Photoacoustic Tomography in Forward-Detection Mode for Monitoring Structural Changes in an Extracted Wisdom Tooth
Abstract
1. Introduction
2. Materials and Methods
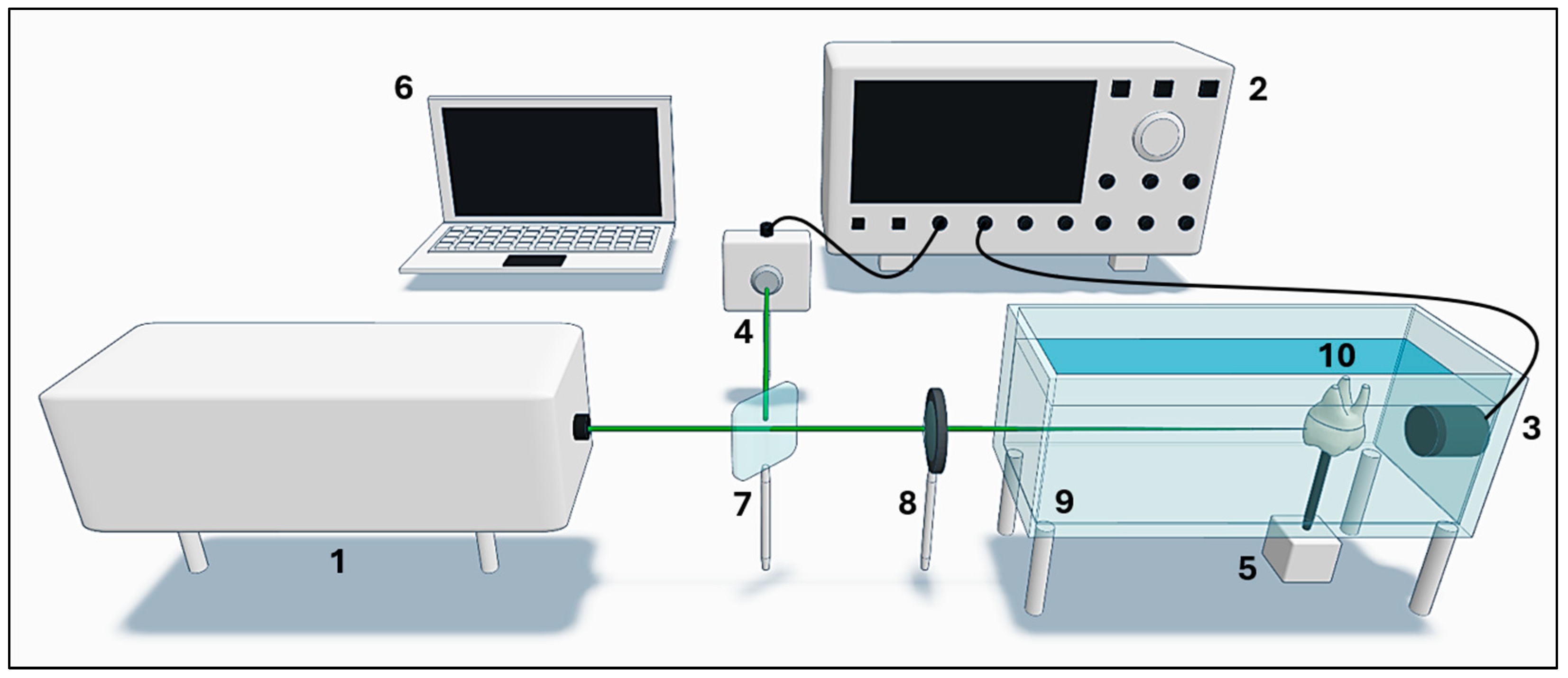
2.1. Signal Acquisition
2.2. Image Reconstruction Algorithm
2.3. Image Analysis and Comparison
3. Results
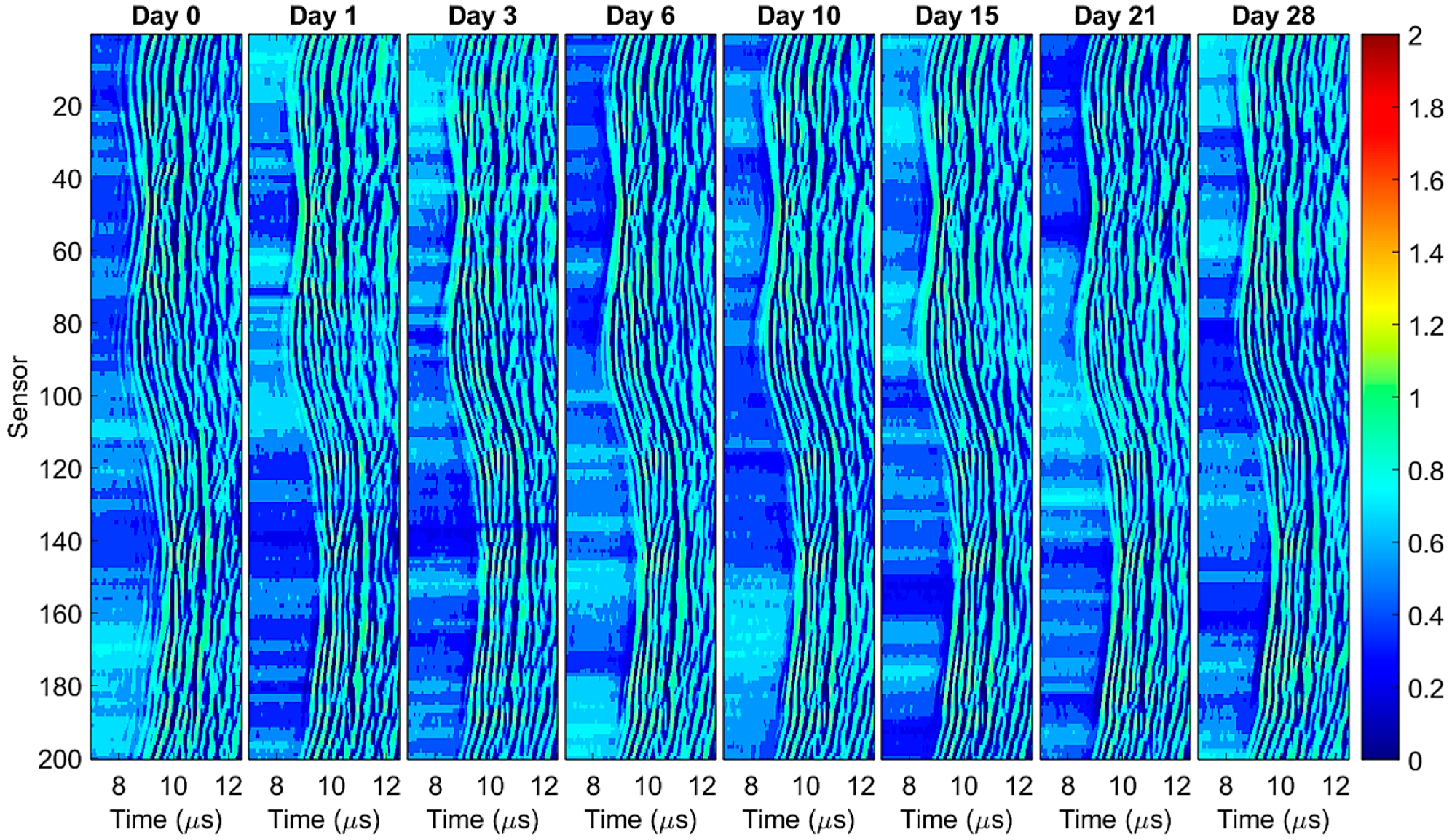
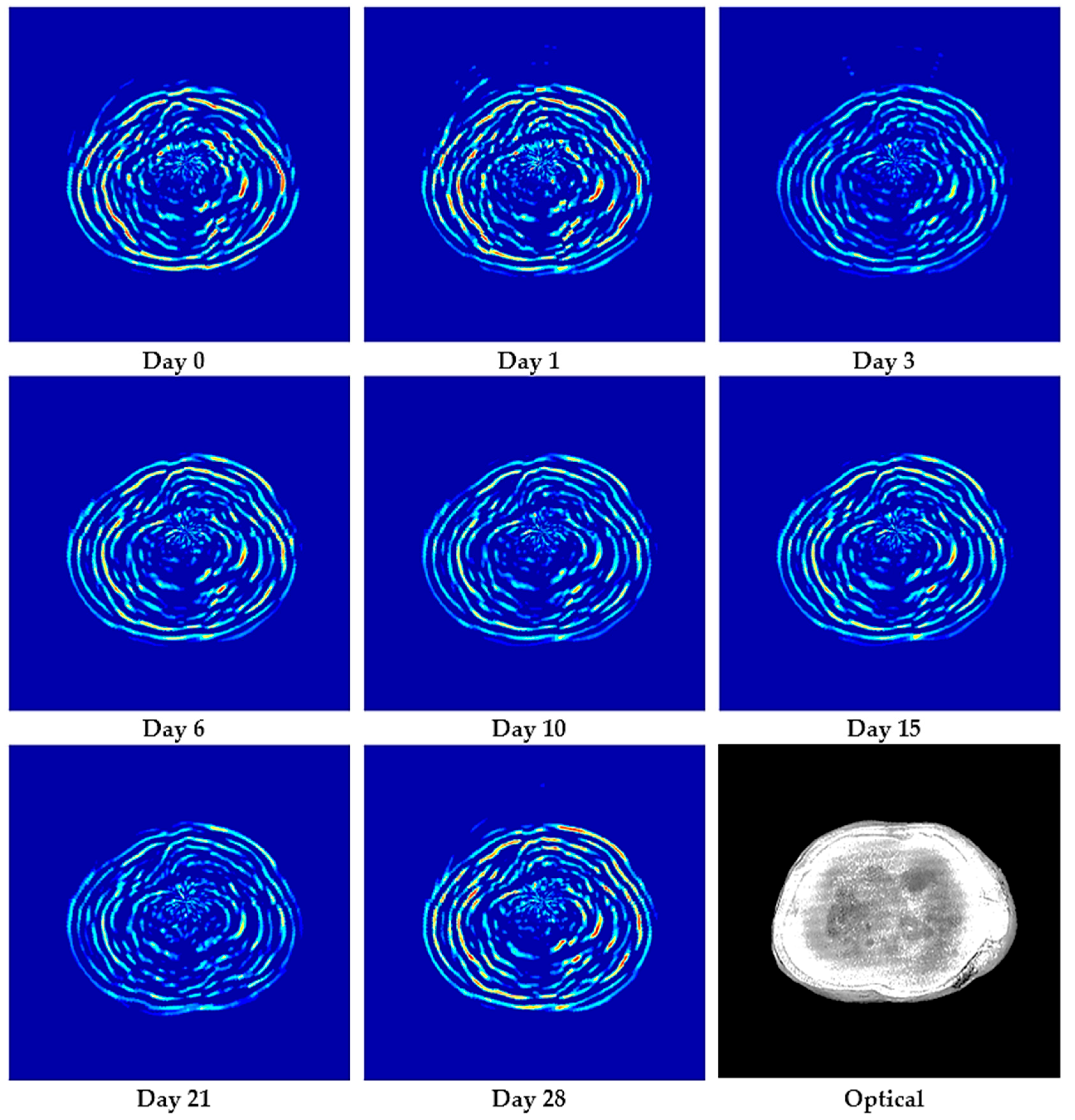
3.1. Photoacoustic Imaging
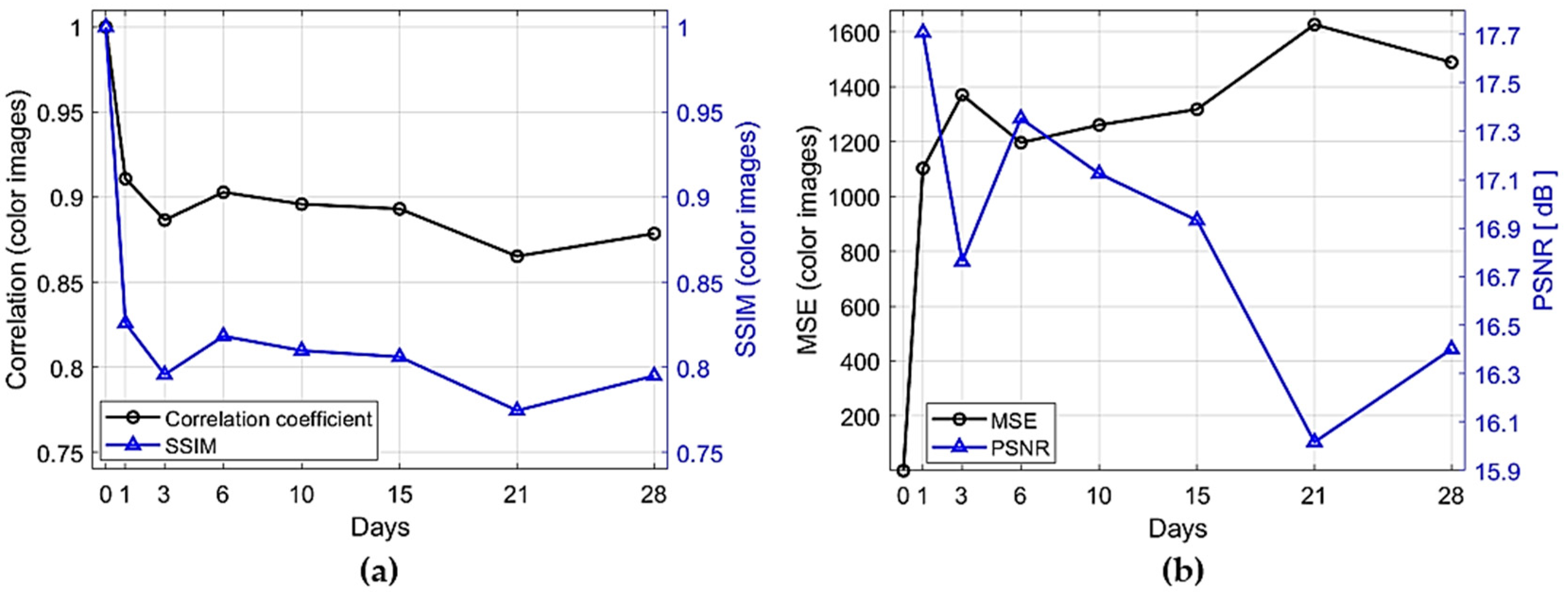
3.2. Image Comparison Metrics
3.3. Image Intensity Profiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSE | Mean Squared Error |
| PAIs | Photoacoustic Images |
| PAT | Photoacoustic Tomography |
| PSNR | Peak Signal-to-Noise Ratio |
| SSC-SAFT | Single Sensor Scanning Synthetic Aperture Focusing Technique |
| SSIM | Structural Similarity Index |
Appendix A
| Reference (Year) | Imaging Modality | Experimental Configuration | Application Focus | Key Findings | Detection Mode | In Vivo Ex Vivo |
|---|---|---|---|---|---|---|
| Cheng et al. (2016) [26] | Dual-contrast PAT (B-mode + S-mode) | Nd:YAG (532 nm, 10 Hz, 80 mJ); single-element transducer (4.39 MHz), circular mechanical scan (120 steps); reconstruction: B-mode (delay and sum) and S-mode (spectral-slope) | Early dental lesion detection | Dual-contrast improves early caries visualization (higher lesion contrast) | Backward | ex vivo |
| Moore et al. (2018) [31] | Clinical PA-US | Nd:YAG (680 nm, 20 Hz, 5ns); linear array transducer (16, 21, 40 MHz); axial scanning; cuttlefish-ink contrast; image J analysis | Periodontal pocket monitoring | 0.01 mm depth precision; full pocket coverage, measurements matched manual probing | Backward | in vivo |
| da Silva et al. (2021) [30] | Visible and NIR PAM | Nd:YAG (532/1064 nm, 10 Hz, 17 mJ/cm2); transducer (5 MHz); scanning X-Y (0.5 mm); color-map amplitude | Incipient occlusal caries detection | 1064 nm gives stronger PA signal than 532 nm | Backward | ex vivo |
