Hyperthermia and Chemotherapy Combination in Triple-Negative Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Measurements
2.2.1. Hyperthermia Treatment
2.2.2. Drug Treatment
2.2.3. Hyperthermia Treatments in Combination with PTX or DOX
2.3. Cell Cycle Analysis
2.4. Analysis of Intracellular ROS
2.5. Cell Apoptosis Assay
2.6. Statistical Analysis
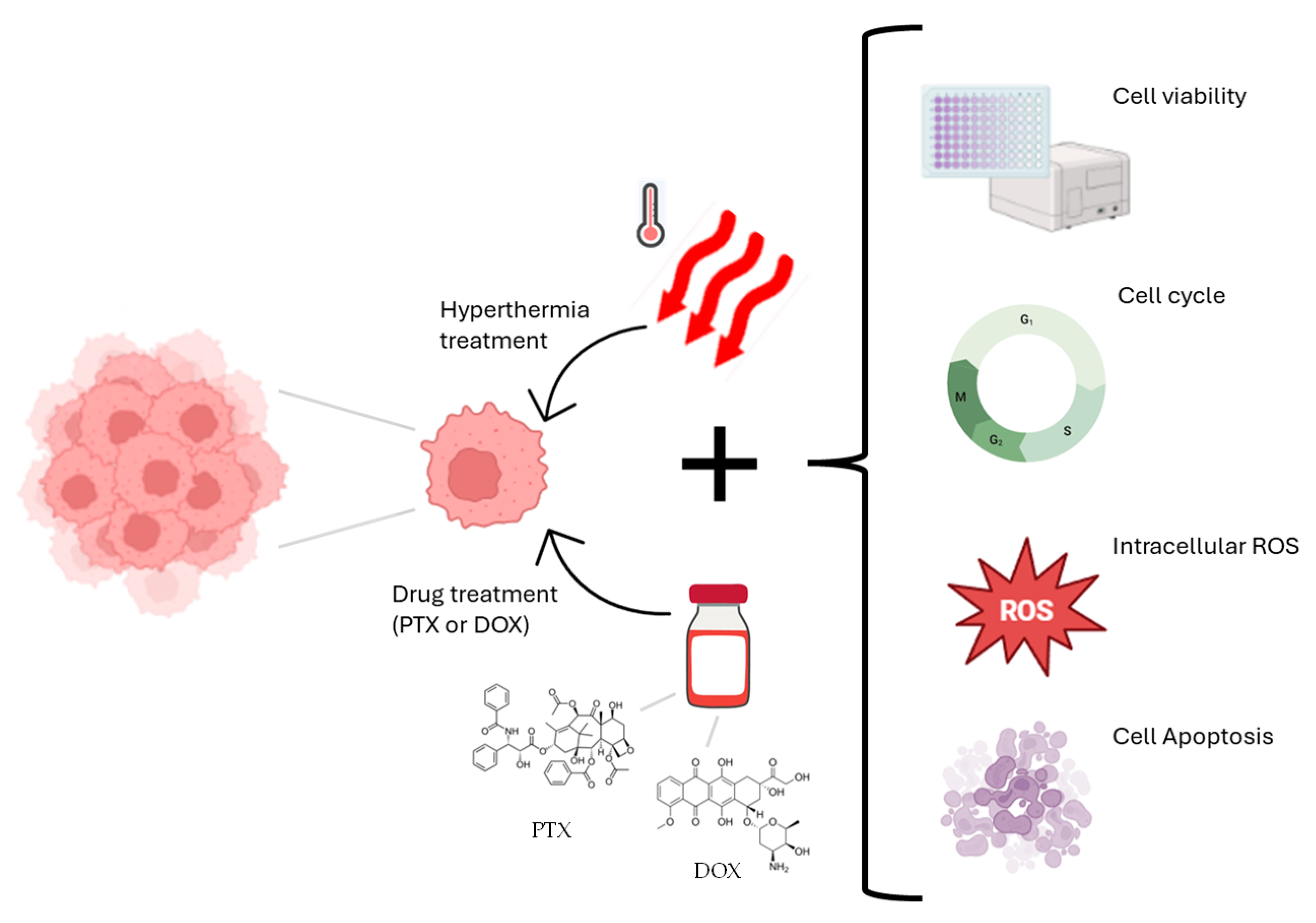
3. Results and Discussion
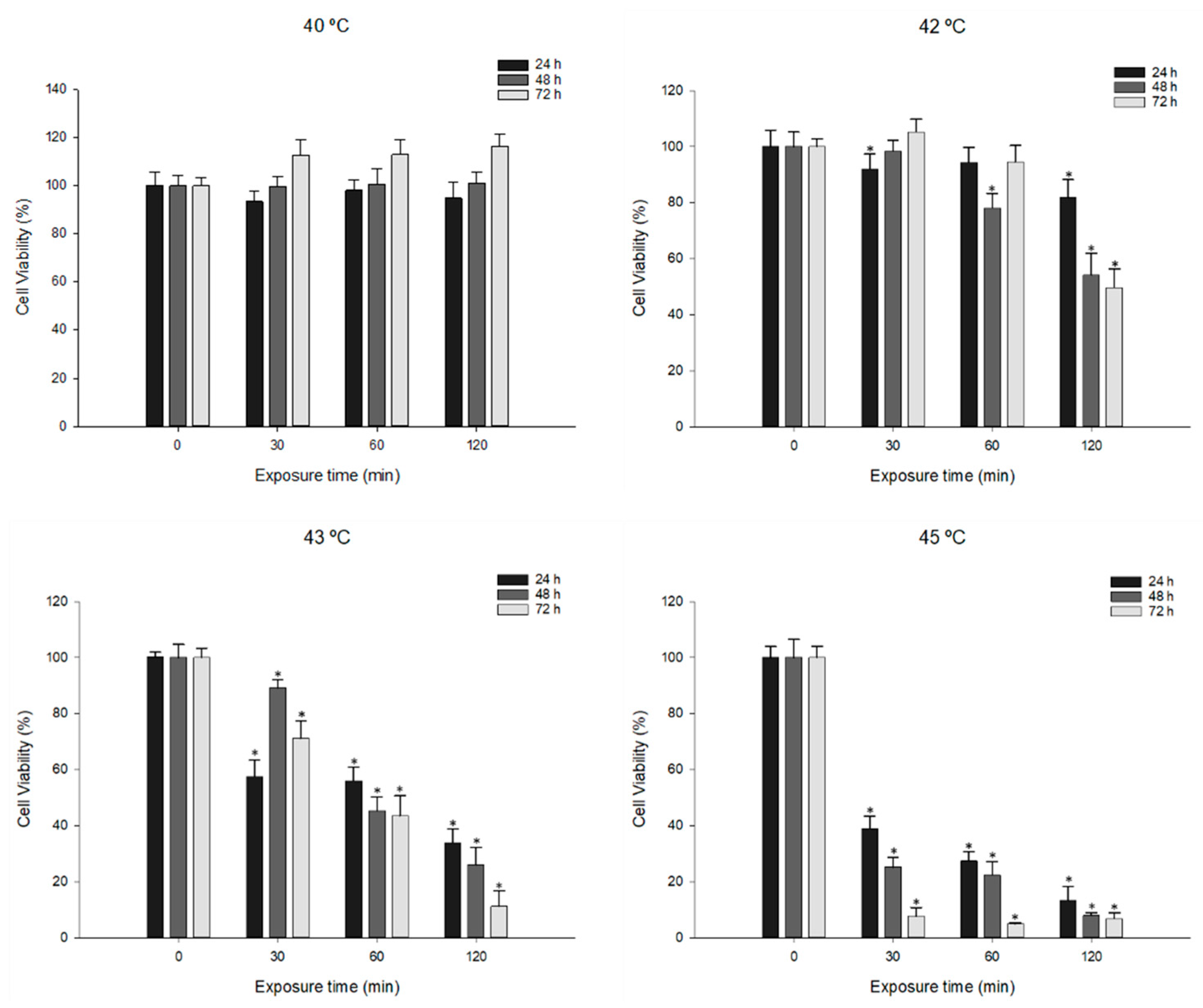
3.1. Effects of Hyperthermia Treatment on MDA-MB-231 Cell Viability
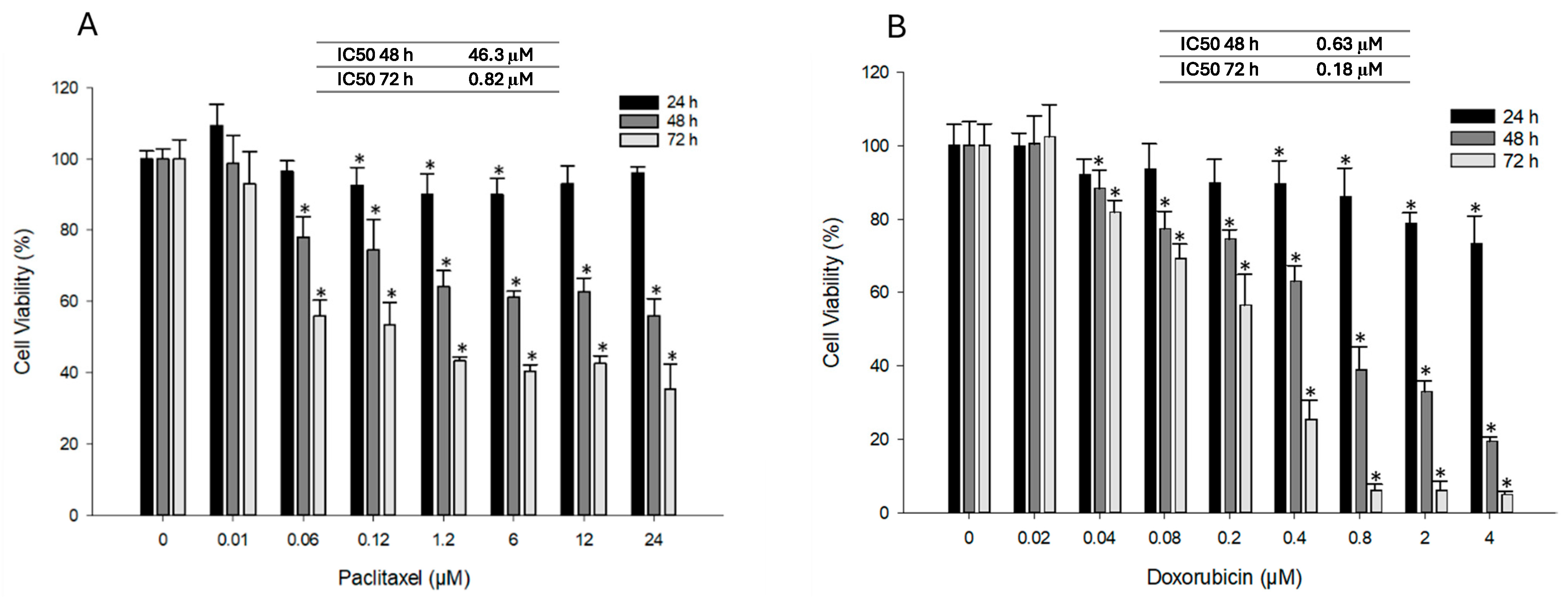
