From Pathogens to Partners: The Beginnings of Gut Microbiota Research
Abstract
1. Introduction
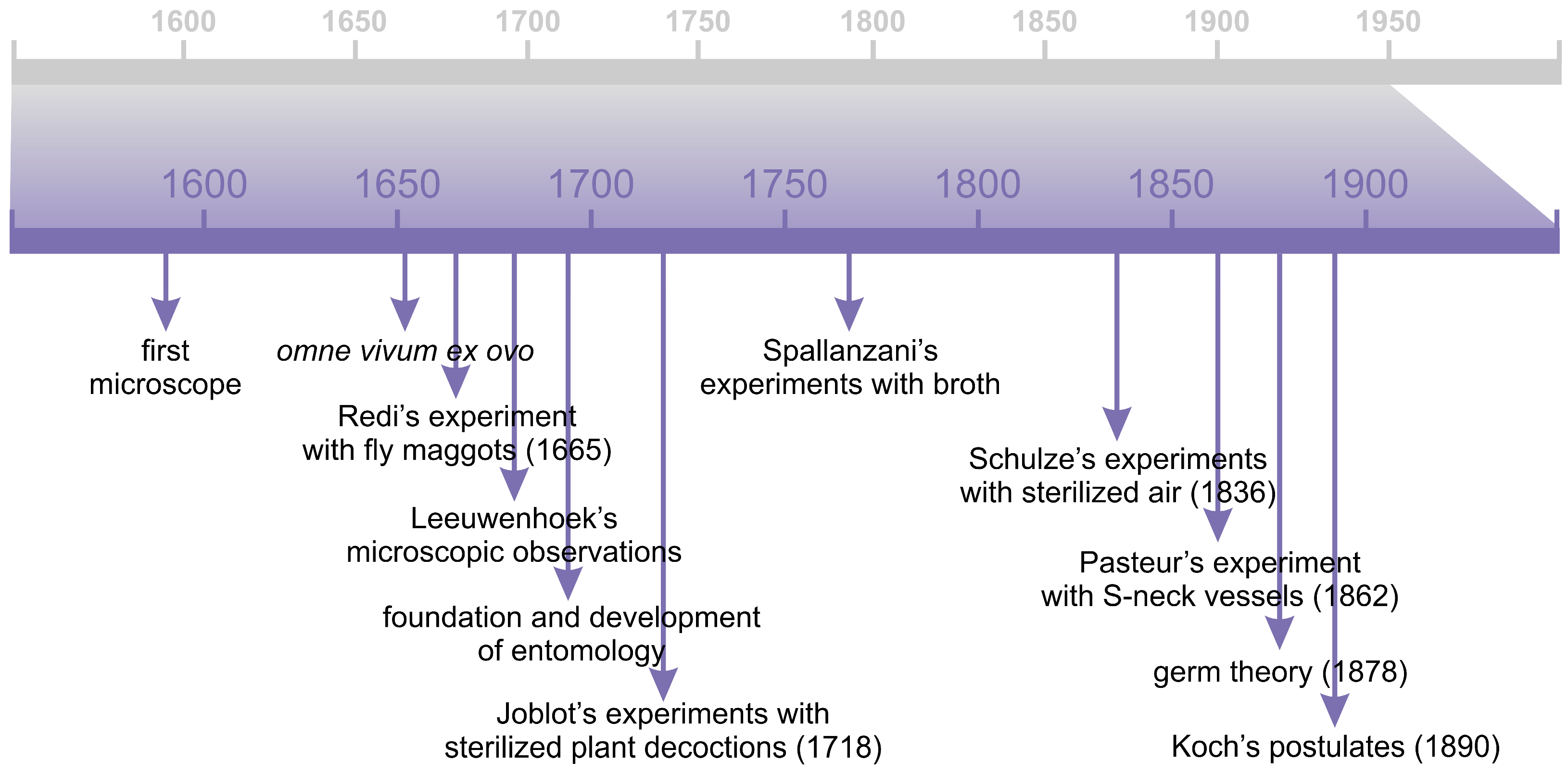
2. From the Spontaneous Generation to the Germ Theory
2.1. Microscopy
2.2. The Theory of Spontaneous Generation
2.3. Infectious Diseases and the Rise of the Germ Theory
- The organism must be shown to be invariably present in characteristic form and arrangement in the diseased tissue.
- The organism, which from its relationship to the diseased tissue appears to be responsible for the disease, must be isolated and grown in pure culture.
- The pure culture must be shown to induce the disease experimentally.
- The organism should be re-isolated from the experimentally infected subject.
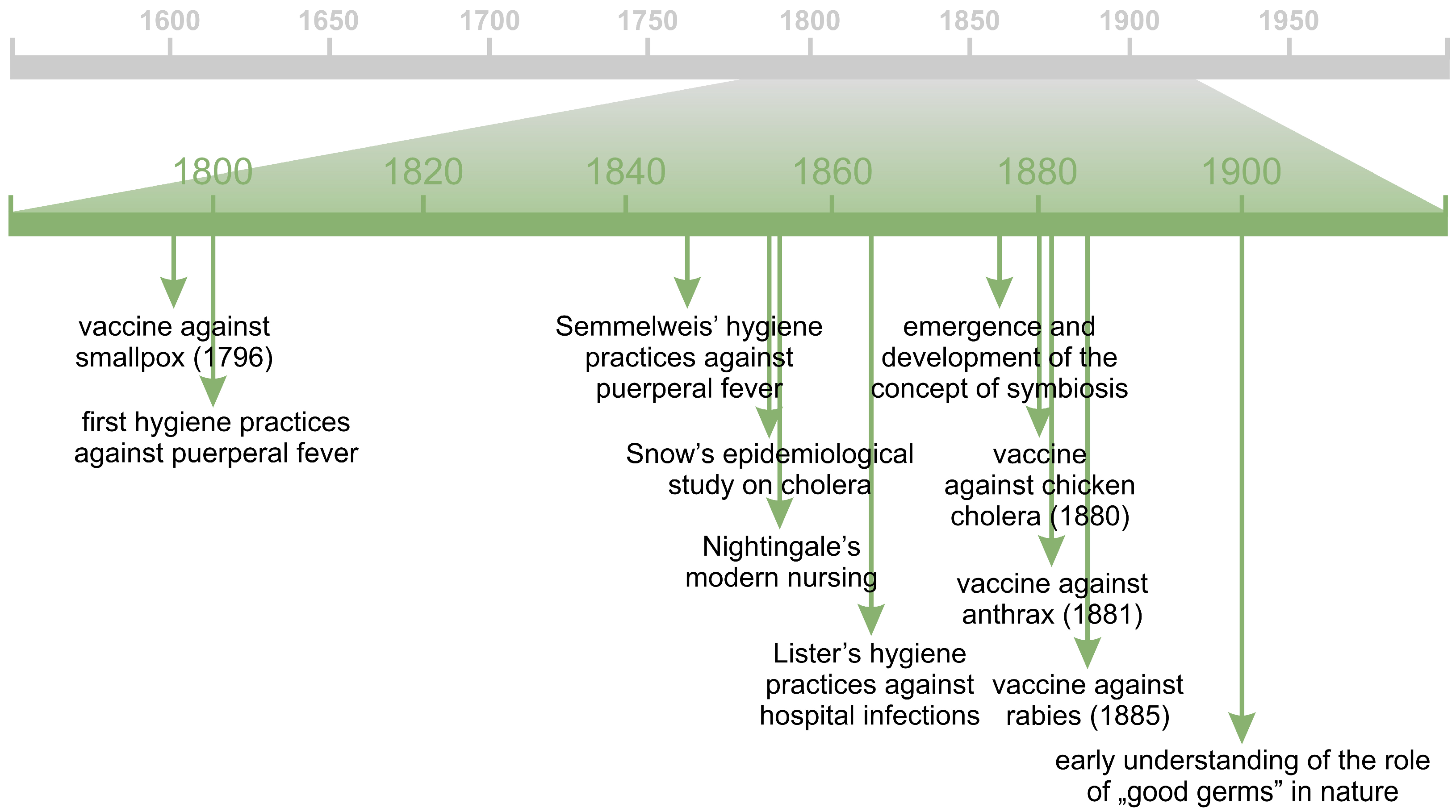
3. “Bad Germs” and “Good Germs”
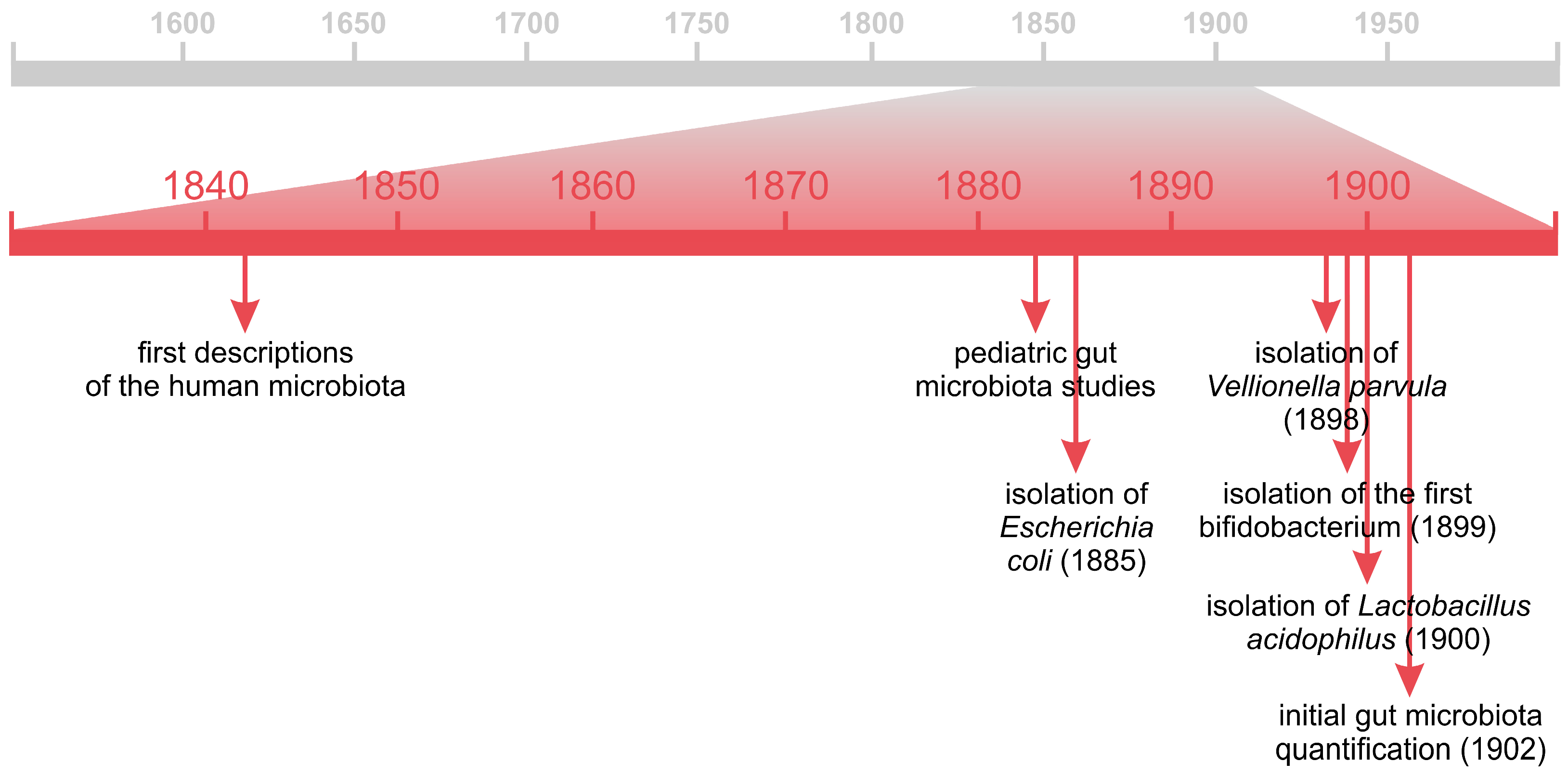
4. Beginnings of the Human Gut Microbiota Studies
5. “Good Germs” in the Service of Human Health
5.1. Probiotics
5.1.1. From the Theory of Autointoxication to Probiotic Bacteria
5.1.2. Current Understanding of Probiotics, Prebiotics, Synbiotics and Postbiotics
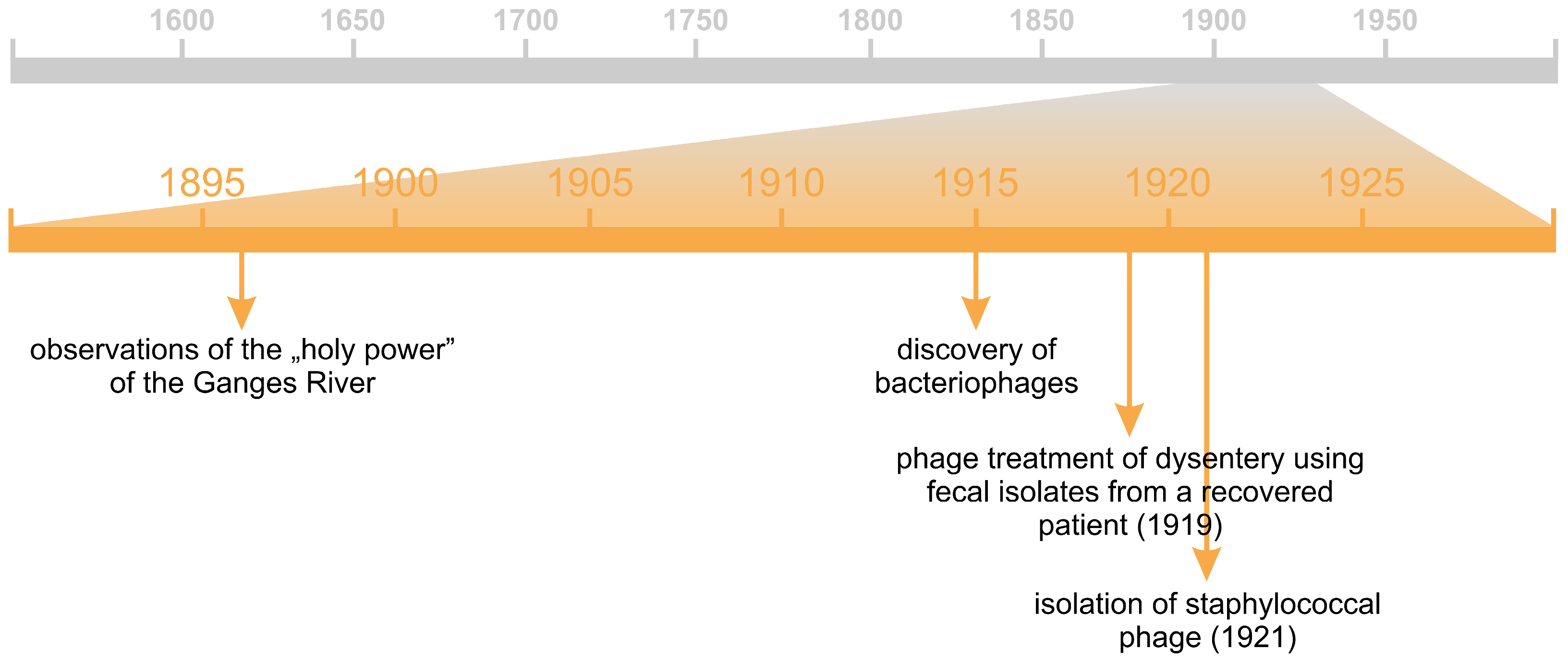
5.2. Phages
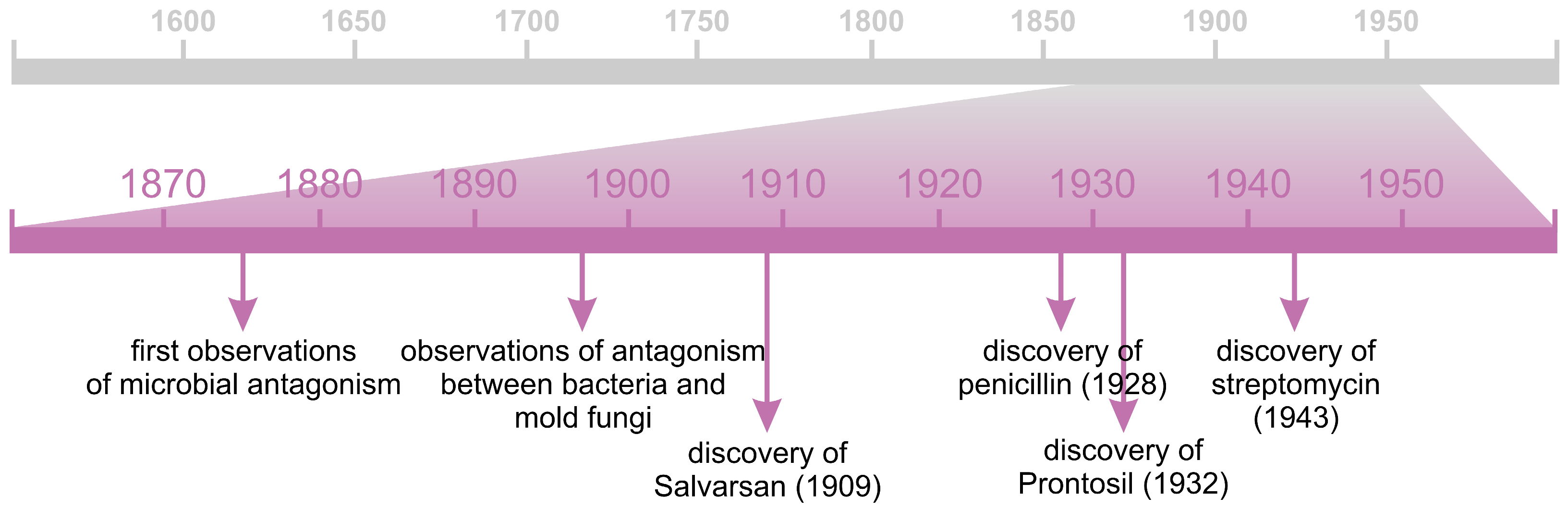
5.3. Antibiotics and Other Antimicrobial Compounds
5.3.1. Antibiotics
5.3.2. Chemotherapeutics
6. Summary and Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Grice, E.A.; Segre, J.A. The Human Microbiome: Our Second Genome. Annu. Rev. Genom. Hum. Genet. 2012, 13, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the human oral microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Lu, Z. Microbiota Research: From History to Advances. E3S Web Conf. 2020, 145, 01014. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Dai, L.; Zhao, Q.; Zhang, X. A review on the effect of gut microbiota on metabolic diseases. Arch. Microbiol. 2022, 204, 192. [Google Scholar] [CrossRef]
- Hooks, K.B.; O’Malley, M.A. Contrasting Strategies: Human Eukaryotic Versus Bacterial Microbiome Research. J. Eukaryot. Microbiol. 2020, 67, 279–295. [Google Scholar] [CrossRef]
- Bocci, V. The neglected organ: Bacterial flora has a crucial immunostimulatory role. Perspect. Biol. Med. 1992, 35, 251–260. [Google Scholar] [CrossRef]
- Gershon, M. The Second Brain: A Groundbreaking New Understanding of Nervous Disorders of the Stomach and Intestine; Harper Collins: New York, NY, USA, 1998. [Google Scholar]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Lindell, A.E.; Zimmermann-Kogadeeva, M.; Patil, K.R. Multimodal interactions of drugs, natural compounds and pollutants with the gut microbiota. Nat. Rev. Microbiol. 2022, 20, 431–443. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Champomier Vergès, M.-C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Vemuri, R.; Shankar, E.M.; Chieppa, M.; Eri, R.; Kavanagh, K. Beyond Just Bacteria: Functional Biomes in the Gut Ecosystem Including Virome, Mycobiome, Archaeome and Helminths. Microorganisms 2020, 8, 483. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Gilbert, J.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Weyrich, L.S. The evolutionary history of the human oral microbiota and its implications for modern health. Periodontology 2020, 85, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Amaral, P.; Carbonell-Sala, S.; De La Vega, F.M.; Faial, T.; Frankish, A.; Gingeras, T.; Guigo, R.; Harrow, J.L.; Hatzigeorgiou, A.G.; Johnson, R.; et al. The status of the human gene catalogue. Nature 2023, 622, 41–47. [Google Scholar] [CrossRef]
- Yang, X.; Xie, L.; Li, Y.; Wei, C. More than 9,000,000 Unique Genes in Human Gut Bacterial Community: Estimating Gene Numbers Inside a Human Body. PLoS ONE 2009, 4, e6074. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, X.; Li, L. Human gut microbiome: The second genome of human body. Protein Cell 2010, 1, 718–725. [Google Scholar] [CrossRef] [PubMed]
- The Nobel Prize. Available online: https://www.nobelprize.org (accessed on 1 September 2025).
- Prescott, S.L. History of medicine: Origin of the term microbiome and why it matters. Hum. Microbiome J. 2017, 4, 24–25. [Google Scholar] [CrossRef]
- Whipps, J.; Lewis, K.; Cooke, R. Mycoparasitism and plant disease control. In Fungi in Biological Control Systems; Burge, M., Ed.; Manchester University Press: Manchester, UK, 1988; pp. 161–187. [Google Scholar]
- Suzuki, T.A.; Fitzstevens, J.L.; Schmidt, V.T.; Enav, H.; Huus, K.E.; Ngwese, M.M.; Grießhammer, A.; Pfleiderer, A.; Adegbite, B.R.; Zinsou, J.F.; et al. Codiversification of gut microbiota with humans. Science 2022, 377, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, I.A.; Goryacheva, I.I. Hereditary Symbionts: Genomic Integration. Russ. J. Genet. 2020, 56, 639–654. [Google Scholar] [CrossRef]
- Beaumont, M.; Roura, E.; Lambert, W.; Turni, C.; Michiels, J.; Chalvon-Demersay, T. Selective nourishing of gut microbiota with amino acids: A novel prebiotic approach? Front. Nutr. 2022, 9, 1066898. [Google Scholar] [CrossRef]