| Schneider et al. (2022) [27] | Multispectral optoacoustic tomography | MSOT inVision-256 (680–960 nm); teeth embedded in agarose/intralipid; back projection reconstruction | 3D reconstruction of teeth vs micro-CT /CBCT | Best PAT contrast at 680 nm; PAT 3D reconstructions comparable to CBCT vs. micro-CT reference | Ring array | ex vivo |
| Periyasamy et al. (2024) [29] | Circular and linear PAT | Circular PAT: Nd:YAG (532/1064 nm, 3.18 mJ/cm2), transducer (2.25 MHz); delay and sum reconstruction Linear PAT: LED (850 nm, 0.4 mJ); transducer (7 MHz), 128 channels; delay and sum reconstruction | Teeth with caries/pigmentation; comparison to X-ray CT | Circular PAT at 1064 nm better captured surface than 532 nm; Linear PAT detected dentin cavities and strong signals from pigmented caries | Forward | ex vivo |
| This work (2025) | Single-sensor PAT | Nd:YAG (532 nm, 10 Hz, 9.5 mJ); single-element transducer (5 MHz), sample rotation (1.8° × 200 steps) to emulate circular array; SSC-SAFT reconstruction | Monitoring dehydration-driven structural changes over 28 days | Largest structural change on day 1; progressive inner-region alterations; radial profiles localize inner changes. | Forward | ex vivo |
References
- Zhou, Y.; Yao, J.; Wang, L.V. Tutorial on Photoacoustic Tomography. J. Biomed. Opt. 2016, 21, 061007. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Yao, J.; Wang, L.V. Photoacoustic Tomography: Principles and Advances. Electromagn. Waves 2014, 147, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Daniel, R. Photoacoustics: A Historical Review. Adv. Opt. Photonics 2016, 8, 586–617. [Google Scholar] [CrossRef]