3.2. Effect of PTX and DOX Treatment on Cell Viability
3.3. Effect of Combined PTX and Hyperthermia Treatment on Cell Viability
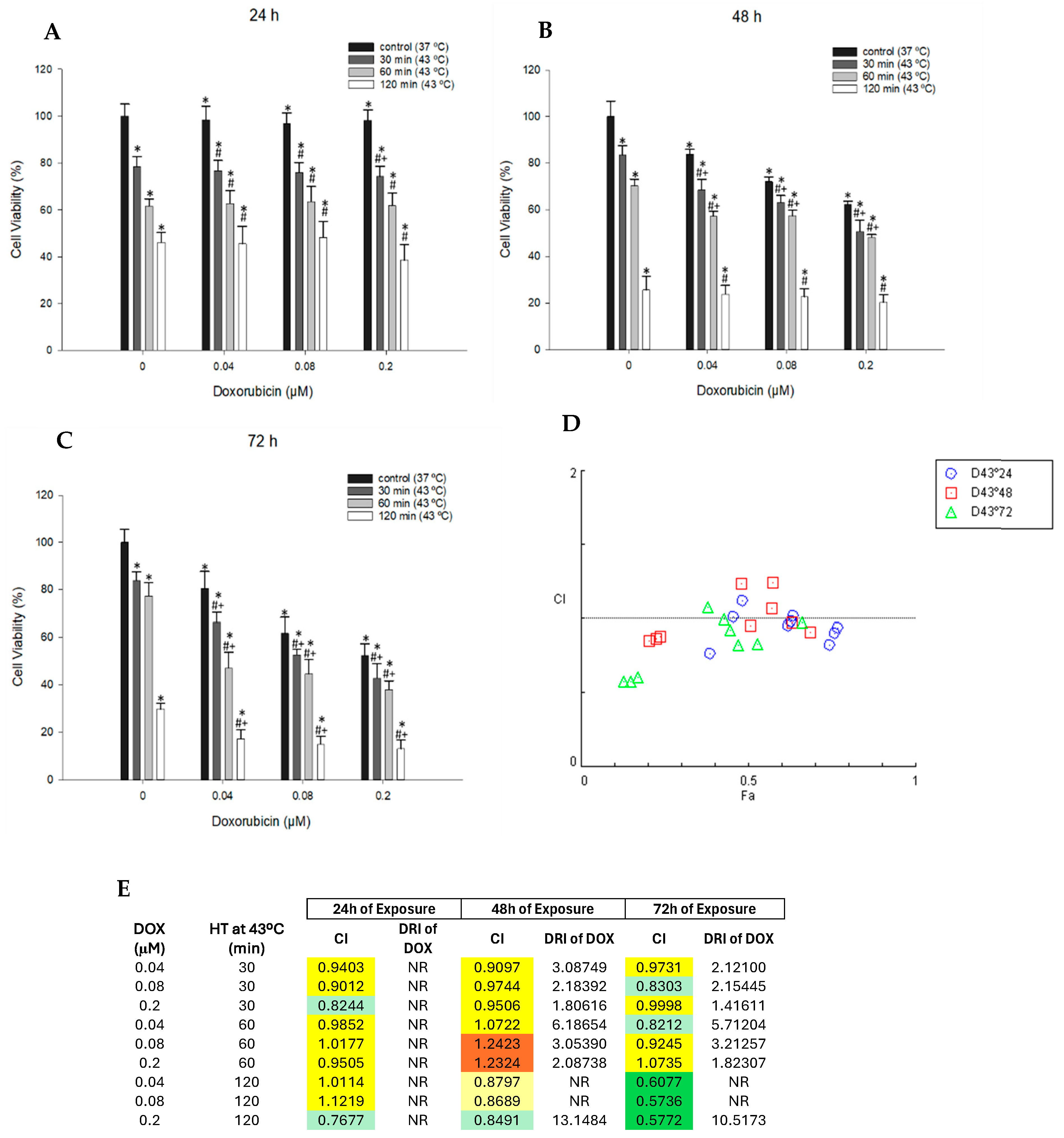
3.4. Effect of Combined DOX and Hyperthermia Treatment on Cell Viability
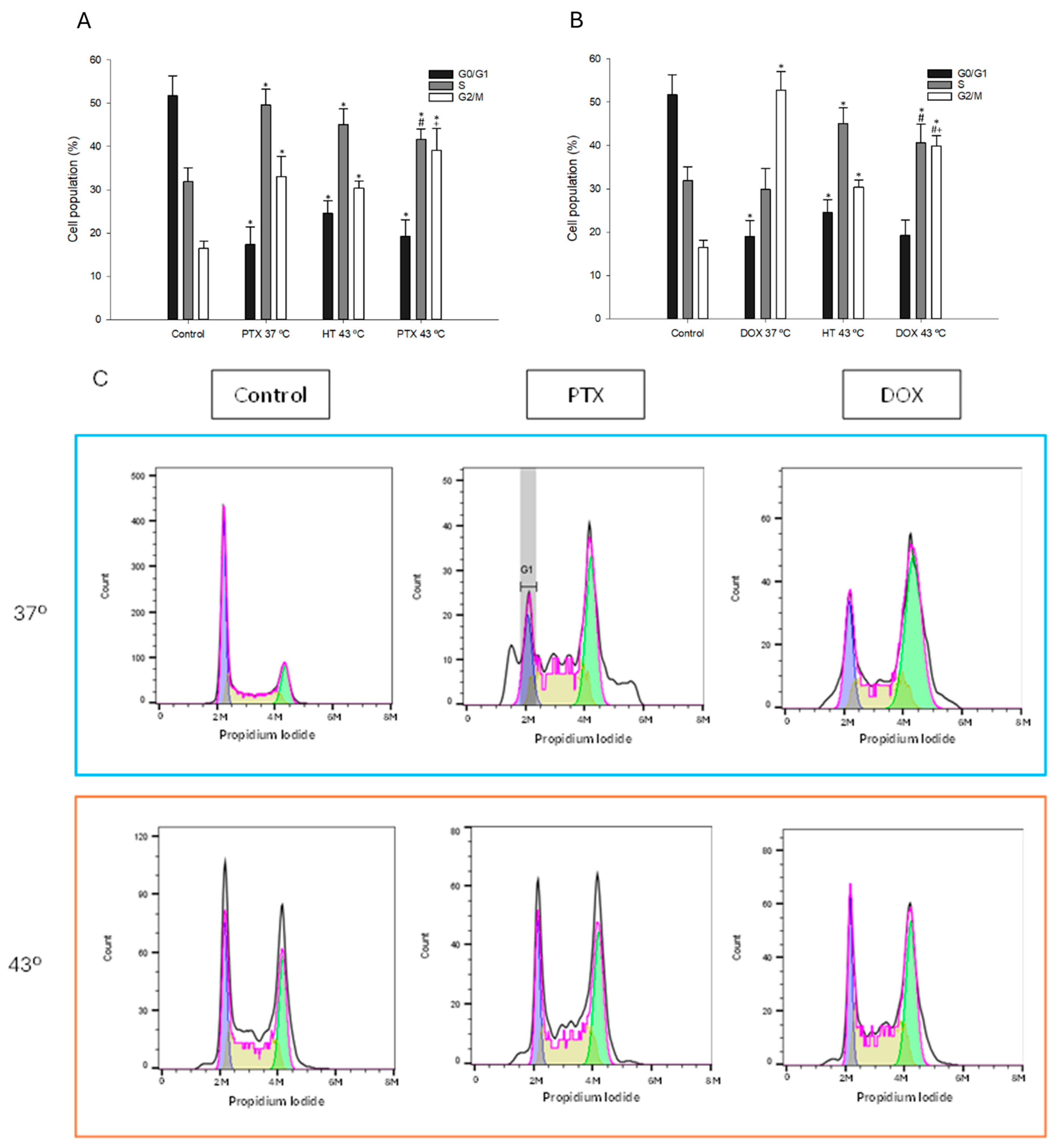