- Abeltino, A.; Hatem, D.; Serantoni, C.; Riente, A.; De Giulio, M.M.; De Spirito, M.; De Maio, F.; Maulucci, G. Unraveling the Gut Microbiota: Implications for Precision Nutrition and Personalized Medicine. Nutrients 2024, 16, 3806. [Google Scholar] [CrossRef]
- Dawson, S.L.; Todd, E.; Ward, A.C. The Interplay of Nutrition, the Gut Microbiota and Immunity and Its Contribution to Human Disease. Biomedicines 2025, 13, 329. [Google Scholar] [CrossRef] [PubMed]
- Sanz, Y.; Cryan, J.F.; Deschasaux-Tanguy, M.; Elinav, E.; Lambrecht, R.; Veiga, P. The gut microbiome connects nutrition and human health. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 534–555. [Google Scholar] [CrossRef]
- Hooks, K.B.; O’Malley, M.A. Dysbiosis and Its Discontents. mBio 2017, 8, 1492. [Google Scholar] [CrossRef]
- Hanišáková, N.; Vítězová, M.; Rittmann, S.K.R. The Historical Development of Cultivation Techniques for Methanogens and Other Strict Anaerobes and Their Application in Modern Microbiology. Microorganisms 2022, 10, 412. [Google Scholar] [CrossRef]
- Fiebiger, U.; Bereswill, S.; Heimesaat, M.M. Dissecting the Interplay Between Intestinal Microbiota and Host Immunity in Health and Disease: Lessons Learned from Germfree and Gnotobiotic Animal Models. Eur. J. Microbiol. Immunol. 2016, 6, 253–271. [Google Scholar] [CrossRef]
- Wang, W.L.; Xu, S.Y.; Ren, Z.G.; Tao, L.; Jiang, J.W.; Zheng, S.S. Application of metagenomics in the human gut microbiome. World J. Gastroenterol. 2015, 21, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Chen, Y. Transcriptomics: Advances and approaches. Sci. China Life Sci. 2013, 56, 960–967. [Google Scholar] [CrossRef]
- Lai, L.A.; Tong, Z.; Chen, R.; Pan, S. Metaproteomics Study of the Gut Microbiome. Methods Mol. Biol. 2019, 1871, 123–132. [Google Scholar] [CrossRef]
- Al-Amrani, S.; Al-Jabri, Z.; Al-Zaabi, A.; Alshekaili, J.; Al-Khabori, M. Proteomics: Concepts and applications in human medicine. World J. Biol. Chem. 2021, 12, 57–69. [Google Scholar] [CrossRef]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef] [PubMed]
- Straus, E.W.; Straus, A. Medical Marvels: The 100 Greatest Advances in Medicine; Prometheus Books: Amherst, NY, USA, 2006. [Google Scholar]
- Hempelmann, E.; Krafts, K. Bad air, amulets and mosquitoes: 2000 years of changing perspectives on malaria. Malar. J. 2013, 12, 232. [Google Scholar] [CrossRef]
- Deichmann, U. Origin of life. The role of experiments, basic beliefs, and social authorities in the controversies about the spontaneous generation of life and the subsequent debates about synthesizing life in the laboratory. Hist. Philos. Life Sci. 2012, 34, 341–359. [Google Scholar]
- Davidson, M.W. Pioneers in Optics: Zacharias Janssen and Johannes Kepler. Microsc. Today 2009, 17, 44–47. [Google Scholar] [CrossRef]
- Chapman, A. England’s Leonardo—Robert Hooke (1635–1703) and the art of experiment in Restoration England. Proc. R. Inst. Great Br. 1996, 67, 239–275. [Google Scholar]
- Gest, H. The discovery of microorganisms by Robert Hooke and Antoni van Leeuwenhoek, Fellows of the Royal Society. Notes Rec. R. Soc. 2004, 58, 187–201. [Google Scholar] [CrossRef]
- Lane, N. The unseen world: Reflections on Leeuwenhoek (1677) ‘Concerning little animals’. Philos. Trans. R. Soc. B 2015, 370, 20140344. [Google Scholar] [CrossRef] [PubMed]
- Dobell, C. The Discovery of the Intestinal Protozoa of Man. Proc. R. Soc. Med. (Sect. Hist Med.) 1920, 13, 1–15. [Google Scholar] [CrossRef]
- Foote, E.T. Harvey: Spontaneous generation and the egg. Ann. Sci. 1969, 25, 139–163. [Google Scholar] [CrossRef]
- Redi, F. Esperienze Intorno Alla Generazione Degl’ Insetti; Accademia della Crusca: Firenze, Italy, 1668. [Google Scholar]
- Bardell, D. Francesco Redi’s Description of the Spontaneous Generation of Gall Flies. Am. Biol. Teach. 1985, 47, 237–238. [Google Scholar] [CrossRef]
- Hawgood, B.J. Francesco Redi (1626–1697): Tuscan philosopher, physician and poet. J. Med. Biogr. 2003, 11, 28–34. [Google Scholar] [CrossRef]
- Van Lenteren, J.C.; Godfray, H.C.J. European science in the Enlightenment and the discovery of the insect parasitoid life cycle in The Netherlands and Great Britain. Biol. Control 2005, 32, 12–24. [Google Scholar] [CrossRef]
- Vidal, S. The history of Hymenopteran parasitoid research in Germany. Biol. Control 2005, 32, 25–33. [Google Scholar] [CrossRef]
- Todd, K. Maria Sibylla Merian (1647–1717): An early investigator of parasitoids and phenotypic plasticity. Terr. Arthropod Rev. 2011, 4, 131–144. [Google Scholar] [CrossRef]
- Joblot, L. Descriptions et Usages de Plusieurs Nouveaux Microscopes, Tant Simples Que Composez: Avec de Nouvelles Observations Faites Sur une Multitude Innombrable D’insectes, et D’autres Animaux de Diverses Especes, Qui Naissent Dans des Liqueurs Préparées, & Dans Celles Qui ne le Sont Point; Chez Jacques Collombat: Paris, France, 1718. [Google Scholar]
