Abstract
Background: Recent research has focused on diet as a potential source of antioxidants in the context of both human health and disease prevention. Among the many plant-derived antioxidants, sulforaphane (SFN) has emerged as a potent phytochemical in the recent literature for sustaining health and combating cancerous, inflammatory, and neurodegenerative diseases. Thus, the market for supplements and functional foods has been quick to adapt to this new market niche. We aimed to investigate the phytochemical profile of broccoli sprouts and evaluate their antioxidant capacity through biochemical assays. Methods: UHPLC and MS/MS were used to analyse the phytochemical characteristics of broccoli sprout extracts. Antioxidant tests, including the DPPH test, ferrous iron chelation, hydroxyl radical neutralisation, and lipoxygenase inhibition, were used to evaluate their antioxidant potential. Results: The broccoli sprout extracts emerged as an adequate source of SFN, as well as other biologically active compounds such as xanthorhamnin. Moreover, biochemical assays showcased their antioxidant capacity. Conclusions: Broccoli sprouts could constitute an important source of dietary antioxidants with high bioavailability and high accessibility, helping sustain health and even combat various diseases.
1. Introduction
In recent years, sulforaphane (SFN) has gained popularity as a powerful nutraceutical with increased antioxidant potential. Numerous studies have illustrated the beneficial effects of SFN in various diseases, varying from neurodegenerative and inflammatory diseases to oncological pathologies, either as a single treatment or as an adjunct to established drug therapy [1].
SFN, 4-methylsulfinylbutyl isothiocyanate (C6H11NOS2), was first obtained by Schmid and Karrer in 1948 [2]. SFN is a chemical that can be obtained naturally from Brassica vegetable species, classified as cruciferous vegetables, of which the most important source is broccoli. Obtaining SFN from these species depends on the concomitant presence of the molecule glucoraphanin (GRP), the glucosinolate that acts as a precursor, and the enzyme that hydrolyses it, myrosinase [3]. As a result of this hydrolysis reaction, the biologically inert GRP is transformed into biologically active isothiocyanates, such as SFN, and/or nitrile derivatives, which are considered biologically inert [4]. Its relatively low molecular weight and lipophilicity offer SFN unique advantages, compared to other polyphenols with large hydrophilic structures [5].
In intact plant tissues, glucosinolates and myrosinase are stored separately [6]. Only when these plant tissues are damaged do they come into contact, and hydrolysis can take place, leading to the formation of biologically active SFN [7]. This glucosinolate-myrosinase system is considered an inducible defence system and is also referred to as the “mustard-oil bomb” [8]. In the cruciferous vegetables present in the human diet, SFN is thus stored in the form of its inert precursor GRP, which is then transformed into SFN after the degradation of plant tissues through digestion [9]. Therefore, SFN is a biologically active compound that is essentially absent in intact plants [4].
In a study published by Fahey and colleagues that evaluated the glucoraphanin content of foods, the authors found that the glucoraphanin content of broccoli sprout extract was 16.6 μmol per gram of fresh weight. In contrast, mature broccoli extract contained 1.08 μmol per gram of fresh weight [10]. Therefore, opting for small quantities of broccoli sprouts may prove to be a more effective dietary intervention than consuming large quantities of mature cruciferous vegetables.
Regarding human health, numerous clinical benefits of SFN have been observed. By means of inducing glutathione synthesis, SFN can help combat oxidative stress [11] and protect against toxicity and disease [12]. SFN is also considered to be the most powerful inducer of phase II enzymes involved in xenobiotic detoxification; it helps mitigate the effects of respiratory pollutants on epithelial cells and may have chemopreventive effects [13,14]. It also helps mitigate the effects of aflatoxins on hepatic cells [15] and enhances immune function [11]. SFN can also help address different gastric diseases through its bactericidal action against Helicobacter pylori and by blocking gastric tumour formation [16]. SFN has also proven useful in the context of neurodegenerative diseases and can help ameliorate symptoms in diseases like Alzheimer’s, Parkinson’s and epilepsy [17].
SFN also exhibits a hormetic effect, meaning that at low doses, it can have beneficial stimulatory effects, but at high doses, it can become inhibitory. A review by Calabrese and colleagues illustrated the hormetic effect of SFN, showing that SFN induces dose-dependent hormetic responses through the amplification of the Nrf2/ARE (Nuclear factor E2-related factor 2/Antioxidant Response Element) pathway. This biphasic response is well integrated, concentration-dependent, and specific to the targeted cell types. The hormetic response of SFN may reduce the incidence and severity of various human pathologies, and it has also been found to be involved in enhancing stem cell proliferation [18].
As a result, given the emerging therapeutic potential of SFN in various diseases and its potential benefits in sustaining health, the dietary supplement market has been quick to capitalise on this opportunity.
Considering the extensive data on the beneficial effects of consumption of broccoli and other dietary sources of SFN, we aimed to assess whether sprouts from commercially available broccoli seeds offer an optimal spectrum of plant metabolites with favourable antioxidant potential.
2. Materials and Methods
2.1. Broccoli Sprouts
Two 150 g bags of Raab broccoli (Brassica rapa cymosa) seeds from organic farming in Italy were purchased from the same market chain. According to the instructions on the broccoli seed packaging, we chose two clean crystallizers of different sizes, and we allowed the seeds to hydrate overnight. The ratio we used was one tablespoon of seeds (approximately 22 g) with 200 mL of water at room temperature. The next day, the seeds were rinsed, and then water was added to each crystallizer to cover the seeds. A perforated sheet of filter paper was placed on top of each crystallizer to maintain proper humidity. This procedure was repeated daily for 5 days.
The seeds were allowed to germinate for 7 days, at which point they were considered to have reached their optimum point of development. The obtained sprouts were divided into three equal parts, weighed and then placed in three Erlenmeyer flasks for maceration and extraction of the active principles.
2.2. Extracts
In order to better assess the concentration of bioflavonoids, total polyphenols and SFN, we aimed for three different types of extract. The use of different solvents would ensure the selective extraction of both aglycones (lipophilic structures) and glycosides (hydrophilic structures).
Therefore, the solvents we opted for consisted of:
- methanol—commonly used for analysing the chemical profile of medicinal plants;
- a methanol-acetone mixture (1:1) that would increase the solubility of the more lipophilic components;
- an acidic solution obtained by diluting acetic acid until we reached pH = 3.
Approximately 8 g of the obtained sprouts were weighed and transferred into flasks, and 30 mL of solvent was added to each flask. The flasks were covered with parafilm to prevent solvent evaporation. Extraction was carried out by continuous shaking at room temperature using a magnetic stirrer.
After 72 h, the extracts were brought to the mark and then filtered, and three samples were obtained:
- Sample 1. Green-coloured methanolic extract (showing chlorophyll extraction);
- Sample 2. Extract in acid solution (glacial acetic acid diluted in water to pH = 3)—almost colourless with an odour reminiscent of pickled cabbage;
- Sample 3. Extract in acetone: methanol mixture (1:1)—greenish-yellow colour.
Extracts thus prepared were used for qualitative (identification reactions) and quantitative (spectrophotometric assay) chemical analysis, as well as biochemical assays.
2.3. Bioactive Components Identification
Based on data existing in the literature and the expected characteristics of the secondary metabolites extracted in alcoholic or aqueous solutions, we opted for the following analyses:
2.3.1. Ultrahigh-Performance Liquid Chromatography-Photodiode Array Detection (UPLC-PDA)
An Ultimate 3000 Thermo Fisher system, coupled with a photodiode array (PDA) detector, was used for mass spectrometry (MS) analysis. The mobile phase included a gradient of A (acetonitrile with 0.1% phosphoric acid) and B (0.1% phosphoric acid) as follows: 0–4 min, 10–15%; the next 4 min, isocratic at 15%; 8–15 min, 30%; 15–18 min, 40%; 18–22 min, 55%; then for the next 3 min, the system returned to the initial conditions. The column used was Luna Omega 5 µm Polar C18 (100 A, 150 × 4.6 mm, Part No. 00F-4754-E0), manufactured by Phenomenex (Torrance, CA, USA). The sample injection was 2 µL with a flow rate of 0.8 mL. Detection was performed at seven different band wavelengths, ranging from 220 nm to 800 nm, using the four detection bands: 245 nm, 280 nm, 330 nm, and 521 nm. These wavelengths correspond to the maximum absorption bands of flavonoids, phenolic acids, and anthocyanidins. Every peak was identified based on UV spectra and the available library data, and the identifications were compared to the standards (caffeic acid, sinapic acid, gallic acid, rutoside, apigenin, luteolin, catechin, all of pure quality and acquired from Sigma-Aldrich, Steinheim, Germany).
2.3.2. MS Liquid Chromatography (UPLC-ESI/MS)
A system comprising a Vanquish UHPLC system, manufactured by Thermo Scientific (Germering, Germany), and coupled with an Orbitrap Exploris 480 mass spectrometer, was used further to assess the chemical composition of the investigated extract. The full scan evaluation was carried out on a Hypersil Gold C18 column (50 × 2.1 mm, 1.9 µm), manufactured by Thermo Scientific (Vilnius, Lithuania), thermostated at 40 °C. The separation of the active compounds was achieved with a gradient mixture of A (acetonitrile with 0.1% phosphoric acid) and B (0.1% phosphoric acid) over 30 min, from 7% to 93% A in B. The flow rate was 0.3 mL. Positive and negative electrospray ionisation (ESI) were employed for mass spectrometric detection. Acquired spectra were recorded within a mass range of m/z 100 to 1500 at a frequency of 5 Hz, using a positive voltage of 4.5 kV, a negative voltage of 3.0 kV, and an ionisation temperature of 350 °C. Aliquots of 5 µL from each sample were injected automatically. Integration and detection were performed using Compound Discoverer 3.3 software for evaluation in both targeted and untargeted modes. The peaks with a rating of at least 7.4 were considered and verified against the Thermo m/z Vault, NIST and ChemSpider databases. All the chromatogram figures included are direct exports from the instrument.