3.5. Effect of Combination of Hyperthermia and PTX/DOX on Cell Cycle Profile
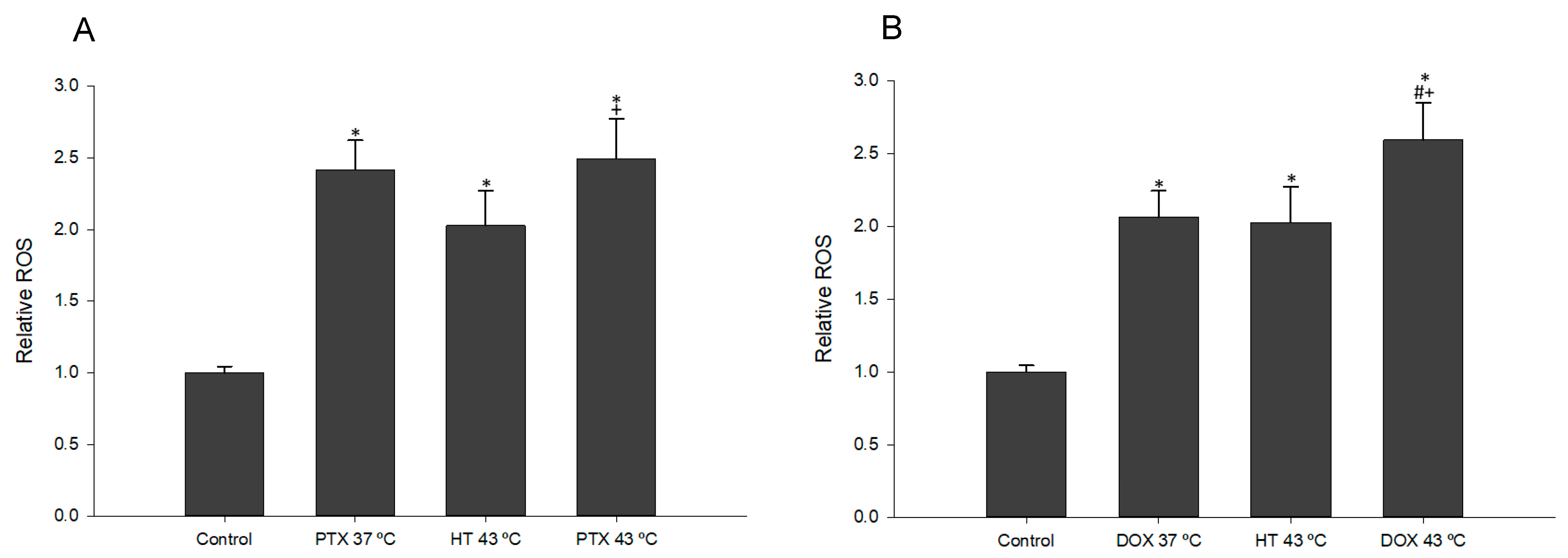
3.6. Effect of Combined Treatment with Chemotherapy and Hyperthermia on Intracellular ROS Levels
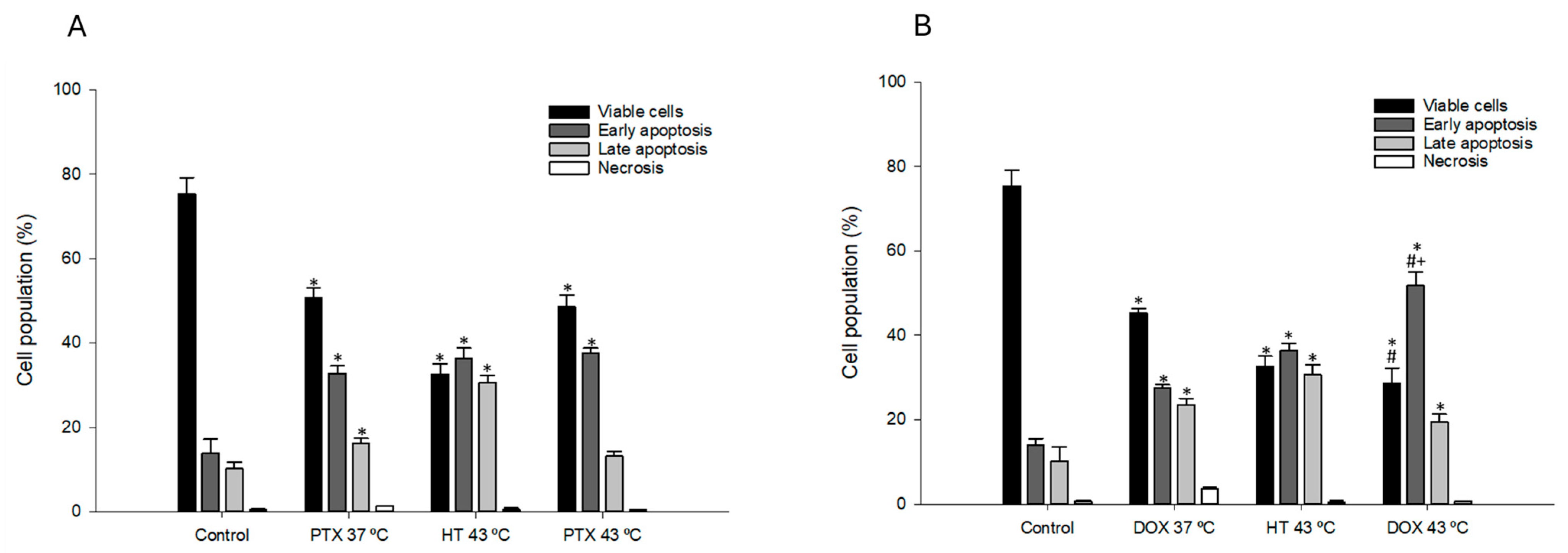
3.7. Effect of Combined Chemotherapy and Hyperthermia Treatment in Apoptosis Induction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Launches New Roadmap on Breast Cancer 2023. Available online: https://www.who.int/news/item/03-02-2023-who-launches-new-roadmap-on-breast-cancer (accessed on 21 April 2023).