- Gutiérrez-Juárez, G.; Sims, M.J.; Gupta, S.K.; Viator, J.A. Application of the Pulsed Photoacoustic Spectroscopy in Biomedicine. AIP Conf. Proc. 2008, 1032, 70–78. [Google Scholar] [CrossRef]
- Neprokin, A.; Broadway, C.; Myllylä, T.; Bykov, A.; Meglinski, I. Photoacoustic Imaging in Biomedicine and Life Sciences. Life 2022, 12, 588. [Google Scholar] [CrossRef]
- Beard, P. Biomedical Photoacoustic Imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Windra Sari, A.; Widyaningrum, R.; Setiawan, A. Mitrayana Recent Development of Photoacoustic Imaging in Dentistry: A Review on Studies over the Last Decade. Saudi Dent. J. 2023, 35, 423–436. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental Enamel Formation and Implications for Oral Health and Disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef]
- Gutiérrez-Salazar, M.d.P.; Reyes-Gasga, J. Microhardness and Chemical Composition of Human Tooth. Mater. Res. 2003, 6, 367–373. [Google Scholar] [CrossRef]
- Raum, K.; Kempf, K.; Hein, H.J.; Schubert, J.; Maurer, P. Preservation of Microelastic Properties of Dentin and Tooth Enamel in Vitro-A Scanning Acoustic Microscopy Study. Dent. Mater. 2007, 23, 1221–1228. [Google Scholar] [CrossRef]
- Featherstone, J.D.B.; Fried, D. Fundamental Interactions of Lasers with Dental Hard Tissues. Med. Laser Appl. 2001, 16, 181–194. [Google Scholar] [CrossRef]
- Colín-García, M.P.; Ruiz-Veloz, M.; Polo-Parada, L.; Castañeda-Guzmán, R.; Gutiérrez-Juárez, G.; Pérez-Pacheco, A.; Ramírez-Chavarría, R.G. Photoacoustic Image Analysis of Dental Tissue Using Two Wavelengths: A Comparative Study. Photonics 2024, 11, 678. [Google Scholar] [CrossRef]
- Kang, J.; Kim, E.K.; Young Kwak, J.; Yoo, Y.; Song, T.K.; Ho Chang, J. Optimal Laser Wavelength for Photoacoustic Imaging of Breast Microcalcifications. Appl. Phys. Lett. 2011, 99, 153702. [Google Scholar] [CrossRef]
