Broccoli Sprouts as Functional Food: Phytochemical Profile and Antioxidant Activity Linked to Human Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Broccoli Sprouts
2.2. Extracts
- methanol—commonly used for analysing the chemical profile of medicinal plants;
- a methanol-acetone mixture (1:1) that would increase the solubility of the more lipophilic components;
- an acidic solution obtained by diluting acetic acid until we reached pH = 3.
- Sample 1. Green-coloured methanolic extract (showing chlorophyll extraction);
- Sample 2. Extract in acid solution (glacial acetic acid diluted in water to pH = 3)—almost colourless with an odour reminiscent of pickled cabbage;
- Sample 3. Extract in acetone: methanol mixture (1:1)—greenish-yellow colour.
2.3. Bioactive Components Identification
2.3.1. Ultrahigh-Performance Liquid Chromatography-Photodiode Array Detection (UPLC-PDA)
2.3.2. MS Liquid Chromatography (UPLC-ESI/MS)
2.3.3. Qualitative Tests
2.4. Quantitative Analysis
2.4.1. Flavonoids Determination
2.4.2. Total Polyphenols Determination
2.4.3. SFN Determination
2.5. Biochemical Assays
2.5.1. Antioxidant Activity as Determined by DPPH
2.5.2. Ferrous Iron Chelation Test
2.5.3. Hydroxyl Radical Neutralisation Capacity
2.5.4. Determination of Lipoxygenase Inhibition Capacity
2.5.5. Reagents
3. Results
3.1. Qualitative and Quantitative Analysis
3.1.1. Qualitative Analysis
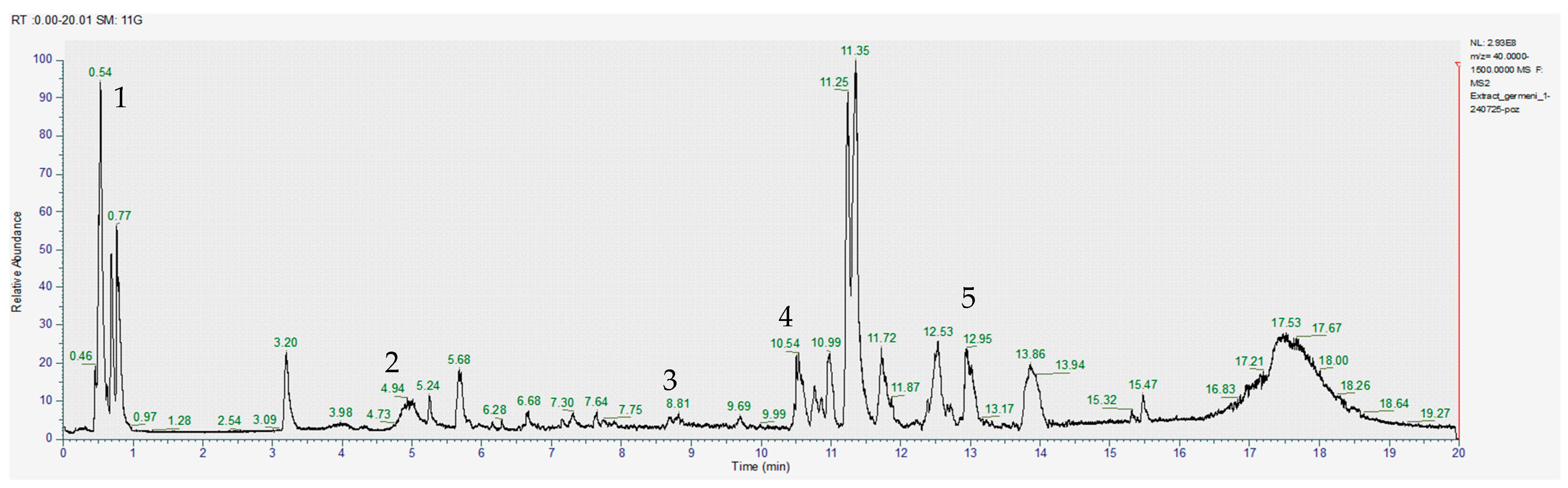
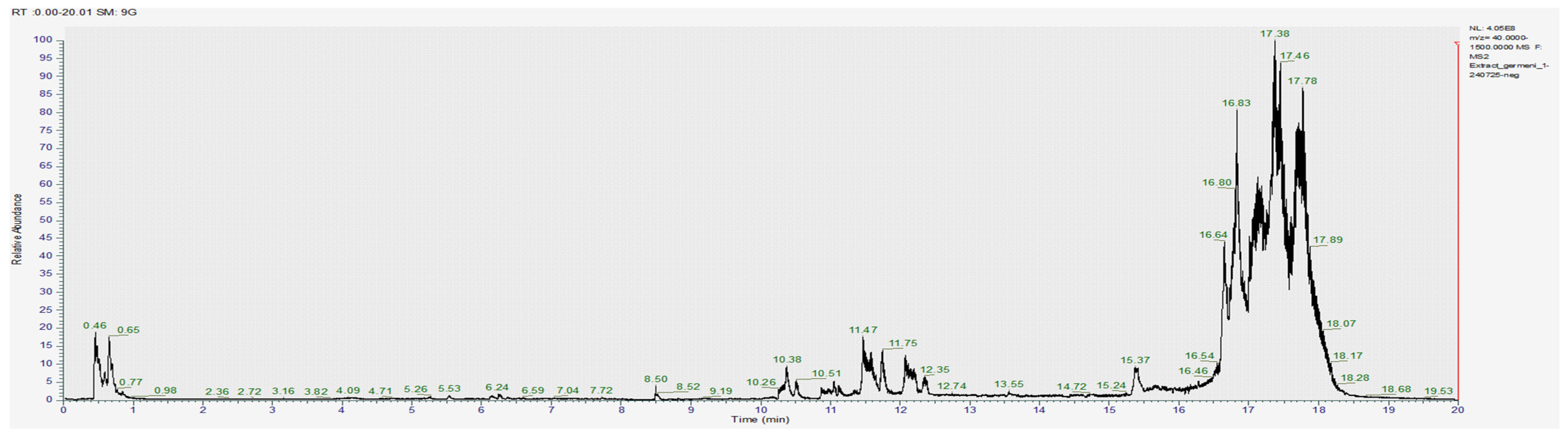
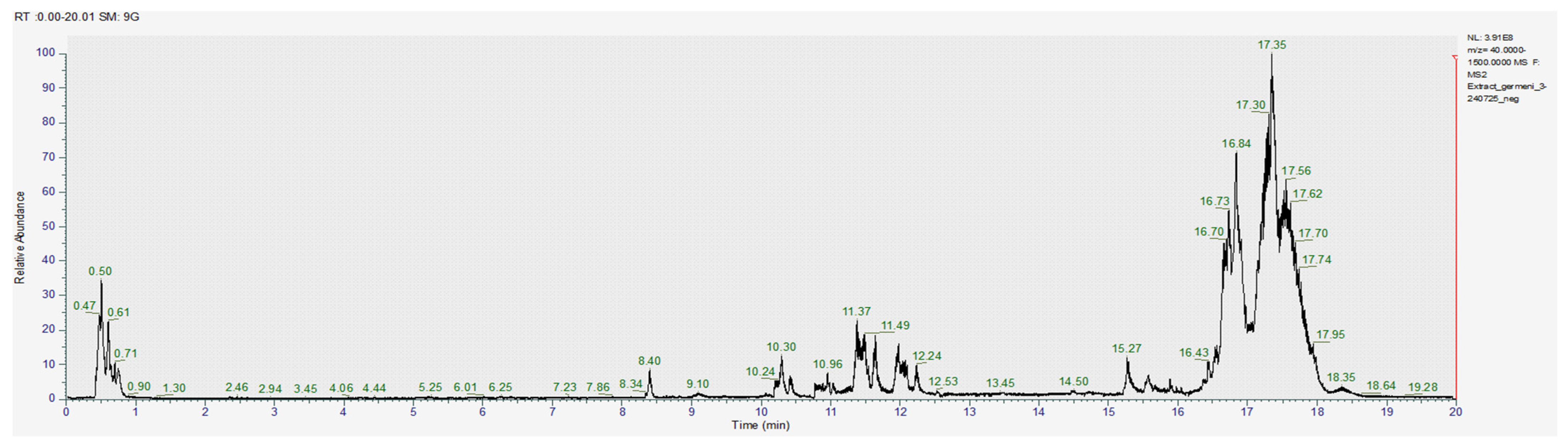
3.1.2. Chromatography Results
3.2. Biochemical Assays Results
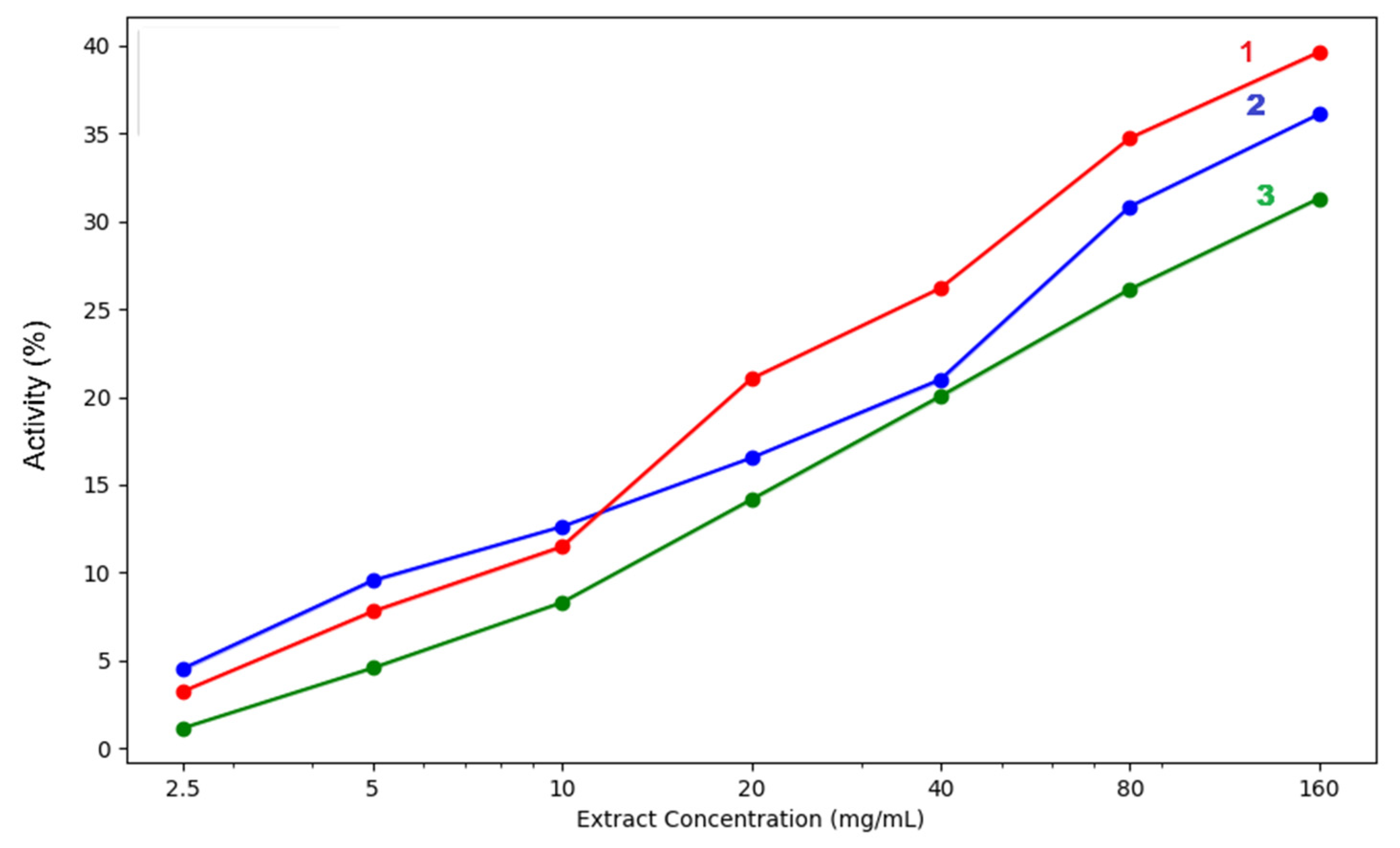
3.2.1. Antioxidant Activity
3.2.2. Ferrous Iron Chelation Test Results
3.2.3. Hydroxyl Radical Neutralisation Capacity Results
