Sun-Drying and Melatonin Treatment Effects on Apricot Color, Phytochemical, and Antioxidant Properties
Abstract
1. Introduction
2. Materials and Methods
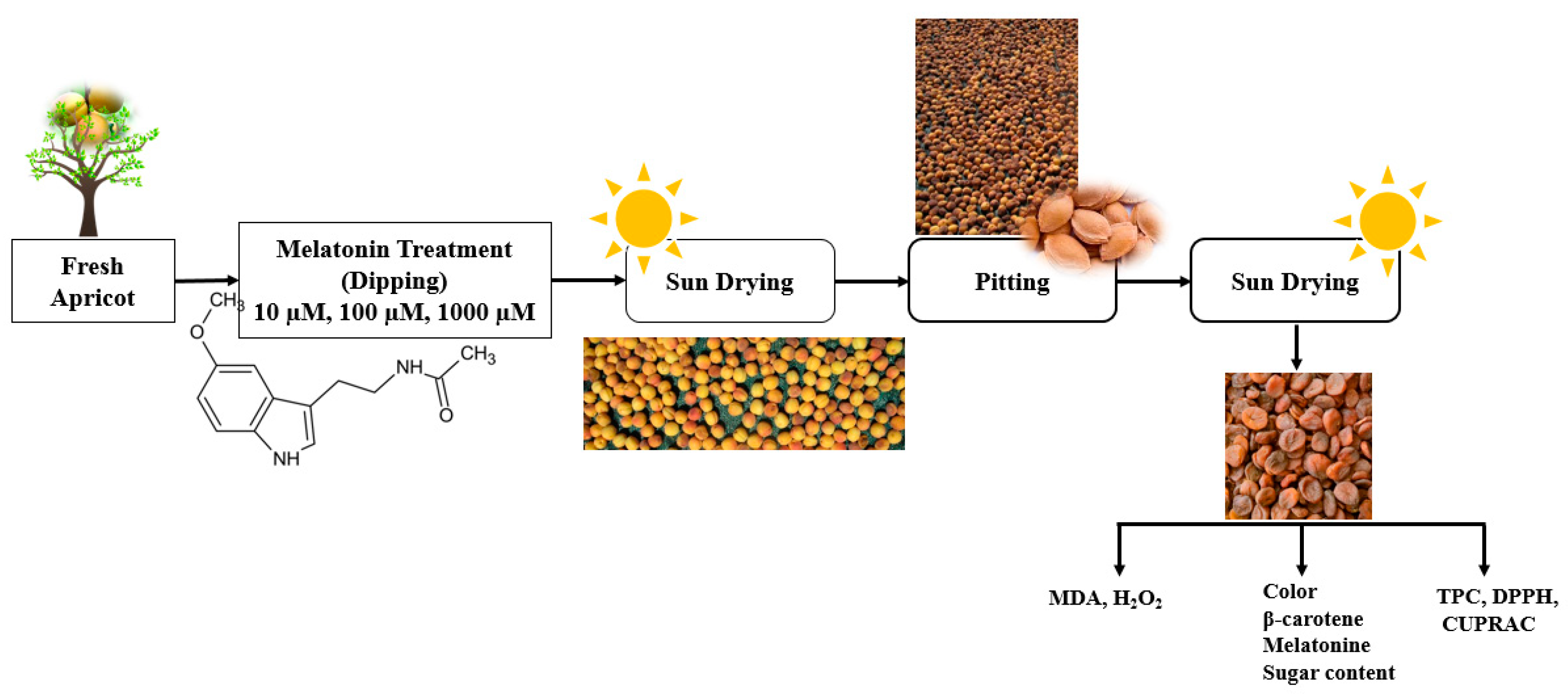
2.1. Plant Material and Treatments
2.2. Sun-Dried Apricot Production
2.3. Some Physicochemical and Biochemical Analysis of Apricot Fruits
2.3.1. Color Measurement
2.3.2. Determination of Total Phenolic Content (TPC) and Antioxidant Capacity
2.3.3. Sugar Content in Fruits
2.3.4. Melatonin Analysis with UFLC-FD in Fruits
2.3.5. Beta-Carotene Determination in HPLC System in Fruits
2.3.6. Malondialdehyde (MDA) Content in Fruits
2.3.7. H2O2 Content in Fruits
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effect of Exogenous Melatonin on Sun-Dried Apricot Color
3.2. Evaluation of Oxidative Stress Markers and Antioxidant Content in Melatonin-Treated Dried Apricots
3.3. Effects of Exogenous Melatonin Application on Sugar Composition, Total Phenolic Content and Antioxidant Capacity in Dried Apricots
3.4. General Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karabulut, I.; Topcu, A.; Duran, A.; Turan, S.; Ozturk, B. Effect of hot air drying and sun drying on color values and β-carotene content of apricot (Prunus armenica L.). LWT 2007, 40, 753–758. [Google Scholar] [CrossRef]
- Güngör, D. Kuru Kayısının Türkiye Ekonomisinde İhracattaki Yeri ve Önemi. Master’s Thesis, Beykent University, Department of Economics, Istanbul, Turkey, 2020. [Google Scholar]
- Juhnevica-Radenkova, K.; Krasnova, I.; Seglina, D.; Kaufmane, E.; Gravite, I.; Valdovska, A.; Radenkovs, V. Biochemical profile and antioxidant activity of dried fruit produced from apricot cultivars grown in Latvia. Horticulturae 2024, 10, 205. [Google Scholar] [CrossRef]
- Miranda, G.; Berna, A.; Salazar, D.; Mulet, A. Sulphur dioxide evolution during dried apricot storage. LWT 2009, 42, 531–533. [Google Scholar] [CrossRef]
- Uğur, Y.; Erdoğan, S. The effect of hydrogen peroxide used in desulfurization of dried apricot on the antioxidant capacity and phenolic compound content of the fruit. J. Food Meas. Charact. 2021, 15, 3708–3719. [Google Scholar] [CrossRef]
- Özbek, H.N.; Elik, A.; Koçak Yanık, D.; Işınay, B.; Sever, M.; Bulut, E.; Göğüş, F. Effect of sequential-combined solar energy assisted hot air and hot air assisted radio frequency drying on the physical and chemical properties of dried apricots. J. Food Sci. Technol. 2022, 59, 2894–2904. [Google Scholar] [CrossRef] [PubMed]
- Güçlü, K.; Altun, M.; Özyürek, M.; Karademir, S.E.; Apak, R. Antioxidant capacity of fresh, sun- and sulphited-dried Malatya apricot (Prunus armeniaca) assayed by CUPRAC, ABTS/TEAC and folin methods. Int. J. Food Sci. Technol. 2006, 41, 76–85. [Google Scholar] [CrossRef]
