Abstract
The role of soy isoflavones, which are an example of functional food, is increasingly emphasised in the context of multiple diseases, including those related to fertility. One of the most common causes of female infertility is polycystic ovary syndrome (PCOS), which, in addition to hormonal disorders, is usually accompanied by metabolic and systemic disorders. Soy isoflavones may be helpful in treating conditions such as insulin resistance, inflammation, cardiovascular disease, and intestinal dysbiosis. The current article is a narrative review aimed at summarizing the available scientific reports on the impact of soy isoflavones on endocrine and metabolic mechanisms in women with PCOS. Despite the limitations, current scientific evidence suggests that soy isoflavones can significantly contribute to lowering metabolic risk and affect hormonal balance in women with PCOS. Promising results have come from studies on PCOS and the gut microbiota, where a bidirectional relationship is suggested: the influence of the gut microbiota on the bioavailability of soy isoflavones and the modulation of microbiota composition by these compounds. However, further interventional studies involving humans are needed to better understand the endocrine and metabolic relationships regarding the role and importance of soy isoflavones in the pathomechanisms of the development and treatment of these disorders.
1. Introduction
Polycystic ovary syndrome (PCOS) affects about 1 in 10 women of reproductive age, making it the most common endocrinopathy in this patient group [1,2,3,4,5]. Many women with PCOS remain undiagnosed or are misdiagnosed, and thus treatment is often delayed [5]. The importance and role of diet, functional foods, supplementation, or properly selected physical activity is also often overlooked or marginalised during the treatment of PCOS [1,2,3,4,5]. The role of soy isoflavones, an example of functional foods, is increasingly highlighted in the context of diseases and conditions, including those related to fertility, such as PCOS. Soy isoflavones are considered phytoestrogens, the active components of plants that have an affinity for oestrogen receptors [6,7]. Depending on, among other things such as type, dose, type of oestrogen receptor they bind to, and levels of endogenous hormones, soy isoflavones can have pro-oestrogenic or anti-oestrogenic effects [8,9,10]. In PCOS, when oestrogen deficiency is present, soy isoflavones bind to oestrogen receptors (ERβ and, to a lesser extent, ERα), offsetting the symptoms of low endogenous oestrogen levels [11,12]. Soy phytoestrogens also affect oestrogen levels by inhibiting the synthesis and activity of selected enzymes involved in the metabolism of both oestrogens and androgens [11,12,13]. The biological activity of androgens is mainly reflected in the reduction of hyperandrogenism, which is associated with high concentrations of testosterone and dihydrotestosterone (DHT), a stronger metabolite of testosterone [13,14,15]. However, not all studies confirm the positive effect of soy isoflavones on testosterone, DHT, or the free androgen index [16,17]. The significant role of soy products is also seen in the context of improving metabolic parameters (lipidogram, blood pressure, glucose and insulin levels, tissue resistance to insulin, and markers of inflammatory status). These disorders are often associated with certain PCOS phenotypes [18,19,20,21,22,23,24]. The scope of the effect of soy isoflavones in PCOS is therefore very wide due to the many different symptoms accompanying the syndrome, such as reproductive, dermatological, microbiotic, or metabolic disorders, as discussed in the various sections of this article [1,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. Recently published studies indicate a link between gut microbiota and the pathogenesis and treatment of PCOS. A major role in this topic is attributed to both the involvement of soy isoflavones in modulating the composition of the host’s gut microbiota and supporting its ability to convert isoflavones into forms that may support positive hormonal and metabolic effects in women with PCOS [17,25,26,27].
Therefore, the main objectives of this narrative review were (1) to review the existing literature on the relationship between soy isoflavones and their effect on hormonal and metabolic pathomechanisms associated with PCOS, (2) to analyse the potential of isoflavone–microbiota interactions in modulating symptoms associated with PCOS, and (3) to indicate limitations in research and the direction of future research in this area.
2. Materials and Methods
We conducted a computerised search using the electronic databases PubMed/MEDLINE, CINAHL, Embase, Scopus, Cochrane Library, and Web of Science. Research was conducted using English-language meta-analyses, systematic reviews, randomised clinical trials, and observational studies from all over the world. The literature review was performed up to April 2025. The following terms related to hormonal and metabolic mechanisms were linked to the phrases “soy isoflavones” and “PCOS” OR “polycystic ovary syndrome”: “hyperandrogenism”, “testosterone level”, dihydrotestosterone level” OR “DHT level”, “androgens level”, “free androgens level”, “oestrogen level”, “insulin resistance” OR “IR”, “risk type 2 diabetes”, “glucose level”, “cardiovascular risk”, “cardiovascular disease” OR “CVD”, “hypertension”, “overweight” OR “obesity”, “metabolic syndrome”, “antioxidants”, “antioxidant effect”, “anti-inflammatory”, “inflammation”, “microbiota” OR “gut–microbiome”. The search was supplemented by using the names of the main soy isoflavones: genistein, daidzein, and the metabolite of daidzein: equol. The literature search and study selection were conducted independently by three researchers (IM, MZ, and AD). Discrepancies were resolved through discussion.
Studies that met the following criteria were eligible for review: articles in English, meta-analyses, systematic reviews, randomised clinical trials, observational studies, in vivo studies, in vitro studies, animal studies, and recommendation and guidance documents. The exclusion criteria were as follows: articles in a language other than English, books, case studies, commentaries, letters to the editor, narrative reviews, review of reviews, research involving adolescents (<18 years old), adult postmenopausal women, and research on cancers (see Figure 1).
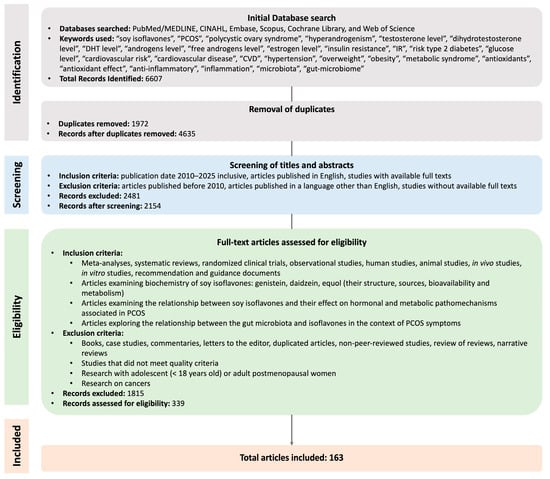
Figure 1.
Methodology selection flowchart.
This review primarily included publications from the years 2010 to 2025 to ensure the relevance and currency of the data and research findings discussed. Older sources were cited only when essential for a comprehensive understanding of the context or when they constituted foundational work on the topic. Additionally, to broaden the scope of the analysis and address potential gaps arising from database searches, the reference lists of selected articles were also examined. This approach allowed for the identification of further valuable and relevant sources. The search process for this narrative review is illustrated in Figure 1.
3. Biochemistry of Soy Isoflavones
3.1. Genistein
Genistein, also known as 5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one, is one of the best-researched compounds in the isoflavone group [28]. It was first isolated from Genista tinctoria L., a member of the Fabaceae family, from which its name is derived [29]. Genistein occurs naturally in various plant-derived foods, including legumes, fruits (e.g., apples, grapes, strawberries), seeds (e.g., sunflower), and vegetables such as cauliflower, broccoli, onions, spinach, cabbage, and tomatoes, as well as in clover and alfalfa sprouts. However, the main dietary source remains soybeans and their products [30,31,32]. In foods, genistein is usually found in a glycosidic form—as genistin. For intestinal absorption to occur, it must first be hydrolysed into its active aglycone form (genistein), a process facilitated by the enzyme lactase-phlorizin hydrolase or by gut microbiota [33], primarily from the genera Dorea, Sutterella, Parabacteroides, Bifidobacterium, and Lactobacillus [34]. The genistein and genistin content in soy products varies depending on the degree of processing. In non-fermented soy products—such as raw soybeans, soy milk, soy nuts, or tofu—the glycosidic form predominates. Genistin levels in such products can range from approximately 200.6 to 968.1 µg/g, whereas biologically active genistein is present at significantly lower levels, typically between 4.6 and 18.2 µg/g. Similar proportions are observed in soy milk and tofu (1.9–13.9 µg/g genistein; 94.8–137.7 µg/g genistin). Fermentation significantly alters isoflavone content. Fermented soy products such as miso and natto contain markedly higher levels of free genistein—ranging from 38.5 to 229.1 µg/g [32], with raw tempeh containing up to approximately 280 ± 110 µg/g [35]. This increase results from the enzymatic activity of fermentative microorganisms, which cleave β-glycosidic bonds in genistin molecules, removing the sugar moiety and converting it into free, active genistein. Although genistin remains present in fermented products, the proportion of aglycone increases substantially [32].
Although genistein is efficiently absorbed from the gastrointestinal tract, its oral bioavailability is limited to approximately 10% [36,37,38]. This is attributed, among other factors, to its lipophilic nature, which results in poor water solubility, extensive metabolic transformations [39,40,41,42], and the activity of efflux transporters responsible for removing the compound from cells [37]. The vast majority of ingested genistein undergoes accumulation and biotransformation primarily in the intestine and liver. The main metabolic pathways involved in this process are glucuronidation and sulfation. These reactions lead to the formation of conjugated metabolites—glucuronides and sulphates—which dominate in the systemic circulation and exhibit limited biological activity [37]. The involvement of cytochrome P450 (CYP) enzymes in genistein metabolism is relatively minor [38,43] and appears to be confined mainly to less prominent hydroxylation pathways [44]. Following prior hydrolysis of genistin (the glycosidic form of GEN) by microbial enzymes, free genistein can either be absorbed or further metabolised in the colon by gut bacteria. In this environment, secondary microbial metabolites such as dihydrogenistein (DH-GEN), 6′-hydroxy-O-desmethylangolensin (6′OH-ODMA), and 4-ethylphenol (4EP) can be produced. These transformations occur exclusively through the activity of the intestinal microbiota. However, there is substantial variability between individuals in their ability to generate these metabolites, primarily due to differences in gut microbiota composition. Not all individuals harbour microbial species capable of catalysing specific steps in these metabolic pathways. This results in marked variations in the post-ingestion metabolite profile of isoflavones between individuals and across species [44]. Importantly, the course and efficiency of genistein metabolism may be further modulated by a range of environmental and physiological factors, including intestinal transit time, gastrointestinal pH, redox potential, as well as the nutritional and immune status of the host [44]. From a biological activity perspective, the microbial degradation of genistein to DH-GEN, 6′OH-ODMA, and/or 4EP is associated with a reduction in oestrogenic potential. Genistein itself has a higher affinity for oestrogen receptor β (ERβ) than α-type (ERα). However, 4EP has no detectable affinity for either receptor. Meanwhile, 6′OH-ODMA binds to ERα and ERβ with comparable potency [45]. Since only about 9–22% of ingested genistein is excreted in the urine, it is presumed that the remaining portion is either eliminated through alternative pathways or distributed among various tissues in the body [46].
The oestrogen-like activity of genistein arises from its chemical structure (Figure 2), particularly the presence of hydroxyl groups at the carbon positions 4 and 7 of the ring structure. Their spatial arrangement is similar to that of the hydroxyl groups in the 17β-oestradiol molecule, which enables genistein to bind to oestrogen receptors—both ERα and ERβ. Due to this structural similarity to oestradiol, genistein can exert cellular effects via receptor activation; however, its potency is considerably weaker than that of 17β-oestradiol [47]. Importantly, genistein exhibits approximately 20-fold higher affinity for the ERβ receptor compared to ERα [48,49]. This is particularly significant from both physiological and safety perspectives. The adverse effects of estrogenic activity are largely associated with ERα activation, whereas ERβ activation is believed to exert protective and health-promoting effects. Consequently, genistein is classified as a phytoestrogen with a favourable action profile [50]. The effects of genistein are influenced by the localisation of oestrogen receptors, which can vary temporally, across tissues, between individuals, and even across populations [51,52,53]. Notably, the biological activity of genistein is not limited to interactions with oestrogen receptors. It has also been shown to modulate the activity of various other targets, including 5α-reductase [54], tyrosine kinase [55], peroxisome proliferator-activated receptor gamma (PPAR-γ) [56,57], glucose transporters (GLUT1 and GLUT4) [58,59], as well as topoisomerases type I and II [60]. These interactions broaden the range of genistein’s potential biological effects.

Figure 2.
Skeletal structures of selected soy isoflavones (genistein, daidzein, and equol) and oestradiol.
3.2. Daidzein and Equol
Daidzein (7-hydroxy-3-(4-hydroxyphenyl)chromen-4-one) is, alongside genistein, one of the principal representatives of isoflavones [61]. It occurs predominantly in leguminous plants, with soy and soy-based products—such as miso, tempeh, tofu, soy flour, soy yogurt, soy drink, and sufu—being the richest dietary sources. This compound is also present in other plants, including common beans, mung beans, fava beans, red clover, broccoli, and asparagus, as well as in nuts and seeds [62]. Similar to genistein, daidzein’s lipophilicity and extensive intestinal-hepatic metabolism result in its low bioavailability—approximately 6% [63,64]. Like other isoflavones, it cannot be absorbed in the human intestine in its conjugated form; it must first undergo hydrolysis into its active aglycone form via β-glucuronidase in the small intestine [65]. The resulting aglycone can be directly absorbed and transported to the liver, where it is conjugated by hepatic enzymes to form sulphated, glycosylated, and glucuronidated isoflavones [66]. Alternatively, it may undergo further metabolic transformations mediated by the gut microbiota, including hydroxylation, demethylation, methylation, reduction, or C-ring cleavage [67]. These transformations can lead to the formation of secondary metabolites such as equol, dihydrodaidzein, and O-desmethylangolenin [68,69].
Equol, also known as (3S)-3-(4-hydroxyphenyl)-3,4-dihydro-2H-chromen-7-ol, is the most metabolically active derivative of daidzein and exhibits higher biological activity than both daidzein and genistein [70]. Studies indicate that only a subset of the population possesses the ability to synthesize equol microbiologically. It is estimated that approximately 80–90% of individuals produce O-desmethylangolensin—a compound devoid of hormonal activity—whereas only 25–50% of the population can convert daidzein into equol via intestinal bacteria [71,72,73]. The highest prevalence of equol producers is observed among Asian populations, suggesting a significant influence of environmental factors, including dietary patterns [74]. Notably, one study found that 59% of vegetarians were equol producers, compared to only 29% among meat-eaters, implying that dietary composition may substantially affect the gut microbiota’s capacity to metabolise daidzein [72]. Bacteria responsible for equol production primarily belong to the family Coriobacteriaceae, including species such as Adlercreutzia equolifaciens, Asaccharobacter celatus, Enterorhabdus mucosicola, Slackia isoflavoniconvertens, and Slackia equolifaciens. Additionally, certain strains from the genera Lactobacillus, Lactococcus, and Bifidobacterium have also demonstrated the ability to produce equol [67].
Both daidzein and equol bind to ERα and ERβ, with a greater affinity for the ERβ isoform. Equol displays a higher affinity for oestrogen receptors than its precursor daidzein, reaching a level comparable to that of genistein [75]. Due to the unequal expression of ERα and ERβ in different tissues, the effect of equol may vary and depend on the local ratio of receptor isoforms. ERα is primarily expressed in the uterus, and to a lesser extent in the ovaries, intestines, and skin. In contrast, ERβ expression is found in the ovaries, colon, kidneys, central nervous system (CNS), and cardiovascular system [76]. In addition, the effect of equol as an agonist or antagonist may be modulated by the concentration of endogenous oestrogens, which have a higher affinity for both receptor types [67]. Both daidzein and equol exhibit a range of therapeutic properties, including the ability to neutralize reactive oxygen species and prevent oxidative stress, primarily through the activation of antioxidant enzymes [77]. In addition, these compounds may inhibit DNA fragmentation and apoptosis, partly through the activation of the Akt signalling pathway [78], and may also modulate epigenetic processes by influencing DNA methylation, histone modification, and miRNA expression [77,79].