- Blodgett, D.W. Ultrasonic Assessment of Tooth Structure. In Laser Tissue Interaction XIII: Photochemical, Photothermal, and Photomechanical; SPIE: Bellingham, WA, USA, 2002; Volume 4617, pp. 284–288. [Google Scholar] [CrossRef]
- Fried, D.; Glena, R.E.; Featherstone, J.D.B.; Seka, W. Nature of Light Scattering in Dental Enamel and Dentin at Visible and Near-Infrared Wavelengths. Appl. Opt. 1995, 34, 1278–1285. [Google Scholar] [CrossRef]
- Da Silva, T.M.; De Oliveira, H.P.M.; Severino, D.; Balducci, I.; Huhtala, M.F.R.L.; Gonçalves, S.E.P. Direct Spectrometry: A New Alternative for Measuring the Fluorescence of Composite Resins and Dental Tissues. Oper. Dent. 2014, 39, 407–415. [Google Scholar] [CrossRef]
- Luk, K.; Zhao, I.S.; Gutknecht, N.; Chu, C.H. Use of Carbon Dioxide Lasers in Dentistry. Lasers Dent. Sci. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Dogandzhiyska, V.; Angelov, I.; Dimitrov, S.; Uzunov, T. In Vitro Study of Light Radiation Penetration through Dentin, According to the Wavelength. Acta Medica Bulg. 2015, 42, 16–22. [Google Scholar] [CrossRef]
- Zhang, D.; Mao, S.; Lu, C.; Romberg, E.; Arola, D. Dehydration and the Dynamic Dimensional Changes within Dentin and Enamel. Dent. Mater. 2009, 25, 937–945. [Google Scholar] [CrossRef]
- Suliman, S.; Sulaiman, T.A.; Olafsson, V.G.; Delgado, A.J.; Donovan, T.E.; Heymann, H.O. Effect of Time on Tooth Dehydration and Rehydration. J. Esthet. Restor. Dent. 2019, 31, 118–123. [Google Scholar] [CrossRef]
- Alamé, C.; Mehanna Zogheib, C. The Effect of Dehydration on Tooth Color: A Prospective In Vivo Study. Cureus 2023, 15, e48140. [Google Scholar] [CrossRef]
- Almas Chowdhury, A.F.; Alam, A.; Islam, R.R.; Yamauti, M.; Alam, S.; Rahman, M.; Álvarez, P.; Sano, H. The Influences of Dehydration on the Mechanical Properties of Human Dentin. Minerals 2021, 11, 336. [Google Scholar] [CrossRef]