2.3.3. Qualitative Tests
The first qualitative test we performed was the identification of flavonoids and flavonoid glycosides. In this case, 1 mL of extract was treated with 2.5% aluminium chloride in a 10% sodium acetate medium and observed to see if the yellow/yellow-green fluorescence colour was highlighted in the test tubes.
Secondly, we identified the free hydroxyl groups on the flavonoid nucleus. As in the first reaction, a small amount of the extract was treated with 10% sodium hydroxide; the appearance of an intense yellow (to orange) colour indicates the presence of hydroxyl groups in the flavonoid structure (bathochromic—colour-changing effect).
We also conducted the identification of flavonoid aglycones (R. Shibata). In this case, 3 mL of each extract was evaporated in a capsule over a water bath. The residue obtained was mixed with 2 mL of methanol (at 50 °C), and we observed whether a yellow colouration of the solution occurred. Afterwards, a few fragments of magnesium filings and 4–5 drops of concentrated hydrochloric acid were added. If a brick-red colour (cyanidol formation) appeared, the reaction was considered positive, and it indicates the presence of flavonoid aglycones.
Lastly, we used the Folin–Ciocalteu reagent to determine the total polyphenols. This reaction was carried out by mixing 1 mL of extract, 0.25 mL of Folin–Ciocalteu reagent, and an equal amount of sodium carbonate in a test tube, followed by vigorous shaking. The change in colour of the solution from yellowish-green to blue confirms the presence of polyphenols.
2.4. Quantitative Analysis
2.4.1. Flavonoids Determination
In the presence of the aluminium cation (from aluminium chloride), flavonoid derivatives form intense yellow complexes, for which the absorbance is determined at λ = 430 nm and expressed in rutoside concentrations.
For this determination, 5 mL of the extract was diluted with methanol to a total volume of 25 mL and then filtered. Afterwards, over 5 mL of the filtrate, 5 mL of sodium acetate, and 3 mL of aluminium chloride were added, and the total volume was brought to 25 mL with methanol. After 15 min, the absorbances were determined using a spectrophotometer (UV-VIS V-550 Jasco) at a wavelength of λ = 430 nm. Since the initial solution was stained, a solution obtained under the same conditions, i.e., 5 mL filtrate, 8 mL distilled water and methanol at 25 mL, was used as a control.
The flavonoid concentration of the analysed broccoli extracts was calculated using a calibration curve, established in parallel and under the same conditions as the sample solutions, using 0.5–5 mL of 0.1 g/L rutoside standard solution in methanol, 5 mL of 10% sodium acetate, 3 mL of 2.5 g/L aluminium chloride and methanol in 25 mL in each volumetric flask.
After stirring for 15 min, absorbances were determined at λ = 430 nm on a Jasco V-550 UV-VIS V-550 UV-VIS spectrophotometer using two 1 mL quartz cuvettes against the corresponding control solutions.
2.4.2. Total Polyphenols Determination
In an alkaline medium (sodium carbonate), the polyphenols present in broccoli samples can react with the Folin–Ciocalteu reagent, leading to the formation of intense blue-coloured complexes that can be quantified spectrophotometrically at 765 nm.
Each broccoli sample was diluted with distilled water, depending on the initial colour intensity of the extract analysed. Over 0.04 mL of sample and 3.16 mL of distilled water, a total volume of 0.2 mL of Folin–Ciocalteu reagent was added. After homogenization, a 5 min waiting time was allowed. Afterwards, a volume of 0.6 mL of 20% sodium carbonate was added (for the colour control, the carbonate was replaced by 0.6 mL of distilled water). After homogenization, the samples were left in the dark for 2 h to allow for colour development. Absorbances were read at a wavelength of 765 nm against a colour control prepared individually for each extract.
The calculation of the total polyphenol concentration of the broccoli extracts was carried out using a gallic acid calibration curve that was performed in parallel and under the same conditions as the broccoli samples.
2.4.3. SFN Determination
SFN and its glycosidic derivatives show a specific absorption curve at 325 nm, which is also confirmed by the infrared spectra, which correspond to specific groups in the range 1990–2500 cm−1. Absolute ethanol and methanol were used for the dilutions, and no other reagents were necessary. The scale was calibrated based on an SFN-containing supplement with a known SFN concentration.
2.5. Biochemical Assays
2.5.1. Antioxidant Activity as Determined by DPPH
DPPH, 2,2-diphenyl-1-picrylhydrazyl, is a stable synthetic radical; as a result of the non-participating electron pair of its two nitrogen atoms, the solution is dark violet in colour. In reaction with a free radical scavenger or an extract with antioxidant action (due to compounds such as sulforaphane that are present in the analysed samples), the DPPH solution changes its colour from purple to yellow by the formation of diphenyl picryl hydrazine (DPPH-H) [19]. The colour variations are read with a spectrophotometer at 512 nm. A decrease in absorbance intensity indicates the antioxidant effect of the extracts investigated.
Initially, stock solutions in methanol at a concentration of 40 mg/mL were obtained from each of the 3 samples of broccoli sprout extracts, from which subsequent dilutions in methanol were made. To 1 mL of each dilution, 1 mL of 0.004% DPPH methanolic solution was added. The mixture was thoroughly homogenised, and the absorbance of the sample (Asample) was determined at λ = 517 nm. For proper calculation, the absorbance of the DPPH solution (Acontrol) was initially read to be between 0.95 and 1.15.
DPPH radical scavenger activity (%) was calculated using the formula:
Activity% = 100 × (Acontrol − Asample)/Acontrol
2.5.2. Ferrous Iron Chelation Test
Fe2+ in the presence of ferrozine forms a pink complex with maximum absorbance at 562 nm. The presence of a chelating agent in the reaction medium results in a reduction in the absorbance of the formed complex [20].
In this procedure, 0.74 mL of 0.1 M acetate buffer solution (pH 5.25) and 0.02 mL of 2 mM ferrous sulphate solution in 0.2 M hydrochloric acid were added to 0.2 mL of the sample solution. After shaking for 10–15 s, 0.04 mL of 5 mM ferrozine solution was added. After 10 min of rest in the dark, the absorbance of the solution was determined at 562 nm against a control prepared under the same conditions as the sample (the ferrous sulphate solution was replaced with double-distilled water). In parallel, the control solution and its respective control were prepared. The control contained 0.2 mL of double-distilled water, 0.74 mL of 0.1 M acetate buffer solution (pH 5.25), and 0.02 mL of 2 mM ferrous sulphate solution in 0.2 M hydrochloric acid. After shaking for 10–15 s, 0.04 mL of 5 mM ferrozine solution was added.
All determinations were performed in triplicate, with the results expressed as the average of three determinations ± standard deviation.
The ferrous ion chelation capacity was calculated according to the formula:
where
Chelation% = 100 × (Acontrol − Asample)/Acontrol
Acontrol—absorbance of the control solution,
Asample—absorbance of the sample solution.
2.5.3. Hydroxyl Radical Neutralisation Capacity
The hydroxyl radical is synthesised under the action of ferrous ions or copper ions through the Fenton and Haber-Weiss reactions. Its increased reactivity is favoured by its small size and ability to cross biological membranes. Its synthesis occurs not only in cells, but also in the extracellular environment. The hydroxyl radical has increased reactivity and can initiate chain oxidation reactions that affect the structure of lipids, proteins, and nucleic acids [21,22].
The hydroxyl radical, formed in the reaction between the ferrous ion and hydrogen peroxide, will hydroxylate salicylic acid to form a pink-purple compound with maximum absorbance at 562 nm.
Compounds with hydroxyl radical neutralising effects cause a reduction in the colour intensity of the solution, with a reduction in absorbance [23].
To 0.225 mL of the sample solution, 0.750 mL of 1.5 mM iron (II) sulphate solution, 0.9 mL of 20 mM sodium salicylate solution, and 0.525 mL of 6 mM hydrogen peroxide solution were added. The mixture was kept at 37 °C for 30 min. After cooling to room temperature, the absorbance of the sample and that of the control were read at 562 nm against their respective blanks (controls) in which the ferrous sulphate solution was replaced with double-distilled water. The positive control was processed under the same conditions as the samples, using double-distilled water instead of the sample solution.
All determinations were performed in triplicate, with the results expressed as the average of three determinations ± standard deviation.
The hydroxyl radical neutralisation capacity was calculated according to the formula:
where
Activity% = 100 × (Acontrol − Asample)/Acontrol
Acontrol—absorbance of the control solution,
Asample—absorbance of the sample solution.
2.5.4. Determination of Lipoxygenase Inhibition Capacity
Lipoxygenases are oxidoreductases that catalyse the synthesis of lipid peroxides from unsaturated fatty acids. The most important are 5-, 12-, and 15-lipoxygenase. The synthesised peroxides are involved in inflammatory and allergic reactions and cause the degradation of membrane lipids and protein macromolecules [24].
Flavanones and silver nanoparticles block 15-lipoxygenase by inhibiting linoleic acid oxidation and reducing absorbance at 234 nm [25].
During this test, 0.05 mL of 15-lipoxygenase solution in borate buffer (pH 9) was treated with 0.05 mL of sample solution, and the mixture was incubated for 10 min at room temperature. Afterwards, 2 mL of 0.16 mM linoleic acid solution in 0.1 M borate buffer (pH 9) was added. The absorbance of the solution was measured at 234 nm over a range of 0–120 s. In parallel, the positive control was made, in which the sample solution was replaced with double-distilled water.
All determinations were performed in triplicate; the results were expressed as the average of three determinations ± standard deviation.
The lipoxygenase inhibition capacity was calculated according to the formula:
where
Activity% = (AEFI − AECI) · 100/AEFI
AEFI—represents the difference between the absorbance of the enzyme solution without inhibitor at 90 s and the absorbance of the same solution at 30 s.
AECI—represents the difference between the absorbance of the enzyme solution treated with the sample at 90 s and the absorbance of the same solution at 30 s.