3.2.4. Determination of Lipoxygenase Inhibition Capacity Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Sulforaphane | SFN |
| GRP | Glucoraphanin |
| Nrf2/ARE | Nuclear factor E2-related factor 2/Antioxidant Response Element |
References
- Shams, R.; Abu-Khudir, R.; Ali, E. Sulforaphane, Polyphenols and Related Anti-Inflammatory and Antioxidant Activities Changes of Egyptian Broccoli during Growth. J. Food Meas. Charact. 2017, 11, 2061. [Google Scholar] [CrossRef]
- Schmid, H.; Karrer, P. Synthese der racemischen und der optisch aktiven Formen des Sulforaphans. Helv. Chim. Acta 1948, 31, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Atwell, L.L.; Hsu, A.; Wong, C.P.; Stevens, J.F.; Bella, D.; Yu, T.-W.; Pereira, C.B.; Löhr, C.V.; Christensen, J.M.; Dashwood, R.H.; et al. Absorption and Chemopreventive Targets of Sulforaphane in Humans Following Consumption of Broccoli Sprouts or a Myrosinase-Treated Broccoli Sprout Extract. Mol. Nutr. Food Res. 2015, 59, 424–433. [Google Scholar] [CrossRef]
- Dmytriv, T.R.; Lushchak, O.; Lushchak, V.I. Glucoraphanin Conversion into Sulforaphane and Related Compounds by Gut Microbiota. Front. Physiol. 2025, 16. [Google Scholar] [CrossRef]
- Houghton, C.A. Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxidative Med. Cell. Longev. 2019, 2019, 2716870. [Google Scholar] [CrossRef]
- Bones, A.; Rossiter, J. The Myrosinase-Glucosinolate System, Its Organisation and Biochemistry. Physiol. Plant. 1996, 97, 194–208. [Google Scholar] [CrossRef]
- Ratzka, A.; Vogel, H.; Kliebenstein, D.J.; Mitchell-Olds, T.; Kroymann, J. Disarming the Mustard Oil Bomb. Proc. Natl. Acad. Sci. USA 2002, 99, 11223–11228. [Google Scholar] [CrossRef]
- Lüthy, B.; Matile, P. The Mustard Oil Bomb: Rectified Analysis of the Subcellular Organisation of the Myrosinase System. Biochem. Physiol. Pflanz. 1984, 179, 5–12. [Google Scholar] [CrossRef]