- Dolan, J.R. Re-visiting the ridiculed rival of Leeuwenhoek: Louis Joblot (1645–1723). Protist 2022, 173, 125882. [Google Scholar] [CrossRef]
- Ariatti, A.; Mandrioli, P. Lazzaro Spallanzani. A blow against spontaneous generation. Aerobiologia 1993, 9, 101–107. [Google Scholar] [CrossRef]
- Schulze, F. Vorläufige Mittheilung der Resultate einer experimentellen Beobachtung über Generatio aequivoca. Poggendorff’s Annal. D Phys. U Chem. 1836, 115, 487–489. [Google Scholar] [CrossRef]
- Müller-Wille, S. Cell Theory, Specificity, and Reproduction, 1837–1870. Stud. Hist. Philos. Biol. Biomed. Sci. 2010, 41, 225–231. [Google Scholar] [CrossRef]
- Weed, L.A. John Tyndall and His Contribution to the Theory of Spontaneous Generation. Ann. Med. Hist. 1942, 4, 55–62. [Google Scholar] [PubMed]
- Krzysztofik, M. The Image of Disease in Religious, Medical-Astrological and Social Discourses: Old Polish Literature as an Example of Early Modern European Mentality. J. Relig. Health 2022, 61, 3340–3349. [Google Scholar] [CrossRef]
- Halliday, S. Death and miasma in Victorian London: An obstinate belief. BMJ 2001, 323, 22–29. [Google Scholar] [CrossRef]
- Jouanna, J. The Legacy of the Hippocratic Treatise The Nature of Man: The Theory of the Four Humours. In Greek Medicine from Hippocrates to Galen; van der Eijk, P., Ed.; Brill: Leiden-Boston, MA, USA, 2012; pp. 335–359. [Google Scholar]
- Sumrall, L.; O’Malley, M.A. Conceptual parallels: Microbiome research and ancient medicine. Perspect. Biol. Med. 2024, 67, 406–423. [Google Scholar] [CrossRef] [PubMed]
- Huremović, D. Brief History of Pandemics (Pandemics Throughout History). In Psychiatry of Pandemics; Huremović, D., Ed.; Springer: Cham, Switzerland, 2019; pp. 7–35. [Google Scholar] [CrossRef]
- Smith, T. Pasteur and insect pathogens. Nat. Struct. Biol. 1999, 6, 720. [Google Scholar] [CrossRef]
- Berche, P. Louis Pasteur, from crystals of life to vaccination. Clin. Microbiol. Infect. 2012, 18, 1–6. [Google Scholar] [CrossRef]
- Ozen, M.; Dinleyici, E.C. The history of probiotics: The untold story. Benef. Microbes 2014, 6, 159–165. [Google Scholar] [CrossRef]
- Roll-Hansen, N. Revisiting the Pouchet-Pasteur controversy over spontaneous generation: Understanding experimental method. Hist. Philos. Life Sci. 2018, 40, 68. [Google Scholar] [CrossRef]
- Cavaillon, J.-M.; Legout, S. Louis Pasteur: Between Myth and Reality. Biomolecules 2022, 12, 596. [Google Scholar] [CrossRef]
- Pesapane, F.; Marcelli, S.; Nazzaro, G. Hieronymi Fracastorii: The Italian scientist who described the “French disease”. An. Bras. Dermatol. 2015, 90, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Torrey, H.B. Athanasius Kircher and the Progress of Medicine. Osiris 1938, 5, 246–275. [Google Scholar] [CrossRef]
- Galea, J. Doctors during the 1837 Cholera Epidemic in Malta: Unearthing the Truth. Malta Med. J. 2018, 30, 32–41. [Google Scholar]
- Holland, H. On the hypothesis of insect life as a cause of disease? In Medical Notes and Reflections; Holland, H., Ed.; Longman, Orme, Brown, Green, and Longmans: London, UK, 1839; pp. 560–589. [Google Scholar] [CrossRef]
- Appert, N. L’art de Conserver Pendant Plusieurs Années Toutes les Substances Animales et Végétales; Patris: Paris, France, 1810. [Google Scholar]
- Garcia, R.; Adrian, J. Nicolas Appert: Inventor and Manufacturer. Food Rev. Int. 2009, 25, 115–125. [Google Scholar] [CrossRef]
- Currier, R.W.; Widness, J.A. A Brief History of Milk Hygiene and Its Impact on Infant Mortality from 1875 to 1925 and Implications for Today: A Review. J. Food Prot. 2018, 81, 1713–1722. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E. Robert Koch, the Nobel Prize, and the Ongoing Threat of Tuberculosis. N. Engl. J. Med. 2005, 353, 2423–2426. [Google Scholar] [CrossRef]
- Blevins, S.M.; Bronze, M.S. Robert Koch and the ‘golden age’ of bacteriology. Int. J. Infect. Dis. 2010, 14, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Tabrah, F.L. Koch’s Postulates, Carnivorous Cows, and Tuberculosis Today. Hawaii Med. J. 2011, 70, 144–148. [Google Scholar]
- Bentivoglio, M.; Pacini, P. Filippo Pacini: A determined observer. Brain Res. Bull. 1995, 38, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Riedel, S. Anthrax: A continuing concern in the era of bioterrorism. Bayl. Univ. Med. Cent. Proc. 2005, 18, 234–243. [Google Scholar] [CrossRef]
- Davaine, C.J. Recherche sur les infusoires du sang dans la maladie connue sous le nom de sang de rate. Comptes Rendus De L’académie Des Sci. 1863, 57, 220–223. [Google Scholar]
- Estey, R.H. A Note on Casimir-Joseph Davaine 1812-1882. Agric. Hist. 1975, 49, 549–552. [Google Scholar]
- Belongia, E.A.; Naleway, A.L. Smallpox Vaccine: The Good, the Bad, and the Ugly. Clin. Medi Res. 2003, 1, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef]
- Saleh, A.; Qamar, S.; Tekin, A.; Singh, R.; Kashyap, R. Vaccine Development Throughout History. Cureus 2021, 13, 16635. [Google Scholar] [CrossRef]