2.5.5. Reagents
The reagents used for iron chelation tests were: 0.1 M acetate buffer pH 5.25 (Fluka, Sigma-Aldrich, Steinheim, Germany), iron (II) sulphate heptahydrate (Merck, KgaA, Darmstadt, Germany), 2 mM iron (II) sulphate solution in 0.2 M HCl, ferrozine (sodium salt of 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4′′ disulfonic acid) (Fluka, Sigma-Aldrich, Steinheim, Germany), 5 mM ferrozine solution: ferrozine is placed in a 25 mL volumetric flask, approx. 5 mL of purified water is added, and after dissolution, water is added up to the mark, hydrochloric acid (Merck).
The reagents used for hydroxyl radical neutralisation tests were: dimethyl sulfoxide (DMSO) (Merck, KgaA, Darmstadt, Germany), iron (II) sulphate heptahydrate (Merck, KgaA, Darmstadt, Germany), 1.5 mM iron (II) sulphate solution in double-distilled water, 6 mM hydrogen peroxide solution in double-distilled water (Sigma-Aldrich, Steinheim, Germany), 20 mM sodium salicylate in double-distilled water, ABL & E Jasco V-550 UV-VIS spectrophotometer (Tokyo, Japan).
Reagents used for lipoxygenase inhibition tests consisted of: 0.1 M borate buffer, pH 9, linoleic acid (Sigma-Aldrich, Steinheim, Germany), 0.16 mM in 0.1 M borate buffer pH 9, soybean 15-lipoxygenase (Sigma-Aldrich, Steinheim, Germany) in 0.1 M borate buffer, pH 9, UV-VIS spectrophotometer ABL & E Jasco V-550 (Tokyo, Japan).
3. Results
3.1. Qualitative and Quantitative Analysis
3.1.1. Qualitative Analysis
Qualitative analysis of the extracts obtained from broccoli sprouts revealed compounds from the class of aglycones and glycosylated flavonoids and polyphenols, as shown in Table 1.

Table 1.
Qualitative determination of secondary metabolites in the analysed broccoli sprout extracts.
3.1.2. Chromatography Results