4. Polycystic Ovary Syndrome and Soy Isoflavones
Polycystic ovary syndrome is a disease that is one of the most common causes of female infertility [80]. It is estimated that PCOS affects 10–13% of women of reproductive age if the diagnosis is based on the Rotterdam criteria, and the incidence rate is steadily increasing [1,2,3,4,5]. PCOS, frequently, in addition to hormonal and typically related to the reproductive system, can be accompanied by numerous metabolic and systemic disorders, such as tissue insulin resistance (IR), cardiovascular diseases (CVD), chronic inflammation, dermatological and psychological symptoms, and intestinal dysbiosis [1,17,18,19,20,21,22,23,24,81,82,83,84,85,86]. The characteristic PCOS features and associated disorders of the various systems are shown in the figure below (see Figure 3).
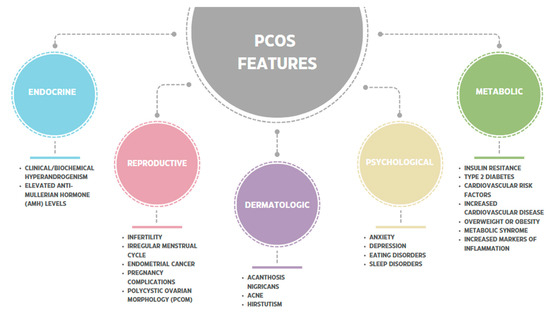
Figure 3.
The characteristic PCOS features and associated disorders of the various systems [17,18,19,20,21,22,23,24,80,81,82,83,84,85,86,87,88].
According to the latest guidelines by Tedee et al. from 2023, the diagnosis of polycystic ovary syndrome is recommended based on the updated and expanded Rotterdam criteria [1]. These criteria assume the presence of two of the following characteristics: (1) clinical or biochemical hyperandrogenism, (2) ovulation disorders, and (3) polycystic ovary syndrome (PCOS) on ultrasound [1]. In the 2023 recommendations, an elevated anti-Müllerian hormone (AMH) level was included as an auxiliary marker in the PCOM assessment in adult women, if other causes of the above ailments were excluded [1].
International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome 2023 (extended Rotterdam criteria 2003) allows us to distinguish four basic phenotypes of PCOS, with phenotypes A and B not differing significantly in terms of endocrine or metabolic characteristics [1,89] (see Figure 4). According to the 2014 consensus of the European Society of Endocrinology (ESE) and other researchers, it is possible to change from one phenotype to another, especially in the transition from ovulatory to anovulatory cycles, which is associated, among other things, with weight gain, poor diet, and low physical activity [90,91,92,93].

Figure 4.
PCOS phenotypes in adult women based on International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome 2023 (extended Rotterdam criteria 2003) [1]. ✓—indicates the presence of a symptom; ✗—indicates the absence of a symptom.
Experts from all over the world strongly recommend implementing a healthy lifestyle that combines a healthy diet and physical activity [1]. Both women trying to get pregnant and those who are not planning to do so in the near future will benefit from this solution. The main advantages include the following: improvement of general health, body composition, and quality of life. An additional, and for many women key, advantage of lifestyle changes is weight control, including maintaining weight, preventing weight gain, or achieving controlled weight reduction [1,94].
Although the pathogenesis of polycystic ovary syndrome is still unclear, it has been proven that inflammation deteriorates its course [95,96,97,98]. Therefore, improvement can be achieved by following diets with high anti-inflammatory potential, while the Western diet worsens the condition of patients [94,96]. Significant benefits in both the prevention and management of PCOS and its metabolic and hormonal complications are also seen in functional foods, particularly soy isoflavones [17,19,21,25,26,27,99]. Nutritional models in which soy products or legumes play an important role are primarily plant-based diets, e.g., the portfolio low-carbohydrate diet or the Mediterranean diet, which also have high anti-inflammatory potential [96,99]. The benefits of using plant-based models containing soy isoflavones, in addition to their potential to affect oestrogen receptors in PCOS, may also have other benefits. These include a low degree of food processing and the associated lower caloric content of the diet, which can facilitate the reduction of excessive body weight or maintain it at a healthy level [100]. The effect of individual soy isoflavones on various mechanisms occurring in PCOS is discussed in the following chapters of this article.
4.1. Endocrine Mechanisms
4.1.1. Hyperandrogenism
The main feature of the three PCOS phenotypes (A-C) is an above-normal level of androgens, which triggers other characteristic symptoms such as menstrual disorders, infertility, excessive hair growth, acne, and many others [94,101,102]. In women of reproductive age without hormonal disorders, androgen production occurs in similar amounts in the ovaries and adrenal glands, and the main androgens are androstenedione and testosterone [101,102]. When steroidogenesis is disrupted, as in PCOS, the ovaries and, less commonly, the adrenal glands produce excessive amounts of androgens [101]. The causes of ovarian dysfunction in this regard remain unclear [95,101,102,103,104]. Some studies indicate that the cause of functional ovarian hyperandrogenism (FOH) is the overexpression of mRNA of steroidogenic enzymes in isolated theca cells, especially cytochrome P450c17 and luteinizing hormone (LH) receptors [105,106]. This defect in theca cells leads to excessive androgen production due to higher activity of steroidogenic enzymes [105,106]. Increased androgen secretion through various mechanisms is also observed in response to IR occurring in PCOS [95]. This condition affects both slim women with PCOS and those who are overweight or obese [107]. Excess insulin increases LH production, which is often elevated in women with PCOS. High LH levels stimulate excessive androgen production mainly in the ovaries (to a lesser extent in the adrenal glands), activating steroidogenic enzymes, including 17α-hydroxylase, 17,20c-lyase, and cytochrome P450c17 [95,108]. Due to the increase in LH levels, relatively low FSH levels lead to an increase in the imbalance between LH-dependent androgen synthesis and follicle-stimulating hormone (FSH)-stimulated oestrogen synthesis [108,109]. Hyperinsulinemia also affects the decrease in the production of sex hormone-binding globulin (SHBG) in the liver, which in turn increases the level of free androgens [96,110,111]. Selected pathways leading to hyperandrogenism in PCOS are shown in the figure below (see Figure 5).
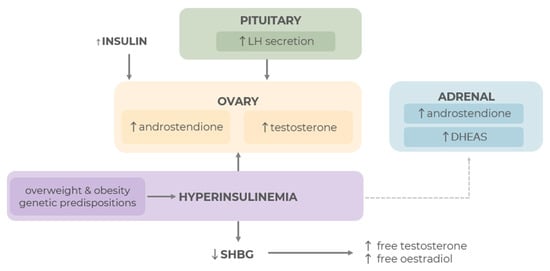
Figure 5.
Selected pathways leading to hyperandrogenism in PCOS [38,45,46,47,48,49,50,51,52,53,54,55,56], ↑ indicates an increase; ↓ indicates a decrease.
Ovarian hyperandrogenism is considered to be the main mechanism leading to excess androgens in PCOS. However, functional adrenal hyperandrogenism (FAH) may also play a role, although it is more common in lean women with PCOS [101,104,112]. In FAH, serum levels of dihydroepiandrosterone sulphate (DHEAS), secreted by the adrenal cortex, are elevated. The causes, similar to FOH, are believed to be related to disturbances in steroidogenesis pathways through hyperinsulinemia, but also increased adrenal sensitivity to ACTH and, consequently, increased activity of the enzymes 17a-hydroxylase and 17,20-lyase [113].
4.1.2. Hypothalamic–Pituitary–Ovarian Axis
In addition to hyperandrogenism resulting from ovarian factors, non-ovarian factors also influence the pathogenesis of PCOS and the increase in androgen levels, including abnormalities in the hypothalamic–pituitary–ovarian (HPO) axis [114]. Under physiological conditions, this axis regulates normal hormone concentrations and function, including ovulation [115]. Gonadotropin-releasing hormone (GnRH) is released in pulses in the hypothalamus, stimulating the pituitary gland to release follicle-stimulating hormone (FSH) and luteinizing hormone. Follicle-stimulating hormone and lutropin regulate ovarian function. The former is responsible for the formation of ovarian follicles (Graafian follicles) and stimulating the production of oestrogen in the granulosa cells of the ovaries and progesterone [115]. FSH also increases the activity of aromatase, an enzyme that helps convert androgens into oestrogens. The second hormone, LH, plays a key role in ovulation. If ovulation proceeds normally—in the production of the corpus luteum and thus progesterone, and also stimulates the theca cells of the ovaries to produce androgens (testosterone, androstenedione, dehydroepiandrosterone (DHEA)—in small amounts) [115]. FSH and LH in each phase of the menstrual cycle are precisely regulated and controlled by variations in the amplitude and frequency of GnRH pulses [114]. In women with polycystic ovary syndrome, LH and FSH levels deviate significantly from normal values. Usually, LH levels increase significantly, while FSH levels decrease, although they may also remain largely unchanged. This situation causes an increased LH/FSH ratio, which is observed in most patients with PCOS [115,116,117,118]. The cause of this condition is thought to be increased frequency and amplitude of LH pulses caused by increased frequency of GnRH pulses. On the other hand, increased GnRH secretion in PCOS may be caused by hyperinsulinemia, which increases the pituitary gland’s response to GnRH. Low progesterone levels caused by anovulation or hyperandrogenism affecting the hypothalamus through a negative feedback mechanism may play a similar role [116]. There are also reports concerning kisspeptin, a neuropeptide produced in the hypothalamus, which shows elevated levels in women with PCOS. It affects HPO axis dysfunction by binding to the GPR54 (KISS1R) receptor on GnRH neurons and causing an increase in the frequency and amplitude of GnRH pulses. As a result, there is an increase in LH and androgen levels [119,120]. Another neurotransmitter, gamma-aminobutyric acid (GABA), which has an inhibitory effect in other regions of the CNS, may have a stimulating effect in the hypothalamus, stimulating the release of GnRH and thus leading to an increase in LH/FSH [121].
4.1.3. Aromatase and Oestrogens
Under physiological conditions, androgens produced by theca cells are converted to oestrogens by the enzyme aromatase (CYP19A1) [122]. Aromatase is active in many tissues, including the gonads, brain, adrenal glands, adipose tissue, and bones [60]. In PCOS, reduced expression of this enzyme in the ovaries and decreased production of oestradiol (E2) in the granulosa cells of the ovary to lower normal values or slightly lower values are observed [123]. Researchers suggest that this is most likely due to hypermethylation of the CYP19A1 promoter region, resulting in excessive accumulation of androgens and E2 deficiencies in the ovaries [124]. In turn, in patients with PCOS and excessive adipose tissue, its increased amount is responsible for the increased conversion of androgens to estrone (E1) [125]. However, there are also reports suggesting that high leptin levels, visceral obesity, and related inflammatory processes and hyperinsulinemia may lead to a decrease in the methylation of this gene and a decrease in aromatase activity in adipocytes [126,127]. Studies show that AMH levels are higher in women with PCOS, correlate with hyperandrogenism, and may explain reduced oestrogen levels [128,129,130]. Elevated AMH may also directly stimulate theca cells to overproduce androgens [130]. It has been proven in both animal models and studies involving women that PCOS may have its origins in foetal life. High AMH and hyperandrogenism in pregnant women led to a decrease in aromatase activity in the placenta and foetal exposure to excess oestrogen [128,129]. Research also suggests that there is a link between PCOM and hormonal dysregulation, including elevated AMH levels [131].
Depending on the phenotype of PCOS, its cause, and the accompanying complications, pharmacological therapy, nutritional interventions, supplementation, or the use of functional foods may have varying degrees of effectiveness. In the case of hyperandrogenism, soy isoflavones can be an effective support method (Table 1). Studies involving women diagnosed with PCOS suggest a reduction in biochemical markers of hyperandrogenism, including a decrease in hormone levels such as total and free T, LH, and DHEAS, and a reduction in FAI in response to soy isoflavone supplementation or consumption of functional foods (soy bread) [14,15,16,132]. One study also reported an increase in SHBG levels [132]. These studies showed an improvement in hormonal parameters, but for most parameters, the differences were not statistically significant [14,15,16,132]. Previous studies conducted with PCOS patients confirm an improvement in the levels of certain hormones compared to the period before soy isoflavone supplementation [14,15,16,132].
In summary, previous studies conducted with PCOS patients confirm a slight or significant improvement in the levels of certain hormones compared to the placebo group, despite the lack of statistical significance of the results [14,132]. One of them confirms a significant improvement in this regard in female patients compared to the period before soy isoflavone supplementation [15]. However, in the case of consumption of bread containing soy isoflavones, long-term consumption is suggested in order to observe statistically significant benefits [132]. In a pilot study by Romuladi et al. [16], no statistical significance was found in terms of the hormonal profile. Nevertheless, there was a significant and statistically significant improvement in lipid profile parameters, which may also indirectly affect disease control and improve patient outcomes [16].
A larger number of studies on the relationship between soy isoflavones and endocrine mechanisms in PCOS have been conducted on animal models (Table 2) [133,134,135,136,137,138]. Preclinical studies have shown a reduction in hormonal parameters such as total T, DHT, and LH [133,137,138]. However, the main mechanism of action of soy isoflavones in the context of preclinical studies seems to be related to their high antioxidant potential and positive effect on inflammatory markers in the body [134,135,136,137]. A decrease in parameters such as IL-6, TNF-a, and TOS, and an increase in TAC, GSH, and SOD have been reported [134,135]. Benefits were also seen in the context of enzymes modulating steroid hormone metabolism: ↓3β-HSD, 17β-HSD, and aromatase activity [133]. Modulation of the activity of the latter enzyme may help reduce the concentration of androgens, including testosterone, in peripheral blood, thereby alleviating the symptoms of hyperandrogenism and the severity of PCOS [71,72,73,133]. Significant benefits of soy isoflavone supplementation are also seen in the context of improving egg morphology and ovarian histology, thereby normalizing ovarian function [133,134,136,137]. The main conclusions from studies on rats concern the ability of soy isoflavones to modulate aromatase enzyme activity and the associated reduction in some androgen levels, as well as their strong antioxidant effect and anti-inflammatory properties.
One of the most commonly used drugs in the treatment of PCOS and associated metabolic disorders is metformin [136]. There was a statistically significant decrease in the number of cysts in groups treated with both metformin and genistein, resulting in the appearance of normal follicles and corpus luteum. However, despite treatment, the number of follicles remained significantly higher than in the control group [136]. Ma et al. [137] report that the reduction in ovarian weight and volume, the reduction in the number of atretic follicles, and the improvement in follicle quality in the groups treated with soy isoflavones and metformin were similar. At the same time, the reduction in T, LH, and LH/FSH ratios, as well as the increase in E2 and FSH concentrations, were similar between the metformin and soy isoflavone groups [137].
However, it is important to remember that studies on animal models rely on pharmacologically induced PCOS. In reality, the course of this disease in women can vary significantly, if only because of its complex and still not fully understood etiology.
Some of the studies looked at both hormonal and metabolic or inflammatory parameters, and their results are split between different tables (Table 1, Table 2, Table 3 and Table 4). The tables contain references to other tables for supplementary results or conclusions. Data such as study design, duration, and characteristics of the study group have been repeated to facilitate familiarisation with the full characteristics of the study.

Table 1.
Overview of clinical trials evaluating the effect of soy isoflavones on hormonal parameters in women with PCOS.
Table 1.