- Neuhauser, D. Surgical experience, hospital size and severity adjusted mortality: James Y Simpson, 1869. Qual. Saf. Health Care 2005, 14, 67–68. [Google Scholar] [CrossRef][Green Version]
- Fitzharris, L. The Butchering Art: Joseph Lister’s Quest to Transform the Grisly World of Victorian Medicine; Farrar, Straus and Giroux: New York, NY, USA, 2017. [Google Scholar][Green Version]
- Roberts, W.C. Facts and ideas from anywhere. Baylor Univ. Med. Cent. Proc. 2018, 31, 257–267. [Google Scholar] [CrossRef][Green Version]
- Amr, S.S.; Tbakhi, A.T. Abu Bakr Muhammad Ibn Zakariya Al Razi (Rhazes): Philosopher, Physician and Alchemist. Ann. Saudi Med. 2007, 27, 305–307. [Google Scholar] [CrossRef]
- Hajar, R. The Air of History (Part IV): Great Muslim Physicians Al Rhazes. Hist. Med. 2013, 14, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Selwyn, S. Sir John Pringle: Hospital reformer, moral philosopher and pioneer of antiseptics. Med. Hist. 1966, 10, 266–274. [Google Scholar] [CrossRef][Green Version]
- Bennet, P.N. Alexander Gordon (1752–1799) and his writing: Insights into medical thinking in the late eighteenth century. J. R. Coll. Physicians Edinb. 2012, 42, 165–171. [Google Scholar] [CrossRef]
- Dunn, P.M. Oliver Wendell Holmes (1809–1894) and his essay on puerperal fever. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Best, M.; Neuhauser, D. Ignaz Semmelweis and the birth of infection control. Qual. Saf. Health Care 2004, 13, 233–234. [Google Scholar] [CrossRef]
- Pittet, D.; Allegranzi, B. Preventing sepsis in healthcare—200 years after the birth of Ignaz Semmelweis. Euro Surveill. 2018, 23, 18-00222. [Google Scholar] [CrossRef] [PubMed]
- Karimi, H.; Alavi, N.M. Florence Nightingale: The Mother of Nursing. Nurs. Midwifery Stud. 2015, 4, e29475. [Google Scholar] [PubMed]
- Pitt, D.; Aubin, J.M. Joseph Lister: Father of modern surgery. Can. J. Surg. 2012, 55, 8–9. [Google Scholar] [CrossRef]
- Ramsey, M.A.E. John Snow, MD: Anaesthetist to the Queen of England and pioneer epidemiologist. Baylor Univ. Med. Cent. Proc. 2006, 19, 24–28. [Google Scholar] [CrossRef]
- Moorhead, R. William Budd and typhoid fever. J. R. Soc. Med. 2002, 95, 561–564. [Google Scholar] [CrossRef][Green Version]
- Darwin, C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life; John Murray: London, UK, 1859. [Google Scholar][Green Version]
- Bos, L. Beijerinck’s work on tobacco mosaic virus: Historical context and legacy. Philos. Trans. R. Soc. B-J. 1999, 354, 675–685. [Google Scholar] [CrossRef]
- Burton, A. It’s life, Martinus, but not as we know it. Front. Ecol. Environ. 2011, 9, 84. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Dworkin, M.; Gutnick, D. Sergei Winogradsky: A founder of modern microbiology and the first microbial ecologist. FEMS Microbiol. Rev. 2012, 36, 364–379. [Google Scholar] [CrossRef]
- Frank, A.B. Über die biologischen Verhältnisse des Thallus einiger Krustflechten. Cohn Beitr. Biol. Pflanz. 1877, 2, 123–200. [Google Scholar]
- Frank, A.B. Ueber die auf Wurzelsymbiose beruhende Ernährung gewisser Bäume durch unterirdische Pilze. Ber. Deutsch. Bot. Ges. 1885, 3, 128–145. [Google Scholar]
- Trappe, J.M. A.B. Frank and mycorrhizae: The challenge to evolutionary and ecologic theory. Mycorrhiza 2005, 15, 277–281. [Google Scholar] [CrossRef]
- Sapp, J. Saltational symbiosis. Theory Biosci. 2010, 129, 125–133. [Google Scholar] [CrossRef]
- Kutschera, U.; Hossfeld, U. Physiological phytopathology: Origin and evolution of a scientific discipline. J. Appl. Bot. Food Qual. 2012, 85, 1–5. [Google Scholar]
- Oulhen, N.; Schulz, B.J.; Carrier, T.J. English translation of Heinrich Anton de Bary’s 1878 speech, ‘Die Erscheinung der Symbiose’ (‘De la symbiose’). Symbiosis 2016, 69, 131–139. [Google Scholar] [CrossRef]
- In this issue. Nat. Rev. Microbiol. 2008, 6, 709. [CrossRef]
- Bary, A. De la symbiose. Rev. Int. Sci. 1879, 3, 301–309. [Google Scholar]
- Bary, A. Die Erscheinung der Symbiose: Vortrag; Verlag von Karl, J. Trübner: Strassburg, Germany, 1879. [Google Scholar]
- Farré-Maduell, E.; Casals-Pascual, C. The origins of gut microbiome research in Europe: From Escherich to Nissle. Hum. Microbiome J. 2019, 14, 100065. [Google Scholar] [CrossRef]
- Goodsir, J.; Wilson, G. History of a Case in Which a Fluid Periodically Ejected from the Stomach Contained Vegetable Organisms of an Undescribed Form. Edinb. Med. Surg. J. 1842, 57, 430–443. [Google Scholar] [CrossRef]
- Donaldson, K.; Henry, C. John Goodsir: Discovering Sarcina ventriculi and diagnosing Darwin’s dyspepsia. Scott. Med. J. 2020, 65, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Leidy, J. A Flora and Fauna Within Living Animals; Smithsonian Institution: Washington, DC, USA, 1853. [Google Scholar]
- Smith Beale, L. The Microscope in Its Application to Practical Medicine; Lindsay and Blakiston: Philadelphia, PA, USA, 1867. [Google Scholar]