- Hua, L.C.; Wang, W.Y.; Swain, M.V.; Zhu, C.L.; Huang, H.B.; Du, J.K.; Zhou, Z.R. The Dehydration Effect on Mechanical Properties of Tooth Enamel. J. Mech. Behav. Biomed. Mater. 2019, 95, 210–214. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.V. Photoacoustic Imaging in Biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef]
- Cheng, R.; Shao, J.; Gao, X.; Tao, C.; Ge, J.; Liu, X. Noninvasive Assessment of Early Dental Lesion Using a Dual-Contrast Photoacoustic Tomography. Sci. Rep. 2016, 6, 21798. [Google Scholar] [CrossRef]
- Schneider, S.J.M.; Höhne, C.; Schneider, M.; Schmitter, M. Photoacoustic Tomography versus Cone-Beam Computed Tomography versus Microcomputed Tomography: Accuracy of 3D Reconstructions of Human Teeth. PLoS ONE 2022, 17, e0274818. [Google Scholar] [CrossRef]
- Tasmara, F.A.; Widyaningrum, R.; Setiawan, A.; Mitrayana, M. Photoacoustic Imaging of Hidden Dental Caries Using Visible–Light Diode Laser. J. Appl. Clin. Med. Phys. 2023, 24, e13935. [Google Scholar] [CrossRef]
- Periyasamy, V.; Gisi, K.; Pramanik, M. Ex Vivo Human Teeth Imaging with Various Photoacoustic Imaging Systems. Biomed. Opt. Express 2024, 15, 5479–5490. [Google Scholar] [CrossRef]
- da Silva, E.J.; de Miranda, E.M.; de Oliveira Mota, C.C.B.; Das, A.; Gomes, A.S.L. Photoacoustic Imaging of Occlusal Incipient Caries in the Visible and Near-Infrared Range. Imaging Sci. Dent. 2021, 51, 107–115. [Google Scholar] [CrossRef]
- Moore, C.; Bai, Y.; Hariri, A.; Sanchez, J.B.; Lin, C.Y.; Koka, S.; Sedghizadeh, P.; Chen, C.; Jokerst, J.V. Photoacoustic Imaging for Monitoring Periodontal Health: A First Human Study. Photoacoustics 2018, 12, 67–74. [Google Scholar] [CrossRef]
- Sari, A.W.; Widyaningrum, R.; Mitrayana, M. Photoacoustic Imaging for Periodontal Disease Examination. J. Lasers Med. Sci. 2022, 13, e37. [Google Scholar] [CrossRef]