The chromatographic analysis confirmed that all samples contained various amounts of the expected compounds, and the MS2 tentative identification was assessed by comparing the obtained spectra to those of the standard and well-recognised databases.
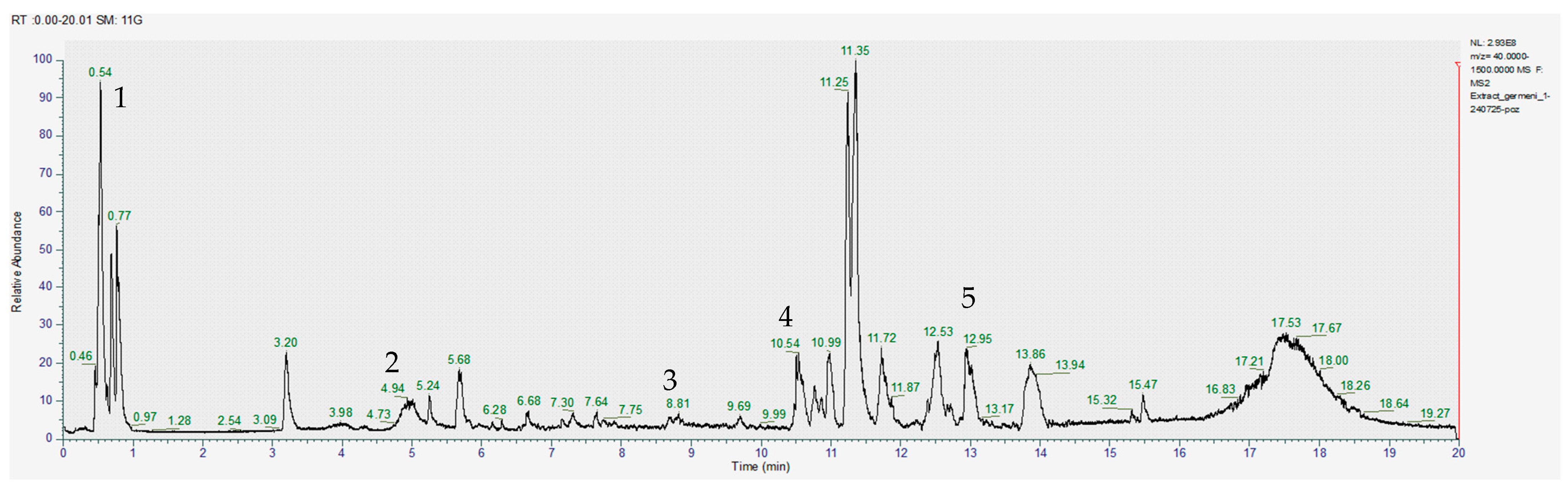
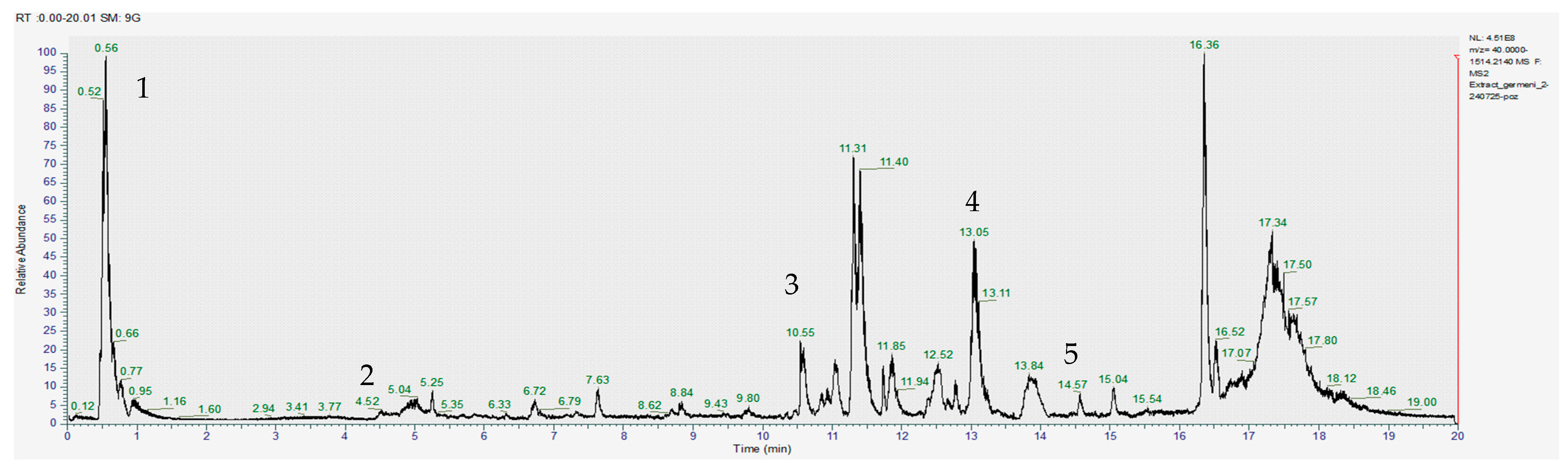
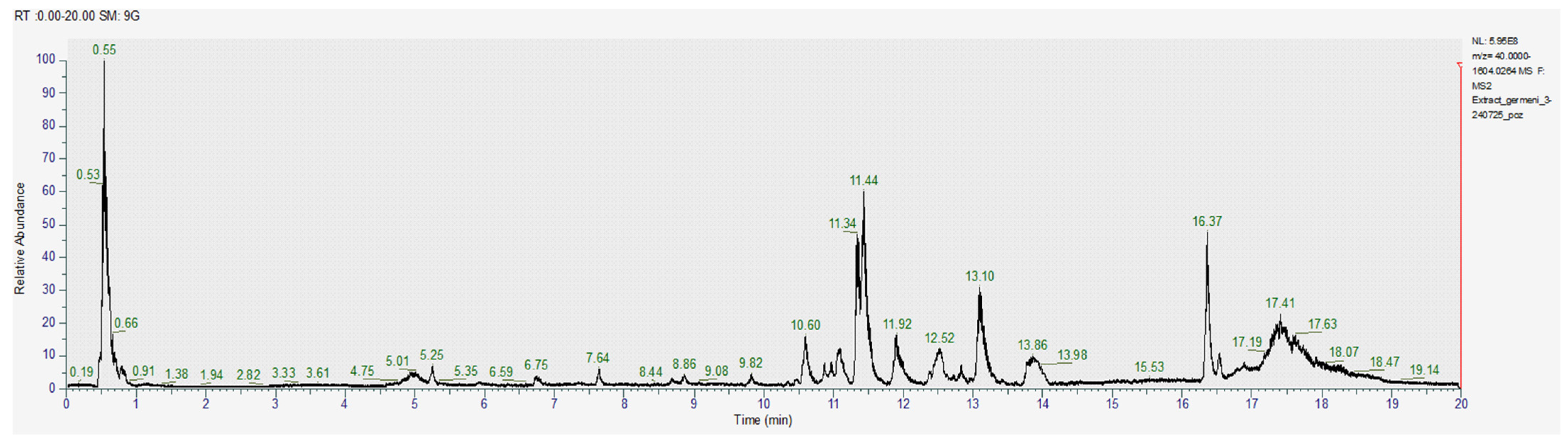
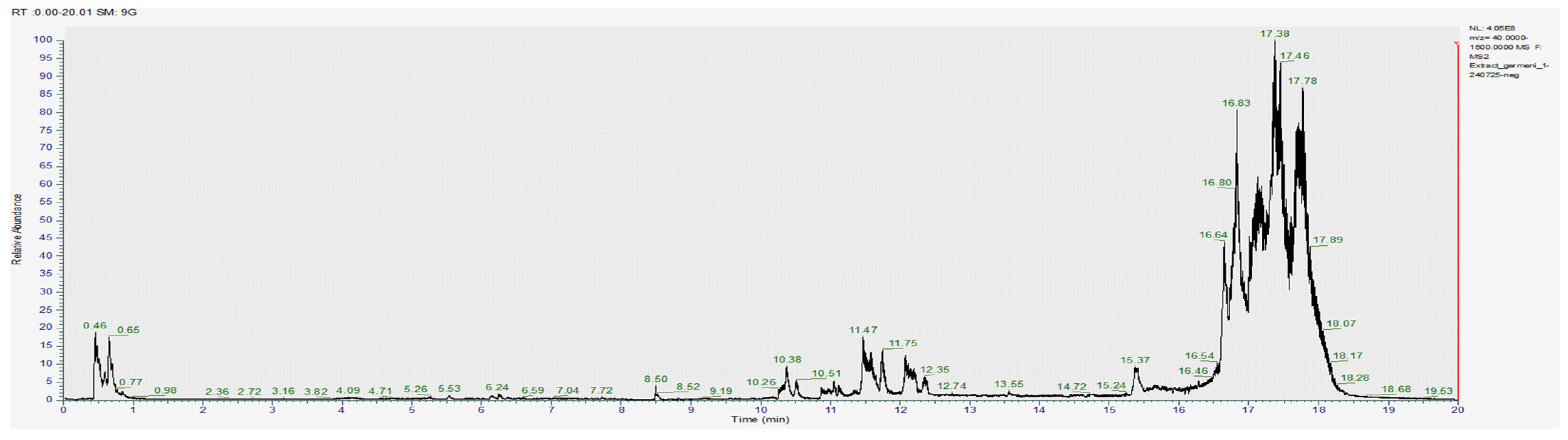
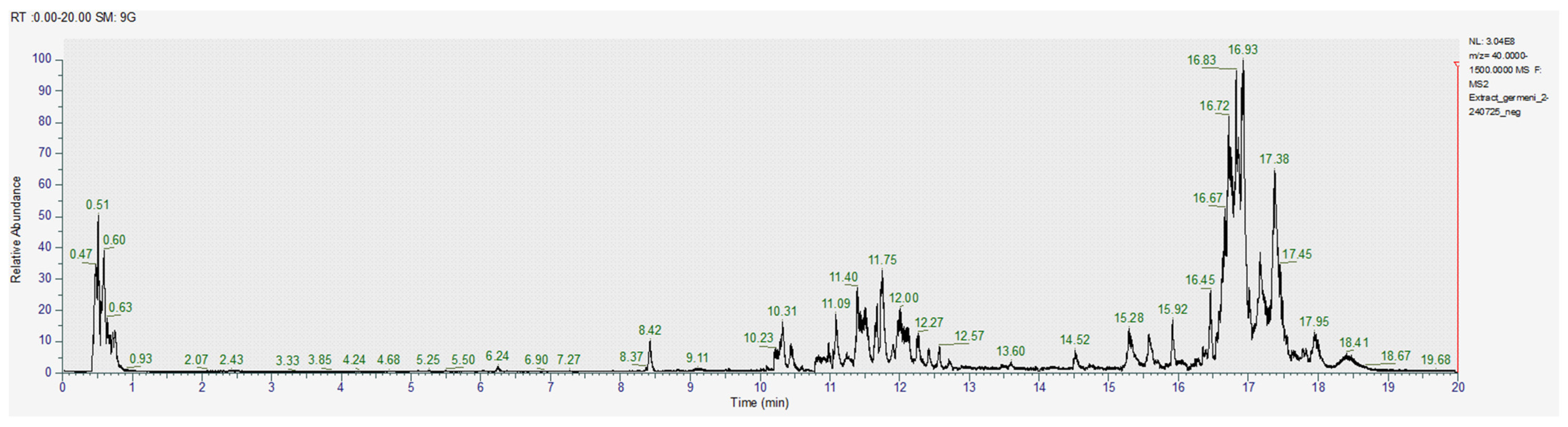
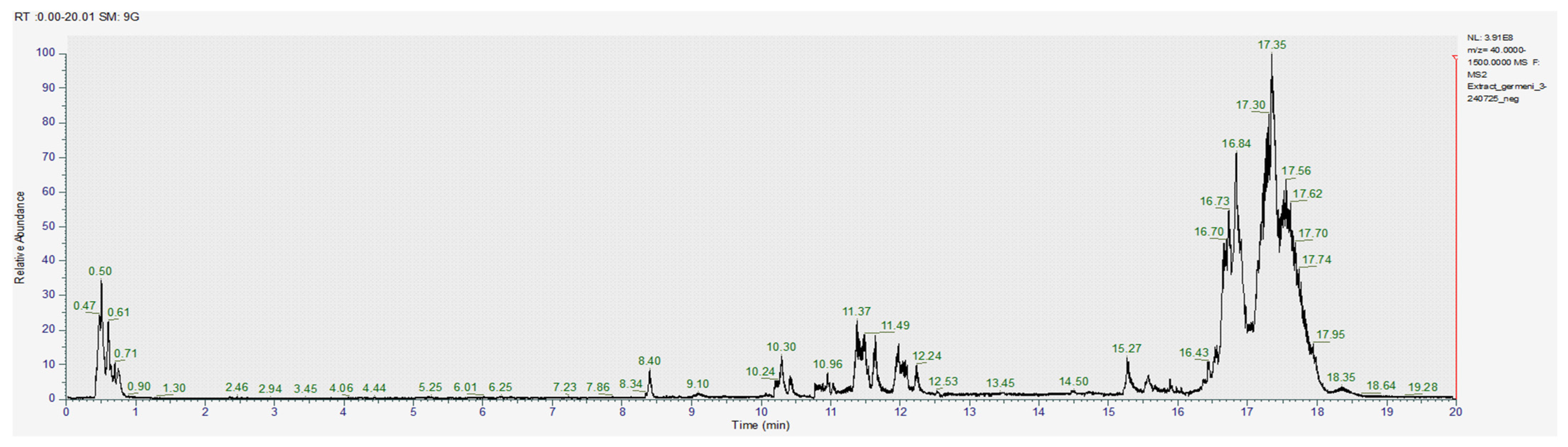
The obtained images of the chromatograms of full MS in both positive and negative modules are shown below. The main compounds identified were sulforaphane, glucoraphanin, sinapic acid, caffeic acid, lycopene, lutein and zeaxanthin (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6, Table 2).
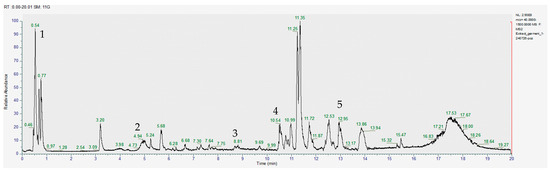
Figure 1.
Chromatogram for the methanol extract (positive), where 1–gallic acid, 2–caffeic acid, 3–quercetin, 4–apigenin, 5–glucoraphanin.
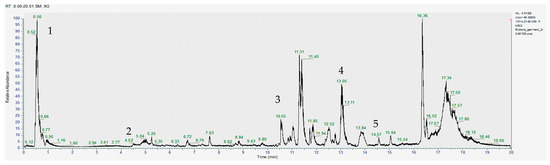
Figure 2.
Chromatogram for the acetic acid extract (positive) where 1–gallic acid, 2–sinapic acid, 3–apigenin, 4–glucoraphanin, 5–sulforaphane.
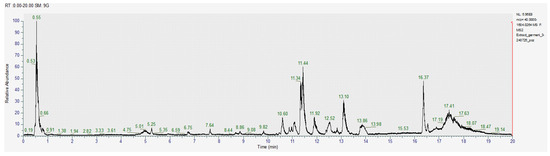
Figure 3.
Chromatogram for the acetone-methanol extract (positive).
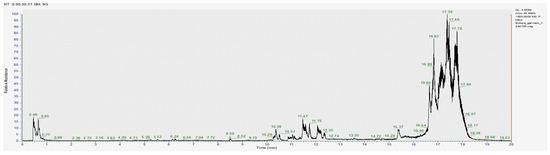
Figure 4.
Chromatogram for the methanol extract (negative).
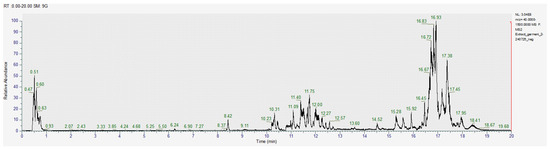
Figure 5.
Chromatogram for the acetic acid extract (negative).
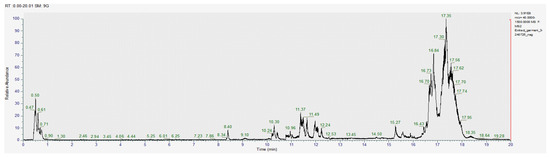
Figure 6.
Chromatogram for the acetone-methanol extract (negative).

Table 2.
Identified compounds in the broccoli sprouts extracts.
With the help of MS2, we were able to identify sulforaphane, glucoraphanin, lycopene and zeaxanthin in all investigated extracts; however, the amounts varied significantly between the acetic acid extract and the other two samples (Table 2).