- Janczewski, Ł. Sulforaphane and Its Bifunctional Analogs: Synthesis and Biological Activity. Molecules 2022, 27, 1750. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli Sprouts: An Exceptionally Rich Source of Inducers of Enzymes That Protect against Chemical Carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L. Discovery and Development of Sulforaphane as a Cancer Chemopreventive Phytochemical. Acta Pharmacol. Sin. 2007, 28, 1343–1354. [Google Scholar] [CrossRef]
- Pastore, A.; Federici, G.; Bertini, E.; Piemonte, F. Analysis of Glutathione: Implication in Redox and Detoxification. Clin. Chim. Acta 2003, 333, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A Major Inducer of Anticarcinogenic Protective Enzymes from Broccoli: Isolation and Elucidation of Structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef] [PubMed]
- Ritz, S.A.; Wan, J.; Diaz-Sanchez, D. Sulforaphane-Stimulated Phase II Enzyme Induction Inhibits Cytokine Production by Airway Epithelial Cells Stimulated with Diesel Extract. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L33–L39. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Chen, J.-G.; Egner, P.A.; Fahey, J.W.; Jacobson, L.P.; Stephenson, K.K.; Ye, L.; Coady, J.L.; Wang, J.-B.; Wu, Y.; et al. Effects of Glucosinolate-Rich Broccoli Sprouts on Urinary Levels of Aflatoxin-DNA Adducts and Phenanthrene Tetraols in a Randomized Clinical Trial in He Zuo Township, Qidong, People’s Republic of China. Cancer Epidemiol. Biomark. Prev. 2005, 14 Pt 1, 2605–2613. [Google Scholar] [CrossRef]
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane Inhibits Extracellular, Intracellular, and Antibiotic-Resistant Strains of Helicobacter Pylori and Prevents Benzo[a]Pyrene-Induced Stomach Tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615. [Google Scholar] [CrossRef]