- Hallier, E. Die Parasiten der Infectionskrankheiten. In Zeitschrift für Parasitenkunde; Hallier, E., Zürn, F.A., Eds.; Mauke’s Verlag: Jena, Germany, 1869; pp. 117–184. [Google Scholar]
- Barnes, J.K.; Woodward, J.J. The Medical and Surgical History of the War of the Rebellion. Part II. Volume 1. Medical History; Government Printing Office: Washington, DC, USA, 1879. [Google Scholar]
- Uffelman, J. Untersuchungen über das mikroskopische und chemische Verhaltender Fäces natürlicher nährter Säuglinge und über die Verdauung der einzelnen Nahrungsbestandtheile Seitens derselben. Dtsch. Arch. Klin. Med. 1881, 28, 437–475. [Google Scholar]
- Macfadyen, A.; Nencki, M.; Sieber, N. Research into the Chemical Processes in the Small Intestine of Man. J. Anat. Physiol. 1891, 25, 390–427. [Google Scholar]
- Shulman, S.T. Theodor Escherich: The First Pediatric Infectious Disease Physician? Clin. Infect. Dis. 2007, 45, 1025–1029. [Google Scholar] [CrossRef]
- Blount, Z.D. The Natural History of Model Organisms: The unexhausted potential of E. coli. eLife 2015, 4, e05826. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, N.; Silhavy, T.J. How Escherichia coli Became the Flagship Bacterium of Molecular Biology. J. Bacteriol. 2022, 204, e00230-22. [Google Scholar] [CrossRef]
- Lee, J.-H.; O’Sullivan, D.J. Genomic Insight into Bifidobacteria. Microbiol. Mol. Biol. Rev. 2010, 74, 378–416. [Google Scholar] [CrossRef]
- Orla-Jensen, S. La classification des bacteries lactiques. LAIT 1924, 4, 468–474. [Google Scholar] [CrossRef]
- Weirich, A.; Hoffmann, G.F. Ernst Moro (1874–1951)—A great pediatric career started at the rise of university-based pediatric research but was curtailed in the shadows of Nazi laws. Eur. J. Pediatr. 2005, 164, 599–606. [Google Scholar] [CrossRef]
- Moro, E. Untersuchungen über diastatisches Enzym in den Stühlen von Säuglingen und in der Muttermilch. Jahrb. Kinderheilkd. 1898, 47, 342–361. [Google Scholar]
- Moro, E. Über den Bacillus acidophilus n. spec.: Ein Beitrag zur Kenntnis der normalen Darmbakterien des Säuglings. Jahrb. Kinderheilkd. 1900, 52, 38–55. [Google Scholar]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Oberg, T.S.; McMahon, D.J.; Culumber, M.D.; McAuliffe, O.; Oberg, C.J. Invited review: Review of taxonomic changes in dairy-related lactobacilli. J. Dairy. Sci. 2022, 105, 2750–2770. [Google Scholar] [CrossRef] [PubMed]
- Veillon, A.; Zuber, A. Recherches sur quelques microbes strictement anaérobies et leur rôle en pathologie. Arch. Med. Exp. 1898, 10, 517–545. [Google Scholar]
- Prévot, A.R. Études de systématique bactérienne. I: Lois générale. II: Cocci anaérobies. Ann. Sci. Nat. Bot. 1933, 15, 23–260. [Google Scholar]
- Strasburger, J. Untersuchungen über die Bakterienmenge in menschlichen Fäces. Z. Klin. Med. 1902, 48, 413–444. [Google Scholar]
- Jacobson, W. Gram’s discovery of his staining technique. J. Infect. 1983, 7, 97–98. [Google Scholar] [CrossRef]
- Ernst, E. Colonic irrigation and the theory of autointoxication: A triumph of ignorance over science. J. Clin. Gastroenterol. 1997, 24, 196–198. [Google Scholar] [CrossRef]
- Senator, H. Über einen Fall von Hydrothionämie und über Selbstinfection durch abnorme Verdauungs vorgänge. Berl. Klin. Wochenschr. 1868, 24, 254–256. [Google Scholar]
- Bell, R. Constipation viewed as a disease ‘per se’ and as an exciting cause of disease. Lancet 1880, 115, 243–244. [Google Scholar] [CrossRef]
- Bouchard, C. Leçons Sur les Auto-Intoxications Dans les Maladies; Librairie, F. Savy: Paris, France, 1887. [Google Scholar]
- Mathias, M. Autointoxication and historical precursors of the microbiome–gut–brain axis. Microb. Ecol. Health Dis. 2018, 29, 1548249. [Google Scholar] [CrossRef]
- Lewandowska-Pietruszka, Z.; Figlerowicz, M.; Mazur-Melewska, K. The History of the Intestinal Microbiota and the Gut-Brain Axis. Pathogens 2022, 11, 1540. [Google Scholar] [CrossRef]
- Beaumont, W. Experiments and Observations on the Gastric Juice and the Physiology of Digestion; Maclachlan and Stewart: Edinburgh, UK, 1838. [Google Scholar]
- Podolsky, S.H. Cultural Divergence: Elie Metchnikoff’s Bacillus bulgaricus Therapy and His Underlying Concept of Health. Bull. Hist. Med. 1998, 72, 1–27. [Google Scholar] [CrossRef]
- Whorton, J. Civilisation and the colon: Constipation as the “disease of diseases”. BMJ 2000, 321, 1586–1589. [Google Scholar] [CrossRef]
- Lane, W.A. Remarks on The Results of the Operative Treatment of Chronic Constipation. BMJ 1908, 1, 126–130. [Google Scholar] [CrossRef][Green Version]
- Morris, M.; Price, T.; Cowan, S.W.; Yeo, C.J.; Phillips, B. William Arbuthnot Lane (1856–1943): Surgical Innovator and His Theory of Autointoxication. Am. Surg. 2017, 83, 1–2. [Google Scholar] [CrossRef]
- Cavaillon, J.-M.; Legout, S. Centenary of the death of Elie Metchnikoff: A visionary and an outstanding team leader. Microbes Infect. 2016, 18, 577–594. [Google Scholar] [CrossRef]
