (CKD) (Table 3), thereby suggesting that a massive β2M excretion could be a consequence of nephron destruction due to the cytotoxicity of cumulative Cd in the proximal tubular cells of the kidneys [41]. A fall in eGFR at a high rate (≥3 mL/min/1.73 m2 per year) among cohort participants has been causally linked to Cd excretion in a longitudinal study from Switzerland [42]. In another prospective cohort study from Japan, a large decrease in eGFR was observed over a 5-year observation period among participants who had β2M excretion rates above 300 µg/g cr, but environmental exposure to toxic metals was not investigated [43].
4.3. Urinary Cd Benchmarks for the β2M Excretion and eGFR Reduction Endpoints
Most conventional BMD methods rely on a single dose–response model and arbitrary cut-off values to define departure from normalcy (a point of departure, POD). In toxicological risk assessment, POD represents the dose at which a specific adverse effect is first observed or at which a response deviates from a baseline or control [18,21]. It is a starting point to evaluate the potential health impact of exposure to any health-hazardous substance. PODs are used to estimate permissible exposure levels for chemicals in both environmental and occupational exposure settings.
Herein, we report urinary Cd excretion levels that link to the conventional toxic endpoint, an elevation of β2M excretion, and a newly proposed toxic endpoint, a reduction in eGFR, through advanced BMD modeling, involving multiple dose–response models, model weights, and averaging (Section 2.3). The use of AIC to assess performance of different models is another advantage of advanced BMD modeling. In addition, exposure benchmark levels can be derived for continuous and quantal endpoints.
We were unable to determine urinary Cd excretion benchmarks for the β2M endpoint (Figure 2), but we have found a urinary Cd excretion rate of 0.17 µg/g cr to be the benchmark level for an early effect of Cd on eGFR (Figure 1). Our findings cast considerable doubts on the use of β2M excretion in the assessment of the health risk from environmental Cd exposure. As discussed above, β2M excretion rates ≥ 300 µg/g cr did not appear to be an early effect of the nephrotoxicity of Cd but a consequence of severe tubular dysfunction leading to nephron destruction and a fall in eGFR. Furthermore, a recent indebt analysis on the homeostasis of β2M has indicated that an elevated urinary excretion β2M is not a reliable indicator of tubular dysfunction [44]. Apparently, it is inappropriate to use β2M excretion rates above 300 µg/g cr in the assessment of the health risk from environmental Cd exposure.
4.4. Dietary Cd Exposure Carrying a Negligible Health Risk
It is recommended that eGFR reduction is used as an indicator of an adverse effect of Cd on the kidney. A reduction in eGFR is a clinically relevant outcome, and a Cd excretion benchmark of 0.17 µg/g cr could serve as a basis to compute a meaningful dietary exposure limit for Cd. This figure is 3.2% of the threshold level at 5.24 µg/g cr of the JECFA derived from the β2M endpoint. A crude analysis of the prevalence rates of CKD among those with ECd/Ecr above and below 0.2 µg/g cr showed 9.2% and 5.1%, respectively. Logistic regression with adjusting for confounders revealed a significant elevation in odds of CKD even at ECd/Ecr of 0.2 µg/g cr (Table S1).
Typically, a translation of a threshold to exposure limits involves reverse dosimetry with the physiologically based pharmacokinetic model [45,46]. In the absence of a translational figure, Cd exposure levels associated with an increased risk of CKD in a Chinese population study are highlighted herein. Compared with a dietary Cd exposure level of 16.7 μg/d, CKD risk rose 1.73-fold, 2.93-fold, and 4.05-fold at Cd consumption rates of 23.2, 29.6, and 36.9 μg/d, respectively [47]. A Cd exposure level of 23.2 μg/d was only 40% of the JECFA’s tolerable exposure level. For a 60 kg person, a Cd exposure of 23.2 µg/d is comparable to the EFSA’s reference dose (RfD) of 0.36 µg/kg b.w./d [48] and the US FDA’s toxicological reference value (TRV) of 0.21–0.36 µg/kg b.w./d [49].
Existing dietary Cd exposure guidelines based on the β2M endpoint do not appear to be protective of the kidneys. By 2040, CKD is projected to be the fifth-leading cause of years of life lost [14,15]. Cd-induced nephron destruction which causes eGFR to fall is irreversible [50]. Minimization of Cd exposure from all sources may slow eGFR deterioration to < 15 mL/min/1.73 m2, which marks dialysis or a kidney transplant for survival.