3.2. Biochemical Assays Results
3.2.1. Antioxidant Activity
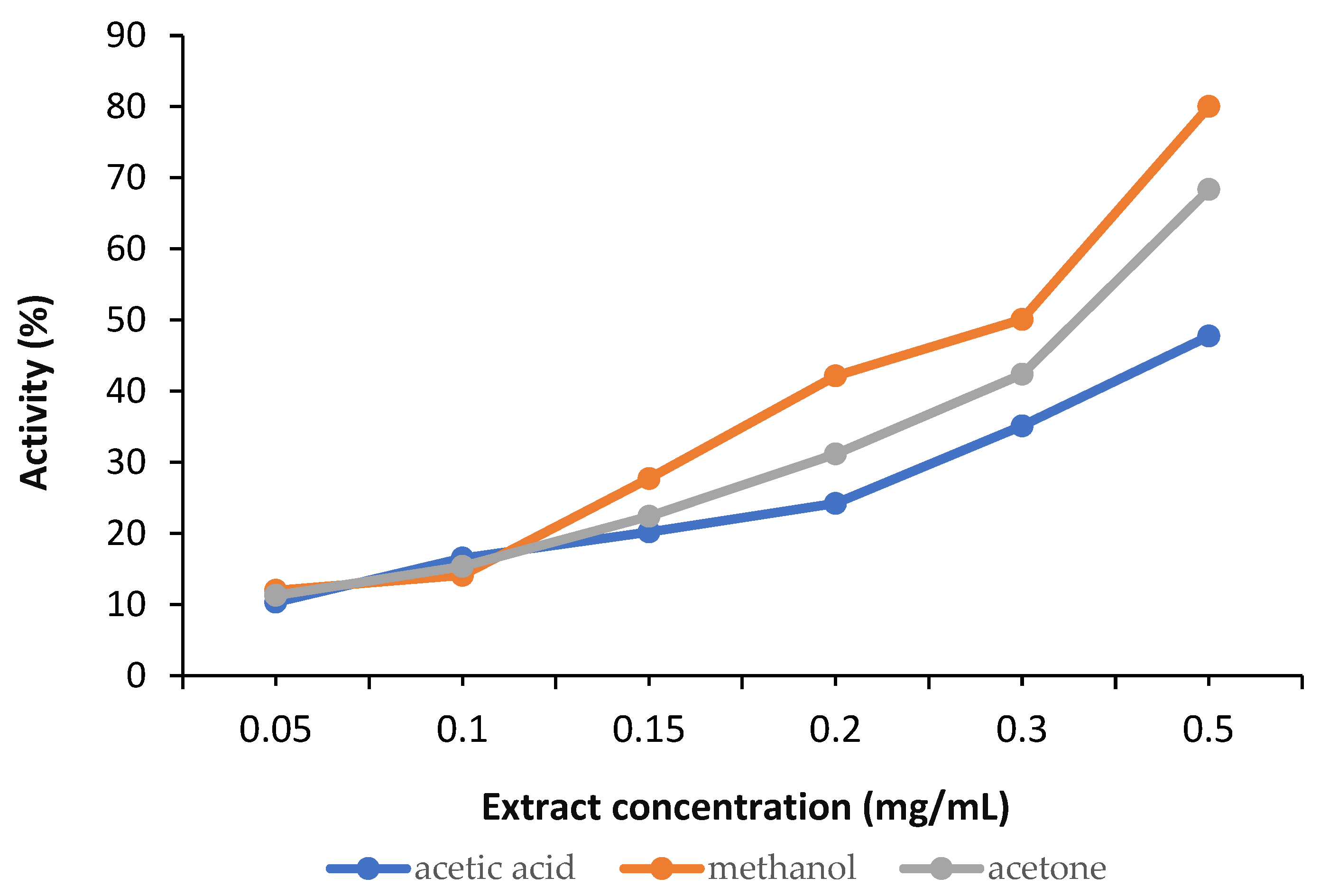
DPPH is a synthetic radical commonly used for a general screening of the antioxidant potential of plant extracts. In our research, the initial testing against DPPH radical indicated that at the lowest concentrations, there is no significant difference in the intensity of the activity of the extracts.
The calculations revealed a variation in the antioxidant activity of the samples, as shown in Figure 7.
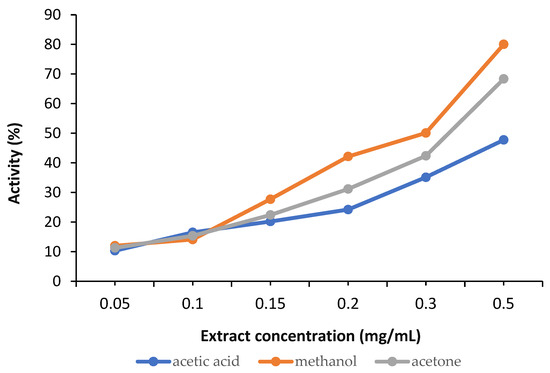
Figure 7.
Antioxidant activity of the analysed samples.
3.2.2. Ferrous Iron Chelation Test Results
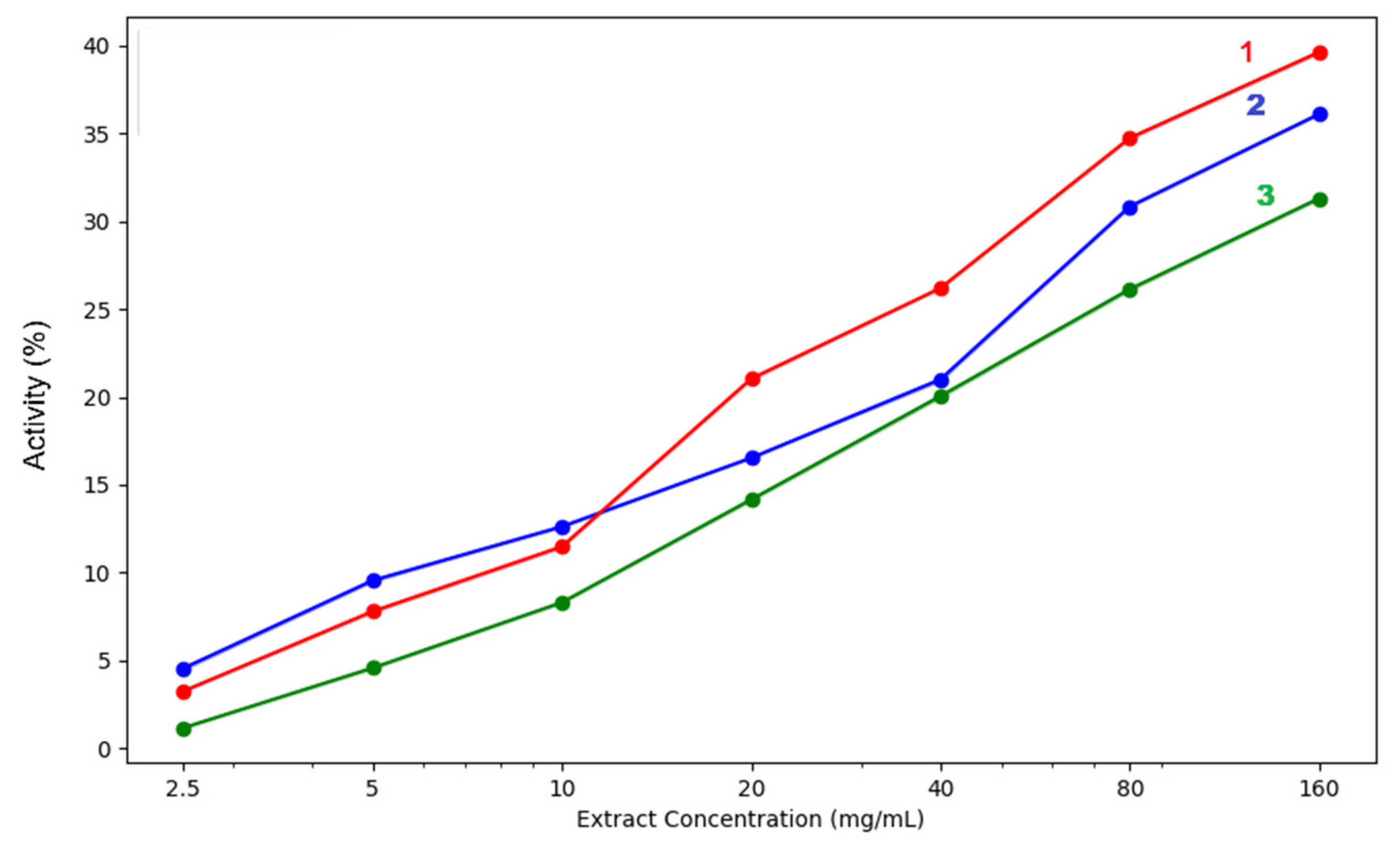
Plant extracts that can chelate iron ions have direct and indirect antioxidant effects because iron ions are involved in the synthesis of hydroxyl radicals with strong oxidising effects [26]. In addition, blocking their oxidising effect contributes to reducing local inflammation stimulated by oxidative stress.
The ability of plant extracts to block ferrous ions depends on the presence of polyphenols and nitrogen compounds in their composition, which form complexes with metal ions. Iron chelation reduces oxidative stress at the cellular level and reduces the risk of inflammation, neurodegenerative diseases, diabetes, liver and heart disease, and cancer. This chelation mechanism is also applicable in the treatment of conditions caused by excessive iron retention in the body or post-transfusion iron overload. Chelation products are more easily eliminated through urine and faeces compared to ferrous ions [27]. The chelation capacity of the three extracts is presented in Table 3 and Figure 8.

Table 3.
Ferrous iron chelation test results.