- Wu, N.; Luo, Z.; Deng, R.; Zhang, Z.; Zhang, J.; Liu, S.; Luo, Z.; Qi, Q. Sulforaphane: An Emerging Star in Neuroprotection and Neurological Disease Prevention. Biochem. Pharmacol. 2025, 233, 116797. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Kozumbo, W.J. The Phytoprotective Agent Sulforaphane Prevents Inflammatory Degenerative Diseases and Age-Related Pathologies via Nrf2-Mediated Hormesis. Pharmacol. Res. 2021, 163, 105283. [Google Scholar] [CrossRef]
- Yeo, J.; Shahidi, F. Critical Re-Evaluation of DPPH Assay: Presence of Pigments Affects the Results. J. Agric. Food Chem. 2019, 67, 7526–7529. [Google Scholar] [CrossRef]
- Dinis, T.C.; Maderia, V.M.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free Radicals and Their Impact on Health and Antioxidant Defenses: A Review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Halliwell, B.; Adhikary, A.; Dingfelder, M.; Dizdaroglu, M. Hydroxyl Radical Is a Significant Player in Oxidative DNA Damage in Vivo. Chem. Soc. Rev. 2021, 50, 8355–8360. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.B.; Hong, S.C.; Jeong, H.J. 3,4-Dihydroxybenzaldehyde Purified from the Barley Seeds (Hordeum vulgare) Inhibits Oxidative DNA Damage and Apoptosis via Its Antioxidant Activity. Phytomedicine 2009, 16, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zou, H. Lipoxygenase Metabolism: Critical Pathways in Microglia-Mediated Neuroinflammation and Neurodevelopmental Disorders. Neurochem. Res. 2022, 47, 3213–3220. [Google Scholar] [CrossRef]
- Malterud, K.E.; Rydland, K.M. Inhibitors of 15-Lipoxygenase from Orange Peel. J. Agric. Food Chem. 2000, 48, 5576–5580. [Google Scholar] [CrossRef]