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignate, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Malhotra, G.K.; Zhao, X.; Band, H.; Band, V. Histological, molecular and functional subtypes of breast cancers 2010. Cancer Biol. Ther. 2010, 10, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.W.S.; Wu, M.; Cho, W.C.S.; To, K.K.W. Recent advances in the treatment of breast cancer. Front Oncol. 2018, 8, 227. [Google Scholar] [CrossRef]
- Bardia, A.; Mayer, I.A.; Diamond, J.R.; Moroose, R.L.; Isakoff, S.J.; Starodub, A.N.; Shah, N.C.; O’Shaughnessy, J.; Kalinsky, K.; Guarino, M.; et al. Efficacy & safety of anti-Trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J. Clin. Oncol. 2017, 35, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Stockmans, G.; Deraedt, K.; Wildiers, H.; Moerman, P.; Paridaens, R. Triple-negative breast cancer. Curr. Opin Oncol. 2008, 20, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, H.; Matsui, H. The Cytotoxicity of Doxorubicin Can Be Accelerated by a Combination of Hyperthermia and 5-Aminolevulinic Acid. Antioxidants 2021, 10, 1531. [Google Scholar] [CrossRef]
- Issels, R.; Kampmann, E.; Kanaar, R.; Lindner, L.H. Hallmarks of hyperthermia in driving the future of clinical hyperthermia as targeted therapy: Translation into clinical application. Int. J. Hyperth. 2016, 32, 89–95. [Google Scholar] [CrossRef]
- Vaupel, P.; Horsman, M.R. Tumour perfusion and associated physiology: Characterization and significance for hyperthermia. Int. J. Hyperth. 2010, 26, 209–210. [Google Scholar] [CrossRef]
- Oei, A.L.; Vriend, L.E.M.; Crezee, J.; Franken, N.A.P.; Krawczyk, P.M. Effects of hyperthermia on DNA repair pathways: One treatment to inhibit them all. Radiat. Oncol. 2015, 10, 1–13. [Google Scholar] [CrossRef]
- Lee, H.; Kim, S.; Choi, B.H.; Park, M.T.; Lee, J.; Jeong, S.Y.; Choi, E.; Lin, B.; Kim, C.; Park, H. Hyperthermia improves therapeutic efficacy of doxorubicin carried by mesoporous silica nanocontainers in human lung cancer cells. Int. J. Hyperth. 2011, 27, 698–707. [Google Scholar] [CrossRef]
- Krawczyk, P.M.; Eppink, B.; Essers, J.; Stap, J.; Rodermond, H.; Odijk, H.; Zelensky, A.; Bree, C.; Stalpers, L.J.; Buist, M.R.; et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc. Natl. Acad. Sci. USA 2011, 108, 9851–9856. [Google Scholar] [CrossRef]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Özayral, S.; Caserto, J.S.; Ten Cate, R.; Anders, N.M.; Barnett, J.D.; Kandala, S.K.; Henderson, E.; Stewart, J.; Liapi, E.; et al. Increased uptake of doxorubicin by cells undergoing heat stress does not explain its synergistic cytotoxicity with hyperthermia. Int. J. Hyperth. 2019, 36, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Harima, Y.; Ohguri, T.; Imada, H.; Sakurai, H.; Ohno, T.; Hiraki, Y.; Tuji, K.; Tanaka, M.; Terashima, H. A multicentre randomised clinical trial of chemoradiotherapy plus hyperthermia versus chemoradiotherapy alone in patients with locally advanced cervical cancer. Int. J. Hyperth. 2016, 32, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.; Dou, Y.N.; Drake, D.M.; Spence, T.; Gontijo, S.M.; Wells, P.G.; Allen, C. Hyperthermia-mediated drug delivery induces biological effects at the tumor and molecular levels that improve cisplatin efficacy in triple negative breast cancer. J. Control. Release 2018, 282, 35–45. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Khan, I.U.; Guo, N.N.; Wang, B.; Guo, Y.; Xiao, B.; Zhang, Y.; Chu, Y.; Chen, J.; et al. The anti-tumor effects of the combination of microwave hyperthermia and lobaplatin against breast cancer cells in vitro and in vivo. Biosci. Rep. 2022, 42, BSR20190878. [Google Scholar] [CrossRef]
- Ammendola, M.; Currò, G.; Memeo, R.; Curto, L.S.; Luposella, M.; Zuccalà, V.; Pessaux, P.; Navarra, G.; Gadaleta, C.D.; Ranieri, G. Targeting Stem Cells with Hyperthermia: Translational Relevance in Cancer Patients. Oncology 2020, 98, 755–762. [Google Scholar] [CrossRef]
- Kulkarni-Dwivedi, N.; Patel, P.R.; Shravage, B.V.; Umrani, R.D.; Paknikar, K.M.; Jadhav, S.H. Hyperthermia and doxorubicin release by Fol-LSMO nanoparticles induce apoptosis and autophagy in breast cancer cells. Nanomedicine 2022, 17, 1929–1949. [Google Scholar] [CrossRef]
- Loboda, A.; Smolanka Sr, I.; Orel, V.E.; Syvak, L.; Golovko, T.; Dosenko, I.; Lyashenko, A.; Smolanka, I.; Dasyukevich, O.; Tarasenko, T.; et al. Efficacy of Combination Neoadjuvant Chemotherapy and Regional Inductive Moderate Hyperthermia in the Treatment of Patients With Locally Advanced Breast Cancer. Technol. Cancer Res. Treat 2020, 19, 1533033820963599. [Google Scholar] [CrossRef]