Overview of clinical trials evaluating the effect of soy isoflavones on hormonal parameters in women with PCOS.
| Authors, Year | Study Design, Duration | Characteristics of the Study Group | Intervention/Aim of the Study | Main Results | Conclusions |
|---|---|---|---|---|---|
| Romualdi et al., 2008 [16] | Pilot prospective study, 6 months | 12 caucasian with obesity, hyperinsulinemic, and dyslipidemic; women with PCOS (Rotterdam criteria); patients received 36 mg/d of genistein. | To investigate the effect of treatment with a standardised dose of soy isoflavone (genistein) on hormonal and metabolic disorders (see Table 3) in a group with obesity, hyperinsulinemic, and dyslipidemic patients with PCOS. | No statistically significant differences were observed in the hormone panel and menstrual cycle. | The possible benefits of the therapeutic use of phytooestrogens in PCOS appear to be limited to improving lipid metabolism. |
| Khani et al., 2011 [15] | Quasi-randomised trial, 3 months | 137 subjects with PCOS (Rotterdam criteria), aged 18–35; completed the study; study group: 69 women, 18 mg genistein twice a day orally; control group: 68 women—placebo, cellulose pills | To investigate the effect of soy phytoestrogens on reproductive hormones and lipid profiles (see Table 3) in women with PCOS. | No statistically significant differences in the hormonal profile between the study group and the placebo group. Significantly ↓ LH, ↓ DHEAS, ↓ T after genistein supplementation compared to hormone levels before intervention in the study group. | Genistein supplementation improved the hormonal profile in women with PCOS compared to the results before the use of soy isoflavones. No statistically significant differences were observed between the study group and the control group. |
| Forouhari et al., 2013 [132] | Crossover randomised controlled trial, 2 months | 42 women, aged 18–35; suffered from PCOS (Rotterdam criteria); (1) experimental group: soy bread (75 g of soy flour, 40 g of wheat flour) (2) control group: wheat bread and their common diet without foods that contained high amounts of soya | To investigate the effects of balanced soy flour bread on some sex hormones in premenopausal women with PCOS. | After consumption of soy bread: a slight ↓ total T, ↓ FSH, ↓ oestradiol concentrations; however not statistically significant. | Long-term consumption of soy bread in women with PCOS may show potential benefits in improving hormonal parameters. |
| Jamilian and Asemi, 2016 [14] | Randomised, double-blinded, placebo-controlled trial, 12 weeks | 70 women diagnosed with PCOS, aged 18–40 years; study group: 36 women, 50 mg/d soy isoflavones (containing 37.5 mg genistein, 10 mg daidzein, and 2.5 mg glycitein); control group: 35 women, placebo | The aim of the present study was to evaluate the effects of soy isoflavone supplementation on metabolic status and hormonal panel in women with PCOS. | ↓ FAI, ↓ free T, ↓ total T, ↓ DHEAS, ↑ SHBG, After adjusting biochemical markers, age, and baseline BMI to baseline values, there were no significant changes in hormone panel results. | Supplementation with soy isoflavones resulted in significant reductions in free androgen index and other metabolic indicators (see Table 3). |
BMI—body mass index; DHEAS—dehydroepiandrosterone sulphate; FAI—free androgen index; FSH—follicle-stimulating hormone; LH—luteinizing hormone; PCOS—polycystic ovary syndrome; SHBG—sex hormone-binding globulin; T—testosterone; ↑ indicates an increase; ↓ indicates a decrease.

Table 2.
Overview of preclinical studies on the effect of soy isoflavones on hormonal parameters in PCOS.
Table 2.
Overview of preclinical studies on the effect of soy isoflavones on hormonal parameters in PCOS.
| Authors, Year | Study Design, Duration | Characteristics of the Study Group | Intervention/Aim of the Study | Main Results | Conclusions |
|---|---|---|---|---|---|
| Rajan et al., 2017 [133] | Experimental animal study; 14-day treatment after 21-day PCOS induction | 24 female Sprague-Dawley rats, including 18 rats with letrozole-induced PCOS (1 mg/kg letrozole, p.o. once daily for 21 consecutive days), treatment 14-day, rats divided into 4 groups (n = 6/each); (1) control received 0.3% w/v CMC, p.o.; (2) served as PCOS control group and received vehicle, p.o.; (3) treated with soy isoflavones 50 mg/kg, p.o.,; (4) PCOS rats treated with soy isoflavones 100 mg/kg, p.o. Soy isoflavones (a mix of genistin, genistein, daidzin, and daidzein, in amounts of 59.91%, 12.05%, 23.53%, and 4.49%, respectively). | To investigate the effect of soy isoflavones on a rat model of PCOS induced by letrozole. | 100 mg/kg soy isoflavones treatment: ↓ body weight gain, ↓ diestrus phase %, ↓ testosterone, ↓ 3β-HSD and 17β-HSD enzyme activity, and improved ovarian histology with healthy antral follicles and granulosa cells. No statistically significant differences in 17β-oestradiol concentrations in study groups after soy isoflavone treatment. | Treatment with soy isoflavones may have a positive effect on rats with PCOS. This effect is attributed to the ability of soy isoflavones to modulate aromatase enzyme activity and reduce testosterone levels in peripheral blood. |
| Alivandi Farkhad and Khazali, 2019 [134] | Experimental animal study, post-induction SISAF treatment for 21 days, after 60-day EV-induction of PCOS | 32 female Wistar rats, including 24 rats with EV-induced; divided into 4 groups (n = 8/each): (1): CON, only received the vehicle, (2) rats with EV-induced PCOS, (3) a treatment group receiving SISAF 50 mg/kg by gavage for 21 days, (4) a treatment group receiving SISAF 100 mg/kg by gavage for 21 days Major compounds in the SISAF were genistein, daidzein, and glycitein. | To investigate the effect of SISAF on oxidative stress markers, inflammatory cytokines, and ovarian histology in rats with EV-induced PCOS. | SISAF treatment: ↓ number of cystic follicles, ↓ thickness of the theca layer, ↑ number of corpus luteum cells, and ↑ granulosa cells in rats with PCOS. Soy isoflavones improved inflammation indicators (see Table 4). | SISAF extract had an impact on ovarian histology in rats with EV-induced PCOS, by reducing oxidative stress markers and increasing TAC in rats with PCOS. |
| Rajaei et al., 2019 [135] | Experimental animal study; post-induction genistein treatment, 14-day treatment after 60-day PCOS EV-induction | 20 female Wistar rats, including 15 rats with EV-induced PCOS; divided into 4 groups (n = 4/each): (1) control group, no intervention; (2) EV-induced PCOS (60 days); (3) rats without PCOS, treated with genistein (14 days); (4): EV-induced PCOS rats (60 days), treated with genistein (14 days) | To evaluate the antioxidant effect of genistein on ovarian tissue morphology and oxidative stress markers (see Table 4) in PCOS rats | Genistein improved follicular morphology comparable to controls, by ↓ ovarian and plasma MDA levels; and other metabolic and inflammatory markers (see Table 4). | Genistein treatment maintained follicle quality by increasing antioxidant activity and scavenging levels of oxidants in PCOS rats. |
| Amanat et al., 2021 [136] | Experimental animal study; 42-day treatment | 40 female Sprague-Dawley rats, including 30 rats with letrozole-induced PCOS, divided into 4 groups (n = 10/each): (1) control group, healthy rats; (2) PCOS-induced rats that received normal saline by gavage; (3) PCOS-induced rats treated with 150 mg/kg of metformin dissolved in normal saline by gavage; (4) PCOS-induced rats supplemented with 20 mg/kg of genistein dissolved in 1% methylcellulose solution | To evaluate the effects of genistein (20 mg/kg) vs metformin on ovarian histology, oxidative stress, and other metabolic panel parameters. | Genistein improved ovarian histology (↑luteinization, ↓cystic follicles) and other metabolic and inflammatory markers (see Table 4). | Genistein supplementation in rats with PCOS induced significant remission of oxidative, inflammatory and histopathological parameters. |
| Ma et al., 2021 [137] | Experimental animal study; 28-day treatment after 21-day PCOS induction | 20 female Sprague-Dawley rats, including 15 rats with letrozole-induced PCOS (1 mg/kg letrozole); divided into 4 groups ((n = 5/each); (1) normal control group, (2) PCOS group (1 mL saline p.o.), (3) MET group (1 mL saline dissolved with 500 mg/kg metformin p.o.), (4) SI group (1 mL saline dissolved with 100 mg/kg soy isoflavones p.o.) | Investigation of the therapeutic effect and molecular activity of soy isoflavones in rats with PCOS. | Soy isoflavones: ↓ body weight, ↓ ovarian weight, ↓ LH, and ↓ testosterone; ↑ FSH, ↑ oestradiol; improved ovarian morphology and estrous cycle; ↓ inflammatory markers (see Table 4). | Soy isoflavones improved ovarian structure, and hormone levels, and reduced inflammation (see Table 4) in PCOS rats. |
| Xiao et al., 2024 [138] | Experimental (two-way randomisation) animal study; 8 weeks | 156 weanling female Sprague-Dawley rats; divided into 6 groups (n = 26/each): (1) control fed diets containing 0 g ISF/kg diet; (2) control fed 0.5 g ISF/kg diet, (3) control fed diets containing 1 g ISF/kg diet; (4) DHT-induced PCOS fed diets containing 0 g ISF/kg diet; (5) DHT-induced PCOS fed diets containing 0.5 g ISF/kg diet; (6) DHT-induced PCOS fed diets containing 1 g ISF/kg diet | To investigate whether ISF alleviates metabolic disorders associated with PCOS in a rat model and the quality of their ovaries. | ISF in both doses reduced DHT-induced changes: normalised ovarian follicle development (↑ primary, ↓ primordial follicles) | The ISF soy diet alleviated DHT-induced inhibition of ovarian follicle development. |
3β-HSD—3β-hydroxysteroid dehydrogenase; 17β-HSD—17β-hydroxysteroid dehydrogenase; CMC—carboxy methyl cellulose; CON—negative control group; CTRL—control; DHT—dihydrotestosterone; EV—oestradiol valerate; FSH—follicle-stimulating hormone; ISF—soy isoflavones; MET—metformin; MDA—malondialdehyde; PCOS—polycystic ovary syndrome; p.o.—per oral; SISAF—soybean isoflavone-aglycone fraction; SOD—superoxide dismutase; TAC—total antioxidant capacity; TOS—total oxidative status, ↑ indicates an increase; ↓ indicates a decrease.
4.2. Metabolic Mechanisms
PCOS is not only an endocrine disorder but also a metabolic disorder. It is often associated with obesity (particularly the visceral phenotype), IR, secondary hyperinsulinemia, and dyslipidaemia. These factors increase the risk of developing metabolic complications and CVD and exacerbate the clinical and hormonal symptoms. They play an important role in the pathophysiology of PCOS [139,140,141].
4.2.1. Central Obesity and Adipocyte Dysfunction in PCOS
In PCOS, the presence of central obesity is associated with an increased risk of metabolic abnormalities, even when body weight is normal [142,143]. Adipose tissue, especially visceral adipose tissue, has high metabolic and endocrine activity. It is a source of pro-inflammatory cytokines (such as IL-6 and TNF-α) and adipokines that promote the development of low-grade chronic inflammation [144,145]. Adipose tissue dysfunction contributes to the development of IR, secondary hyperinsulinemia, and endocrine disruption. This includes increased production of androgens and their conversion to oestrogens as a result of increased aromatase activity [146]. In addition, PCOS shows increased oxidative stress, markers of which are elevated levels of malondialdehyde (MDA) and reduced levels of glutathione (GSH), with impaired activity of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GPx) [147,148]. Adipocyte dysfunction is also associated with the activation of the NF-κB pathway (which regulates the inflammatory response) and dysregulation of PPARγ-dependent gene expression, which can lead to increased inflammatory and metabolic processes [149]. Excess and dysfunctional adipose tissue adversely affects the HPO axis, folliculogenesis, SHBG levels, and androgen concentrations, exacerbating the clinical symptoms of PCOS [150,151]. In this context, a meta-analysis of 30 clinical trials showed that soy and isoflavone intake may favourably affect anthropometric parameters in overweight individuals, including women, leading to reductions in weight, waist circumference, and BMI, particularly with moderate doses and short intervention times [152].
4.2.2. Insulin Resistance and Hyperinsulinaemia
IR involves reduced insulin sensitivity of target tissues, leading to compensatory hyperinsulinemia and impaired carbohydrate metabolism. IR in PCOS includes both classic insulin target tissues, such as adipose tissue and muscle, and non-classic tissues, such as the ovaries. Androgens affect adipocyte function by disrupting the insulin signalling pathway (including GLUT-4 expression) and promoting visceral fat deposition [153]. In addition, there is increased expression of the enzyme AKR1C3, which catalyses the conversion of androstenedione to testosterone, in the adipose tissue of women with PCOS. AKR1C3 expression is stimulated by insulin, which can increase local androgenogenesis and exacerbate IR [154,155]. IR in PCOS mainly involves the PI3K pathway, which is responsible for the metabolic effects of insulin, while the MAPK pathway remains active [156,157]. This explains the phenomenon whereby maintained and often increased insulin sensitivity in the ovaries leads to increased androgen production, independent of systemic IR [158,159]. Insulin enhances the action of LH in tecal cells and lowers SHBG levels, leading to an increase in free androgen levels and exacerbating hyperandrogenism [95,159,160]. Adipokines play an important role in the pathogenesis of IR in PCOS—decreased adiponectin and increased leptin levels correlate with IR and inflammation [161,162]. Regulation of these processes is mediated by nuclear peroxisome proliferator-activated receptors (PPAR), in particular the PPARγ isoform, which controls adipogenesis and insulin sensitivity. Reduced PPARγ activity and its genetic polymorphisms have been associated with increased IR in PCOS [163,164,165]. Long-term hyperinsulinemia can lead to pancreatic β-cell dysfunction and increase the risk of developing type 2 diabetes. IR is therefore considered to be central to the pathophysiology of PCOS and a key risk factor for metabolic complications. Importantly, hyperandrogenemia, as well as hypogonadism, in women with PCOS are closely associated with an increased risk of developing metabolic dysfunction-associated fatty liver disease (MASLD). Although obesity, especially of the visceral type, further exacerbates this risk, studies show that this association also occurs regardless of age and BMI [166]. The main pathogenetic mechanism is IR, which exacerbates both hyperandrogenism and liver fat accumulation [167].
4.2.3. Lipid Profile and Cardiovascular Risk
Metabolic abnormalities in PCOS—including obesity, IR, hepatic steatosis, and dyslipidaemia—increase the risk of CVD, highlighting the systemic nature of PCOS-related complications. Dyslipidemia is more common in women with PCOS than in women without the condition, and according to some studies, it affects up to 50–70% of PCOS patients [168,169]. In a meta-analysis by Wekker et al., women with PCOS were found to have significantly higher total cholesterol levels, lower levels of the HDL fraction, and an increased risk of hypertension, type 2 diabetes, and non-fatal cerebrovascular events [170]. Interestingly, women with PCOS without obesity are more likely to have adverse lipid abnormalities, such as hypertriglyceridemia and reduced HDL fraction cholesterol levels, compared to the control group [171]. This suggests that even in the absence of obesity, PCOS patients remain at increased risk of CVD. In addition to metabolic disorders, hyperandrogenism, which affects sympathetic nervous system activation and oxidative stress, may play an important role in the development of CVD. These may include the MC4R receptor pathway and arachidonic acid metabolites such as 20-HETE [172,173,174]. Soy isoflavones have been suggested to have a moderate protective effect against CVD risk factors. A recent meta-analysis by Lei et al. showed that supplementation with isolated isoflavones can lead to a significant reduction in blood pressure, while Zhan and Ho showed that consumption of soy protein containing isoflavones significantly improves lipid profile—lowering total cholesterol, LDL cholesterol, and triglycerides, and increasing HDL levels—which may contribute to CVD risk reduction [175,176]. In addition, isoflavones may support endothelial function through antioxidant and anti-inflammatory effects, and increased nitric oxide production, an important mechanism in the prevention of atherosclerosis [177,178]. In prospective studies, isoflavone intake was inversely associated with all-cause and cancer-related mortality, although no significant association was confirmed for CVD mortality [18]. There is also growing interest in equol, the active metabolite of daidzein, which may have stronger cardioprotective effects than soy isoflavones alone [74,179]. Interestingly, the results comparing the effectiveness of soy isoflavones to metformin in the context of metabolic and inflammatory disorders may also be of interest [136,137]. In studies by Amanat et al. and Ma et al., both soy isoflavone and metformin combination therapy was used in the treatment of PCOS [136,137]. Amanat et al. report that body weight was reduced in both groups of rats treated with metformin and soy isoflavones, with a greater reduction in the case of isoflavones, although these differences were not statistically significant. Ma et al. reported that both the group of rats administered with soy isoflavones and metformin showed significantly lower body weight than rats in the PCOS group [137]. The HOMA-IR index and glucose levels were statistically significantly lower in the group of rats with induced PCOS treated with metformin or soy isoflavones compared to the PCOS group. After treatment with metformin or genistein, there were slight changes in lipid levels, and statistically significant results were obtained in both groups in terms of LDL cholesterol, which decreased significantly compared to the PCOS group [136]. Both metformin and genistein showed similar results in terms of positive effects on inflammatory markers [136].