- Metchnikoff, E. Essais Optimistes; A. Maloine: Paris, France, 1907. [Google Scholar]
- Metchnikoff, E. Études sur la flore intestinale. Putréfaction intestinale. Ann. Inst. Pasteur 1908, 22, 929–955. [Google Scholar]
- Metchnikoff, E. Études sur la flore intestinale. Poisons intestinaux et scléroses. Ann. Inst. Pasteur 1910, 24, 755–770. [Google Scholar]
- Metchnikoff, E.; Wollman, E. Sur quelques essais de désintoxication intestinale. Ann. Inst. Pasteur 1912, 26, 825–849. [Google Scholar]
- Tsiklinsky, P.V. Sur la flore microbienne termophile du canal intestinal de l’homme. Ann. Inst. Pasteur 1903, 17, 217–240. [Google Scholar]
- Choukévitch, J. Etude de la flore bactérienne du gros intestin du cheval. Ann. Inst. Pasteur 1911, 25, 345–367. [Google Scholar]
- Metchnikoff, O. Note sur l’influence des microbes dans le développement des têtards. Ann. Inst. Pasteur 1901, 15, 631–634. [Google Scholar]
- Wollman, E. Sur l’élevage de mouches stériles. Ann. Inst. Pasteur 1911, 25, 79. [Google Scholar]
- Cohendy, M. Expériences sur la vie sans microbes. Ann. Inst. Pasteur 1912, 26, 106. [Google Scholar]
- Basic, M.; Bleich, A. Gnotobiotics: Past, present and future. Lab. Anim. 2019, 53, 232–243. [Google Scholar] [CrossRef]
- Gasbarrini, G.; Bonvicini, F.; Gramenzi, A. Probiotics History. J. Clin. Gastroenterol. 2016, 50, 116–119. [Google Scholar] [CrossRef]
- Teixeira, P. LACTOBACILLUS|Lactobacillus delbrueckii ssp. bulgaricus. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 425–431. [Google Scholar] [CrossRef]
- Herter, C.A.; Kendall, A.I. An Observation on the Fate of B. bulgaricus (In Bacillac) in the Digestive Tract of a Monkey. J. Biol. Chem. 1908, 5, 293–302. [Google Scholar] [CrossRef]
- Sutula, J.; Coulthwaite, L.; Verran, J. Culture media for differential isolation of Lactobacillus casei Shirota from oral samples. J. Microbiol. Methods 2012, 90, 65–71. [Google Scholar] [CrossRef]
- NDA (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on the substantiation of a health claim related to Lactobacillus casei strain Shirota and maintenance of the upper respiratory tract defence against pathogens by maintaining immune defences pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1860. [Google Scholar] [CrossRef]
- Vitetta, L.; Vitetta, G.; Hall, S. Immunological Tolerance and Function: Associations Between Intestinal Bacteria, Probiotics, Prebiotics, and Phages. Front. Immunol. 2018, 9, 2240. [Google Scholar] [CrossRef]
- Krawczyk, R.T.; Banaszkiewicz, A. Dr. Józef Brudziński—The true ‘Father of probiotics’. Benef. Microbes 2021, 12, 211–213. [Google Scholar] [CrossRef]
- Kendall, A.I. Some Observations on the Study of the Intestinal Bacteria. J. Biol. Chem. 1909, 6, 499–507. [Google Scholar] [CrossRef]
- Aziz, R.K. A hundred-year-old insight into the gut microbiome! Gut Pathog. 2009, 1, 21. [Google Scholar] [CrossRef]
- Kellog, J.H. Autointoxication or Intestinal Toxemia; The Modern Medicine Publishing Co.: Battle Creek, MI, USA, 1919. [Google Scholar]
- Fee, E.; Brown, T.M. John Harvey Kellogg, MD: Health Reformer and Antismoking Crusader. Am. J. Public. Health 2002, 92, 935. [Google Scholar] [CrossRef]
- Sonnenborn, U.; Schulze, J. The non-pathogenic Escherichia coli strain Nissle 1917—Features of a versatile probiotic. Microb. Ecol. Health Dis. 2009, 21, 122–158. [Google Scholar] [CrossRef]
- Vergin, F. Anti- und Probiotica. Hippokrates 1954, 25, 116–119. [Google Scholar]
- Lilly, D.M.; Stillwell, R.H. Probiotics: Growth Promoting Factors Produced by Microorganisms. Science 1965, 147, 747–748. [Google Scholar] [CrossRef]
- Parker, R.B. Probiotics, the Other Half of Antibiotic Story. Anim. Nutr. Health 1974, 29, 4–8. [Google Scholar]
- Fuller, R. Probiotics in man and animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar] [CrossRef]
- Alok, A.; Singh, I.D.; Singh, S.; Kishore, M.; Jha, P.C.; Iqubal, A. Probiotics: A New Era of Biotherapy. Adv. Biomed. Res. 2017, 6, 31. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Accettulli, A.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Campaniello, D.; Racioppo, A.; Altieri, C.; Bevilacqua, A. Psycho-Microbiology, a New Frontier for Probiotics: An Exploratory Overview. Microorganisms 2023, 10, 2141. [Google Scholar] [CrossRef]
- Martin, R.; Langella, P. Emerging Health Concepts in the Probiotics Field: Streamlining the Definitions. Front. Microbiol. 2019, 10, 1047. [Google Scholar] [CrossRef]
- Das, T.K.; Pradhan, S.; Chakrabarti, S.; Mondal, K.C.; Ghosh, K. Current status of probiotic and related health benefits. Appl. Food Res. 2022, 2, 100185. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Van Niekerk, E.; Autran, C.A.; Nel, D.G.; Kirsten, G.F.; Blaauw, R.; Bode, L. Human milk oligosaccharides differ between HIV-infected and HIV-uninfected mothers and are related to necrotizing enterocolitis incidence in their preterm very-low-birth-weight infants. J. Nutr. 2014, 144, 1227–1233. [Google Scholar] [CrossRef]