4.5. Strengths and Limitations
This cross-sectional design with a large Thai cohort (n = 799) employs advanced BMD modeling to reassess the utility of β2M excretion as a basis to derive the reference dose for dietary Cd exposure. It has provided evidence that a reduction in eGFR is a suitable endpoint for such a purpose.
A cohort of participants exposed to a wide range of Cd doses represented its major strength. ECd/Ccr values ranged between 0.0002 and 1.70 µg/L filtrate (geometric mean 0.018 µg/L filtrate), while ECd/Ecr values ranged between 0.03 and 106 µg/g cr (geometric mean 2.15 µg/g cr). A wide range of Cd exposure doses allowed the establishment of a clear dose–response relationship as did a wide age range (18–87 years). Furthermore, age and BMI histograms of cohort participants conformed to a normal distribution. This group could thus be considered to represent the general Thai population exposed to Cd in a normal diet. The BMD estimates are generalizable because the basic mechanism of the cytotoxicity of Cd is the same and because sources of Cd make no difference to its toxicity. The use of multiple mathematical dose–response models for both continuous and quantal endpoints is an additional strength.
The limitations include a one-time-only assessment of Cd exposure and outcomes and inability to adequately adjust potential confounders that included prediabetes status, alcohol consumption, and history of non-steroidal anti-inflammatory drug use. Notably, however, the Swiss prospective cohort study has implicated Cd exposure, even in low doses, as a cause of falling eGFR at a high rate [42]. A Japanese longitudinal cohort study observed an increased risk of falling eGFR at 10 mL/min/1.73 m2 over 5 years among those with a β2M excretion rate of 300 µg/g cr [43]. Use of β2M excretion rate of 300 µg/g cr as a criterion to judge the nephrotoxicity of Cd is questionable.
5. Conclusions
Using multiple mathematical dose–response models and a bootstrap model averaging approach, the benchmark of Cd excretion is 0.17 µg/g creatinine, when a reduction in eGFR is used as a toxic endpoint. The BMDU/BMDL ratio for the BMDL estimate was 16.9. In comparison, the Cd excretion benchmark for the conventional β2M endpoint is unreliable because of a high degree of uncertainty around the BMD estimates, reflected by BMDU/BMDL of 182. For Ccr-based data, an ECd/Ccr value of 0.0088 µg/L filtrate is associated with a 5% reduction in eGFR. This benchmark estimate was of high certainty, given a BMDU/BMDL ratio of 3.4.
The practice of adjusting urinary Cd and β2M concentrations to creatinine excretion creates non-differential errors that bias the dose–response relationships toward the null [20]. Such measurement errors/imprecisions can be attributed to the variation in creatinine excretion because of the differences in muscle mass, especially between men and women. A dose–response relationship could not be established between Cd exposure and the severity of the abnormality in β2M excretion rates (Table 3). Thus, use of β2M excretion at a rate higher than 300 µg/g creatinine in Cd health risk computation is inappropriate.
Excretion of any urinary marker of kidney effects, Cd included, should be adjusted to creatinine clearance. This adjustment is achieved by using a generic mathematical equation: Ex/Ccr = [x]u[cr]p/[cr]u. Hence, a timed urine collection is obviated (SM. This method of adjusting urinary concentration of excreted substances corrects for interindividual differences in both urine dilution and functioning nephrons, and it is not affected by muscle mass).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app152111757/s1, Figure S1: Cadmium excretion rates associated with 5% prevalence of β2M excretion level 1; Figure S2: Cadmium excretion rates associated with 5% prevalence of β2M excretion level 3; Table S1: Logistic regression analysis for an association between the prevalence odds for CKD and a newly identified threshold.
