Functional Evaluation of Fucus vesiculosus Extract: Bioactivity Retention After In Vitro Digestion and Anti-Inflammatory Effects on Murine Peritoneal Macrophages
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Seaweed Biomass
2.2. Chemical Composition
2.3. Extraction Procedure
2.4. In Vitro Digestion
2.5. Evaluation of the Total Polyphenol Content (TPC), Total Flavonoid Content (TFC), and Total Phlorotannin Content (TPhC)
2.6. Evaluation of Functional Properties
2.6.1. Evaluation of ABTS Radical Scavenging Activity
2.6.2. Evaluation of Growth Inhibitory Activity
2.6.3. Evaluation of Minimal Inhibitory Concentration (MIC)
2.6.4. Evaluation of Anti-Inflammatory Bioactivity
Collection and Treatment of Peritoneal Macrophages (PMs)
Cell Viability
RNA Extraction Procedure, Reverse-Transcription and Quantitative RT-PCR (qRT-PCR)
2.7. Statistical Analysis
3. Results
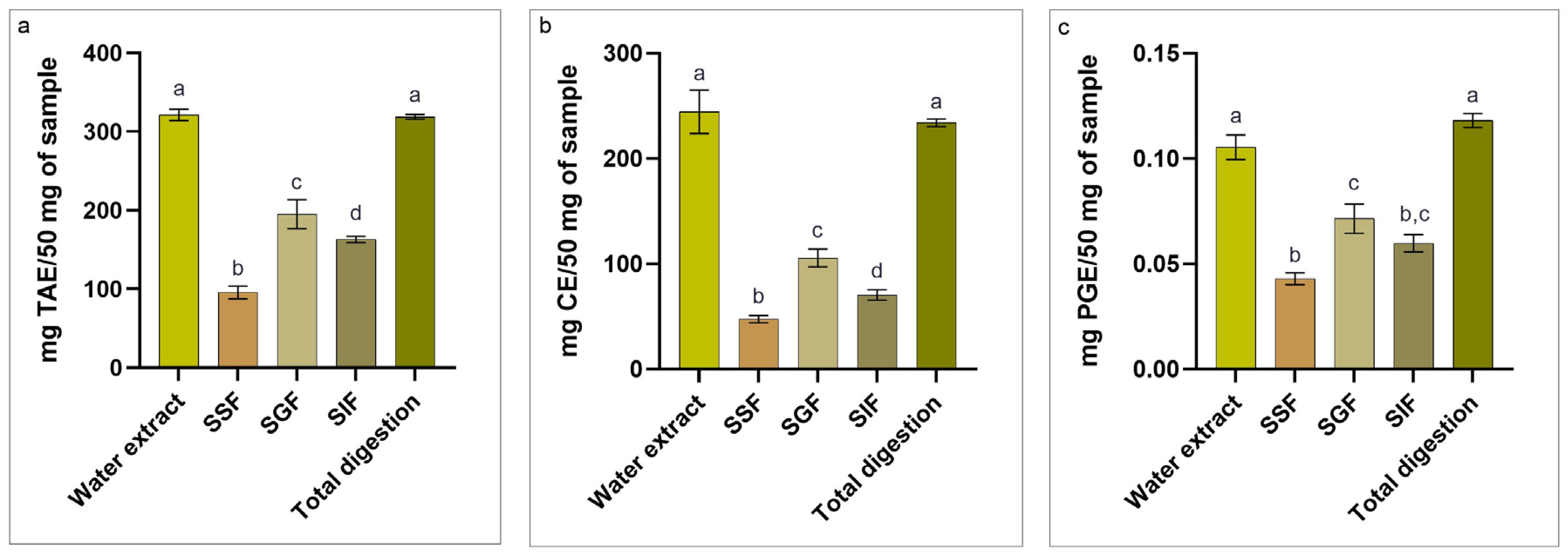
3.1. Chemical Analysis