In conclusion, the metabolic mechanisms present in PCOS, such as obesity, IR, MASLD, and increased CVD risk, work together and may be important targets for intervention with soy isoflavones. To provide an overview of the current state of knowledge regarding the efficacy of soy isoflavones in modulating metabolic parameters in women with PCOS, the results of selected clinical studies are summarised below (Table 3). In addition, Table 4 provides an overview of preclinical studies that provide a mechanistic basis for the observed effects and indicate possible directions for further translational research.

Table 3.
Overview of clinical trials evaluating the effect of soy isoflavones on metabolic and inflammatory parameters in women with PCOS.
Table 3.
Overview of clinical trials evaluating the effect of soy isoflavones on metabolic and inflammatory parameters in women with PCOS.
| Authors, Year | Study Design, Duration | Characteristics of the Study Group | Intervention/Aim of the Study | Main Results | Conclusions |
|---|---|---|---|---|---|
| Romualdi et al., 2008 [16] | Pilot prospective study, 6 months | 12 caucasian with obesity, hyperinsulinemic, and dyslipidemic; women with PCOS (Rotterdam criteria); patients received 36 mg/d of genistein | To investigate the effect of treatment with a standardised dose of soy isoflavone (genistein) on hormonal (see Table 1) and metabolic disorders in a group with obesity, hyperinsulinemic, and dyslipidemia. | ↓ Total cholesterol, ↓ LDL, ↓ LDL/HDL ratio, and HDL did not increase significantly. No effect on IR and glycemia. | Genistein improved lipid profile. The lack of effect on glucose–insulin metabolism and hormonal profile suggests that genistein acts mainly through metabolic mechanisms. |
| Khani et al., 2011 [15] | Quasi-randomised trial, 3 months | 137 subjects with PCOS (Rotterdam criteria), aged 18–35; completed the study; study group: 69 women, 18 mg genistein twice a day orally; control group: 68 women—placebo, cellulose pills. | To investigate the effect of soy phytoestrogens on reproductive hormones (see Table 2) and lipid profiles in women with PCOS. | ↓ LDL, ↓ TG; no effect on HDL. | Genistein supplementation was associated with improvements in lipid parameters, suggesting a potential role in mitigating CVD risk in women with PCOS. |
| Jamilian and Asemi, 2016 [14] | Randomised, double-blind, placebo-controlled study; 12 weeks | 70 women diagnosed with PCOS, aged 18–40 years; study group: 35 women, 50 mg/d soy isoflavones (containing 37.5 mg genistein, 10 mg daidzein, and 2.5 mg glycitein); control group: 35 women, placebo. | To assess the effects of 50 mg/day soy isoflavones for 12 weeks on metabolic, endocrine, inflammatory, and oxidative stress markers in women with PCOS. | ↓ Insulin, ↓ HOMA-IR, ↑ QUICKI, ↓ TG, ↑ total glutathione, ↓ MDA. No significant changes in other lipids or inflammation markers. | Soy isoflavones improved IR, triglycerides, and oxidative stress in women with PCOS. |
LDL—low-density lipoprotein; HDL—high-density lipoprotein; TG—triglycerides; HOMA-IR—homeostasis model assessment of insulin resistance; QUICKI—quantitative insulin sensitivity check index; MDA—malondialdehyde; PCOS—polycystic ovary syndrome; ↑ indicates an increase; ↓ indicates a decrease.

Table 4.
Overview of preclinical studies on the effect of soy isoflavones on metabolic disorders and inflammatory parameters in PCOS.
Table 4.
Overview of preclinical studies on the effect of soy isoflavones on metabolic disorders and inflammatory parameters in PCOS.
| Authors, Year | Study Design, Duration | Characteristics of the Study Group | Intervention/Aim of the Study | Main Results | Conclusions |
|---|---|---|---|---|---|
| Rajan et al., 2017 [133] | Experimental animal study; 14-day treatment after 21-day PCOS induction. | 24 female Sprague-Dawley rats; including 18 rats with letrozole-induced PCOS (1 mg/kg letrozole, p.o. once daily for 21 consecutive days), treatment 14-day, rats divided into 4 groups (n = 6/each); (1) control received 0.3% w/v CMC, p.o.; (2) served as PCOS control group and received vehicle, p.o.; (3) treated with soy isoflavones 50 mg/kg, p.o.; (4) PCOS rats treated with soy isoflavones 100 mg/kg, p.o. | To evaluate the effect of soy isoflavones (50 and 100 mg/kg) on physical, metabolic, hormonal, and histological parameters in a rat PCOS model. | 100 mg/kg soy isoflavones ↓ body weight gain, ↓ 3β-HSD and 17β-HSD enzyme activity, ↓ oxidative stress. | Soy isoflavones improved metabolic abnormalities, anti-inflammatory markers, and hormonal panel (see Table 2) in letrozole-induced PCOS rats, likely via anti-androgenic effects and aromatase activity modulation. |
| Rajaei et al., 2019 [135] | Experimental animal study; 60-day PCOS induction with oestradiol valerate, followed by 14-day genistein treatment. | 20 female Wistar rats, including 15 rats with EV-induced PCOS; divided into 4 groups (n = 4/each): (1) control group, no intervention; (2) EV-induced PCOS (60 days); (3) rats without PCOS, treated with genistein (14 days); (4): EV-induced PCOS rats (60 days), treated with genistein (14 days). | To evaluate the antioxidant effect of genistein on ovarian tissue morphology and oxidative stress markers (MDA, TAC, GPx, SOD) in PCOS rats. | Genistein: ↓MDA (plasma and ovary), ↑ TAC, ↑ GPx, ↑ SOD; improved follicular morphology comparable to controls. | Genistein protected ovarian tissue by enhancing antioxidant defences and reducing oxidative stress, contributing to improved follicular quality in PCOS rats. |
| Alivandi Farkhad and Khazali et al., 2019 [134] | Experimental animal study, post-induction SISAF treatment for 21 days, after 60-day EV-induction of PCOS. | 32 female Wistar rats, including 24 rats with EV-induced; divided into 4 groups (n = 8/each): (1): CON, only received the vehicle, (2) rats with EV-induced PCOS, (3) a treatment group receiving SISAF 50 mg/kg by gavage for 21 days, (4) a treatment group receiving SISAF 100 mg/kg by gavage for 21 days | To assess the effect of soybean isoflavone–aglycone fraction (SISAF; 50 and 100 mg/kg) on inflammation, oxidative stress, and ovarian histology in PCOS rats. | SISAF treatment significantly: ↓ IL-6, ↓ TNF-a levels in ovarian tissue, and ↑ overall oxidative–antioxidant status compared to the PCOS group. Rats in (2) group significantly ↑ TOS in ovarian tissue compared to the (1) group. Rats treated with SISAF significantly ↓ TOS compared to the (2) group. Significant ↓TAC was observed in the (2) group compared to the (1) group. Rats in groups (3) and (4) treated with SISAF at doses of 50 and 100 mg/kg body weight had mean TAC values comparable to those in the CON group. | Soy isoflavone-aglycones reduced inflammatory and oxidative stress markers in PCOS rats, supporting their therapeutic potential. |
| Ma et al., 2021 [137] | Experimental animal study; 28-day treatment (after 21-day PCOS induction). | 20 female Sprague-Dawley rats, including 15 rats with letrozole-induced PCOS (1 mg/kg letrozole); divided into 4 groups (n = 5/each); (1) normal control group, (2) PCOS group (1 mL saline p.o.), (3) MET group (1 mL saline dissolved with 500 mg/kg metformin p.o.), (4) SI group (1 mL saline dissolved with 100 mg/kg soy isoflavones p.o.) | To assess the therapeutic effect and mechanism of soy isoflavones (100 mg/kg) vs metformin (500 mg/kg) in improving ovarian morphology and hormonal imbalance via NF-κB signalling. | Soy isoflavones ↓ body and ovarian weight, ↓ oxidative stress and inflammation; inhibited NF-κB p65 phosphorylation and ↑ IκBα expression. There was also an improvement in hormonal parameters (see Table 2). | Soy isoflavones reduced inflammation and improved ovarian structure and hormone levels (see Table 2) in PCOS rats by inhibiting NF-κB signalling and enhancing antioxidant defence. |
| Amanat et al., 2021 [136] | Experimental animal study; 42-day treatment. | 40 female Sprague-Dawley rats, including 30 rats with letrozole-induced PCOS; divided into 4 groups (n = 10/each): (1) control group, healthy rats; (2) PCOS-induced rats that received normal saline by gavage; (3) PCOS-induced rats treated with 150 mg/kg of metformin dissolved in normal saline by gavage; (4) PCOS-induced rats supplemented with 20 mg/kg of genistein dissolved in 1% methylcellulose solution. | To evaluate the effects of genistein (20 mg/kg) vs metformin on IR, inflammation, oxidative stress, lipid profile, and ovarian histology. | Genistein ↓ fasting insulin, ↓ HOMA-IR, ↓ MDA, ↓ TNF-α; ↑ SOD activity; improved ovarian histology (see Table 2). | Genistein improved metabolic, inflammatory, and histopathological markers in PCOS rats, suggesting beneficial effects comparable to metformin. |
| Watanabe et al., 2023 [180] | In vitro (3T3-L1 adipocytes) and in vivo (ICR mice, 14-day feeding trial with 0.2% genistein); 14 days. | Eight-week-old male ICR mice; fed a standard AIN-93M diet supplemented with 0.2% genistein for 14 days; cultured murine 3T3-L1 preadipocytes differentiated into adipocytes and treated with genistein (10 μM) for 7 days. | To investigate whether genistein promotes NAD⁺ biosynthesis and improves insulin sensitivity by regulating NAMPT expression and PPARγ activity. | Genistein ↑ NAMPT expression, ↑ NAD⁺ levels, ↑ adiponectin, ↓ phosphorylated PPARγ (Ser273), ↑ C/EBPβ stability via ERK–PHB1 pathway; effects abolished by PHB1 or NAMPT knockdown. | Genistein improves adipocyte metabolic function and insulin sensitivity through the PHB1–NAMPT–PPARγ signalling axis; supports nutraceutical role in metabolic disorders. |
| Xiao et al., 2024, [138] | Experimental (two-way randomisation) animal study; 8 weeks. | 156 weanling female Sprague-Dawley rats; divided into 6 groups (n = 26/each); (1) control fed diets containing 0 g ISF/kg diet; (2) control fed 0.5 g ISF/kg diet, (3) control fed diets containing 1 g ISF/kg diet; (4) DHT-induced PCOS fed diets containing 0 g ISF/kg diet; (5) DHT-induced PCOS fed diets containing 0.5 g ISF/kg diet; (6) DHT-induced PCOS fed diets containing 1 g ISF/kg diet. | To evaluate whether dietary soy isoflavones (0, 0.5, or 1 g/kg diet) can mitigate metabolic and reproductive disorders associated with DHT-induced PCOS in rats. | Soya isoflavones at 1 g/kg reduced body weight gain, serum total and free cholesterol, NEFA, leptin, and hepatic TAG; improved insulin sensitivity; decreased liver lipid droplet accumulation; normalised ovarian functions (see Table 2). | Soy isoflavones alleviated metabolic disorders related to PCOS, including IR and fatty liver; they may offer therapeutic potential for managing PCOS-related conditions. |
PCOS—polycystic ovary syndrome; 3β-HSD—3β-hydroxysteroid dehydrogenase; 17β-HSD—17β-hydroxysteroid dehydrogenase; C/EBPβ—CCAAT/enhancer-binding protein beta; IR—insulin resistance; DHT—dihydrotestosterone; ERK—extracellular signal-regulated kinase; TAC—total antioxidant capacity; HOMA-IR—homeostasis model assessment of insulin resistance; IL-6—interleukin-6; IκBα—nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; MDA—malondialdehyde; NAD⁺—nicotinamide adenine dinucleotide; NAMPT—nicotinamide phosphoribosyltransferase; NF-κB—nuclear factor kappa B; NEFA—non-esterified fatty acids; PHB1—prohibitin 1; PPARγ—peroxisome proliferator-activated receptor gamma; SISAF—soybean isoflavone-aglycone fraction; SOD—superoxide dismutase; TAG—triacylglycerols; TNF-α—tumor necrosis factor-alpha; ↑ indicates an increase; ↓ indicates a decrease.
4.3. Gut Microbiota
The gut microbiota is currently an intensively studied element in the pathogenesis of PCOS. Numerous studies have shown significant differences in the composition of the gut microbiota in patients with PCOS compared to healthy women [25,26,27]. A recently published systematic review and meta-analysis [181] confirmed that women with PCOS have reduced microbial evenness and phylogenetic diversity of the gut microbiome. A decreased abundance of bacteria from the genera Lachnospira and Prevotella has been observed, along with an increased presence of Bacteroides, Parabacteroides, Lactobacillus, Fusobacterium, and Escherichia/Shigella. The gut dysbiosis observed in PCOS patients—manifested, among other things, by a lower abundance of bacteria producing short-chain fatty acids (SCFAs), involved in bile acid metabolism and glucose regulation—may indicate an imbalance in the gut microbiota. This imbalance is characterised by a shift towards dominance of pro-inflammatory microorganisms at the expense of anti-inflammatory species [182]. The “Dysbiosis of Gut Microbiota” (DOGMA) hypothesis is one of the conceptual frameworks aiming to explain the mechanisms underlying PCOS development [183]. It proposes that quantitative and qualitative changes in the gut microbiota composition may lead to impaired intestinal barrier integrity, increasing its permeability and enabling the translocation of lipopolysaccharides (LPS) into the systemic circulation. The subsequent activation of the immune system may interfere with insulin receptor function, leading to hyperinsulinemia, which in turn stimulates excessive ovarian androgen production and disrupts follicular development.
Despite the high content of isoflavones in soy products, their bioavailability is limited—only a small fraction is absorbed in the small intestine. The majority reaches the large intestine where it is further metabolised by the intestinal microbiota [24]. The pharmacological effects of isoflavones may be enhanced or diminished depending on the ability of the gut microbiota to produce equol—the biologically active metabolite of daidzein. Individuals capable of equol production are referred to as “equol producers”. It is estimated that approximately 25–50% of the population exhibits this capacity, with a higher prevalence in Asian populations, while in Western populations, the average is around 35% [72,73,74].