- Chuljerm, H.; Paradee, N.; Katekaew, D.; Nantachai, P.; Settakorn, K.; Srichairatanakool, S.; Koonyosying, P. Iron Chelation Property, Antioxidant Activity, and Hepatoprotective Effect of 6-Gingerol-Rich Ginger (Zingiber officinale) Extract in Iron-Loaded Huh7 Cells. Plants 2023, 12, 2936. [Google Scholar] [CrossRef]
- Adjimani, J.P.; Asare, P. Antioxidant and Free Radical Scavenging Activity of Iron Chelators. Toxicol. Rep. 2015, 2, 721–728. [Google Scholar] [CrossRef]
- Gentile, M.T.; Camerino, I.; Ciarmiello, L.; Woodrow, P.; Muscariello, L.; De Chiara, I.; Pacifico, S. Neuro-Nutraceutical Polyphenols: How Far Are We? Antioxidants 2023, 12, 539. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, Q.; Jiang, J.; Khan, M.S. Tannin Complexation with Metal Ions and Its Implication on Human Health, Environment and Industry: An Overview. Int. J. Biol. Macromol. 2023, 253 Pt 7, 127485. [Google Scholar] [CrossRef]
- Ilhan, A.; Akyol, O.; Gurel, A.; Armutcu, F.; Iraz, M.; Oztas, E. Protective Effects of Caffeic Acid Phenethyl Ester against Experimental Allergic Encephalomyelitis-Induced Oxidative Stress in Rats. Free Radic. Biol. Med. 2004, 37, 386–394. [Google Scholar] [CrossRef]
- Biringer, R.G. The Enzymology of Human Eicosanoid Pathways: The Lipoxygenase Branches. Mol. Biol. Rep. 2020, 47, 7189–7207. [Google Scholar] [CrossRef]
- Lončarić, M.; Strelec, I.; Moslavac, T.; Šubarić, D.; Pavić, V.; Molnar, M. Lipoxygenase Inhibition by Plant Extracts. Biomolecules 2021, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Isothiocyanates, Nitriles and Thiocyanates as Products of Autolysis of Glucosinolates in Cruciferae. Available online: https://www.scilit.com/publications/a73ff413331fe1848bc11a4cf71aa624 (accessed on 13 September 2025).