- Park, B.; Oh, D.; Kim, J.; Kim, C. Functional Photoacoustic Imaging: From Nano- and Micro- to Macro-Scale. Nano Converg. 2023, 10, 29. [Google Scholar] [CrossRef]
- Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Functional Photoacoustic Microscopy for High-Resolution and Noninvasive in Vivo Imaging. Nat. Biotechnol. 2006, 24, 848–851. [Google Scholar] [CrossRef]
- Tarvainen, T.; Cox, B. Quantitative Photoacoustic Tomography: Modeling and Inverse Problems. J. Biomed. Opt. 2023, 29, S11509. [Google Scholar] [CrossRef]
- Mallidi, S.; Luke, G.P.; Emelianov, S. Photoacoustic Imaging in Cancer Detection, Diagnosis, and Treatment Guidance. Trends Biotechnol. 2011, 29, 213–221. [Google Scholar] [CrossRef]
- Stan, A.T.; Idorași, L.; Stan, V.F.; Rogobete, A.F.; Sinescu, C.; Negruțiu, M.L.; Romînu, M. Photoacoustic Microscopy in Dental Medicine. J. Interdiscip. Med. 2017, 2, 53–56. [Google Scholar] [CrossRef]
- Ruiz-Veloz, M.; Gutiérrez-Juárez, G.; Polo-Parada, L.; Cortalezzi, F.; Kline, D.D.; Dantzler, H.A.; Cruz-Alvarez, L.; Castro-Beltrán, R.; Hidalgo-Valadez, C. Image Reconstruction Algorithm for Laser-Induced Ultrasonic Imaging: The Single Sensor Scanning Synthetic Aperture Focusing Technique. J. Acoust. Soc. Am. 2023, 153, 560–572. [Google Scholar] [CrossRef]
- Dohmen, M.; Klemens, M.A.; Baltruschat, I.M.; Truong, T.; Lenga, M. Similarity and Quality Metrics for MR Image-to-Image Translation. Sci. Rep. 2025, 15, 3853. [Google Scholar] [CrossRef]
- Al Najjar, Y. Comparative Analysis of Image Quality Assessment Metrics: MSE, PSNR, SSIM and FSIM. Int. J. Sci. Res. 2024, 13, 110–114. [Google Scholar] [CrossRef]
- Wang, Z.; Bovik, A.C.; Sheikh, H.R.; Simoncelli, E.P. Image Quality Assessment: From Error Visibility to Structural Similarity. IEEE Trans. Image Process. 2004, 13, 600–612. [Google Scholar] [CrossRef]