Research suggests a synergistic interaction between soy isoflavones and the gut microbiota [17,184,185]. On one hand, isoflavones may modulate the composition of the gut microbiota by promoting the growth of anti-inflammatory bacteria and limiting the proliferation of potentially pathogenic species. One possible explanation for the beneficial effects of isoflavones lies in their potential prebiotic properties. In an in vitro study using the Simulator of the Human Intestinal Microbial Ecosystem (SHIME), Chen et al. [186] found that soy isoflavones (daidzein, daidzin, genistein, and glycitin) supported the growth of probiotic bacteria such as Bifidobacterium bifidum DNG6, Lactococcus lactis St66, Lactobacillus plantarum 10960, and Lactobacillus rhamnosus L8.8. Furthermore, isoflavones may enhance the production of anti-inflammatory and antimicrobial compounds by these bacteria. When optimal concentrations of isoflavones were applied—40 mg/mL for L. lactis St66, 20 mg/mL for L. plantarum 10960, 30 mg/mL for L. rhamnosus L8.8, and 40 mg/mL for B. bifidum DNG6—the most pronounced zones of inhibition against pathogenic bacteria such as Escherichia coli and Staphylococcus aureus were observed [186]. This antimicrobial effect is likely mediated by the production of organic acids, bacteriostatic peptides, and bacteriocins by the probiotic strains [187]. Moreover, genistein has been shown to inhibit DNA topoisomerase IV activity, thereby potentially disrupting the function of pathogenic bacteria [188]. Chen et al. [186] also demonstrated a synergistic effect between isoflavones and probiotics, resulting in enhanced antimicrobial efficacy. Pathogen-induced dysbiosis of the colonic microbiota led to a significant reduction in beneficial bacteria, such as Bifidobacterium, Bacteroides, Lactobacillus, and Weissella, and a concomitant increase in pathogens, including Klebsiella, Pseudomonas, Acinetobacter, Escherichia-Shigella, and Staphylococcus (p < 0.05). Treatment with isoflavones restored microbial balance by significantly reducing pathogenic bacterial counts and increasing populations of beneficial bacteria (p < 0.05). In addition, isoflavones have been shown to stimulate the production of SCFAs by intestinal bacteria [186]. SCFAs are known not only to play a vital role in glucose homeostasis, lipid metabolism, and appetite regulation but also to exhibit anti-inflammatory and antimicrobial effects. SCFAs are known to play a vital role not only in glucose homeostasis, lipid metabolism, and appetite regulation but also in exerting anti-inflammatory and antimicrobial effects [189]. Inoculation with pathogens resulted in a significant reduction in SCFA concentrations in the colon, from approximately 124–126 mmol/L to 106–108 mmol/L in different segments (p < 0.05). Isoflavone supplementation significantly increased SCFA concentrations (p < 0.05) [186]. On the other hand, a well-functioning microbiota enhances the host’s capacity to convert isoflavones into their bioactive forms, such as equol, potentially amplifying the beneficial metabolic and hormonal effects in women with PCOS [17]. The gut microbiota primarily influences the bioavailability and bioactivity of isoflavones through enzymatic activity, including β-glucosidase. This enzyme catalyses the hydrolysis of glycosidic isoflavone forms to their aglycone forms, which are characterised by higher bioavailability. This enzyme is found in many probiotic bacteria, particularly in the Bifidobacterium and Lactobacillus genera [33,34,65]. Additionally, certain intestinal bacterial strains demonstrate the capacity to further metabolise daidzein into equol, a compound exhibiting stronger oestrogen-like effects and greater bioactivity. These “equol producers” primarily include bacteria from the Coriobacteriaceae family, though selected strains of beneficial bacteria from the Lactobacillus, Lactococcus, and Bifidobacterium genera also demonstrate this ability [67]. Therefore, the ability to produce equol is closely linked to a state of microbial equilibrium characterised by adequate species richness, commensal bacterial dominance, and low levels of potentially pathogenic microorganisms [190]. In the context of PCOS, microbiota disorders (dysbiosis), which are commonly observed in female patients, may limit this metabolic potential. Therefore, interventions that restore eubiosis, such as dietary changes, prebiotics, and probiotics, may enhance the effectiveness of isoflavone therapy. In a mouse study conducted by Ghimire et al. [191], it was observed that switching from a phytoestrogen-deficient diet to one rich in soy isoflavones increased gut microbial diversity and modulated the expression of genes involved in LPS biosynthesis, thereby reducing pro-inflammatory responses. Notably, when the diet was changed from isoflavone-rich to isoflavone-deficient, genes responsible for equol production were lost within just seven days. Reintroduction of the isoflavone-rich diet led to the reappearance of these genes in some mice. This finding suggests that the presence of equol-producing bacteria may depend on dietary composition and can dynamically respond to its changes. During a three-day dietary intervention, involving women with PCOS, it was found that the participants’ gut microbiota began to resemble that of healthy women after just three days of isoflavone consumption [17]. Such a rapid microbiota response suggests that isoflavones may promote the growth of equol-producing bacteria. This indicates that even women with PCOS who are not equol producers may still benefit from isoflavone intake. However, in equol-producing individuals, these effects may be particularly pronounced. Scientific reports also suggest that improvements in diet quality may positively affect both the conversion of daidzein to equol and equol’s bioavailability. A diet high in carbohydrates appears to play a key role, as carbohydrate fermentation products—hydrogen and SCFAs—support the activity of bacteria involved in daidzein conversion [192,193]. In contrast, a high-fat diet has been shown to negatively correlate with bacterial equol synthesis capacity [194].
Despite the growing body of literature on gut microbiota in women with PCOS and the known health benefits of soy isoflavone consumption, there remains a lack of studies that comprehensively analyse the relationships between gut microbiota composition, isoflavone metabolism, and endocrine-metabolic symptoms in PCOS. Selected available studies are presented in Table 5 and Table 6.

Table 5.
Overview of clinical trials evaluating the effects of soy isoflavones on gut microbiota and endocrine-metabolic symptoms in PCOS.

Table 6.
Overview of preclinical studies evaluating the effects of soy isoflavones and probiotics/prebiotics on gut microbiota and endocrine-metabolic symptoms in PCOS.
5. Limitations and Future Research
Given the prevalence of PCOS in the population of reproductive-age women and the current state of knowledge, which is often based on divergent research findings regarding the role of soy isoflavones, this narrative review provides a comprehensive summary of the available literature on this topic. In addition, it has the merit of considering the interaction between the microbiota and the metabolism of soy isoflavones, which is an important addition to previous studies and may point to new directions for research. However, the limitations of the present study must be taken into account. In addition to the limitations due to the heterogeneity of the available studies, the very nature of the narrative review also entails certain methodological limitations—primarily the possibility of subjective selection and interpretation of the literature, despite efforts to keep the approach as objective as possible.
Although most of the scientific studies cited in this narrative review suggest that soy isoflavones have a beneficial effect on endocrine, metabolic, and gut microbiota-related parameters in women with PCOS, it is worth discussing contradictory or inconclusive results for the sake of objectivity. Romualdi et al., Khani et al., and Forouhari et al. found no statistically significant differences in hormonal profiles between groups of women taking isoflavones and control groups [15,16,132]. In a study by Jamilian and Asemi, improvements in certain hormonal parameters were observed; however, these differences were not significant after statistical correction [14]. Divergent results were also reported in terms of metabolic parameters. While Romualdi et al. showed no effect of genistein on IR or glycaemia, Jamilian and Asemi observed a significant reduction in IR, a decrease in triglycerides, and an improvement in biomarkers of oxidative stress. However, they found no significant changes in total cholesterol, LDL cholesterol, HDL cholesterol, or inflammatory markers [14,16]. Regarding the gut microbiota, Liyanage et al. and Bulsara et al. demonstrated in preclinical studies that soy isoflavones alone had limited effects on gut microbiota and PCOS symptoms. They also showed that the therapeutic effects were significantly stronger when isoflavones were administered alongside resistant starch or a probiotic (Bacillus coagulans) [184,185]. The results of the scientific studies conducted to date demonstrate the complex and individualised mechanisms of action of soy isoflavones in PCOS. In addition, the ability of the organism to convert daidzein to equol is an individual issue that may significantly affect clinical efficacy. Further well-designed studies involving women with PCOS that take into account the role of the gut microbiota are needed in order to formulate specific clinical and nutritional recommendations (which are currently lacking in the context of PCOS), indicating the necessary duration of intervention, form of administration, and dose.
In particular, the limited size of clinical trials, their often short duration, and the predominance of preclinical studies, especially in animal models, significantly limit the ability to formulate clear conclusions and apply them in clinical practice. In addition, animal studies are based on pharmacologically induced models of PCOS, which do not fully reflect the complex and heterogeneous nature of this disease in women. In fact, the course of PCOS can vary considerably depending on the individual predisposition and the still incompletely understood aetiology of the condition. Another major limitation is the wide variety of isoflavone preparations used, in terms of composition, dose, form, and route of administration. The lack of standardisation of preparations makes direct comparison of study results difficult. In addition, the heterogeneity of the pathophysiological mechanisms of PCOS, which are related to the diversity of clinical phenotypes among other things, may significantly affect the differential response to soy isoflavone supplementation. This highlights the importance of tailoring the therapeutic approach. For example, since soy isoflavones have been shown to improve insulin sensitivity and lower TG, TC, and LDL levels, it is likely that patients with more pronounced metabolic abnormalities—such as women with phenotypes A and B—may benefit more from supplementation. Key factors contributing to this variability may include differences in the composition of gut microbiota, which affects isoflavone metabolism and equol production; metabolic phenotype, which determines baseline insulin resistance and lipid profile; and hormonal status (e.g., oestrogen and androgen levels). All of these factors may influence the body’s response to phytoestrogens.
Therefore, future research should focus on long-term randomised controlled trials in representative groups of PCOS patients, which will allow a more reliable assessment of the efficacy and safety of soy isoflavone supplementation. Research is also needed on the synergistic effects of soy isoflavones and lifestyle modifications, especially diet and physical activity, and their potential effects on gut microbiome composition and function. Assessing the influence of the gut microbiota, in particular, the ability to produce equol—a biologically active metabolite of isoflavones—on response to therapy will also be an important line of research. It is also worth investigating whether specific PCOS phenotypes differ in the ability of their microbiota to produce equol, and whether these differences may determine the efficacy of supplementation. Another aspect that needs to be clarified is the possibility that isoflavones may act synergistically with probiotics and prebiotics, and their effects on PCOS symptoms and modulation of the gut microbiome in human studies. Further research should determine the optimal dosage and duration of isoflavone supplementation and compare the efficacy of different forms of these compounds—from natural soy sources, fortified foods, and standardised extracts. Finally, it will be of utmost importance to analyse potential interactions between soy isoflavones, and the pharmacokinetics of drugs used in the treatment of PCOS, which may have important implications for the safety and efficacy of therapy. In this context, research on animal models may lead to interesting conclusions. They have shown similar effects after the use of soy isoflavones and metformin in terms of many disorders in PCOS. However, metformin has a well-documented and multifaceted effect in PCOS, and therefore soy isoflavones may have potential applications to support its action, but not replace it. Furthermore, as already emphasised, these conclusions are based on preclinical studies. In the future, a certain advantage of soy isoflavones, especially those derived from food, over metformin may be seen in the reduced number of adverse effects, including gastrointestinal disorders and interactions with other drugs.
Research specifically examining soy isoflavones in the PCOS context has focused primarily on benefits rather than risks, creating a knowledge gap regarding potential adverse endocrine effects in this population. Studies in the general population report that soy or soy product intake is inversely associated with deaths from cardiovascular disease and cancer, including breast cancer. Many studies, especially those of good quality, also indicate a decrease in all-cause mortality in selected subgroups. Potential risks from soy are more likely to be associated with supplementation than dietary intake. Careless selection of supplements may result in low-quality isoflavones, including the presence of contaminants and no guarantee of the actual dose of isoflavones. High doses of soy, both from food sources and supplements, may also be associated with gastrointestinal distress, but these depend on individual tolerance. Interactions with other drugs, particularly thyroid hormones, may also occur in the presence of iodine deficiency, while data on other drugs are inconclusive. Due to their high-phytate content, soy products may interfere with the absorption of certain minerals, including iron and zinc. In this case, supplementation may offer an advantage, as supplements can be purified of these compounds. However, it should be noted that there is a lack of data on the long-term intake of soy isoflavones and their adverse effects on health, especially in the form of dietary supplements. Consideration of the above aspects in future studies is essential for the development of sound clinical recommendations and optimal use of the potential of soy isoflavones in the treatment of endocrine and metabolic disorders in the course of PCOS.
6. Conclusions
Soy isoflavones, active ingredients in functional foods, show potential in regulating the endocrine and metabolic disorders characteristic of PCOS. This review outlines a number of their mechanisms of action, including the reduction of inflammation and oxidative stress, and the modulation of the activity and metabolism of enzymes and hormones involved in the pathogenesis of this disease entity. A bidirectional interaction of isoflavones with the gut microbiota is also described, which may influence both the bioavailability of these functional compounds and the composition of the microbiota, as well as systemic metabolic effects. The interaction between isoflavones and the gut microbiota appears to play a synergistic role in the regulation of symptoms related to PCOS by enhancing the bioactivity of isoflavones, promoting gut homeostasis, and potentially modulating metabolic and hormonal pathways.
Although the available results from preclinical studies and selected clinical trials are promising, their relatively small numbers (especially in women) and significant methodological limitations currently prevent the formulation of clear clinical recommendations. Further carefully designed studies in women with PCOS are therefore needed to reliably assess the efficacy and safety of soy isoflavone supplementation.