- González, F.; Quintero, J.; Del Río, R.; Mahn, A. Optimization of an Extraction Process to Obtain a Food-Grade Sulforaphane-Rich Extract from Broccoli (Brassica oleracea Var. Italica). Molecules 2021, 26, 4042. [Google Scholar] [CrossRef] [PubMed]
- Kall, M.A.; Vang, O.; Clausen, J. Effects of Dietary Broccoli on Human Drug Metabolising Activity. Cancer Lett. 1997, 114, 169–170. [Google Scholar] [CrossRef]
- Mohammed, Q. Extraction of Sulforaphane from Iraqi (Kurdistan Region) Cabbage. J. Koya Univ. 2009, 10, 21–28. [Google Scholar]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef]
- Duque-Soto, C.; Borrás-Linares, I.; Quirantes-Piné, R.; Falcó, I.; Sánchez, G.; Segura-Carretero, A.; Lozano-Sánchez, J. Potential Antioxidant and Antiviral Activities of Hydroethanolic Extracts of Selected Lamiaceae Species. Foods 2022, 11, 1862. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; He, Y.; Zhang, S.; Huang, B.; Wang, Y.; Lin, W.; Xiao, W.; Zou, Z.; Cui, G. GGV Formula Attenuates CCl4-Induced Hepatic Injury in Mice by Modulating the Gut Microbiota and Metabolites. Front. Nutr. 2025, 12, 1564177. [Google Scholar] [CrossRef]
- Ivanochko, M.V.; Fediv, K.V.; Shvadchak, V.V.; Bayliak, M.M.; Lushchak, V.I. Nutritional Analysis of Aqueous and Ethanol Broccoli Sprout Extracts. J. Plant Biochem. Biotechnol. 2025, 34, 749–760. [Google Scholar] [CrossRef]
- Chaudhri, A.A.; Nadeem, M.; Rahman, A.U.; Alam, T.; Sajjad, W.; Hasan, F.; Badshah, M.; Khan, S.; Rehman, F.; Shah, A.A. Antioxidative and Radioprotective Properties of Glycosylated Flavonoid, Xanthorhamnin from Radio-Resistant Bacterium Bacillus Indicus Strain TMC-6. Curr. Microbiol. 2020, 77, 1245–1253. [Google Scholar] [CrossRef]
- Pandi, A.; Kalappan, V.M. Pharmacological and Therapeutic Applications of Sinapic Acid—An Updated Review. Mol. Biol. Rep. 2021, 48, 3733–3745. [Google Scholar] [CrossRef]
- Jiang, C.-H.; Sun, T.-L.; Xiang, D.-X.; Wei, S.-S.; Li, W.-Q. Anticancer Activity and Mechanism of Xanthohumol: A Prenylated Flavonoid From Hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530. [Google Scholar] [CrossRef]
- Oyovwi, M.O.; Ohwin, P.E.; Rotu, A.R.; Tesi, P.E.; Ben-Azu, B.; Naiho, O.A. Lycopene Againsts the Polystyrene Microplastics-Induced Neurotoxicity via Modulation of mTOR/Beclin-1 Activities in Adult Male Wistar Rats. Clin. Tradit. Med. Pharmacol. 2024, 5, 200180. [Google Scholar] [CrossRef]