- Deng, L.Z.; Xiong, C.H.; Pei, Y.P.; Zhu, Z.Q.; Zheng, X.; Zhang, Y.; Xiao, H.W. Effects of various storage conditions on total phenolic, carotenoids, antioxidant capacity, and color of dried apricots. Food Control 2022, 136, 108846. [Google Scholar] [CrossRef]
- Baccichet, I.; Linge, C.D.S.; Tagliabue, A.G.; Chiozzotto, R.; Tura, D.; Bassi, D.; Cirilli, M. Phenotypic characterization and quality perception of unsulfurated dried apricots in a panel of European accessions and breeding selections. Sci. Hortic. 2024, 331, 113095. [Google Scholar] [CrossRef]
- Çoban, E.; Karlıdağ, H.; Kutsal, İ.K. The influence of different ripening stages, harvest and drying methods on quality of unsulfured sun-dried apricots. Turk. J. Agric.-Food Sci. Technol. 2020, 8, 2397–2404. [Google Scholar] [CrossRef]
- Karabulut, İ.; Gökbulut, İ.; Bilenler, T.; Sişlioglu, K.; Ozdemir, İ.S.; Bahar, B.; Çelik, B.; Seyhan, F. Effect of fruit maturity level on quality, sensory properties and volatile composition of two common apricot (Prunus armeniaca L.) varieties. J. Food Sci. Technol. 2018, 55, 2671–2678. [Google Scholar] [CrossRef]
- Sui, X.; Meng, Z.; Dong, T.; Fan, X.; Wang, Q. Enzymatic browning and polyphenol oxidase control strategies. Curr. Opin. Biotechnol. 2023, 81, 102921. [Google Scholar] [CrossRef]
- Bork, L.V.; Stobernack, T.; Rohn, S.; Kanzler, C. Browning reactions of hydroxycinnamic acids and heterocyclic Maillard reaction intermediates–formation of phenol-containing colorants. Food Chem. 2024, 449, 139189. [Google Scholar] [CrossRef] [PubMed]
- Eddine Derardja, A.; Pretzler, M.; Kampatsikas, I.; Barkat, M.; Rompel, A. Inhibition of apricot polyphenol oxidase by combinations of plant proteases and ascorbic acid. Food Chem X 2019, 4, 100053. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a plant biostimulant in crops and during post-harvest: A new approach is needed. J. Sci. Food Agric. 2021, 101, 5297–5304. [Google Scholar] [CrossRef]
- Shekari, A.; Hassani, N.R.; Aghdam, S.M.; Rezaee, M.; Jannatizadeh, A. The effects of melatonin treatment on cap browning and biochemical attributes of Agaricus bisporus during low temperature storage. Food Chem. 2021, 348, 129074. [Google Scholar] [CrossRef]
- Zhang, Y.; Huber, J.D.; Hu, M.; Jiang, G.; Gao, Z.; Xu, X.; Jiang, Y.; Zhang, Z. Melatonin delays postharvest browning in litchi fruit by enhancing anti-oxidative processes and oxidation repair. J. Agric. Food Chem. 2018, 66, 7475–7484. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Roy, S.; Arnao, M.B. Nanovehicles for melatonin: A new journey for agriculture. Trends Plant Sci. 2024, 29, 232–248. [Google Scholar] [CrossRef]