Author Contributions
Conceptualization, I.M., A.D., M.Z., E.Ł. and K.D.; methodology, I.M., A.D. and M.Z.; data curation, E.Ł. and K.D.; writing—original draft preparation, I.M., A.D. and M.Z.; writing—review and editing, I.M., A.D., M.Z., E.Ł. and K.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations From the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2023, 108, 2447–2469. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An Androgen Excess Society guideline. J. Clin. Endocrinol. Metab. 2006, 91, 4237–4245. [Google Scholar] [CrossRef]
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef]
- March, W.; Moore, V.; Willson, K.; Phillips, D.I.; Norman, R.J.; Davies, M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2010, 25, 544–551. [Google Scholar] [CrossRef]
- Fletcher, R.J. Food sources of phyto-oestrogens and their precursors in Europe. Br. J. Nutr. 2003, 89, S39–S43. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Ekena, K.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Determinants of ligand specificity of estrogen receptor-alpha: Estrogen versus androgen discrimination. J. Biol. Chem. 1998, 273, 693–699. [Google Scholar] [CrossRef]
- Gustafsson, J.A. Therapeutic potential of selective estrogen receptor modulators. Curr. Opin. Chem. Biol. 1998, 2, 508–511. [Google Scholar] [CrossRef]
- Wang, L.Q. Mammalian phytoestrogens: Enterodiol and enterolactone. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 777, 289–309. [Google Scholar] [CrossRef]
- Ye, L.; Chan, M.Y.; Leung, L.K. The soy isoflavone genistein induces estrogen synthesis in an extragonadal pathway. Mol. Cell Endocrinol. 2009, 302, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.A.; Cross, J.E.; Burden, C.; Lacey, M. Acute and chronic effects of genistein, tyrphostin and lavendustin A on steroid synthesis in luteinized human granulosa cells. Hum. Reprod. 2002, 17, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.C.; Zhou, C.; Sherman, M.; Laughton, C.A.; Chen, S. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: A site-directed mutagenesis study. Environ. Health Perspect. 1998, 106, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Asemi, Z. The Effects of Soy Isoflavones on Metabolic Status of Patients With Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2016, 101, 3386–3394. [Google Scholar] [CrossRef]
- Khani, B.; Mehrabian, F.; Khalesi, E.; Eshraghi, A. Effect of soy phytoestrogen on metabolic and hormonal disturbance of women with polycystic ovary syndrome. J. Res. Med. Sci. 2011, 16, 297–302. [Google Scholar]
- Romualdi, D.; Costantini, B.; Campagna, G.; Lanzone, A.; Guido, M. Is there a role for soy isoflavones in the therapeutic approach to polycystic ovary syndrome? Results from a pilot study. Fertil. Steril. 2008, 90, 1826–1833. [Google Scholar] [CrossRef]
- Haudum, C.; Lindheim, L.; Ascani, A.; Trummer, C.; Horvath, A.; Münzker, J.; Obermayer-Pietsch, B. Impact of Short-Term Isoflavone Intervention in Polycystic Ovary Syndrome (PCOS) Patients on Microbiota Composition and Metagenomics. Nutrients 2020, 12, 1622. [Google Scholar] [CrossRef]
- Nachvak, S.M.; Moradi, S.; Anjom-Shoae, J.; Rahmani, J.; Nasiri, M.; Maleki, V.; Sadeghi, O. Soy, soy isoflavones, and protein intake in relation to mortality from all causes, cancers, and cardiovascular diseases: A systematic review and dose-response meta-analysis of prospective cohort studies. J. Acad. Nutr. Diet. 2019, 119, 1483–1500.e17. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Zhao, R.; Yi, M.; Wan, Q.; Du, L.; Zhou, Y. Soy and isoflavone consumption and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomized trials in humans. Mol. Nutr. Food Res. 2020, 64, e1900751. [Google Scholar] [CrossRef]
- Mosallanezhad, Z.; Mahmoodi, M.; Ranjbar, S.; Hosseini, R.; Clark, C.C.T.; Carson-Chahhoud, K.; Norouzi, Z.; Abbasian, A.; Sohrabi, Z.; Jalali, M. Soy intake is associated with lowering blood pressure in adults: A systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Complement. Ther. Med. 2021, 59, 102692. [Google Scholar] [CrossRef]
- Liu, X.X.; Li, S.H.; Chen, J.Z.; Sun, K.; Wang, X.J.; Wang, X.G.; Hui, R.T. Effect of soy isoflavones on blood pressure: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Pan, A.; Manson, J.E.; Willett, W.C.; Malik, V.; Rosner, B.; Giovannucci, E.; Hu, F.B.; Sun, Q. Consumption of soy foods and isoflavones and risk of type 2 diabetes: A pooled analysis of three US cohorts. Eur. J. Clin. Nutr. 2016, 70, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G. The Antioxidant Role of Soy and Soy Foods in Human Health. Antioxidants 2020, 9, 635. [Google Scholar] [CrossRef]
- Kumari, N.; Kumari, R.; Dua, A.; Singh, M.; Kumar, R.; Singh, P.; Duyar-Ayerdi, S.; Pradeep, S.; Ojesina, A.I.; Kumar, R. From Gut to Hormones: Unraveling the Role of Gut Microbiota in (Phyto)Estrogen Modulation in Health and Disease. Mol. Nutr. Food Res. 2024, 68, e2300688. [Google Scholar] [CrossRef]
- Wang, B.; Hu, L.; Dong, P. Meta-analysis of gut microbiota biodiversity in patients with polycystic ovary syndrome based on medical images. SLAS Technol. 2024, 29, 100178. [Google Scholar] [CrossRef]
- Lindheim, L.; Bashir, M.; Munzker, J.; Trummer, C.; Zachhuber, V.; Leber, B.; Horvath, A.; Pieber, T.R.; Gorkiewicz, G.; Stadlbauer, V.; et al. Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with Polycystic Ovary Syndrome (PCOS): A Pilot Study. PLoS ONE 2017, 12, e0168390. [Google Scholar] [CrossRef]
- He, F.; Li, Y. The gut microbial composition in polycystic ovary syndrome with insulin resistance: Findings from a normal-weight population. J. Ovarian Res. 2021, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.X.; Jalil, J.; Lam, K.W.; Husain, K.; Premakumar, C.M. Genistein: A review on its anti-inflammatory properties. Front. Pharmacol. 2022, 13, 820969. [Google Scholar] [CrossRef]
- Mei, J.; Chen, X.; Liu, J.; Yi, Y.; Zhang, Y.; Ying, G. A biotransformation process for production of genistein from sophoricoside by a strain of Rhizopus oryza. Sci. Rep. 2019, 9, 6564. [Google Scholar] [CrossRef]
- Liggins, J.; Bluck, L.J.; Runswick, S.; Atkinson, C.; Coward, W.A.; Bingham, S.A. Daidzein and genistein content of fruits and nuts. J. Nutr. Biochem. 2000, 11, 326–331. [Google Scholar] [CrossRef]
- Swathi Krishna, S.; Kuriakose, B.B.; Lakshmi, P.K. Effects of phytoestrogens on reproductive organ health. Arch. Pharm. Res. 2022, 45, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Fukutake, F.; Takahashi, M.; Ishida, K.; Kawamura, H.; Sugimura, T.; Wakabayashi, K. Quantification of genistein and genistin in soybeans and soybean products. Food Chem. Toxicol. 1996, 34, 457–461. [Google Scholar] [CrossRef]
- Walsh, K.R.; Haak, S.J.; Bohn, T.; Tian, Q.; Schwartz, S.J.; Failla, M.L. Isoflavonoid glucosides are deconjugated and absorbed in the small intestine of human subjects with ileostomies. Am. J. Clin. Nutr. 2007, 85, 1050–1056. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Wang, Z.; Wu, D.; Zhao, Y.; Gong, X.; Jiang, Q.; Xia, C. Study on biotransformation and absorption of genistin based on fecal microbiota and Caco-2 cell. Front. Pharmacol. 2024, 15, 1437020. [Google Scholar] [CrossRef]
- Haron, H.; Ismail, A.; Azlan, A.; Shahar, S.; Peng, L.S. Daidzein and genestein contents in tempeh and selected soy products. Food Chem. 2009, 115, 1350–1356. [Google Scholar] [CrossRef]
- Garbiec, E.; Cielecka-Piontek, J.; Kowalówka, M.; Hołubiec, M.; Zalewski, P. Genistein—Opportunities Related to an Interesting Molecule of Natural Origin. Molecules 2022, 27, 815. [Google Scholar] [CrossRef]
- Yang, Z.; Kulkarni, K.; Zhu, W.; Hu, M. Bioavailability and pharmacokinetics of genistein: Mechanistic studies on its ADME. Anticancer. Agents Med. Chem. 2012, 12, 1264–1280. [Google Scholar] [CrossRef]
- Yu, L.; Rios, E.; Castro, L.; Liu, J.; Yan, Y.; Dixon, D. Genistein: Dual Role in Women’s Health. Nutrients 2021, 13, 3048. [Google Scholar] [CrossRef]
- Wu, J.G.; Ge, J.; Zhang, Y.P.; Yu, Y.; Zhang, X.Y. Solubility of Genistein in Water, Methanol, Ethanol, Pro-pan-2-ol, 1-Butanol, and Ethyl Acetate from (280 to 333) K. J. Chem. Eng. Data 2010, 55, 5286–5288. [Google Scholar] [CrossRef]
- Kobayashi, S.; Shinohara, M.; Nagai, T.; Konishi, Y. Transport mechanisms of soy isoflavones and their microbial metabolites dihydrogenistein and dihydrodaidzein across monolayers and membranes. Pharm. Anal. Acta 2013, 77, 2210–2217. [Google Scholar] [CrossRef]
- Braune, A.; Blaut, M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 2016, 7, 216–234. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Nakagawa, H.; Ohnishi-Kameyama, M.; Tsushida, T. Dihydrogenistein-producing bacterium TM-40 isolated from human feces. Food Sci. Technol. Res. 2007, 13, 129–132. [Google Scholar] [CrossRef]
- Doerge, D.R.; Chang, H.C.; Churchwell, M.I.; Holder, C.L. Analysis of Soy Isoflavone Conjugation In Vitro and in Human Blood Using Liquid Chromatography-Mass Spectrometry. Drug Metab. Dispos. 2000, 28, 298. [Google Scholar] [CrossRef] [PubMed]
- Hüser, S.; Guth, S.; Joost, H.G.; Soukup, S.T.; Köhrle, J.; Kreienbrock, L.; Diel, P.; Lachenmeier, D.W.; Eisenbrand, G.; Vollmer, G.; et al. Effects of isoflavones on breast tissue and the thyroid hormone system in humans: A comprehensive safety evaluation. Arch. Toxicol. 2018, 92, 2703–2748. [Google Scholar] [CrossRef]
- Pfitscher, A.; Reiter, E.; Jungbauer, A. Receptor binding and transactivation activities of red clover isoflavones and their metabolites. J. Steroid. Biochem. Mol. Biol. 2008, 112, 87–94. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.; Murphy, P.; Cook, L.; Hendrich, S. Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. J. Nutr. 1994, 124, 825–832. [Google Scholar] [CrossRef]
- Guelfi, G.; Pasquariello, R.; Anipchenko, P.; Capaccia, C.; Pennarossa, G.; Brevini, T.A.L.; Gandolfi, F.; Zerani, M.; Maranesi, M. The Role of Genistein in Mammalian Reproduction. Molecules 2023, 28, 7436. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Carlsson, B.; Grandien, K.; Enmark, E.; Haggblad, J.; Nilsson, S.; Gustafsson, J.A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Pintova, S.; Dharmupari, S.; Moshier, E.; Zubizarreta, N.; Ang, C.; Holcombe, R.F. Genistein Combined with FOLFOX or FOLFOX–Bevacizumab for the Treatment of Metastatic Colorectal Cancer: Phase I/II Pilot Study. Cancer Chemother. Pharmacol. 2019, 84, 591–598. [Google Scholar] [CrossRef]
- Koenig, A.; Buskiewicz, I.; Huber, S.A. Age-Associated changes in estrogen receptor ratios correlate with increased female susceptibility to Coxsackievirus B3-Induced myocarditis. Front. Immunol. 2017, 8, 1585. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Lin, Z.; Cheng, Y.H.; Huang, C.C.; Marsh, E.; Yin, P.; Milad, M.P.; Confino, E.; Reierstad, S.; Innes, J.; et al. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol. Reprod. 2007, 77, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S. Estrogen Receptor-Beta Gene Polymorphism in women with Breast Cancer at the Imam Khomeini Hospital Complex, Iran. BMC Med. Genet. 2010, 11, 109. [Google Scholar] [CrossRef]
- Evans, B.A.; Griffiths, K.; Morton, M.S. Inhibition of 5 alpha-reductase in genital skin fibroblasts and prostate tissue by dietary lignans and isoflavonoids. J. Endocrinol. 1995, 147, 295–302. [Google Scholar] [CrossRef]
- Sobhy, M.M.K.; Mahmoud, S.S.; El-Sayed, S.H.; Rizk, E.M.A.; Raafat, A.; Negm, M.S.I. Impact of treatment with a Protein Tyrosine Kinase Inhibitor (Genistein) on acute and chronic experimental Schistosoma mansoni infection. Exp. Parasitol. 2018, 185, 115–123. [Google Scholar] [CrossRef]
- Valli, V.; Heilmann, K.; Danesi, F.; Bordoni, A.; Gerhäuser, C. Modulation of Adipocyte Differentiation and Proadipogenic Gene Expression by Sulforaphane, Genistein, and Docosahexaenoic Acid as a First Step to Counteract Obesity. Oxid. Med. Cell Longev. 2018, 2018, 1617202. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Xing, K.; Li, G.; Liu, D.; Guo, Y. Dietary Genistein Alleviates Lipid Metabolism Disorder and Inflammatory Response in Laying Hens with Fatty Liver Syndrome. Front. Physiol. 2018, 9, 1493. [Google Scholar] [CrossRef]
- Bazuine, M.; van den Broek, P.J.; Maassen, J.A. Genistein directly inhibits GLUT4-mediated glucose uptake in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2005, 326, 511–514. [Google Scholar] [CrossRef]
- Vera, J.C.; Reyes, A.M.; Cárcamo, J.G.; Velásquez, F.V.; Rivas, C.I.; Zhang, R.H.; Strobel, P.; Iribarren, R.; Scher, H.I.; Slebe, J.C. Genistein Is a Natural Inhibitor of Hexose and Dehydroascorbic Acid Transport through the Glucose Transporter, GLUT1. J. Biol. Chem. 1996, 271, 8719–8724. [Google Scholar] [CrossRef]
- Johnson, A.; Roberts, R.L.; Elkins, G. Complementary and alternative medicine for menopause. J. Evid. Based Integr. Med. 2019, 24, 2515690X19829380. [Google Scholar] [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A. Pharmacokinetics, pharmacodynamics, toxicity, and formulations of daidzein: An important isoflavone. Phytother. Res. 2023, 37, 2578–2604. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.; Hayowitz, D.B.; Holden, J.M. USDA Database for the Isoflavone Content of Selected Foods, Release 2.0; US Department of Agriculture: Washington, DC, USA, 2008.