- Kim, E.-J.; Kim, M.-H. Antioxidant Activity of Solvent Fraction from Broccoli Sprouts Cultivated at the Plant Factory System. Korean J. Food Nutr. 2015, 28, 1–8. [Google Scholar] [CrossRef]





| Secondary Metabolites | Samples | ||
|---|---|---|---|
| Methanol | Acetic Acid | Acetone-Acetic Acid | |
| Flavonoid aglycones: | +++ | ++ | |
| R. Shibata | +++ | ||
| Flavonoids: | ++ | +++ | +++ |
| AlCl3 | |||
| NaOH | ++ | ++ | +++ |
| Total polyphenols: Folin–Ciocalteu | ++++ | +++ | ++++ |
| COMPOUNDS | RT (min) | [M-H]- m/z | Exact Mass (g/mol) | Formula | Concentration (µg/mL) | ||
|---|---|---|---|---|---|---|---|
| Methanol | Acetic Acid | Methanol-Acetone | |||||
| Sinapic acid | 4.65 | 223.0612 | 224.068473 | C11H12O5 | 28.30 ± 0.1 | 1.87 ± 0.12 | 16.30 ± 0.01 |
| Caffeic acid | 4.71 | 179.0350 | 180.042259 | C9H8O4 | 8.48 ± 0.12 | 6.310.33 | 5.18 ± 0.31 |
| Gallic acid | 0.54 | 169.0142 | 170.021523 | C7H6O5 | 3.97 ± 0.01 | 4.10 ± 0.12 | 2.79 ± 0.01 |
| Apigenin | 10.54 | 269.0455 | 270.052823 | C15H10O5 | 1.82 ± 0.01 | 1.27 ± 0.12 | 1.51 ± 0.1 |
| Quercetin | 8.60 | 301.0354 | 302.042653 | C15H10O7 | 1.24 ± 0.13 | 1.84 ± 0.01 | 1.62 ± 0.2 |
| Kaempferol | 10.25 | 285.0403 | 286.047738 | C15H10O6 | 1.16 ± 0.3 | 1.54 ± 0.12 | 2.35 ± 0.12 |
| Sulforaphane | 14.52 | 176.0209 | 177.028206 | C6H11NOS2 | 3.87 ± 0.12 | 3.06 ± 0.11 | 3.43 ± 0.01 |
| Glucoraphanin | 13.06 | 436.0411 | 437.048409 | C12H23NO10S3 | 1.02 ± 0.2 | 0.961 ± 0.01 | 1.05 ± 0.12 |
| Lycopene | 9.16 | 535.4309 | 536.438202 | C40H56 | 7.54 ± 0.12 | 0.283 ± 0.12 | 7.96 ± 0.01 |
| Lutein/Zeaxanthin | 14.12 | 567.4208 | 568.428031 | C40H56O2 | 4.64 ± 0.01 | 0.431 ± 0.01 | 1.22 ± 0.2 |
| Activity (%) ± SD | Extract Concentration | |||||||
| 2.5 mg/mL | 5 mg/mL | 10 mg/mL | 20 mg/mL | 40 mg/mL | 80 mg/mL | 160 mg/mL | ||
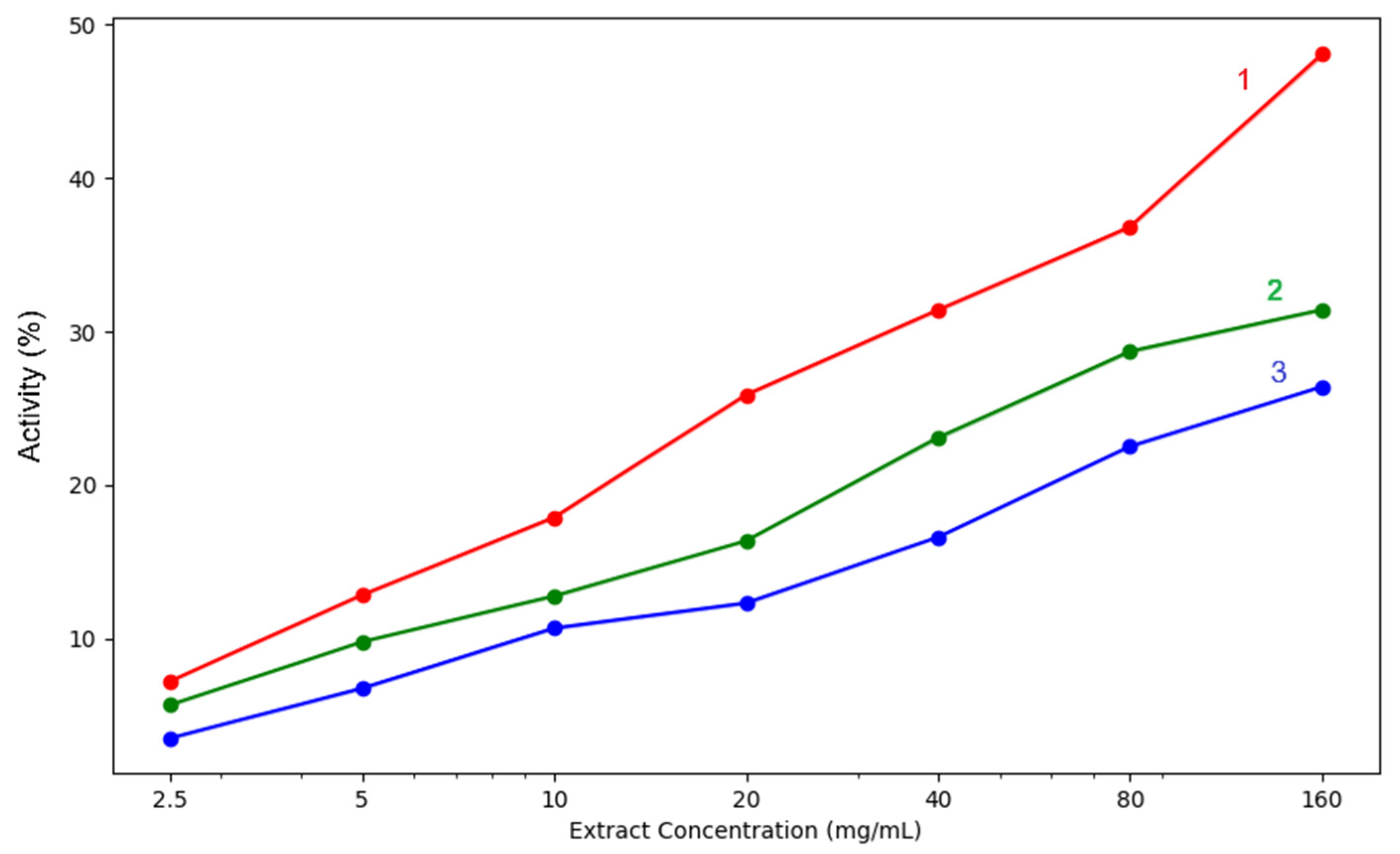
| acetic acid | 4.55% ± 0.10 | 9.55% ± 0.07 | 12.62% ± 0.03 | 16.53% ± 0.01 | 21.00% ± 0.10 | 30.85% ± 0.05 | 36.11% ± 0.04 | |
| methanol | 3.25% ± 0.04 | 7.79% ± 0.07 | 11.48% ± 0.08 | 21.04% ± 0.06 | 26.20% ± 0.04 | 34.75% ± 0.06 | 39.64% ± 0.08 | |
| acetone | 1.16% ± 0.04 | 4.57% ± 0.06 | 8.30% ± 0.05 | 14.16% ± 0.08 | 20.05% ± 0.10 | 26.14% ± 0.05 | 31.31% ± 0.07 | |
| Activity (%) ± SD | Extract Concentration | |||||||