- Ze, Y.; Gao, H.; Li, T.; Yang, B.; Jiang, Y. Insights into the roles of melatonin in maintaining quality and extending shelf life of postharvest fruits. Trends Food Sci. Technol. 2021, 109, 569–578. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Wang, S.Y.; Shi, X.C.; Wang, R.; Wang, H.L.; Liu, F.; Laborda, P. Melatonin in fruit production and postharvest preservation: A review. Food Chem. 2020, 320, 126642. [Google Scholar] [CrossRef] [PubMed]
- Medina-Santamarina, J.; Zapata, P.J.; Valverde, J.M.; Valero, D.; Serrano, M.; Guillén, F. Melatonin treatment of apricot trees leads to maintenance of fruit quality attributes during storage at chilling and non-chilling temperatures. Agron. J. 2021, 11, 917. [Google Scholar] [CrossRef]
- Zengin, R.; Maraş, Z.; Uğur, Y.; Özhan, O.; Karaat, F.E.; Erdoğan, S. Determination of phytochemical composition in fruits and leaves from different origins: Black Mulberry, Chokeberry and Elderberry genotypes. Anal. Lett. 2024, 1–23. [Google Scholar] [CrossRef]
- Gomes, W.F.; França, F.R.M.; Denadai, M.; Andrade, J.K.S.; da Silva Oliveira, E.M.; de Brito, E.S.; Narain, N. Effect of freeze- and spray-drying on physico-chemical characteristics, phenolic compounds and antioxidant activity of papaya pulp. J. Food Sci. Technol. 2018, 55, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Yaman, R. Phytochemical properties of some mulberry genotypes grown in Malatya province. Yuz. Yıl Univ. J. Agric. Sci. 2021, 31, 348–355. [Google Scholar] [CrossRef]
- Uğur, Y.; Zengin, R.; Ernim, C.; Günhan, Z.İ.; Şalva, E.; Erdoğan, S. Changes in the phenolic, melatonin, sugar contents and antioxidant capacity, depending on ripening stage in different Cornelian cherry (Cornus mas L.) fruits. ChemistrySelect 2024, 9, e202304682. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, T.; Zhang, P.; Wang, Z.Y. Melatonin attenuates postharvest physiological deterioration of cassava storage roots. J. Pineal Res. 2016, 60, 424–434. [Google Scholar] [CrossRef]
- Liu, X.; Ma, M.; Yu, H.; Shang, S.; Du, L. Enhanced storage resistance of mulberries using laminated cellulose nanocrystals/chitosan composite coatings. J. Polym. Environ. 2024, 32, 5115–5126. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Yu, R.; Wu, C.; Fan, G.; Li, T. Effects of postharvest application of methyl jasmonate on physicochemical characteristics and antioxidant system of the blueberry fruit. Sci. Hortic. 2019, 258, 108785. [Google Scholar] [CrossRef]