Author Contributions
Conceptualization, S.S. and D.A.V.; methodology, S.S. and A.B.Đ.; formal analysis, S.S. and A.B.Đ.; resources, D.A.V.; data curation, S.S.; writing—original draft preparation, S.S.; writing—review and editing, S.S., D.A.V. and A.B.Đ. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study analyzed archived data, for which the ethical approval was obtained when the original study was conducted, thereby obviating the need for additional ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The work was supported with resources from the Centre for Kidney Disease Research, Translational Research Institute, and the Department of Kidney and Transplant Services, Princess Alexandra Hospital, QLD, Australia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aoshima, K. Epidemiology of renal tubular dysfunction in the inhabitants of a cadmium-polluted area in the Jinzu River basin in Toyama Prefecture. Tohoku J. Exp. Med. 1987, 152, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Aoshima, K.; Katoh, T.; Teranishi, H.; Kasuya, M. Renal tubular dysfunction in male inhabitants of a cadmium-polluted area in Toyama, Japan—An eleven-year follow-up study. J. Epidemiol. 2001, 11, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Horiguchi, H.; Matsukawa, T.; Kobayashi, M.; Omori, Y.; Oguma, E.; Komatsuda, A. A suspected case of “itai-itai disease” in a cadmium-polluted area in Akita prefecture, Japan. Environ. Health Prev. Med. 2024, 29, 40. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Aoshima, K.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Hosoi, Y.; Katoh, T.; Kayama, F. Latest status of cadmium accumulation and its effects on kidneys, bone, and erythropoiesis in inhabitants of the formerly cadmium-polluted Jinzu River Basin in Toyama, Japan, after restoration of rice paddies. Int. Arch. Occup. Environ. Health 2010, 83, 953–970. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Summary and Conclusions. In Proceedings of the Joint FAO/WHO Expert Committee on Food Additives and Contaminants, Seventy-Third Meeting, Geneva, Switzerland, 8–17 June 2010; Available online: https://iris.who.int/items/9b0d9daf-c57a-4051-a9f7-6380ec80fd25 (accessed on 25 September 2025).
- Wong, C.; Roberts, S.M.; Saab, I.N. Review of regulatory reference values and background levels for heavy metals in the human diet. Regul. Toxicol. Pharmacol. 2022, 130, 105122. [Google Scholar] [CrossRef]
- Crump, K.S. A new method for determining allowable daily intakes. Fundam. Appl. Toxicol. 1984, 4, 854–871. [Google Scholar] [CrossRef]
- Gaylor, D.; Ryan, L.; Krewski, D.; Zhu, Y. Procedures for calculating benchmark doses for health risk assessment. Regul. Toxicol. Pharmacol. 1998, 28, 150–164. [Google Scholar] [CrossRef]
- Ginsberg, G.L. Cadmium risk assessment in relation to background risk of chronic kidney disease. J. Toxicol. Environ. Health Part A 2012, 75, 374–390. [Google Scholar] [CrossRef]
- Murton, M.; Goff-Leggett, D.; Bobrowska, A.; Garcia Sanchez, J.J.; James, G.; Wittbrodt, E.; Nolan, S.; Sörstadius, E.; Pecoits-Filho, R.; Tuttle, K. Burden of Chronic Kidney Disease by KDIGO Categories of Glomerular Filtration Rate and Albuminuria: A Systematic Review. Adv. Ther. 2021, 38, 180–200. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Farrell, D.R.; Vassalotti, J.A. Screening, identifying, and treating chronic kidney disease: Why, who, when, how, and what? BMC Nephrol. 2024, 25, 34. [Google Scholar] [CrossRef]
- Doccioli, C.; Sera, F.; Francavilla, A.; Cupisti, A.; Biggeri, A. Association of cadmium environmental exposure with chronic kidney disease: A systematic review and meta-analysis. Sci. Total Environ. 2024, 906, 167165. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- GBD 2021 Forecasting Collaborators. Burden of disease scenarios for 204 countries and territories, 2022–2050: A forecasting analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2204–2256. [Google Scholar] [CrossRef]
- Slob, W.; Setzer, R.W. Shape and steepness of toxicological dose-response relationships of continuous endpoints. Crit. Rev. Toxicol. 2014, 44, 270–297. [Google Scholar] [CrossRef]