| 2.5 mg/mL | 5 mg/mL | 10 mg/mL | 20 mg/mL | 40 mg/mL | 80 mg/mL | 160 mg/mL | ||
| acetic acid | 3.51% ± 0.04 | 6.77% ± 0.07 | 10.68% ± 0.07 | 12.31% ± 0.07 | 16.62% ± 0.03 | 22.52% ± 0.06 | 26.43% ± 0.05 | |
| methanol | 7.24% ± 0.05 | 12.84% ± 0.06 | 17.91% ± 0.08 | 25.90% ± 0.08 | 31.41% ± 0.07 | 36.84% ± 0.10 | 48.08% ± 0.15 | |
| acetone | 5.69% ± 0.05 | 9.79% ± 0.03 | 12.77% ± 0.07 | 16.38% ± 0.04 | 23.10% ± 0.10 | 28.72% ± 0.09 | 31.42% ± 0.07 | |
| Activity (%) ± SD | Extract Concentration | |||||||
| 2.5 mg/mL | 5 mg/mL | 10 mg/mL | 20 mg/mL | 40 mg/mL | 80 mg/mL | 160 mg/mL | ||
| acetic acid | 1.93% ± 0.05 | 4.01% ± 0.15 | 7.81% ± 0.14 | 9.87% ± 0.25 | 15.55% ± 0.41 | 25.29% ± 0.52 | 30.94% ± 0.28 | |
| methanol | 2.65% ± 0.06 | 5.46% ± 0.22 | 12.40% ± 0.18 | 19.70% ± 0.50 | 29.09% ± 0.39 | 36.35% ± 0.48 | 40.80% ± 0.29 | |
| acetone | 2.21% ± 0.06 | 4.55% ± 0.17 | 8.37% ± 0.11 | 14.61% ± 0.32 | 21.88% ± 0.55 | 28.25% ± 0.38 | 34.25% ± 0.33 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitran, A.-M.; Lungu, I.I.; Caba, I.C.; Mircea, C.; Robu, S.; Stefanache, A.; Rusu, P.; Mita-Baciu, I.; Hancianu, M.; Cioanca, O. Broccoli Sprouts as Functional Food: Phytochemical Profile and Antioxidant Activity Linked to Human Health. Appl. Sci. 2025, 15, 11375. https://doi.org/10.3390/app152111375
Mitran A-M, Lungu II, Caba IC, Mircea C, Robu S, Stefanache A, Rusu P, Mita-Baciu I, Hancianu M, Cioanca O. Broccoli Sprouts as Functional Food: Phytochemical Profile and Antioxidant Activity Linked to Human Health. Applied Sciences. 2025; 15(21):11375. https://doi.org/10.3390/app152111375
Chicago/Turabian StyleMitran, Andreea-Maria, Ionut Iulian Lungu, Ioana Cezara Caba, Cornelia Mircea, Silvia Robu, Alina Stefanache, Paula Rusu, Ioana Mita-Baciu, Monica Hancianu, and Oana Cioanca. 2025. "Broccoli Sprouts as Functional Food: Phytochemical Profile and Antioxidant Activity Linked to Human Health" Applied Sciences 15, no. 21: 11375. https://doi.org/10.3390/app152111375
APA StyleMitran, A.-M., Lungu, I. I., Caba, I. C., Mircea, C., Robu, S., Stefanache, A., Rusu, P., Mita-Baciu, I., Hancianu, M., & Cioanca, O. (2025). Broccoli Sprouts as Functional Food: Phytochemical Profile and Antioxidant Activity Linked to Human Health. Applied Sciences, 15(21), 11375. https://doi.org/10.3390/app152111375