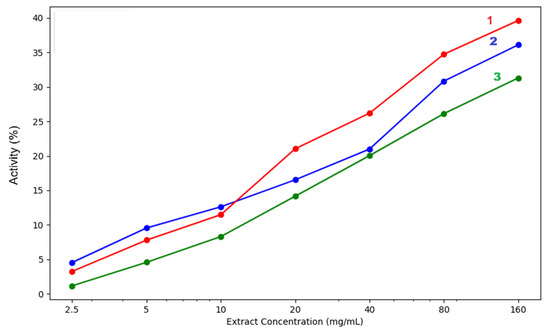
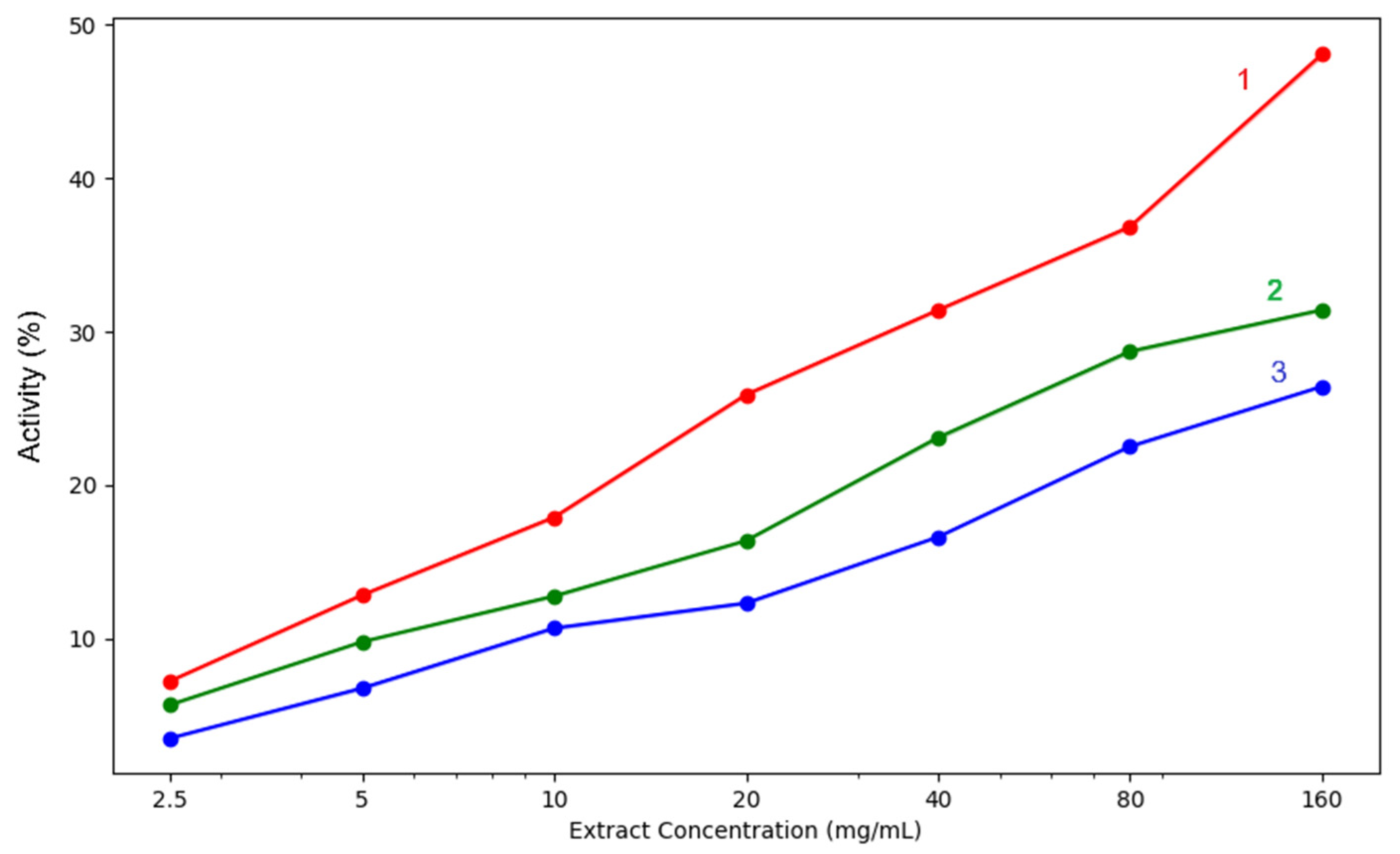
Figure 8.
Iron chelation capacity of the three analysed extracts: 1—acetic acid extract, 2—methanol extract, 3—acetone extract. The lighter contours surrounding the lines indicate the variability of the data, represented by the standard deviation (SD).
3.2.3. Hydroxyl Radical Neutralisation Capacity Results
Polyphenolic compounds, which have hydrogen-donating hydroxyl groups, are very effective at neutralising hydroxyl radicals, stabilising their structure and blocking their oxidising capacity [28]. Polyphenols can also block iron ions by blocking the synthesis of hydroxyl radicals [29].
The compounds present in plant extracts block the ability of hydroxyl radicals to generate DNA oxidation products, protecting genetic material and reducing the risk of cancerous processes [22]. The radical neutralisation capacity of the three extracts is presented in Table 4 and Figure 9.

Table 4.
Hydroxyl radical neutralisation capacity results.
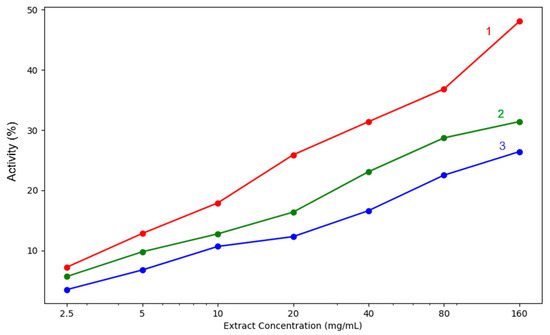
Figure 9.
Hydroxyl radical neutralisation capacity of the analysed extracts: 1—acetic acid extract, 2—methanol extract, 3—acetone extract. The lighter contours surrounding the lines indicate the variability of the data, represented by the standard deviation (SD).
3.2.4. Determination of Lipoxygenase Inhibition Capacity Results
Reducing or blocking lipoxygenase activity leads to a reduction in the synthesis of lipid peroxides, which are involved in inflammatory processes and allergic reactions [30]. Plant extracts containing polyphenols can block iron ions, which are important for enzyme function, block proton and electron exchange, or affect the spatial structure of the enzyme.
The complex mechanism of interaction explains the more intense effect of the extracts for this test compared to the ferrous ion chelation test [31,32]. The results obtained when testing the three broccoli sprouts are presented in Table 5 and Figure 10.

Table 5.
Lipoxygenase inhibition capacity.
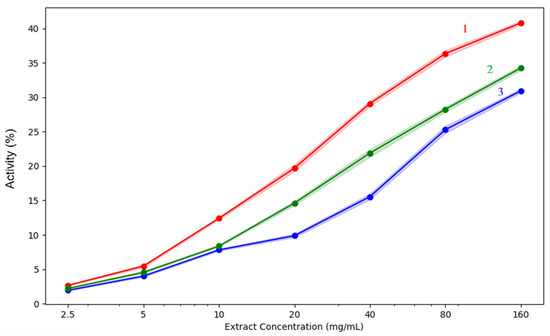
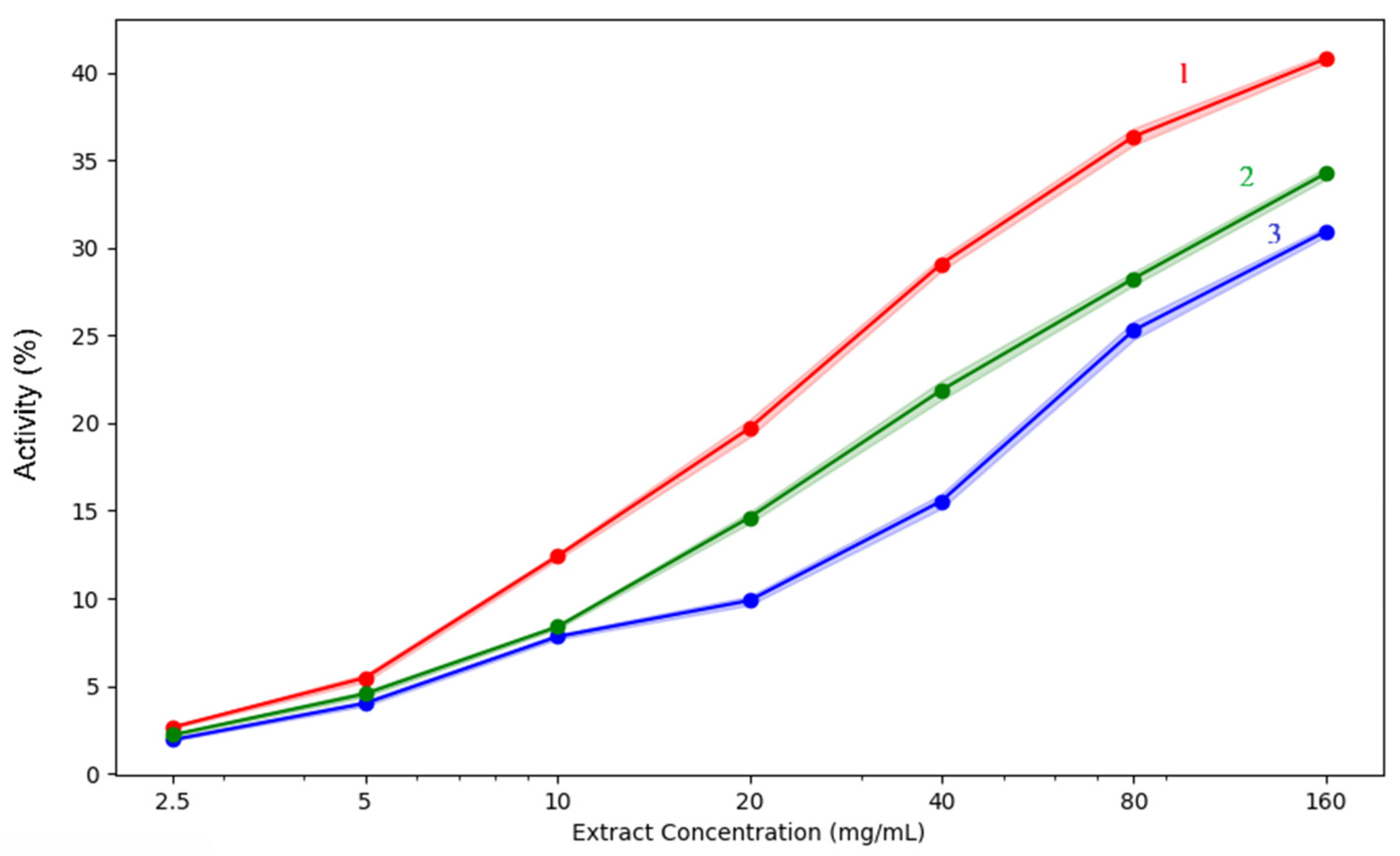
Figure 10.
Lipoxygenase inhibition capacity of the analysed extracts: 1—acetic acid extract, 2—methanol extract, 3—acetone extract. The lighter contours surrounding the lines indicate the variability of the data, represented by the standard deviation (SD).
4. Discussion
Due to the health benefits highlighted in the recent literature, SFN rapidly became a popular interest in scientific research, pharmaceutical formulation, and medical therapy [33,34]. However, SFN is not an essential nutrient, and to date, the necessary doses for daily administration have not been established. Most often, specialists recommend a daily intake of 200–400 µg of SFN (preferably from extracts obtained from broccoli sprouts) [35,36].