- Evgenidis, G.; Traka-Mavrona, E.; Koutsika-Sotiriou, M. Principal component and cluster analysis as a tool in the assessment of tomato hybrids and cultivars. Int. J. Agron. 2011, 2011, 697879. [Google Scholar] [CrossRef]
- Xiao, Y.; Xie, J.; Wu, C.; He, J.; Wang, B. Effects of melatonin treatment on browning alleviation of fresh-cut foods. J. Food Biochem. 2021, 45, e13798. [Google Scholar] [CrossRef]
- Kakaei, S.; Saba, M.K.; Mansouri, S.; Darvishi, H. Melatonin postharvest spray influence on white mulberry browning, storage life, and biochemical changes. Postharvest Biol. Technol. 2024, 213, 112947. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, Z.; Chen, J.; Dong, Y.; Qu, K.; Guo, T.; Li, X. Reactive oxygen species signaling in melatonin-mediated plant stress response. Plant Physiol. Biochem. 2024, 207, 108398. [Google Scholar] [CrossRef]
- Ali, S.; Akbar Anjum, M.; Nawaz, A.; Naz, S.; Ejaz, S.; Shahzad Saleem, M.; Ul Hasan, M. Effect of gum arabic coating on antioxidative enzyme activities and quality of apricot (Prunus armeniaca L.) fruit during ambient storage. J. Food Biochem. 2021, 45, e13656. [Google Scholar] [CrossRef]
- Coşkun, A.L.; Türkyılmaz, M.; Aksu, Ö.T.; Koç, B.E.; Yemiş, O.; Özkan, M. Effects of various sulphuring methods and storage temperatures on the physical and chemical quality of dried apricots. Food Chem. 2013, 141, 3670–3680. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and phytomelatonin: Chemistry, biosynthesis, metabolism, distribution, and bioactivity in plants and animals—An overview. Int. J. Mol. Sci. 2021, 22, 9996. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Niu, Y.; Ding, X.; Zhao, S.; Li, Y.; Fan, G.; Liao, K. Analysis of carotenoid content and diversity in apricots (Prunus armeniaca L.) grown in China. Food Chem. 2020, 330, 127223. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, W.; Liu, S.; Liu, C.; Zheng, L. Effects of melatonin treatment on the enzymatic browning and nutritional quality of fresh-cut pear fruit. Food Chem. 2019, 299, 125116. [Google Scholar] [CrossRef]
- Akin, E.B.; Karabulut, I.; Topcu, A. Some compositional properties of main Malatya apricot (Prunus armeniaca L.) varieties. Food Chem. 2008, 107, 939–948. [Google Scholar] [CrossRef]
- Niu, X.X.; Deng, L.Z.; Wang, H.; Wang, Q.H.; Xu, M.Q.; Li, S.B.; Xiao, H.W. Transformation of cell wall pectin profile during postharvest ripening process alters drying behavior and regulates the sugar content of dried plums. Food Chem. 2024, 458, 140093. [Google Scholar] [CrossRef] [PubMed]
- Erdogan-Orhan, I.; Kartal, M. Insights into research on phytochemistry and biological activities of Prunus armeniaca L. (apricot). Food Res. Int. 2011, 44, 1238–1243. [Google Scholar] [CrossRef]