- Singh, S.; Grewal, S.; Sharma, N.; Behl, T.; Gupta, S.; Anwer, M.K.; Vargas-De-La-Cruz, C.; Mohan, S.; Bungau, S.G.; Bumbu, A. Unveiling the Pharmacological and Nanotechnological Facets of Daidzein: Present State-of-the-Art and Future Perspectives. Molecules 2023, 28, 1765. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.; Erba, D.; Spadafranca, A.; Ciappellano, S.; Bresciani, J.; Testolin, G. Daidzein is absorbed by passive transport in isolated small intestine of rats. Nutr. Res. 2006, 26, 284–288. [Google Scholar] [CrossRef]
- Day, A.J.; Dupont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.C.; Morgan, M.R.A.; Williamson, G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver β-glucosidase activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Alwasel, S.H.; Harrath, A.H. The Influence of Plant Isoflavones Daidzein and Equol on Female Reproductive Processes. Pharmaceuticals 2021, 14, 373. [Google Scholar] [CrossRef]
- Mayo, B.; Vázquez, L.; Flórez, A.B. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef]
- Langa, S.; Curiel, J.A.; de la Bastida, A.R.; Peirotén, Á.; Álvarez, I.; Landete, J.M. Production of equol, dehydroequol, 5-hydroxy-equol and 5-hydroxy-dehydroequol in soy beverages by the action of dihydrodaidzein reductase in Limosilactobacillus fermentum strains. Food Chem. 2025, 464 Pt 2, 141707. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G.H. The antioxidant activity of daidzein metabolites, O-desmethylangolensin and equol, in HepG2 cells. Mol. Med. Rep. 2014, 9, 328–332. [Google Scholar] [CrossRef]
- Muthyala, R.S.; Ju, Y.H.; Sheng, S.; Williams, L.D.; Doerge, D.R.; Katzenellenbogen, B.S.; Helferich, W.G.; Katzenellenbogen, J.A. Equol, a natural estrogenic metabolite from soy isoflavones: Convenient preparation and resolution of R-and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg. Med. Chem. 2004, 12, 1559–1567. [Google Scholar] [CrossRef]
- Song, K.B.; Atkinson, C.; Frankenfeld, C.L.; Jokela, T.; Wähälä, K.; Thomas, W.K.; Lampe, J.W. Prevalence of daidzein-metabolizing phenotypes differs between Caucasian and Korean American women and girls. J. Nutr. 2006, 136, 1347–1351. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Cole, S.J. Method of defining equol-producer status and its frequency among vegetarians. J. Nutr. 2006, 136, 2188–2193. [Google Scholar] [CrossRef]
- Setchell, K.D.; Brown, N.M.; Summer, S.; King, E.C.; Heubi, J.E.; Cole, S.; Guy, T.; Hokin, B. Dietary factors influence production of the soy isoflavone metabolite S-(-)equol in healthy adults. J. Nutr. 2013, 143, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, A.; Ihara, M.; Lopez, O.; Kakuta, C.; Lopresti, B.; Higashiyama, A.; Aizenstein, H.; Chang, Y.-F.; Mathis, C.; Miyamoto, Y.; et al. Effect of S-equol and Soy Isoflavones on Heart and Brain. Curr. Cardiol. Rev. 2019, 15, 114–135. [Google Scholar] [CrossRef]
- Lehmann, L.; Esch, H.L.; Wagner, J.; Rohnstock, L.; Metzler, M. Estrogenic and genotoxic potential of equol and two hydroxylated metabolites of daidzein in cultured human Ishikawa cells. Toxicol. Lett. 2005, 158, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-L.; Deng, S.-L.; Lian, Z.-X.; Yu, K. Estrogen Receptors in Polycystic Ovary Syndrome. Cells 2021, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.; Sotoca, A.M.; Vervoort, J.; Louisse, J. Mechanisms underlying the dualistic mode of action of major soy isoflavones in relation to cell proliferation and cancer risks. Mol. Nutr. Food Res. 2013, 57, 100–113. [Google Scholar] [CrossRef]
- Hu, W.S.; Lin, Y.M.; Kuo, W.W.; Pan, L.F.; Yeh, Y.L.; Li, Y.H.; Kuo, C.H.; Chen, R.J.; Padma, V.V.; Chen, T.S.; et al. Suppression of isoproterenol-induced apoptosis in H9c2 cardiomyoblast cells by daidzein through activation of Akt. Chin. J. Physiol. 2016, 59, 323–330. [Google Scholar] [CrossRef]
- Hilakivi-Clarke, L.; Andrade, J.E.; Helferich, W. Is soy consumption good or bad for the breast? J. Nutr. 2010, 140, 2326S–2334S. [Google Scholar] [CrossRef]
- Boomsma, C.; Eijkemans, M.; Hughes, E.; Visser, G.; Fauser, B.C.; Macklon, N.S. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum. Reprod. Update 2006, 12, 673–683. [Google Scholar] [CrossRef]
- Legro, R.; Kunselman, A.; Dodson, W.; Dunaif, A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: A prospective, controlled study in 254 affected women. J. Clin. Endocrinol. Metab. 1999, 84, 165–168. [Google Scholar] [CrossRef]
- Berni, T.R.; Morgan, C.L.; Rees, D.A. Women with Polycystic Ovary Syndrome Have an Increased Risk of Major Cardiovascular Events: A Population Study. J. Clin. Endocrinol. Metab. 2021, 106, e3369–e3380. [Google Scholar] [CrossRef] [PubMed]
- Apridonidze, T.; Essah, P.; Luorno, M.; Nestler, J. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 1929–1935. [Google Scholar] [CrossRef]
- Moran, L.; Gibson-Helm, M.; Teede, H.; Deeks, A. Polycystic ovary syndrome: A biopsychosocial understanding in young women to improve knowledge and treatment options. J. Psychosom. Obstet. Gynaecol. 2010, 31, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Deeks, A.; Gibson-Helm, M.; Teede, H. Is having polycystic ovary syndrome (PCOS) a predictor of poor psychological function including depression and anxiety. Hum. Reprod. 2011, 26, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef]
- Lee, A.T.; Zane, L.T. Dermatologic manifestations of polycystic ovary syndrome. Am. J. Clin. Dermatol. 2007, 8, 201–219. [Google Scholar] [CrossRef]
- Housman, E.; Reynolds, R.V. Polycystic ovary syndrome: A review for dermatologists: Part I. Diagnosis and manifestations. J. Am. Acad. Dermatol. 2014, 71, 847–857. [Google Scholar] [CrossRef]
- Chang, S.; Dunaif, A. Diagnosis of Polycystic Ovary Syndrome: Which Criteria to Use and When? Endocrinol. Metab. Clin. N. Am. 2021, 50, 11–23. [Google Scholar] [CrossRef]
- Conway, G.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Franks, S.; Gambineri, A.; Kelestimur, F.; Macut, D.; Micic, D.; Pasquali, R.; et al. ESE PCOS Special Interest Group. The polycystic ovary syndrome: A position statement from the European Society of Endocrinology. Eur. J. Endocrinol. 2014, 171, P1–P29. [Google Scholar] [CrossRef]
- Welt, C.K.; Carmina, E. Lifecycle of Polycystic Ovary Syndrome (PCOS): From In Utero to Menopause. J. Clin. Endocrinol. Metab. 2013, 98, 4629–4638. [Google Scholar] [CrossRef]
- Pasquali, R.; Gambineri, A. Polycystic ovary syndrome: A multifaceted disease from adolescence to adult age. Ann. N. Y. Acad. Sci. 2006, 1092, 158–174. [Google Scholar] [CrossRef]
- Wang, Z.; Van Faassen, M.; Groen, H.; Cantineau, A.E.P.; Van Oers, A.; Van der Veen, A.; Hawley, J.M.; Keevil, B.G.; Kema, I.P.; Hoek, A. Resumption of ovulation in anovulatory women with PCOS and obesity is associated with reduction of 11β-hydroxyandrostenedione concentrations. Hum. Reprod. 2024, 39, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Maan, P.; Jyoti, A.; Kumar, A. The Role of Lifestyle Interventions in PCOS Management: A Systematic Review. Nutrients 2025, 17, 310. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Y.; Xu, Q.; Liu, W.; Wang, P.; Yao, J.; Zhao, A.; Chen, Y.; Wang, W. Higher dietary inflammation potential and certain dietary patterns are associated with polycystic ovary syndrome risk in China: A case-control study. Nutr. Res. 2022, 100, 1–18. [Google Scholar] [CrossRef]
- Zeber-Lubecka, N.; Ciebiera, M.; Hennig, E.E. Polycystic Ovary Syndrome and Oxidative Stress-From Bench to Bedside. Int. J. Mol. Sci. 2023, 24, 14126. [Google Scholar] [CrossRef]
- Zuo, T.; Zhu, M.; Xu, W. Roles of Oxidative Stress in Polycystic Ovary Syndrome and Cancers. Oxid. Med. Cell Longev. 2016, 2016, 8589318. [Google Scholar] [CrossRef]
- Sharifi, M.; Saber, A.; Moludi, J.; Salimi, Y.; Jahan-Mihan, A. The effects of portfolio moderate-carbohydrate and ketogenic diets on anthropometric indices, metabolic status, and hormonal levels in overweight or obese women with polycystic ovary syndrome: A randomized controlled trial. Nutr. J. 2024, 23, 152. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 226. [Google Scholar] [CrossRef]
- Tsilchorozidou, T.; Overton, C.; Conway, G.S. The pathophysiology of polycystic ovary syndrome. Clin. Endocrinol. 2004, 60, 1–17. [Google Scholar] [CrossRef]
- Kirschner, M.A.; Bardin, C.W. Androgen production and metabolism in normal and virilized women. Metabolism 1972, 21, 667–688. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C.; Dalkin, A.C.; Haisenleder, D.J.; Paul, S.J. Gonadotropin-releasing hormone pulses: Regulators of gonadotropin synthesis and ovulatory cycles. Recent. Prog. Horm. Res. 1991, 47, 155–189. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Li, S.; Zhou, W.; Ye, L.; Wang, L.; Tao, T.; Gu, J.; Yang, Z.; Zhao, D.; et al. Steroid hormone profiling in obese and nonobese women with polycystic ovary syndrome. Sci. Rep. 2017, 7, 14156. [Google Scholar] [CrossRef]
- Jakimiuk, A.J.; Weitsman, S.R.; Navab, A.; Magoffin, D.A. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J. Clin. Endocrinol. Metab. 2001, 86, 1318–1323. [Google Scholar] [CrossRef][Green Version]
- Nelson, V.L.; Legro, R.S.; Strauss, J.F.; McAllister, J.M. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol. Endocrinol. 1999, 13, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Ciaraldi, T.P.; Aroda, V.; Mudaliar, S.; Chang, R.J.; Henry, R.R. Polycystic ovary syndrome is associated with tissue-specific differences in insulin resistance. J. Clin. Endocrinol. Metab. 2009, 94, 157–163. [Google Scholar] [CrossRef]
- Ehrmann, D.A.; Barnes, R.B.; Rosenfeld, R.L. Policystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endoc. Rev. 1995, 16, 322–353. [Google Scholar] [CrossRef]
- White, D.; Leigh, A.; Wilson, C.; Donaldson, A.; Franks, S. Gonadotropin and gonadal steroid response to a single dose of a long-acting agonist of gonadotrophin-releasing hormone in ovulatory and anovulatory women with polycystic ovary syndrome. Clin. Endocrinol. 1995, 42, 475–481. [Google Scholar] [CrossRef]
- Pugeat, M.; Crave, J.C.; Elmidani, M.; Nicolas, M.H.; Garoscio-Cholet, M.; Lejeune, H.; Déchaud, H.; Tourniaire, J. Pathophysiology of sex hormone binding globulin (SHBG): Relation to insulin. J. Steroid Biochem. Mol. Biol. 1991, 40, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Poretsky, L.; Cataldo, N.A.; Rosenwaks, Z.; Giudice, L.C. The insulin related ovarian regulatory system in health and disease. Endocr. Rev. 1999, 20, 535–582. [Google Scholar] [CrossRef]
- Kumar, A.; Woods, K.S.; Bartolucci, A.A.; Azziz, R. Prevalence of adrenal androgen excess in patients with the polycystic ovary syndrome (PCOS). Clin. Endocrinol. 2005, 62, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.L. Evidence that idiopathic functional adrenal hyperandrogenism is caused by dysregulation of adrenal steroidogenesis and that hyperinsulinemia may be involved. J. Clin. Endocrinol. Metab. 1996, 81, 878–880. [Google Scholar] [CrossRef] [PubMed]
- Clarke, I.J.; Cummins, J.T. GnRH pulse frequency determines LH pulse amplitude by altering the amount of releasable LH in the pituitary glands of ewes. J. Reprod. Fertil. 1985, 73, 425–431. [Google Scholar] [CrossRef]
- Su, P.; Chen, C.; Sun, Y. Physiopathology of polycystic ovary syndrome in endocrinology, metabolism and inflammation. J. Ovarian Res. 2025, 18, 34. [Google Scholar] [CrossRef]
- Liao, B.; Qiao, J.; Pang, Y. Central regulation of PCOS: Abnormal neuronal-reproductive-metabolic circuits in PCOS pathophysiology. Front. Endocrinol. 2021, 12, 667422. [Google Scholar] [CrossRef]
- McArthur, J.W.; Ingersoll, F.M.; Worcester, J. The urinary excretion of interstitial-cell and follicle-stimulating hormone activity by women with diseases of the reproductive system. J. Clin. Endocrinol. Metab. 1958, 18, 1202–1215. [Google Scholar] [CrossRef]
- Yen, S.S.; Vela, P.; Rankin, J. Inappropriate secretion of follicle-stimulating hormone and luteinizing hormone in polycystic ovarian disease. J. Clin. Endocrinol. Metab. 1970, 30, 435–442. [Google Scholar] [CrossRef]
- Tang, R.; Ding, X.; Zhu, J. Kisspeptin and polycystic ovary syndrome. Front. Endocrinol. 2019, 10, 298. [Google Scholar] [CrossRef]
- Ohtaki, T.; Shintani, Y.; Honda, S.; Matsumoto, H.; Hori, A.; Kanehashi, K.; Terao, Y.; Kumano, S.; Takatsu, Y.; Masuda, Y.; et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001, 411, 613–617. [Google Scholar] [CrossRef]
- Ruddenklau, A.; Campbell, R.E. Neuroendocrine impairments of polycystic ovary syndrome. Endocrinology 2019, 160, 2230–2242. [Google Scholar] [CrossRef]
- Payne, A.H.; Hales, D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef] [PubMed]
- DeVane, G.W.; Czekala, N.M.; Judd, H.L.; Yen, S.S. Circulating gonadotropins, estrogens, and androgens in polycystic ovarian disease. Am. J. Obstet. Gynecol. 1975, 121, 496–500. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Sun, C.X.; Liu, Y.K.; Li, Y.; Wang, L.; Zhang, W. Promoter methylation of CYP19A1 gene in Chinese polycystic ovary syndrome patients. Gynecol. Obstet. Investig. 2013, 76, 209–213. [Google Scholar] [CrossRef]
- Kim, N.; Chun, S. Association between the serum estrone-to-estradiol ratio and parameters related to glucose metabolism and insulin resistance in women with polycystic ovary syndrome. Clin. Exp. Reprod. Med. 2021, 48, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Liu, L.; He, D.; Wang, Y.; Sheng, M. Integrated analysis of DNA methylation and transcriptome profling of polycystic ovary syndrome. Mol. Med. Rep. 2020, 21, 2138–2150. [Google Scholar] [CrossRef]
- Piltonen, T.T.; Giacobini, P.; Edvinsson, Å.; Hustad, S.; Lager, S.; Morin-Papunen, L.; Tapanainen, J.S.; Sundström-Poromaa, I.; Arffman, R.K. Circulating antimüllerian hormone and steroid hormone levels remain high in pregnant women with polycystic ovary syndrome at term. Fertil. Steril. 2019, 111, 588–596.e1. [Google Scholar] [CrossRef] [PubMed]
- Tata, B.; Mimouni, N.E.H.; Barbotin, A.L.; Malone, S.A.; Loyens, A.; Pigny, P.; Dewailly, D.; Catteau-Jonard, S.; Sundström-Poromaa, I.; Piltonen, T.T.; et al. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat. Med. 2018, 24, 834–846. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E. Polycystic ovarian syndrome: Pathophysiology, molecular aspects and clinical implications. Expert Rev. Mol. Med. 2008, 10, e3. [Google Scholar] [CrossRef]
- Homburg, R.; Ray, A.; Bhide, P.; Gudi, A.; Shah, A.; Timms, P.; Grayson, K. The relationship of serum anti-mullerian hormone with polycystic ovarian morphology and polycystic ovary syndrome: A prospective cohort study. Hum. Reprod. 2013, 28, 1077–1083. [Google Scholar] [CrossRef]
- Forouhari, S.; Heidari, Z.; Tavana, Z.; Salehi, M.; Sayadi, M. The effect of soya on some hormone levels in women with polycystic ovary syndrome (balance diet): A cross over randomized clinical trial. Bull. Environ. Pharmacol. Life Sci. 2013, 3, 246–250. [Google Scholar] [CrossRef]