- Slob, W. A general theory of effect size, and its consequences for defining the benchmark response (BMR) for continuous endpoints. Crit. Rev. Toxicol. 2017, 47, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Moffett, D.B.; Mumtaz, M.M.; Sullivan, D.W., Jr.; Whittaker, M.H. Chapter 13, General Considerations of Dose-Effect and Dose-Response Relationships. In Handbook on the Toxicology of Metals, 5th ed.; Volume I: General Considerations; Nordberg, G., Costa, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 299–317. [Google Scholar]
- EFSA Scientific Committee. Update: Use of the benchmark dose approach in risk assessment. EFSA J. 2017, 15, 4658. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Budtz-Jørgensen, E. Total imprecision of exposure biomarkers: Implications for calculating exposure limits. Am. J. Ind. Med. 2007, 50, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Byber, K.; Lison, D.; Verougstraete, V.; Dressel, H.; Hotz, P. Cadmium or cadmium compounds and chronic kidney disease in workers and the general population: A systematic review. Crit. Rev. Toxicol. 2016, 46, 191–240. [Google Scholar] [CrossRef]
- Jalili, C.; Kazemi, M.; Cheng, H.; Mohammadi, H.; Babaei, A.; Taheri, E.; Moradi, S. Associations between exposure to heavy metals and the risk of chronic kidney disease: A systematic review and meta-analysis. Crit. Rev. Toxicol. 2021, 51, 165–182. [Google Scholar] [CrossRef]
- Swaddiwudhipong, W.; Nguntra, P.; Kaewnate, Y.; Mahasakpan, P.; Limpatanachote, P.; Aunjai, T.; Jeekeeree, W.; Punta, B.; Funkhiew, T.; Phopueng, I. Human health effects from cadmium exposure: Comparison between persons living in cadmium-contaminated and non-contaminated areas in northwestern Thailand. Southeast Asian J. Trop. Med. Public Health 2015, 46, 133–142. [Google Scholar]
- Nishijo, M.; Suwazono, Y.; Ruangyuttikarn, W.; Nambunmee, K.; Swaddiwudhipong, W.; Nogawa, K.; Nakagawa, H. Risk assessment for Thai population: Benchmark dose of urinary and blood cadmium levels for renal effects by hybrid approach of inhabitants living in polluted and non-polluted areas in Thailand. BMC Public Health 2014, 14, 702. [Google Scholar] [CrossRef]
- Suwatvitayakorn, P.; Ko, M.S.; Kim, K.W.; Chanpiwat, P. Human health risk assessment of cadmium exposure through rice consumption in cadmium-contaminated areas of the Mae Tao sub-district, Tak, Thailand. Environ. Geochem. Health 2020, 42, 2331–2344. [Google Scholar] [CrossRef]
- Swaddiwudhipong, W.; Mahasakpan, P.; Limpatanachote, P.; Krintratun, S. Correlations of urinary cadmium with hypertension and diabetes in persons living in cadmium-contaminated villages in northwestern Thailand: A population study. Environ. Res. 2010, 110, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Đorđević, A.B.; Yimthiang, S.; Vesey, D.A.; Gobe, G.C. The NOAEL Equivalent of Environmental Cadmium Exposure Associated with GFR Reduction and Chronic Kidney Disease. Toxics 2022, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Zarcinas, B.A.; Pongsakul, P.; McLaughlin, M.J.; Cozens, G. Heavy metals in soils and crops in Southeast Asia. 2. Thailand. Environ. Geochem. Health 2004, 26, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Sripaoraya, K.; Siriwong, W.; Pavittranon, S.; Chapman, R.S. Environmental arsenic exposure and risk of diabetes type 2 in Ron Phibun subdistrict, Nakhon Si Thammarat Province, Thailand: Unmatched and matched case-control studies. Risk Manag Healthc. Policy 2017, 10, 41–48. [Google Scholar] [CrossRef]
- Apple, F.; Bandt, C.; Prosch, A.; Erlandson, G.; Holmstrom, V.; Scholen, J.; Googins, M. Creatinine clearance: Enzymatic vs. Jaffé determinations of creatinine in plasma and urine. Clin. Chem. 1986, 32, 388–390. [Google Scholar] [CrossRef]
- Bernard, A.M.; Vyskocil, A.; Lauwerys, R.R. Determination of beta 2-microglobulin in human urine and serum by latex immunoassay. Clin. Chem. 1981, 27, 832–837. [Google Scholar] [CrossRef]
- Trzcinka-Ochocka, M.; Brodzka, R.; Janasik, B. Useful and Fast Method for Blood Lead and Cadmium Determination Using ICP-MS and GF-AAS; Validation Parameters. J. Clin. Lab. Anal. 2016, 30, 130–139. [Google Scholar] [CrossRef]