- Brodbelt, R.H.W.; O’brien, W.J.; Fan, P.L.; Frazer-Dib, J.G.; Yu, R. Translucency of Human Dental Enamel. J. Dent. Res. 1981, 60, 1749–1753. [Google Scholar] [CrossRef]
- Demirkan, I.; Yaprak, G.; Ceylan, C.; Algul, E.; Tomruk, C.O.; Bilen, B.; Unlu, M.B. Acoustic Diagnosis of Elastic Properties of Human Tooth by 320 MHz Scanning Acoustic Microscopy after Radiotherapy Treatment for Head and Neck Cancer. Radiat. Oncol. 2020, 15, 38. [Google Scholar] [CrossRef]
- Ajith Singh, M.K. LED-Based Photoacoustic Imaging; Progress in Optical Science and Photonics; Springer: Singapore, 2020; Volume 7, p. 393. [Google Scholar] [CrossRef]
- Francis, K.J.; Boink, Y.E.; Dantuma, M.; Kuniyil Ajith Singh, M.; Manohar, S.; Steenbergen, W. Light Emitting Diodes Based Photoacoustic and Ultrasound Tomography: Imaging Aspects and Applications; Springer: Singapore, 2020; Volume 7, ISBN 9789811539848. [Google Scholar]
- Allen, T.J.; Beard, P.C. High Power Visible Light Emitting Diodes as Pulsed Excitation Sources for Biomedical Photoacoustics. Biomed. Opt. Express 2016, 7, 1260–1270. [Google Scholar] [CrossRef]
- Shemesh, H.; Lindtner, T.; Portoles, C.A.; Zaslansky, P. Dehydration Induces Cracking in Root Dentin Irrespective of Instrumentation: A Two-Dimensional and Three-Dimensional Study. J. Endod. 2018, 44, 120–125. [Google Scholar] [CrossRef]
- Tatchev, D.; Tsenova-Ilieva, I.; Vassilev, T.; Karova, E. The Effect of Experimental Conditions in Root Dentin Microcracks Detection by Micro-Computed Tomography. J. Mech. Behav. Biomed. Mater. 2022, 128, 105108. [Google Scholar] [CrossRef]

| Metric | Category | Comparison Basis | MATLAB Function | Formula | Scale Interpretation |
|---|---|---|---|---|---|