- Rajan, R.K.; Kumar, M.S.S.; Balaji, B. Soy isoflavones exert beneficial effects on letrozole-induced rat polycystic ovary syndrome (PCOS) model through anti-androgenic mechanism. Pharm. Biol. 2017, 55, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Alivandi Farkhad, S.; Khazali, H. Therapeutic effects of isoflavone-aglycone fraction from soybean (Glycine max L. Merrill) in rats with estradiol valerate-induced polycystic ovary syndrome as an inflammatory state. Gynecol. Endocrinol. 2019, 35, 1078–1083. [Google Scholar] [CrossRef]
- Rajaei, S.; Alihemmati, A.; Abedelahi, A. Antioxidant effect of genistein on ovarian tissue morphology, oxidant and antioxidant activity in rats with induced polycystic ovary syndrome. Int. J. Reprod. Biomed. 2019, 17, 11–22. [Google Scholar] [CrossRef]
- Amanat, S.; Ashkar, F.; Eftekhari, M.H.; Tanideh, N.; Doaei, S.; Gholamalizadeh, M.; Koohpeyma, F.; Mokhtari, M. The effect of genistein on insulin resistance, inflammatory factors, lipid profile, and histopathologic indices in rats with polycystic ovary syndrome. Clin. Exp. Reprod. Med. 2021, 48, 236–244. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Ma, L.; Chen, Y.; He, S. Soy isoflavones alleviate polycystic ovary syndrome in rats by regulating NF-κB signaling pathway. Bioengineered 2021, 12, 7215–7223. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.W.; Carbonel, A.A.; Lima, P.D.A.; Hendry, A.; Tsang, B.K. Consumption of soya isoflavones improved polycystic ovary syndrome-associated metabolic disorders in a rat model. Br. J. Nutr. 2024, 132, 1–9. [Google Scholar] [CrossRef]
- Wan, Z.; Zhao, J.; Ye, Y.; Sun, Z.; Li, K.; Chen, Y.; Fang, Y.; Zhang, Y.; Lin, J.; Sun, P.; et al. Risk and incidence of cardiovascular disease associated with polycystic ovary syndrome. Eur. J. Prev. Cardiol. 2024, 31, 1560–1570. [Google Scholar] [CrossRef]
- Lim, S.S.; Norman, R.J.; Davies, M.J.; Moran, L.J. The effect of obesity on polycystic ovary syndrome: A systematic review and meta-analysis. Obes Rev. 2013, 14, 95–109. [Google Scholar] [CrossRef]
- Cassar, S.; Misso, M.L.; Hopkins, W.G.; Shaw, C.S.; Teede, H.J.; Stepto, N.K. Insulin resistance in polycystic ovary syndrome: A systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum. Reprod. 2016, 31, 2619–2631. [Google Scholar] [CrossRef]
- Satyaraddi, A.; Cherian, K.E.; Kapoor, N.; Kunjummen, A.T.; Kamath, M.S.; Thomas, N.; Paul, T.V. Body Composition, Metabolic Characteristics, and Insulin Resistance in Obese and Nonobese Women with Polycystic Ovary Syndrome. J. Hum. Reprod. Sci. 2019, 12, 78–84. [Google Scholar] [CrossRef]
- Mu, L.; Zhao, Y.; Li, R.; Lai, Y.; Qiao, J. Metabolic characteristics of normal weight central obesity phenotype polycystic ovary syndrome women: A large-scale national epidemiological survey. Reprod. Biomed. Online 2018, 37, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Repaci, A.; Gambineri, A.; Pasquali, R. The role of low-grade inflammation in the polycystic ovary syndrome. Mol. Cell Endocrinol. 2011, 335, 30–41. [Google Scholar] [CrossRef]
- Aboeldalyl, S.; James, C.; Seyam, E.; Ibrahim, E.M.; Shawki, H.E.; Amer, S. The Role of Chronic Inflammation in Polycystic Ovarian Syndrome—A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2021, 22, 2734. [Google Scholar] [CrossRef]
- Chen, J.; Shen, S.; Tan, Y.; Xia, D.; Xia, Y.; Cao, Y.; Wang, W.; Wu, X.; Wang, H.; Yi, L.; et al. The correlation of aromatase activity and obesity in women with or without polycystic ovary syndrome. J. Ovarian Res. 2015, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Uçkan, K.; Demir, H.; Turan, K.; Sarıkaya, E.; Demir, C. Role of Oxidative Stress in Obese and Nonobese PCOS Patients. Int. J. Clin. Pract. 2022, 2022, 4579831. [Google Scholar] [CrossRef]
- Murri, M.; Luque-Ramírez, M.; Insenser, M.; Ojeda-Ojeda, M.; Escobar-Morreale, H.F. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): A systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 268–288. [Google Scholar] [CrossRef]
- Shin, S. Regulation of Adipose Tissue Biology by Long-Chain Fatty Acids: Metabolic Effects and Molecular Mechanisms. J. Obes. Metab. Syndr. 2022, 31, 147–160. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, W.; Zhou, P.; Shi, L.; Chen, Z.; Yang, Y.; Lu, Y.; Zhou, L.; Zhang, H.; Cheng, M.; et al. Obesity is associated with SHBG levels rather than blood lipid profiles in PCOS patients with insulin resistance. BMC Endocr. Disord. 2024, 24, 254. [Google Scholar] [CrossRef]
- Lonardo, M.S.; Cacciapuoti, N.; Guida, B.; Di Lorenzo, M.; Chiurazzi, M.; Damiano, S.; Menale, C. Hypothalamic-Ovarian axis and Adiposity Relationship in Polycystic Ovary Syndrome: Physiopathology and Therapeutic Options for the Management of Metabolic and Inflammatory Aspects. Curr. Obes. Rep. 2024, 13, 51–70. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Zare, M.; Nouripour, F. Effect of Soy and Soy Isoflavones on Obesity-Related Anthropometric Measures: A Systematic Review and Meta-analysis of Randomized Controlled Clinical Trials. Adv. Nutr. 2017, 8, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Corbould, A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J. Endocrinol. 2007, 192, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Paulukinas, R.D.; Mesaros, C.A.; Penning, T.M. Conversion of Classical and 11-Oxygenated Androgens by Insulin-Induced AKR1C3 in a Model of Human PCOS Adipocytes. Endocrinology 2022, 163, bqac068. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.W.; Kempegowda, P.; Walsh, M.; Taylor, A.E.; Manolopoulos, K.N.; Allwood, J.W.; Semple, R.K.; Hebenstreit, D.; Dunn, W.B.; Tomlinson, J.W.; et al. AKR1C3-Mediated Adipose Androgen Generation Drives Lipotoxicity in Women With Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2017, 102, 3327–3339. [Google Scholar] [CrossRef]
- Rajkhowa, M.; Brett, S.; Cuthbertson, D.J.; Lipina, C.; Ruiz-Alcaraz, A.J.; Thomas, G.E.; Logie, L.; Petrie, J.R.; Sutherland, C. Insulin resistance in polycystic ovary syndrome is associated with defective regulation of ERK1/2 by insulin in skeletal muscle in vivo. Biochem. J. 2009, 418, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Corbould, A.; Zhao, H.; Mirzoeva, S.; Aird, F.; Dunaif, A. Enhanced mitogenic signaling in skeletal muscle of women with polycystic ovary syndrome. Diabetes 2006, 55, 751–759. [Google Scholar] [CrossRef]
- Poretsky, L. On the paradox of insulin-induced hyperandrogenism in insulin-resistant states. Endocr. Rev. 1991, 12, 3–13. [Google Scholar] [CrossRef]
- Baillargeon, J.P.; Carpentier, A. Role of insulin in the hyperandrogenemia of lean women with polycystic ovary syndrome and normal insulin sensitivity. Fertil. Steril. 2007, 88, 886–893. [Google Scholar] [CrossRef]
- Nestler, J.E.; Powers, L.P.; Matt, D.W.; Steingold, K.A.; Plymate, S.R.; Rittmaster, R.S.; Clore, J.N.; Blackard, W.G. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1991, 72, 83–89. [Google Scholar] [CrossRef]
- Patil, S.; Veerabhadra Goud, G.K.; Shivashankar, R.N.; Anusuya, S.K.; Ganesh, V. Association of adiponectin levels with polycystic ovary syndrome among Indian women. Bioinformation 2022, 18, 864–869. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, H.; Song, J.; Feng, D.; Na, Z.; Jiang, H.; Meng, Y.; Shi, B.; Li, D. Elevated Serum Leptin Levels as a Predictive Marker for Polycystic Ovary Syndrome. Front. Endocrinol. 2022, 13, 845165. [Google Scholar] [CrossRef] [PubMed]
- Psilopatis, I.; Vrettou, K.; Nousiopoulou, E.; Palamaris, K.; Theocharis, S. The Role of Peroxisome Proliferator-Activated Receptors in Polycystic Ovary Syndrome. J. Clin. Med. 2023, 12, 2912. [Google Scholar] [CrossRef]
- Komar, C.M. Peroxisome proliferator-activated receptors (PPARs) and ovarian function--implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod. Biol. Endocrinol. 2005, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Sirisha, P.; Neelaveni, K.; Anuradha, K.; Sudhakar, G.; Reddy, B.M. Polymorphisms in the IRS-1 and PPAR-γ genes and their association with polycystic ovary syndrome among South Indian women. Gene 2012, 503, 140–146. [Google Scholar] [CrossRef]
- Yao, K.; Zheng, H.; Peng, H. Association between polycystic ovary syndrome and risk of non-alcoholic fatty liver disease: A meta-analysis. Endokrynol. Pol. 2023, 74, 520–527. [Google Scholar] [CrossRef]
- Hong, X.; Guo, Z.; Yu, Q. Hepatic steatosis in women with polycystic ovary syndrome. BMC Endocr. Disord. 2023, 23, 207. [Google Scholar] [CrossRef]
- Legro, R.S.; Kunselman, A.R.; Dunaif, A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am. J. Med. 2001, 111, 607–613. [Google Scholar] [CrossRef]
- Kim, J.J.; Choi, Y.M. Dyslipidemia in women with polycystic ovary syndrome. Obstet. Gynecol. Sci. 2013, 56, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Wekker, V.; van Dammen, L.; Koning, A.; Heida, K.Y.; Painter, R.C.; Limpens, J.; Laven, J.S.E.; Roeters van Lennep, J.E.; Roseboom, T.J.; Hoek, A. Long-term cardiometabolic disease risk in women with PCOS: A systematic review and meta-analysis. Hum. Reprod. Update 2020, 26, 942–960. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, B.; Jiang, X.; Li, Z.; Zhao, S.; Cui, L.; Chen, Z.J. Metabolic disturbances in non-obese women with polycystic ovary syndrome: A systematic review and meta-analysis. Fertil. Steril. 2019, 111, 168–177. [Google Scholar] [CrossRef]
- Maranon, R.; Lima, R.; Spradley, F.T.; do Carmo, J.M.; Zhang, H.; Smith, A.D.; Bui, E.; Thomas, R.L.; Moulana, M.; Hall, J.E.; et al. Roles for the sympathetic nervous system, renal nerves, and CNS melanocortin-4 receptor in the elevated blood pressure in hyperandrogenemic female rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R708–R713. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Schwartzman, M.L. The role of 20-HETE in androgen-mediated hypertension. Prostaglandins Other Lipid Mediat. 2011, 96, 45–53. [Google Scholar] [CrossRef]
- Dalmasso, C.; Maranon, R.; Patil, C.; Moulana, M.; Romero, D.G.; Reckelhoff, J.F. 20-HETE and CYP4A2 ω-hydroxylase contribute to the elevated blood pressure in hyperandrogenemic female rats. Am. J. Physiol. Renal Physiol. 2016, 311, F71–F77. [Google Scholar] [CrossRef]
- Lei, L.; Hui, S.; Chen, Y.; Yan, H.; Yang, J.; Tong, S. Effect of soy isoflavone supplementation on blood pressure: A meta-analysis of randomized controlled trials. Nutr. J. 2024, 23, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Ho, S.C. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am. J. Clin. Nutr. 2005, 81, 397–408. [Google Scholar] [CrossRef]
- Squadrito, F.; Altavilla, D.; Squadrito, G.; Saitta, A.; Cucinotta, D.; Minutoli, L.; Deodato, B.; Ferlito, M.; Campo, G.M.; Bova, A.; et al. Genistein supplementation and estrogen replacement therapy improve endothelial dysfunction induced by ovariectomy in rats. Cardiovasc. Res. 2000, 45, 454–462. [Google Scholar] [CrossRef]
- Honoré, E.K.; Williams, J.K.; Anthony, M.S.; Clarkson, T.B. Soy isoflavones enhance coronary vascular reactivity in atherosclerotic female macaques. Fertil. Steril. 1997, 67, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Birru, R.L.; Ahuja, V.; Vishnu, A.; Evans, R.W.; Miyamoto, Y.; Miura, K.; Usui, T.; Sekikawa, A. The impact of equol-producing status in modifying the effect of soya isoflavones on risk factors for CHD: A systematic review of randomised controlled trials. J. Nutr. Sci. 2016, 5, e30. [Google Scholar] [CrossRef]
- Watanabe, S.; Haruyama, R.; Umezawa, K.; Tomioka, I.; Nakamura, S.; Katayama, S.; Mitani, T. Genistein enhances NAD+ biosynthesis by upregulating nicotinamide phosphoribosyltransferase in adipocytes. J. Nutr. Biochem. 2023, 121, 109433. [Google Scholar] [CrossRef]
- Li, P.; Shuai, P.; Shen, S.; Zheng, H.; Sun, P.; Zhang, R.; Lan, S.; Lan, Z.; Jayawardana, T.; Yang, Y. Perturbations in gut microbiota composition in patients with polycystic ovary syndrome: A systematic review and meta-analysis. BMC Med. 2023, 21, 302. [Google Scholar] [CrossRef]
- Li, C.; Cheng, D.; Ren, H.; Zhang, T. Unraveling the gut microbiota’s role in PCOS: A new frontier in metabolic health. Front. Endocrinol. 2025, 16, 1529703. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)—A novel theory for the development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, G.S.G.; Inoue, R.; Fujitani, M.; Ishijima, T.; Shibutani, T.; Abe, K.; Kishida, T.; Okada, S. Effects of Soy Isoflavones, Resistant Starch and Antibiotics on Polycystic Ovary Syndrome (PCOS)-Like Features in Letrozole-Treated Rats. Nutrients 2021, 13, 3759. [Google Scholar] [CrossRef]
- Bulsara, J.; Soni, A.; Patil, P.; Halpati, K.; Desai, S.; Acharya, S. Bio-enhancement of Soy Isoflavones (Genistein & Daidzein) Using Bacillus coagulans in Letrozole Induced Polycystic Ovarian Syndrome by Regulating Endocrine Hormones in Rats. Probiotics Antimicrob. Proteins 2022, 14, 560–572. [Google Scholar] [CrossRef]
- Chen, P.; Sun, J.; Liang, Z.; Xu, H.; Du, P.; Li, A.; Meng, Y.; Reshetnik, E.I.; Liu, L.; Li, C. The bioavailability of soy isoflavones in vitro and their effects on gut microbiota in the simulator of the human intestinal microbial ecosystem. Food Res. Int. 2022, 152, 110868. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Mukne, A.P.; Viswanathan, V.; Phadatare, A.G. Structure pre-requisites for isoflavones as effective antibacterial agents. Pharmacogn. Rev. 2011, 5, 13. [Google Scholar] [CrossRef]
- Xiong, R.-G.; Zhou, D.-D.; Wu, S.-X.; Huang, S.-Y.; Saimaiti, A.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef]
- Yoshikata, R.; Myint, K.Z.; Ohta, H.; Ishigaki, Y. Inter-relationship between diet, lifestyle habits, gut microflora, and the equol-producer phenotype: Baseline findings from a placebo-controlled intervention trial. Menopause 2019, 26, 273–285. [Google Scholar] [CrossRef]
- Ghimire, S.; Cady, N.M.; Lehman, P.; Peterson, S.R.; Shahi, S.K.; Rashid, F.; Giri, S.; Mangalam, A.K. Dietary Isoflavones Alter Gut Microbiota and Lipopolysaccharide Biosynthesis to Reduce Inflammation. Gut Microbes 2022, 14, 2127446. [Google Scholar] [CrossRef]
- Bolca, S.; Verstraete, W. Microbial equol production attenuates colonic methanogenesis and sulphidogenesis in vitro. Anaerobe 2010, 16, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Decroos, K.; Vanhemmens, S.; Cattoir, S.; Boon, N.; Verstraete, W. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch. Microbiol. 2004, 183, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, T.E.; Maroni, P.D.; Ferucci, P.G.; Dayton, R.; Barnes, S.; Jones, K.; Moore, R.; Ogden, L.G.; Wähälä, K.; Sackett, H.M.; et al. Long-term dietary habits affect soy isoflavone metabolism and accumulation in prostatic fluid in caucasian men. J. Nutr. 2005, 135, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).