As a result, the dietary supplement market has been flooded with numerous products with extremely varied formulations and dosages. Unfortunately, very few of these supplements are available in the form of standardised extract formulations. Thus, the actual dose of SFN in these is unknown, and consumers have to rely on label statements.
The presence of myrosinase, which ensures the conversion of glucoraphanin (precursor) into SFN, is necessary, especially in the context of capsule formulations [37]. SFN is the pharmacologically active compound, while glucoraphanin is the inactive but more stable form. SFN is unstable in aqueous solution, which is why it is preferable to consume sprouts, seeds, or dried broccoli extracts rich in SFN, although their bioavailability is extremely variable. Thus, the bioavailability of isothiocyanates found in cruciferous vegetables can be influenced by various factors, including the mode of administration and individual characteristics, such as the composition and function of the consumer’s intestinal microbiome.
In our analysed broccoli sprouts extracts, the richest SFN content was observed in the methanolic solution, followed by the acetone extract and then the acetic acid extract, although the differences were not significant. The antioxidant activity of all samples included in the study was evaluated by assessing their ability to block the formation of the DPPH radical. Although this radical is synthetic and does not exist naturally, antiradical activity against DPPH is the most widely used method for assessing the antioxidant potential of plant extracts and compounds [1,19,38]. Oxygen reactive species have a strong oxidising capacity and initiate chain oxidation reactions involving unsaturated compounds in cellular and subcellular structures. These processes are involved in the onset and development of oxidative stress, a phenomenon that can lead to numerous diseases such as atherosclerosis, diabetes and cancer [39]. Plant extracts with DPPH radical scavenging capacity could block the harmful effects of free radicals while also helping to restore the antioxidant/pro-oxidant balance in various pathological conditions. Thus, the potential of SFN extracts to ameliorate diseases with an increasing prevalence, especially chronic non-communicable diseases such as diabetes, cancer, obesity, or even neurodegenerative diseases, has become clear.
The in vitro biochemical assays used to test the antioxidant capacity of our extracts highlighted differences between the various solvents used for extraction. In our samples, the methanolic extract proved to be the most efficient in both lipoxygenase inhibition and hydroxyl radical neutralisation. Moreover, regarding iron chelation potential, only at the lowest concentrations of 2.5–10mg/mL did acetic acid surpass the methanol extract. At concentrations over 10 mg/mL, the methanol extract proved superior in ferrous iron chelation potential. All three extracts had concentration-dependent activity, increasing steadily as the concentration rose. Methanol extract showed the highest chelation activity at 160 mg/mL (39.64%), followed closely by acetic acid (36.11%), while acetone had the lowest effect (31.31%). Regarding the hydroxyl radical inhibition capacity, a strong concentration-dependent activity was again observed. The methanol extract consistently yields the strongest extract, reaching 48.08% at 160 mg/mL, which is significantly higher than the values of acetone extracts (31.42%) and acetic acid extracts (26.43%). Even at lower concentrations of 5–10 mg/mL, methanol extracts outperform the others, indicating that stronger hydroxyl radical scavengers are extracted by methanol. The acetone mixture performs moderately, while acetic acid is the weakest.
Acetone-methanol extracts are moderately effective, better than acetic acid in hydroxyl radical neutralisation and lipoxygenase inhibition but weaker in iron chelation. Acetic acid extracts are least effective overall, though they did show some chelation activity at lower concentrations.
Once again, when analysing the lipoxygenase inhibition results, activity also rose with concentration in all extracts, reaching a maximum at 160 mg/mL. With 40.8% inhibition, the methanol extract is once more the most effective, followed by acetone (34.25%) and acetic acid (30.94%). All extracts exhibit relatively poor activity at low doses between 2.5 and 10 mg/mL, but methanol clearly exhibits a higher increase with concentration. This suggests that methanol could be the most effective solvent for the extraction of phytochemicals that inhibit lipoxygenase, such as flavonoids or phenolics.
These results confirm that extraction in an acidic environment ensures an optimal concentration of nutrients from broccoli sprouts. However, it is also worth noting that our investigations were conducted over a relatively short period of time. After obtaining the extracts from plant products, spectrophotometric analyses were performed within 48 h, which did not allow for SFN degradation.
The importance of the solvent used for extraction was also observed by Ivanochko and his colleagues, who highlighted that aqueous extracts from broccoli sprouts have the lowest efficiency compared to alcoholic extracts [40]. In their case, too, it was observed that it is essential to use the whole sprouts, not just the seeds. There were also contradictory results depending on the type of reaction used to highlight the antioxidant potential. However, working conditions, seed age, solvent, and processing/extraction methods will influence both the concentration of secondary metabolites and antioxidant potential.
Although this is only a rough assessment, differences in colour intensity could be observed between the three extracts, which was to be expected since each type of solvent has an affinity for certain compounds. For example, glycosides are more easily extracted in the acetone-methanol mixture and in the acidified solution, whereas aglycones are more soluble in methanol. Based on these observations, we can assume that the methanol extract contains fewer flavonoid glycosides, while the acetic acid and acetone-methanol extracts contain more glycosidic flavonoid derivatives.
Regarding the quantity of total flavonoids and polyphenols, the acetic acid extract and the acetone-methanol extract had a much lower content than the methanol extract, most likely because the sprouts contain flavonoid aglycones in greater quantities than glycosides. Additionally, the acetic acid sample had the highest concentration of total polyphenols compared to the other two samples, although the differences were not considered significant.
One of the compounds identified during phytochemical analyses was xanthorhamnin, a quercetin glycoside with multiple health benefits, including anti-inflammatory, antioxidant, and radioprotective action [41]. In addition, sinapic acid, one of the compounds found in the highest quantity in our extracts, has been shown to exhibit antioxidant, neuroprotective, cardioprotective, and anti-cancer properties. Moreover, like SFN, it could prove helpful in the management of cancers [42]. Its biological activity can be attributed to the existence of the α, β-unsaturated ketone group [41]. Furthermore, our analysis identified a diverse set of secondary metabolites, including carotenoids (zeaxanthin, lutein, lycopene), phenolic acids (sinapic acid, gallic acid, caffeic acid), flavonoids (kaempferol, luteolin), and glucosinolates (glucoraphanin). The presence of this wide range of compounds reflects the phytochemical complexity of broccoli sprouts and supports their value as a potential functional food. Carotenoids, such as zeaxanthin, lutein, and lycopene, are pigments with significant benefits for human health [43,44], while phenolic acids and flavonoids are abundant plant secondary metabolites with complex biological functions. Carotenoids were abundant in the methanol and acetone-methanol extracts, but scarce in the acetic acid one. This was to be expected, as carotenoids are lipophilic molecules and acetic acid is an extremely hydrophilic solvent.
Our results are similar to those in the literature. However, the solvents we used are not usually found in the studies reviewed, most of which evaluated ethanol and aqueous extracts comparatively. Of these, aqueous extracts were the poorest in bioactive principles, both in polyphenols and SFN. Such data suggest that, in general, bioactive compounds in broccoli sprouts are relatively lipophilic.
Furthermore, sprouts, compared to broccoli seeds, are also influenced by the germination period, with some authors highlighting that the germination process leads to a decrease in polyphenol concentration [40,45]. Although the present study did not evaluate the influence of other factors on the concentration of bioactive principles, some studies have shown that the concentration is higher in whole seeds compared to already germinated seeds from which the seedling has been separated. Moreover, polyphenols and flavonoids are compounds that play a role in plants’ defence. Therefore, their biosynthesis and accumulation in seeds aim to protect the new seedling and ensure resistance during the germination process. Thus, in mature plants, compared to sprouts, the concentration of these secondary metabolites will be much lower [42]. Other factors that can influence the concentration of polyphenols include the variety and type of cultivar, soil and climate conditions, the age of the plant at the time of harvest, and the timing of harvest.
Author Contributions
Conceptualisation, M.H.; methodology, S.R.; validation, I.M.-B.; formal analysis, I.C.C.; investigation, P.R.; resources, A.S.; data curation, C.M.; writing—original draft preparation, P.R. and A.-M.M.; writing—review and editing, O.C. and I.I.L.; visualisation, I.C.C.; supervision, C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study.