| Correlation | Statistical | Pixel by pixel | corr2(A, B) | Range: [−1, 1] 1 = perfect linear correlation 0 = nonlinear correlation | |
| SSIM | Structural | Local window | ssim(A, B) | Range: [0, 1] 1 = identical <1 = local degradation | |
| MSE | Error | Pixel by pixel | immse(A, B) | Range: [0, ∞) 0 = identical >0 = more error | |
| PSNR | Error | Pixel by pixel | psnr(A, B) | Range: [0, ∞) dB ∞ = identical <∞ = more error |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colín-García, M.P.; Ruiz-Veloz, M.; Gutiérrez-Juárez, G.; Montoya-Ayala, G.; Ramírez-Chavarría, R.G.; Castañeda-Guzmán, R.; Pérez-Pacheco, A. Photoacoustic Tomography in Forward-Detection Mode for Monitoring Structural Changes in an Extracted Wisdom Tooth. Appl. Sci. 2025, 15, 9146. https://doi.org/10.3390/app15169146
Colín-García MP, Ruiz-Veloz M, Gutiérrez-Juárez G, Montoya-Ayala G, Ramírez-Chavarría RG, Castañeda-Guzmán R, Pérez-Pacheco A. Photoacoustic Tomography in Forward-Detection Mode for Monitoring Structural Changes in an Extracted Wisdom Tooth. Applied Sciences. 2025; 15(16):9146. https://doi.org/10.3390/app15169146
Chicago/Turabian StyleColín-García, Marco P., Misael Ruiz-Veloz, Gerardo Gutiérrez-Juárez, Gonzalo Montoya-Ayala, Roberto G. Ramírez-Chavarría, Rosalba Castañeda-Guzmán, and Argelia Pérez-Pacheco. 2025. "Photoacoustic Tomography in Forward-Detection Mode for Monitoring Structural Changes in an Extracted Wisdom Tooth" Applied Sciences 15, no. 16: 9146. https://doi.org/10.3390/app15169146
APA StyleColín-García, M. P., Ruiz-Veloz, M., Gutiérrez-Juárez, G., Montoya-Ayala, G., Ramírez-Chavarría, R. G., Castañeda-Guzmán, R., & Pérez-Pacheco, A. (2025). Photoacoustic Tomography in Forward-Detection Mode for Monitoring Structural Changes in an Extracted Wisdom Tooth. Applied Sciences, 15(16), 9146. https://doi.org/10.3390/app15169146