- Underwood, M.A.; Gaerlan, S.; De Leoz, M.L.A.; Dimapasoc, L.; Kalanetra, K.M.; Lemay, D.G.; German, J.B.; Mills, D.A.; Lebrilla, C.B. Human milk oligosaccharides in premature infants: Absorption, excretion, and influence on the intestinal microbiota. Pediatr. Res. 2015, 78, 670–677. [Google Scholar] [CrossRef]
- Šuligoj, T.; Vigsnæs, L.K.; Van den Abbeele, P.; Apostolou, A.; Karalis, K.; Savva, G.M.; McConnell, B.; Juge, N. Effects of Human Milk Oligosaccharides on the Adult Gut Microbiota and Barrier Function. Nutrients 2020, 12, 2808. [Google Scholar] [CrossRef]
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of Human Milk Bioactives on Infants’ Gut and Immune Health. Front. Immunol. 2021, 12, 604080. [Google Scholar] [CrossRef]
- Mills, D.A.; German, J.B.; Lebrilla, C.B.; Underwood, M.A. Translating neonatal microbiome science into commercial innovation: Metabolism of human milk oligosaccharides as a basis for probiotic efficacy in breast-fed infants. Gut Microbes 2023, 15, 2192458. [Google Scholar] [CrossRef]
- Ali, S.; Hamayun, M.; Siraj, M.; Khan, S.A.; Kim, H.-Y.; Lee, B. Recent advances in prebiotics: Classification, mechanisms, and health applications. Future Foods 2025, 12, 100680. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Gomez Quintero, D.F.; Kok, C.R.; Hutkins, R. The Future of Synbiotics: Rational Formulation and Design. Front. Microbiol. 2022, 13, 919725. [Google Scholar] [CrossRef]
- Smolinska, S.; Popescu, F.-D.; Zemelka-Wiacek, M. A Review of the Influence of Prebiotics, Probiotics, Synbiotics, and Postbiotics on the Human Gut Microbiome and Intestinal Integrity. J. Clin. Med. 2025, 14, 3673. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Hatoum, R.; Labrie, S.; Fliss, I. Antimicrobial and probiotic properties of yeasts: From fundamental to novel applications. Front. Microbiol. 2012, 3, 421. [Google Scholar] [CrossRef]
- Van Tilburg Bernardes, E.; Kuchařová Pettersen, V.; Gutierrez, M.W.; Laforest-Lapointe, I.; Jendzjowsky, N.G.; Cavin, J.-B.; Vicentini, F.A.; Keenan, C.M.; Ramay, H.R.; Samara, J.; et al. Intestinal fungi are causally implicated in microbiome assembly and immune development in mice. Nat. Commun. 2020, 11, 2577. [Google Scholar] [CrossRef]
- Chiantera, V.; Laganà, A.S.; Basciani, S.; Nordio, M.; Bizzarri, M. A Critical Perspective on the Supplementation of Akkermansia muciniphila: Benefits and Harms. Life 2023, 13, 1247. [Google Scholar] [CrossRef]
- Zeng, S.-Y.; Liu, Y.-F.; Liu, J.-H.; Zeng, Z.-L.; Xie, H.; Liu, J.-H. Potential effects of Akkermansia muciniphila in aging and aging-related diseases: Current evidence and perspectives. Aging Dis. 2023, 14, 2015–2027. [Google Scholar] [CrossRef]
- He, Q.; Niu, M.; Bi, J.; Du, N.; Liu, S.; Yang, K.; Li, H.; Yao, J.; Du, Y.; Duan, Y. Protective effects of a new generation of probiotic Bacteroides fragilis against colitis in vivo and in vitro. Sci. Rep. 2023, 13, 15842. [Google Scholar] [CrossRef]
- Maioli, T.U.; Borras-Nogues, E.; Torres, L.; Barbosa, S.C.; Martins, V.D.; Langella, P.; Azevedo, V.A.; Chatel, J.-M. Possible Benefits of Faecalibacterium prausnitzii for Obesity-Associated Gut Disorders. Front. Pharmacol. 2021, 12, 740636. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. PNAS 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.G.P. The Treatment of Melancholia by the Lactic Acid Bacillus. J. Mental Sci. 1910, 56, 422–430. [Google Scholar] [CrossRef]
- Ma, J.; Lyu, Y.; Liu, X.; Jia, X.; Cui, F.; Wu, X.; Deng, S.; Yue, C. Engineered probiotics. Microb. Cell Factor. 2022, 21, 72. [Google Scholar] [CrossRef]
- Summers, W.C. On the Origins of the Science in Arrowsmith: Paul de Kruif, Felix d’Herelle, and Phage. J. Hist. Med. Allied Sci. 1991, 46, 315–332. [Google Scholar] [CrossRef]
- Abedon, S.T.; Thomas-Abedon, C.; Thomas, A.; Mazure, H. Bacteriophage prehistory. Bacteriophage 2011, 1, 174–178. [Google Scholar] [CrossRef]
- Twort, F.W. An Investigation on the Nature of Ultra-Microscopic Viruses. Lancet 1915, 186, 1241–1243. [Google Scholar] [CrossRef]
- Fruciano, D.E.; Bourne, S. Phage as an antimicrobial agent: d’Herelle’s heretical theories and their role in the decline of phage prophylaxis in the West. Can. J. Infect. Dis. Med. Microbiol. 2017, 18, 19–26. [Google Scholar] [CrossRef]
- Summers, W.C. Félix Hubert d’Herelle (1873–1949): History of a scientific mind. Bacteriophage 2016, 6, 1270090. [Google Scholar] [CrossRef] [PubMed]
- d’Hérelle, F. Sur un microbe invisible antagoniste des bacilles dysentériques. Comptes Rendus De L’académie Des Sci. 1917, 165, 373–375. [Google Scholar]
- Phage Therapy Unit (Hirszfeld Institute of Immunology and Experimental Therapy of the Polish Academy of Sciences). Available online: https://hirszfeld.pl/en/structure/iitd-pan-medical-center/phage-therapy-unit (accessed on 1 September 2025).
- Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Łusiak-Szelachowska, M.; Górski, A. Phage Therapy in Poland—A Centennial Journey to the First Ethically Approved Treatment Facility in Europe. Front. Microbiol. 2020, 11, 1056. [Google Scholar] [CrossRef]
- Barr, J.J.; Auro, R.; Furlan, M.; Whiteson, K.L.; Erb, M.L.; Pogliano, J.; Stotland, A.; Wolkowicz, R.; Cutting, A.S.; Doran, K.S.; et al. Bacteriophage adhering to mucus provide a non–host-derived immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 10771–10776. [Google Scholar] [CrossRef] [PubMed]
- Gratia, J.-P. André Gratia: A forerunner in microbial and viral genetics. Genetics 2000, 156, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Gratia, A. Preliminary report on a Staphylococcus bacteriophage. Proc. Soc. Exp. Biol. Med. 1921, 18, 192–193. [Google Scholar] [CrossRef]
- Gratia, A. Studies on the d’Hérelle phenomenon. J. Exp. Med. 1921, 34, 115–126. [Google Scholar] [CrossRef][Green Version]
- Gratia, A. Sur un remarquable exemple d’antagonisme entre deux souches de colibacille. Comptes Rendus Biol. 1925, 93, 1040–1042. [Google Scholar][Green Version]
- Gratia, A.; Dath, S. Propriétés bacteriolytiques de certaines moisissures. Comptes Rendus Biol. 1925, 91, 1442–1443. [Google Scholar][Green Version]
- Foster, W.; Raoult, A. Early descriptions of antibiosis. J. R. Coll. Gen. Pract. 1974, 24, 889–894. [Google Scholar] [PubMed][Green Version]
- Tyndall, J. The optical deportment of the atmosphere in relation to the phenomena of putrefaction and infection. Philos. Trans. R. Soc. 1876, 166, 27–74. [Google Scholar] [CrossRef]
- Duckett, S. Ernest Duchesne and the concept of fungal antibiotic therapy. Lancet 1999, 354, 2068–2071. [Google Scholar] [CrossRef]
- Fleming, A. On the antibacterial action of cultures of a Penicillium with special reference to their use in the isolation of B. influenza. Br. J. Exp. Pathol. 1929, 10, 226–236. [Google Scholar] [CrossRef]
- Gaynes, R. The Discovery of Penicillin—New Insights After More Than 75 Years of Clinical Use. Emerg. Infect. Dis. 2017, 23, 849–853. [Google Scholar] [CrossRef]
- Chain, E.; Florey, H.W.; Gardner, A.G.; Heatley, N.G.; Jennings, M.A.; Orr-Ewing, J.; Sanders, A.G. Penicillin as a chemotherapeutic agent. Lancet 1940, 236, 226–228. [Google Scholar] [CrossRef]
- Woodruff, H.B.; Selman, A. Waksman, Winner of the 1952 Nobel Prize for Physiology or Medicine. Appl. Environ. Microbiol. 2014, 80, 2–8. [Google Scholar] [CrossRef]
- Clardy, J.; Fischbach, M.; Currie, C. The natural history of antibiotics. Curr. Biol. 2009, 19, R437–R441. [Google Scholar] [CrossRef]
- Sánchez, S.; Demain, A.L. (Eds.) Antibiotics: Current Innovations and Future Trends; Caister Academic Press: Poole, UK, 2015. [Google Scholar] [CrossRef]
- Honigsbaum, M. Antibiotic antagonist: The curious career of René Dubos. Lancet 2015, 387, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, N. René Dubos, the Autochthonous Flora, and the Discovery of the Microbiome. J. Hist. Biol. 2022, 55, 537–558. [Google Scholar] [CrossRef] [PubMed]
- Lennox Thorburn, A. Paul Ehrlich: Pioneer of chemotherapy and cure by arsenic (1854–1915). Br. J. Vener. Dis. 1983, 59, 404–405. [Google Scholar] [CrossRef]
- Tampa, M.; Sarbu, I.; Matei, C.; Benea, V.; Georgescu, S.R. Brief History of Syphilis. J. Med. Life 2014, 7, 4–10. [Google Scholar] [PubMed]
- Gelpi, A.; Gilbertson, A.; Tucker, J.D. Magic bullet: Paul Ehrlich, Salvarsan and the birth of venereology. Sex. Transm. Infect. 2015, 91, 68–69. [Google Scholar] [CrossRef]
- Van Miert, A.S. The sulfonamide-diaminopyrimidine story. J. Vet. Pharmacol. Ther. 1994, 17, 309–316. [Google Scholar] [CrossRef]
- Watkinson-Deane, A. From dyes to D-Day: The first antibacterial drugs. Bull. R. Coll. Surg. Engl. 2024, 106, 42. [Google Scholar] [CrossRef]
- Vitorino, L.C.; Bessa, L.A. Technological Microbiology: Development and Applications. Front. Microbiol. 2017, 8, 827. [Google Scholar] [CrossRef]
- Rosebury, T. Microorganisms Indigenous to Man; McGraw-Hill Book Company: New York, NY, USA, 1962. [Google Scholar]
- Fine, D.H. Dr. Theodor Rosebury: Grandfather of modern oral microbiology. J. Dent. Res. 2006, 85, 990–995. [Google Scholar] [CrossRef]
- Yong, E. I Contain Multitudes: The Microbes Within Us and a Grander View of Life; HarperCollins—Ecco: New York, NY, USA, 2016. [Google Scholar]
- Milestones in Human Microbiota Research. Nature 2019. Available online: https://www.nature.com/immersive/d42859-019-00041-z/index.html (accessed on 1 September 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostka, A. From Pathogens to Partners: The Beginnings of Gut Microbiota Research. Appl. Sci. 2025, 15, 11376. https://doi.org/10.3390/app152111376
Kostka A. From Pathogens to Partners: The Beginnings of Gut Microbiota Research. Applied Sciences. 2025; 15(21):11376. https://doi.org/10.3390/app152111376
Chicago/Turabian StyleKostka, Anna. 2025. "From Pathogens to Partners: The Beginnings of Gut Microbiota Research" Applied Sciences 15, no. 21: 11376. https://doi.org/10.3390/app152111376
APA StyleKostka, A. (2025). From Pathogens to Partners: The Beginnings of Gut Microbiota Research. Applied Sciences, 15(21), 11376. https://doi.org/10.3390/app152111376