Acknowledgments
To the Operational Program for Competitiveness 2014–2020, Axis 1, under POC/448/1/1 Research infrastructure projects for public R&D institutions/universities, project Multidisciplinary platform for medical research-development in N-E region, CENEMED, grant agreement No. 127606.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Sulforaphane | SFN |
| GRP | Glucoraphanin |
| Nrf2/ARE | Nuclear factor E2-related factor 2/Antioxidant Response Element |
References
- Shams, R.; Abu-Khudir, R.; Ali, E. Sulforaphane, Polyphenols and Related Anti-Inflammatory and Antioxidant Activities Changes of Egyptian Broccoli during Growth. J. Food Meas. Charact. 2017, 11, 2061. [Google Scholar] [CrossRef]
- Schmid, H.; Karrer, P. Synthese der racemischen und der optisch aktiven Formen des Sulforaphans. Helv. Chim. Acta 1948, 31, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Atwell, L.L.; Hsu, A.; Wong, C.P.; Stevens, J.F.; Bella, D.; Yu, T.-W.; Pereira, C.B.; Löhr, C.V.; Christensen, J.M.; Dashwood, R.H.; et al. Absorption and Chemopreventive Targets of Sulforaphane in Humans Following Consumption of Broccoli Sprouts or a Myrosinase-Treated Broccoli Sprout Extract. Mol. Nutr. Food Res. 2015, 59, 424–433. [Google Scholar] [CrossRef]
- Dmytriv, T.R.; Lushchak, O.; Lushchak, V.I. Glucoraphanin Conversion into Sulforaphane and Related Compounds by Gut Microbiota. Front. Physiol. 2025, 16. [Google Scholar] [CrossRef]
- Houghton, C.A. Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxidative Med. Cell. Longev. 2019, 2019, 2716870. [Google Scholar] [CrossRef]
- Bones, A.; Rossiter, J. The Myrosinase-Glucosinolate System, Its Organisation and Biochemistry. Physiol. Plant. 1996, 97, 194–208. [Google Scholar] [CrossRef]
- Ratzka, A.; Vogel, H.; Kliebenstein, D.J.; Mitchell-Olds, T.; Kroymann, J. Disarming the Mustard Oil Bomb. Proc. Natl. Acad. Sci. USA 2002, 99, 11223–11228. [Google Scholar] [CrossRef]
- Lüthy, B.; Matile, P. The Mustard Oil Bomb: Rectified Analysis of the Subcellular Organisation of the Myrosinase System. Biochem. Physiol. Pflanz. 1984, 179, 5–12. [Google Scholar] [CrossRef]
- Janczewski, Ł. Sulforaphane and Its Bifunctional Analogs: Synthesis and Biological Activity. Molecules 2022, 27, 1750. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli Sprouts: An Exceptionally Rich Source of Inducers of Enzymes That Protect against Chemical Carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L. Discovery and Development of Sulforaphane as a Cancer Chemopreventive Phytochemical. Acta Pharmacol. Sin. 2007, 28, 1343–1354. [Google Scholar] [CrossRef]
- Pastore, A.; Federici, G.; Bertini, E.; Piemonte, F. Analysis of Glutathione: Implication in Redox and Detoxification. Clin. Chim. Acta 2003, 333, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A Major Inducer of Anticarcinogenic Protective Enzymes from Broccoli: Isolation and Elucidation of Structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef] [PubMed]
- Ritz, S.A.; Wan, J.; Diaz-Sanchez, D. Sulforaphane-Stimulated Phase II Enzyme Induction Inhibits Cytokine Production by Airway Epithelial Cells Stimulated with Diesel Extract. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L33–L39. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Chen, J.-G.; Egner, P.A.; Fahey, J.W.; Jacobson, L.P.; Stephenson, K.K.; Ye, L.; Coady, J.L.; Wang, J.-B.; Wu, Y.; et al. Effects of Glucosinolate-Rich Broccoli Sprouts on Urinary Levels of Aflatoxin-DNA Adducts and Phenanthrene Tetraols in a Randomized Clinical Trial in He Zuo Township, Qidong, People’s Republic of China. Cancer Epidemiol. Biomark. Prev. 2005, 14 Pt 1, 2605–2613. [Google Scholar] [CrossRef]
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane Inhibits Extracellular, Intracellular, and Antibiotic-Resistant Strains of Helicobacter Pylori and Prevents Benzo[a]Pyrene-Induced Stomach Tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615. [Google Scholar] [CrossRef]
- Wu, N.; Luo, Z.; Deng, R.; Zhang, Z.; Zhang, J.; Liu, S.; Luo, Z.; Qi, Q. Sulforaphane: An Emerging Star in Neuroprotection and Neurological Disease Prevention. Biochem. Pharmacol. 2025, 233, 116797. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Kozumbo, W.J. The Phytoprotective Agent Sulforaphane Prevents Inflammatory Degenerative Diseases and Age-Related Pathologies via Nrf2-Mediated Hormesis. Pharmacol. Res. 2021, 163, 105283. [Google Scholar] [CrossRef]
- Yeo, J.; Shahidi, F. Critical Re-Evaluation of DPPH Assay: Presence of Pigments Affects the Results. J. Agric. Food Chem. 2019, 67, 7526–7529. [Google Scholar] [CrossRef]
- Dinis, T.C.; Maderia, V.M.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free Radicals and Their Impact on Health and Antioxidant Defenses: A Review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Halliwell, B.; Adhikary, A.; Dingfelder, M.; Dizdaroglu, M. Hydroxyl Radical Is a Significant Player in Oxidative DNA Damage in Vivo. Chem. Soc. Rev. 2021, 50, 8355–8360. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.B.; Hong, S.C.; Jeong, H.J. 3,4-Dihydroxybenzaldehyde Purified from the Barley Seeds (Hordeum vulgare) Inhibits Oxidative DNA Damage and Apoptosis via Its Antioxidant Activity. Phytomedicine 2009, 16, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zou, H. Lipoxygenase Metabolism: Critical Pathways in Microglia-Mediated Neuroinflammation and Neurodevelopmental Disorders. Neurochem. Res. 2022, 47, 3213–3220. [Google Scholar] [CrossRef]
- Malterud, K.E.; Rydland, K.M. Inhibitors of 15-Lipoxygenase from Orange Peel. J. Agric. Food Chem. 2000, 48, 5576–5580. [Google Scholar] [CrossRef]
- Chuljerm, H.; Paradee, N.; Katekaew, D.; Nantachai, P.; Settakorn, K.; Srichairatanakool, S.; Koonyosying, P. Iron Chelation Property, Antioxidant Activity, and Hepatoprotective Effect of 6-Gingerol-Rich Ginger (Zingiber officinale) Extract in Iron-Loaded Huh7 Cells. Plants 2023, 12, 2936. [Google Scholar] [CrossRef]
- Adjimani, J.P.; Asare, P. Antioxidant and Free Radical Scavenging Activity of Iron Chelators. Toxicol. Rep. 2015, 2, 721–728. [Google Scholar] [CrossRef]
- Gentile, M.T.; Camerino, I.; Ciarmiello, L.; Woodrow, P.; Muscariello, L.; De Chiara, I.; Pacifico, S. Neuro-Nutraceutical Polyphenols: How Far Are We? Antioxidants 2023, 12, 539. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, Q.; Jiang, J.; Khan, M.S. Tannin Complexation with Metal Ions and Its Implication on Human Health, Environment and Industry: An Overview. Int. J. Biol. Macromol. 2023, 253 Pt 7, 127485. [Google Scholar] [CrossRef]
- Ilhan, A.; Akyol, O.; Gurel, A.; Armutcu, F.; Iraz, M.; Oztas, E. Protective Effects of Caffeic Acid Phenethyl Ester against Experimental Allergic Encephalomyelitis-Induced Oxidative Stress in Rats. Free Radic. Biol. Med. 2004, 37, 386–394. [Google Scholar] [CrossRef]
- Biringer, R.G. The Enzymology of Human Eicosanoid Pathways: The Lipoxygenase Branches. Mol. Biol. Rep. 2020, 47, 7189–7207. [Google Scholar] [CrossRef]
- Lončarić, M.; Strelec, I.; Moslavac, T.; Šubarić, D.; Pavić, V.; Molnar, M. Lipoxygenase Inhibition by Plant Extracts. Biomolecules 2021, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Isothiocyanates, Nitriles and Thiocyanates as Products of Autolysis of Glucosinolates in Cruciferae. Available online: https://www.scilit.com/publications/a73ff413331fe1848bc11a4cf71aa624 (accessed on 13 September 2025).
- González, F.; Quintero, J.; Del Río, R.; Mahn, A. Optimization of an Extraction Process to Obtain a Food-Grade Sulforaphane-Rich Extract from Broccoli (Brassica oleracea Var. Italica). Molecules 2021, 26, 4042. [Google Scholar] [CrossRef] [PubMed]
- Kall, M.A.; Vang, O.; Clausen, J. Effects of Dietary Broccoli on Human Drug Metabolising Activity. Cancer Lett. 1997, 114, 169–170. [Google Scholar] [CrossRef]
- Mohammed, Q. Extraction of Sulforaphane from Iraqi (Kurdistan Region) Cabbage. J. Koya Univ. 2009, 10, 21–28. [Google Scholar]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef]
- Duque-Soto, C.; Borrás-Linares, I.; Quirantes-Piné, R.; Falcó, I.; Sánchez, G.; Segura-Carretero, A.; Lozano-Sánchez, J. Potential Antioxidant and Antiviral Activities of Hydroethanolic Extracts of Selected Lamiaceae Species. Foods 2022, 11, 1862. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; He, Y.; Zhang, S.; Huang, B.; Wang, Y.; Lin, W.; Xiao, W.; Zou, Z.; Cui, G. GGV Formula Attenuates CCl4-Induced Hepatic Injury in Mice by Modulating the Gut Microbiota and Metabolites. Front. Nutr. 2025, 12, 1564177. [Google Scholar] [CrossRef]
- Ivanochko, M.V.; Fediv, K.V.; Shvadchak, V.V.; Bayliak, M.M.; Lushchak, V.I. Nutritional Analysis of Aqueous and Ethanol Broccoli Sprout Extracts. J. Plant Biochem. Biotechnol. 2025, 34, 749–760. [Google Scholar] [CrossRef]
- Chaudhri, A.A.; Nadeem, M.; Rahman, A.U.; Alam, T.; Sajjad, W.; Hasan, F.; Badshah, M.; Khan, S.; Rehman, F.; Shah, A.A. Antioxidative and Radioprotective Properties of Glycosylated Flavonoid, Xanthorhamnin from Radio-Resistant Bacterium Bacillus Indicus Strain TMC-6. Curr. Microbiol. 2020, 77, 1245–1253. [Google Scholar] [CrossRef]
- Pandi, A.; Kalappan, V.M. Pharmacological and Therapeutic Applications of Sinapic Acid—An Updated Review. Mol. Biol. Rep. 2021, 48, 3733–3745. [Google Scholar] [CrossRef]
- Jiang, C.-H.; Sun, T.-L.; Xiang, D.-X.; Wei, S.-S.; Li, W.-Q. Anticancer Activity and Mechanism of Xanthohumol: A Prenylated Flavonoid From Hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530. [Google Scholar] [CrossRef]
- Oyovwi, M.O.; Ohwin, P.E.; Rotu, A.R.; Tesi, P.E.; Ben-Azu, B.; Naiho, O.A. Lycopene Againsts the Polystyrene Microplastics-Induced Neurotoxicity via Modulation of mTOR/Beclin-1 Activities in Adult Male Wistar Rats. Clin. Tradit. Med. Pharmacol. 2024, 5, 200180. [Google Scholar] [CrossRef]
- Kim, E.-J.; Kim, M.-H. Antioxidant Activity of Solvent Fraction from Broccoli Sprouts Cultivated at the Plant Factory System. Korean J. Food Nutr. 2015, 28, 1–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).