- Eddine Derardja, A.; Pretzler, M.; Kampatsikas, I.; Radovic, M.; Fabisikova, A.; Zehl, M.; Rompel, A. Polyphenol oxidase and enzymatic browning in apricot (Prunus armeniaca L.): Effect on phenolic composition and deduction of main substrates. Curr. Res. Food Sci. 2022, 5, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Madrau, M.A.; Piscopo, A.; Sanguinetti, A.M.; Del Caro, A.; Poiana, M.; Romeo, F.V.; Piga, A. Effect of drying temperature on polyphenolic content and antioxidant activity of apricots. Euro. Food Res. Technol. 2009, 228, 441–448. [Google Scholar] [CrossRef]
- Alajil, O.; Sagar, V.R.; Kaur, C.; Rudra, S.G.; Sharma, R.R.; Kaushik, R.; Mekhemar, M. Nutritional and phytochemical traits of apricots (Prunus armeniaca L.) for application in nutraceutical and health industry. Foods 2021, 10, 1344. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, D.; Wang, J.; Tian, B.; Li, Y.; Sun, G.; Zhang, H. Exogenous melatonin alleviates NO2 damage in tobacco leaves by promoting antioxidant defense, modulating redox homeostasis, and signal transduction. J. Hazard. Mater. 2022, 424, 127265. [Google Scholar] [CrossRef]
- Boutin, J.A.; Liberelle, M.; Yous, S.; Ferry, G.; Nepveu, F. Melatonin facts: Lack of evidence that melatonin is a radical scavenger in living systems. J. Pineal Res. 2024, 76, e12926. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.S.; Kang, D.C.; Sun, J.; Leng, P.; Liu, L.X.; Wang, L.; Liu, Y.G. Research on melatonin in fruits and vegetables and the mechanism of exogenous melatonin on postharvest preservation. Food Biosci. 2022, 50, 102196. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Killiny, N.; Dutt, M. Melatonin supplementation enhances browning suppression and improves transformation efficiency and regeneration of transgenic rough lemon plants (Citrus × jambhiri). PLoS ONE 2024, 19, e0294318. [Google Scholar] [CrossRef] [PubMed]
- Arabia, A.; Muñoz, P.; Munné-Bosch, S. Fruit-specific effects of tryptophan and melatonin as active components to extend the functionality of red fruits during post-harvest processing. Food Chem. 2025, 463, 141487. [Google Scholar] [CrossRef] [PubMed]
- Rahmanzadeh-Ishkeh, S.; Shirzad, H.; Tofighi, Z.; Fattahi, M.; Ghosta, Y. Exogenous melatonin prolongs raspberry postharvest life quality by increasing some antioxidant and enzyme activity and phytochemical contents. Sci. Rep. 2024, 14, 11508. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Fuentes-Broto, L.P.S.D.; Paredes, S.D.; Reiter, R.J. Significance and application of melatonin in the regulation of brown adipose tissue metabolism: Relation to human obesity. Obes. Rev. 2011, 12, 167–188. [Google Scholar] [CrossRef] [PubMed]