- Klimanov, M.Y.; Syvak, L.A.; Orel, V.E.; Lavryk, G.V.; Tarasenko, T.Y.; Orel, V.B.; Rykhalskyi, A.Y.; Stegnii, V.V.; Nesterenko, A.O. Efficacy of Combined Regional Inductive Moderate Hyperthermia and Chemotherapy in Patients With Multiple Liver Metastases From Breast Cancer. Technol. Cancer Res. Treat 2018, 17, 1533033818806003. [Google Scholar] [CrossRef]
- Othman, T.; Goto, S.; Lee, J.B.; Taimura, A.; Matsumoto, T.; Kosaka, M. Hyperthermic enhancement of the apoptotic and antiproliferative activities of paclitaxel. Pharmacology 2001, 62, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, Z.; Li, Y.; Liao, X.; Liao, S.; Cen, S.; Yang, L.; Wei, J.; Hu, X. Short-term hyperthermia promotes the sensitivity of MCF-7 human breast cancer cells to paclitaxel. Biol. Pharm Bull. 2013, 36, 376–383. [Google Scholar] [CrossRef]
- Liao, S.; Hu, X.; Liu, Z.; Lin, Y.; Liang, R.; Zhang, Y.; Li, Q.; Li, Y.; Liao, X. Synergistic action of microwave-induced mild hyperthermia and paclitaxel in inducing apoptosis in the human breast cancer cell line MCF-7. Oncol. Lett. 2019, 17, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Rodriguez, A.; Chiu-Lam, A.; Morozov, V.M.; Ishov, A.M.; Rinaldi, C. Magnetic nanoparticle hyperthermia potentiates paclitaxel activity in sensitive and resistant breast cancer cells. Int. J. Nanomed. 2018, 13, 4771–4779. [Google Scholar] [CrossRef] [PubMed]
- Michalakis, J.; Georgatos, S.D.; De Bree, E.; Polioudaki, H.; Romanos, J.; Georgoulias, V.; Tsiftsis, D.D.; Theodoropoulos, P.A. Short-term exposure of cancer cells to micromolar doses of paclitaxel, with or without hyperthermia, induces long-term inhibition of cell proliferation and cell death in vitro. Ann. Surg. Oncol. 2007, 14, 1220–1228. [Google Scholar] [CrossRef]
- Cai, D.; Liu, L.; Han, C.; Ma, X.; Qian, J.; Zhou, J.; Zhu, W. Cancer cell membrane-coated mesoporous silica loaded with superparamagnetic ferroferric oxide and Paclitaxel for the combination of Chemo/Magnetocaloric therapy on MDA-MB-231 cells. Sci. Rep. 2019, 9, 14475. [Google Scholar] [CrossRef]
- Su, S.; Tian, Y.; Li, Y.; Ding, Y.; Ji, T.; Wu, M.; Nie, G. “Triple-punch” strategy for triple negative breast cancer therapy with minimized drug dosage and improved antitumor efficacy. ACS Nano 2015, 9, 1367–1378. [Google Scholar] [CrossRef]
- Wu, S.K.; Chiang, C.F.; Hsu, Y.H.; Lin, T.H.; Liou, H.C.; Fu, W.M.; Lin, W.L. Short-time focused ultrasound hyperthermia enhances liposomal doxorubicin delivery and antitumor efficacy for brain metastasis of breast cancer. Int. J. Nanomed. 2014, 9, 4485–4494. [Google Scholar] [CrossRef][Green Version]
- Lokerse, W.J.; Bolkestein, M.; Dalm, S.U.; Eggermont, A.M.; de Jong, M.; Grüll, H.; Koning, G.A. Comparing the therapeutic potential of thermosensitive liposomes and hyperthermia in two distinct subtypes of breast cancer. J. Control. Release 2017, 258, 34–42. [Google Scholar] [CrossRef]
- Dragojevic, S.; Ryu, J.S.; Hall, M.E.; Raucher, D. Targeted Drug Delivery Biopolymers Effectively Inhibit Breast Tumor Growth and Prevent Doxorubicin-Induced Cardiotoxicity. Molecules 2022, 27, 3371. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Ma, H.; Yu, F.; Hu, F.; Yuan, H. Water-Responsive Hybrid Nanoparticles Codelivering ICG and DOX Effectively Treat Breast Cancer via Hyperthermia-aided DOX Functionality and Drug Penetration. Adv. Healthc. Mater 2019, 8, 1801486. [Google Scholar] [CrossRef]
- Piehler, S.; Dähring, H.; Grandke, J.; Göring, J.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Courty, J.; Latorre, A.; Somoza, A.; et al. Iron Oxide Nanoparticles as Carriers for DOX and Magnetic Hyperthermia after Intratumoral Application into Breast Cancer in Mice: Impact and Future Perspectives. Nanomaterials 2020, 10, 1016. [Google Scholar] [CrossRef]
- Vujaskovic, Z.; Kim, D.W.; Jones, E.; Lan, L.; McCall, L.; Dewhirst, M.W.; Craciunescu, O.; Stauffer, P.; Liotcheva, V.; Betof, A.; et al. A phase I/II study of neoadjuvant liposomal doxorubicin, paclitaxel, and hyperthermia in locally advanced breast cancer. Int. J. Hyperthermia 2010, 26, 514–521. [Google Scholar] [CrossRef]