- Hornung, R.W.; Reed, L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Levey, A.S.; Becker, C.; Inker, L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 2015, 313, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Phelps, K.R.; Gosmanova, E.O. A generic method for analysis of plasma concentrations. Clin. Nephrol. 2020, 94, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Sand, S.J.; von Rosen, D.; Filipsson, A.F. Benchmark calculations in risk assessment using continuous dose-response information: The influence of variance and the determination of a cut-off value. Ris Anal. 2003, 23, 1059–1068. [Google Scholar] [CrossRef]
- Slob, W.; Moerbeek, M.; Rauniomaa, E.; Piersma, A.H. A statistical evaluation of toxicity study designs for the estimation of the benchmark dose in continuous endpoints. Toxicol. Sci. 2005, 84, 167–185. [Google Scholar] [CrossRef]
- Sand, S.; Filipsson, A.F.; Victorin, K. Evaluation of the benchmark dose method for dichotomous data: Model dependence and model selection. Regul. Toxicol. Pharmacol. 2002, 36, 184–197. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, T.; Jelsovsky, J.Z. Bootstrap estimation of benchmark doses and confidence limits with clustered quantal data. Risk. Anal. 2007, 27, 447–465. [Google Scholar] [CrossRef]
- Horiguchi, H.; Oguma, E.; Sasaki, S.; Okubo, H.; Murakami, K.; Miyamoto, K.; Hosoi, Y.; Murata, K.; Kayama, F. Age-relevant renal effects of cadmium exposure through consumption of home-harvested rice in female Japanese farmers. Environ. Int. 2013, 56, 1–9. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Nishijo, M.; Ruangyuttikarn, W.; Gobe, G.C. The inverse association of glomerular function and urinary β2-MG excretion and its implications for cadmium health risk assessment. Environ. Res. 2019, 173, 40–47. [Google Scholar] [CrossRef]
- Xie, S.; Perrais, M.; Golshayan, D.; Wuerzner, G.; Vaucher, J.; Thomas, A.; Marques-Vidal, P. Association between urinary heavy metal/trace element concentrations and kidney function: A prospective study. Clin. Kidney J. 2024, 18, sfae378. [Google Scholar] [CrossRef]
- Kudo, K.; Konta, T.; Mashima, Y.; Ichikawa, K.; Takasaki, S.; Ikeda, A.; Hoshikawa, M.; Suzuki, K.; Shibata, Y.; Watanabe, T.; et al. The association between renal tubular damage and rapid renal deterioration in the Japanese population: The Takahata study. Clin. Exp. Nephrol. 2011, 15, 235–241. [Google Scholar] [CrossRef]
- Phelps, K.R.; Yimthiang, S.; Pouyfung, P.; Khamphaya, T.; Vesey, D.A.; Satarug, S. Homeostasis of β2-microglobulin in diabetics and non-diabetics with modest cadmium intoxication. J. Environ. Expo. Assess. 2025, 4, 23. [Google Scholar] [CrossRef]
- Satarug, S.; Swaddiwudhipong, W.; Ruangyuttikarn, W.; Nishijo, M.; Ruiz, P. Modeling cadmium exposures in low- and high-exposure areas in Thailand. Environ. Health Perspect. 2013, 121, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S. Benchmark dose modeling to define permissible exposure levels for environmental cadmium. J. Environ. Expo. Assess. 2025, 4, 28. [Google Scholar] [CrossRef]
- Shi, Z.; Taylor, A.W.; Riley, M.; Byles, J.; Liu, J.; Noakes, M. Association between dietary patterns, cadmium intake and chronic kidney disease among adults. Clin. Nutr. 2018, 37, 276–284. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Statement on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar] [CrossRef]
- Schaefer, H.R.; Flannery, B.M.; Crosby, L.M.; Pouillot, R.; Farakos, S.M.S.; Van Doren, J.M.; Dennis, S.; Fitzpatrick, S.; Middleton, K. Reassessment of the cadmium toxicological reference value for use in human health assessments of foods. Regul. Toxicol. Pharmacol. 2023, 144, 105487. [Google Scholar] [CrossRef]
- Swaddiwudhipong, W.; Limpatanachote, P.; Mahasakpan, P.; Krintratun, S.; Punta, B.; Funkhiew, T. Progress in cadmium-related health effects in persons with high environmental exposure in northwestern Thailand: A five-year follow-up. Environ. Res. 2012, 112, 194–198. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).