| Treatments | L* | a* | b* |
|---|---|---|---|
| Control | 26.91 ± 0.77 b | 4.98 ± 0.76 c | 9.44 ± 0.97 b |
| 10 µM-MT | 27.40 ± 0.79 ab | 5.55 ± 0.60 bc | 10.15 ± 0.76 ab |
| 100 µM-MT | 28.74 ± 0.47 a | 6.67 ± 0.13 a | 11.33 ± 0.26 a |
| 1000 µM-MT | 28.84 ± 1.30 a | 6.35 ± 0.60 ab | 11.15 ± 1.04 a |
| Treatments | MDA µmol/L | H2O2 µmol/g | Melatonin ng/mL | β-Carotene mg/kg |
|---|---|---|---|---|
| Control | 23.44 ± 3.03 a | 1.03 ± 0.11 a | Nd | 181.84 ± 2.11 b |
| 10 µM-MT | 22.75 ± 5.33 ab | 1.00 ± 0.13 a | Nd | 190.57 ± 8.31 ab |
| 100 µM-MT | 16.55 ± 3.45 b | 0.76 ± 0.07 b | Nd | 223.07 ± 32.05 a |
| 1000 µM-MT | 18.50 ± 1.67 ab | 0.66 ± 0.01 b | Nd | 173.76 ± 26.02 b |
| Treatments | Glucose (g/100 g) | Fructose (g/100 g) | Sucrose (g/100 g) | Sorbitol (g/100 g) |
|---|---|---|---|---|
| Control | 16.97 ± 0.12 b | 12.01 ± 0.46 b | 12.12 ± 0.26 c | 23.46 ± 0.56 ab |
| 10 µM-MT | 17.16 ± 0.19 b | 12.11 ± 0.70 ab | 13.98 ± 1.31 b | 23.54 ± 0.21 ab |
| 100 µM-MT | 18.99 ± 0.01 a | 12.58 ± 0.03 ab | 15.52 ± 0.11 a | 23.94 ± 0.24 a |
| 1000 µM-MT | 18.84 ± 0.30 a | 12.86 ± 0.29 a | 14.82 ± 0.10 ab | 22.99 ± 0.37 b |
| Treatments | TPC (mg GAE/100 g) | DPPH (mg TE/100 g) | CUPRAC (mg TE/100 g) |
|---|---|---|---|
| Control | 243.68 ± 14.02 a | 100.13 ± 10.01 ab | 634.72 ± 26.98 a |
| 10 µM-MT | 243.52 ± 5.68 a | 111.30 ± 11.13 a | 542.13 ± 8.10 b |
| 100 µM-MT | 201.20 ± 19.59 b | 86.48 ± 7.75 b | 489.76 ± 26.67 c |
| 1000 µM-MT | 207.71 ± 16.85 b | 90.34 ± 9.03 ab | 510.30 ± 29.13 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zengin, R.; Uğur, Y.; Levent, Y.; Erdoğan, S.; Hatterman-Valenti, H.; Kaya, O. Sun-Drying and Melatonin Treatment Effects on Apricot Color, Phytochemical, and Antioxidant Properties. Appl. Sci. 2025, 15, 508. https://doi.org/10.3390/app15020508
Zengin R, Uğur Y, Levent Y, Erdoğan S, Hatterman-Valenti H, Kaya O. Sun-Drying and Melatonin Treatment Effects on Apricot Color, Phytochemical, and Antioxidant Properties. Applied Sciences. 2025; 15(2):508. https://doi.org/10.3390/app15020508
Chicago/Turabian StyleZengin, Rukiye, Yılmaz Uğur, Yasemin Levent, Selim Erdoğan, Harlene Hatterman-Valenti, and Ozkan Kaya. 2025. "Sun-Drying and Melatonin Treatment Effects on Apricot Color, Phytochemical, and Antioxidant Properties" Applied Sciences 15, no. 2: 508. https://doi.org/10.3390/app15020508
APA StyleZengin, R., Uğur, Y., Levent, Y., Erdoğan, S., Hatterman-Valenti, H., & Kaya, O. (2025). Sun-Drying and Melatonin Treatment Effects on Apricot Color, Phytochemical, and Antioxidant Properties. Applied Sciences, 15(2), 508. https://doi.org/10.3390/app15020508