- de Maar, J.S.; MSuelmann, B.B.; GJABraat, M.N.; van Diest, P.J.; BVaessen, H.H.; Witkamp, A.J.; Linn, S.C.; Moonen, C.T.; van der Wall, E.; Deckers, R. Phase I feasibility study of Magnetic Resonance guided High Intensity Focused Ultrasound-induced hyperthermia, Lyso-Thermosensitive Liposomal Doxorubicin and cyclophosphamide in de novo stage IV breast cancer patients: Study protocol of the i-GO study. BMJ Open 2020, 10, 040162. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, A.; Kurokawa, H.; Ito, H.; Komatsu, Y.; Matano, D.; Terasaki, M.; Bando, H.; Hara, H.; Matsui, H. Elevated Production of Mitochondrial Reactive Oxygen Species via Hyperthermia Enhanced Cytotoxic Effect of Doxorubicin in Human Breast Cancer Cell Lines MDA-MB-453 and MCF-7. Int. J. Mol. Sci. 2020, 219, 522. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.A.; Graham, E.; Macneill, C.M.; Young, M.; Donati, G.; Wailes, E.M.; Jones, B.T.; Levi-Polyachenko, N.H. Differential response of MCF7, MDA-MB-231, and MCF 10A cells to hyperthermia, silver nanoparticles and silver nanoparticle-induced photothermal therapy. Int. J. Hyperth. 2014, 30, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Bu, J.; Kim, B.H.; Poellmann, M.J.; Hong, S.; Hyun, S.H. Sub-lethal hyperthermia promotes epithelial-to-mesenchymal-like transition of breast cancer cells: Implication of the synergy between hyperthermia and chemotherapy. RSC Adv. 2019, 9, 52–57. [Google Scholar] [CrossRef]
- Chang, J.C.; Chang, H.S.; Wu, Y.C.; Cheng, W.L.; Lin, T.T.; Chang, H.J.; Kuo, S.; Chen, S.-T.; Liu, C.-S. Mitochondrial transplantation regulates antitumour activity, chemoresistance and mitochondrial dynamics in breast cancer. J. Exp. Clin. Cancer Res. 2019, 38, 1–16. [Google Scholar] [CrossRef]
- Ghafari, F.; Rajabi, M.R.; Mazoochi, T.; Taghizadeh, M.; Nikzad, H.; Atlasi, M.A.; Taherian, A. Comparing apoptosis and necrosis effects of Arctium lappa root extract and doxorubicin on MCF7 and MDA-MB-231 cell lines. Asian Pac. J. Cancer Prev. 2017, 18, 795–802. [Google Scholar] [CrossRef]
- Guerreiro, P.S.; Fernandes, A.S.; Costa, J.G.; Castro, M.; Miranda, J.P.; Oliveira, N.G. Differential effects of methoxyamine on doxorubicin cytotoxicity and genotoxicity in MDA-MB-231 human breast cancer cells. Mutat Res. Genet. Toxicol. Environ. Mutagen 2013, 757, 140–147. [Google Scholar] [CrossRef]
- Pilco-Ferreto, N.; Calaf, G.M. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int. J. Oncol. 2016, 49, 753–762. [Google Scholar] [CrossRef]
- Leal, B.Z.; Meltz, M.L.; Mohan, N.; Kuhn, J.; Prihoda, T.J.; Herman, T.S. Interaction of hyperthermia with taxol in human MCF-7 breast adenocarcinoma cells. Int. J. Hyperth. 1999, 15, 225–236. [Google Scholar] [CrossRef]
- Ahmed, K.; Tabuchi, Y.; Kondo, T. Hyperthermia: An effective strategy to induce apoptosis in cancer cells. Apoptosis 2015, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Iizumi, T.; Fujiwara, Y.; Zhao, Q.L.; Tabuchi, Y.; Nomura, T.; Kondo, T. Inhibition of checkpoint kinase 1 abrogates G2/M checkpoint activation and promotes apoptosis under heat stress. Apoptosis 2011, 17, 102–112. [Google Scholar] [CrossRef]
- Hevener, K.E.; Verstak, T.A.; Lutat, K.E.; Riggsbee, D.L.; Mooney, J.W. Recent developments in topoisomerase-targeted cancer chemotherapy. Acta Pharm Sin. B 2018, 8, 844–861. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, O.; Shapira, M.; Hershko, D.D. Differential effects of doxorubicin treatment on cell cycle arrest and Skp2 expression in breast cancer cells. Anticancer. Drugs 2007, 18, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.G.; Pereira-Smith, O.M. Primary and Compensatory Roles for RB Family Members at Cell Cycle Gene Promoters That Are Deacetylated and Downregulated in Doxorubicin-Induced Senescence of Breast Cancer Cells. Mol. Cell. Biol. 2006, 26, 2501–2510. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2012, 65, 157–170. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calçona, A.; Bastos, V.; Oliveira, H. Hyperthermia and Chemotherapy Combination in Triple-Negative Breast Cancer Cells. Appl. Sci. 2025, 15, 9883. https://doi.org/10.3390/app15189883
Calçona A, Bastos V, Oliveira H. Hyperthermia and Chemotherapy Combination in Triple-Negative Breast Cancer Cells. Applied Sciences. 2025; 15(18):9883. https://doi.org/10.3390/app15189883
Chicago/Turabian StyleCalçona, Ana, Verónica Bastos, and Helena Oliveira. 2025. "Hyperthermia and Chemotherapy Combination in Triple-Negative Breast Cancer Cells" Applied Sciences 15, no. 18: 9883. https://doi.org/10.3390/app15189883
APA StyleCalçona, A., Bastos, V., & Oliveira, H. (2025). Hyperthermia and Chemotherapy Combination in Triple-Negative Breast Cancer Cells. Applied Sciences, 15(18), 9883. https://doi.org/10.3390/app15189883