3.2. Evaluation of Bioactive Compounds
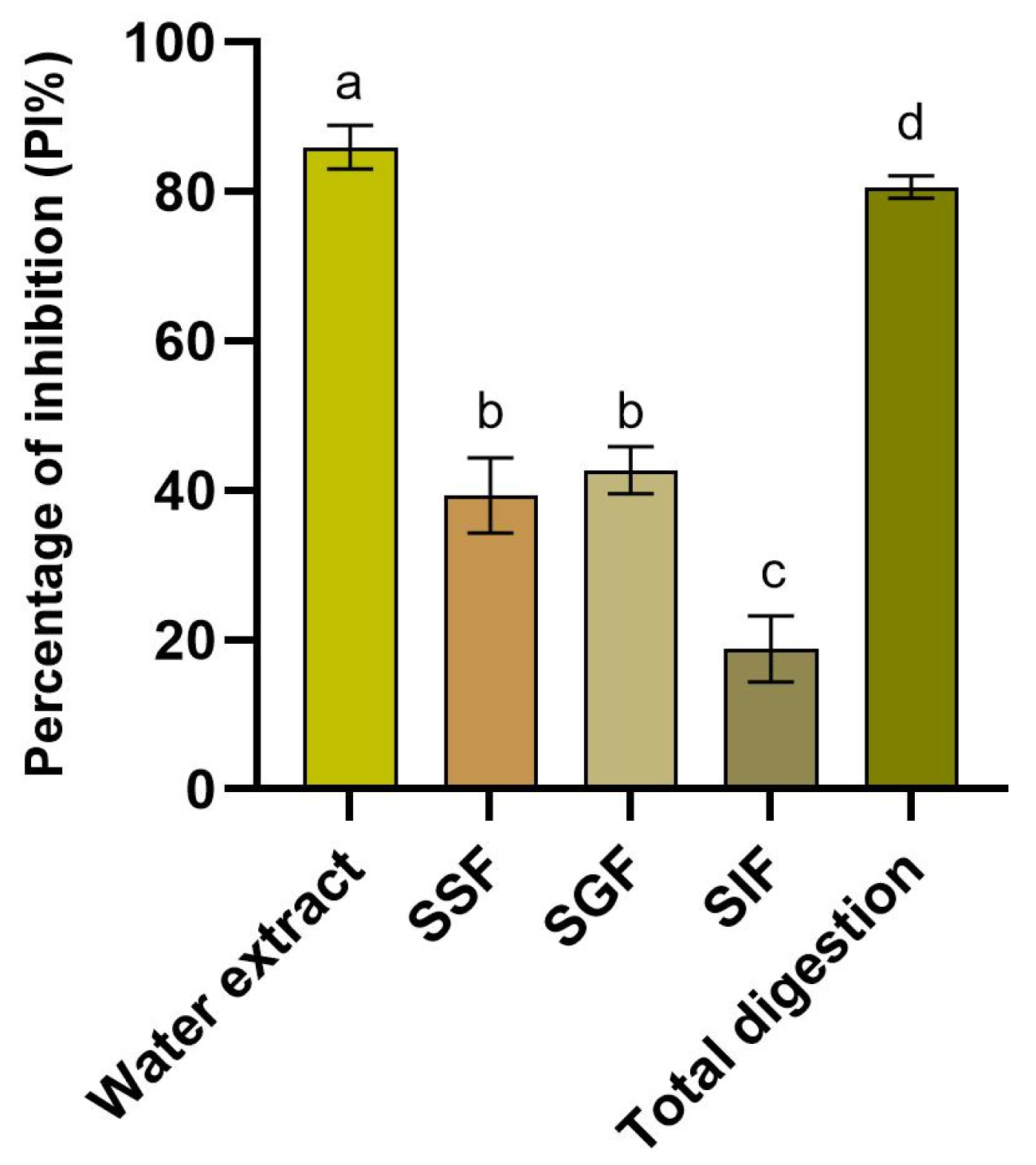
3.3. Evaluation of Antioxidant Capacity
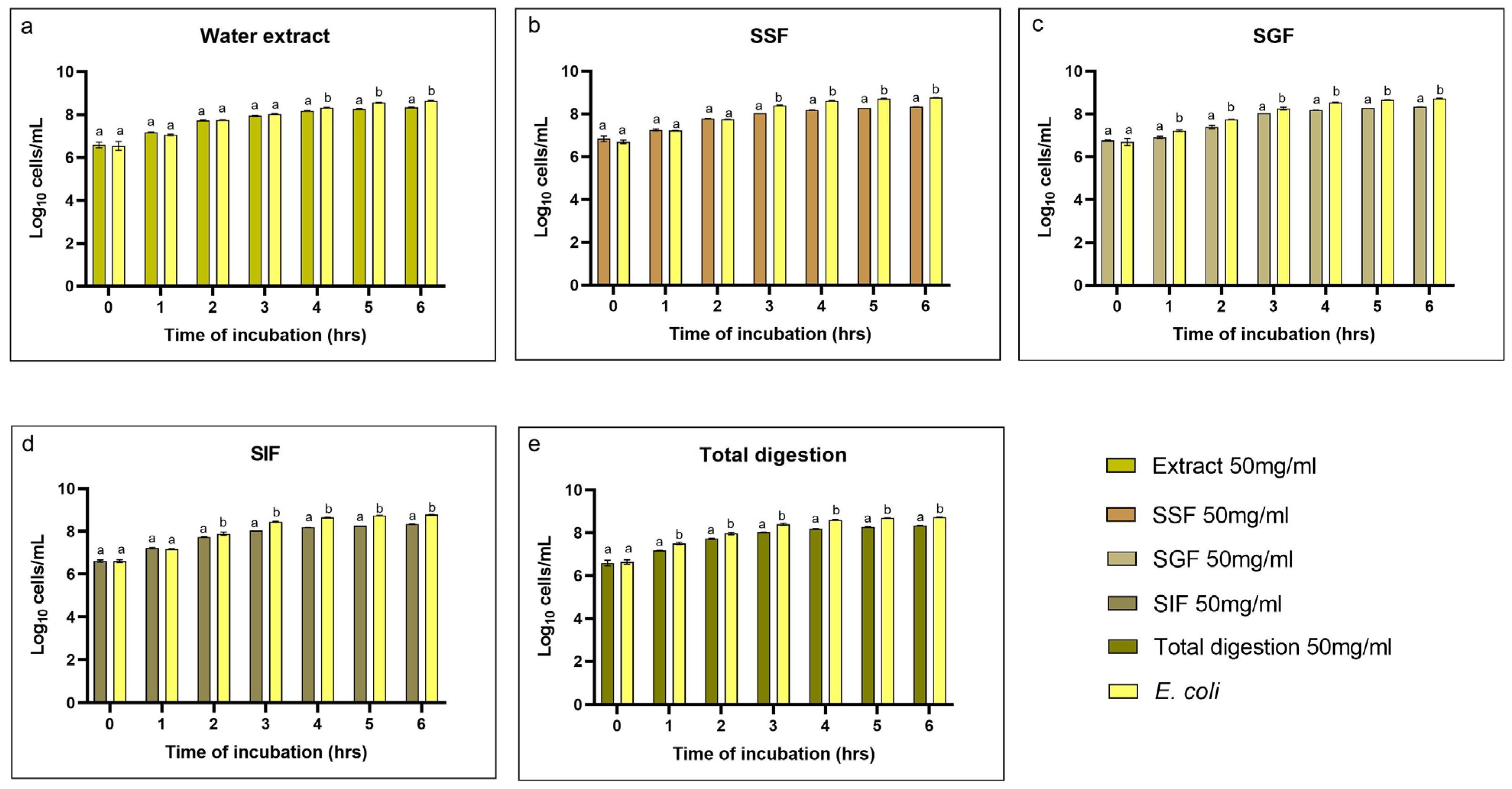
3.4. Evaluation of the Growth Inhibitory Activity of Escherichia coli F18+
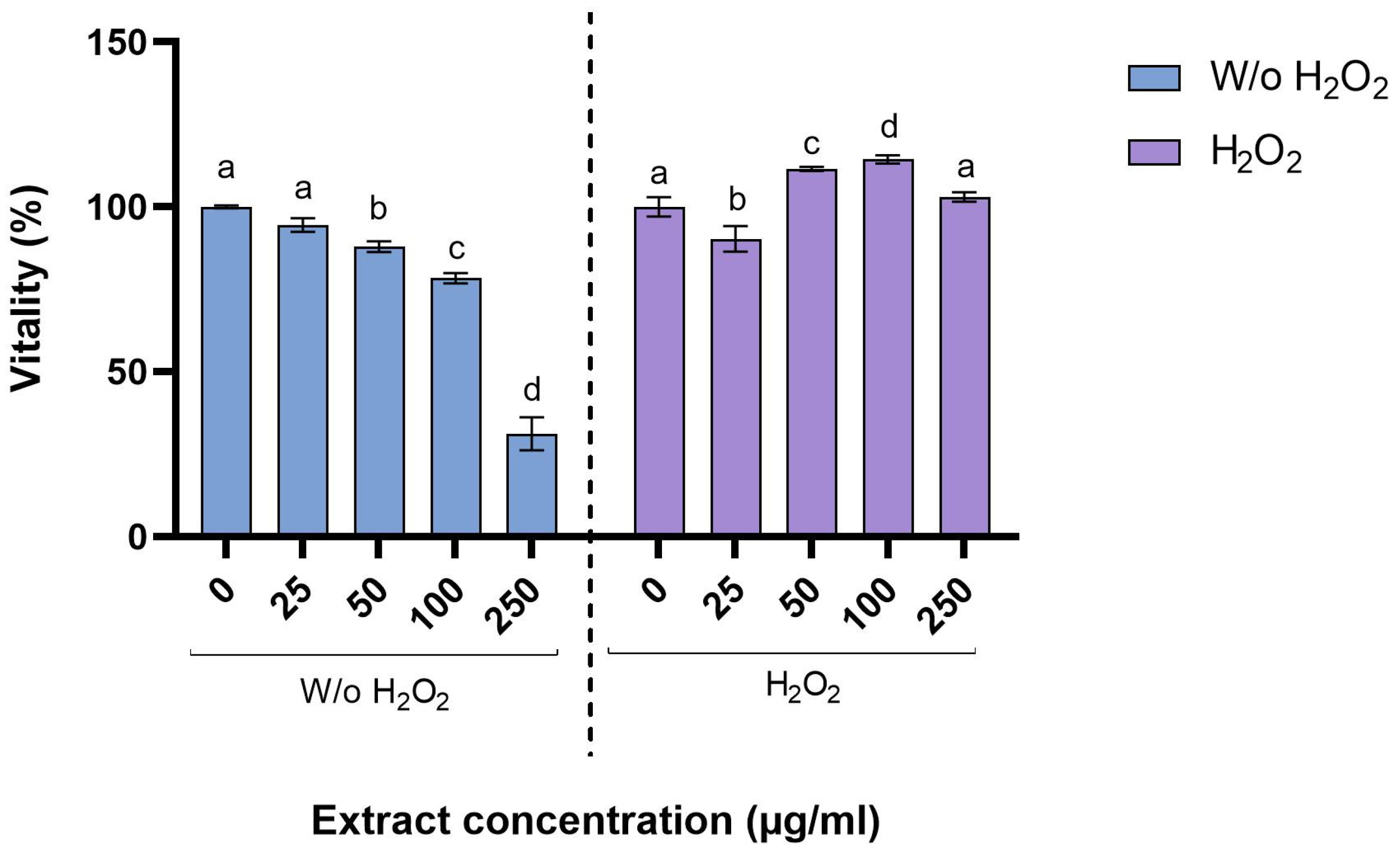
3.5. Cytotoxicity on Mice Peritoneal Macrophages (PMs)
3.6. Evaluation of Anti-Inflammatory Properties
4. Discussion
4.1. Bromatological Composition
4.2. Evaluation of Polyphenols, Flavonoids, and Phlorotannins During the Simulated In Vitro Digestion Process
4.3. Evaluation of the Antioxidant and Growth Inhibition Capacity During the Simulated In Vitro Digestion Process
4.4. Evaluation of the Effect of Fucus vesiculosus Extract on Peritoneal Macrophages Vitality
4.5. Evaluation of the Anti-Inflammatory Properties of Fucus vesiculosus Extract
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jata, N.; Swain, R.; Raula, A.; Priyadarshini, N.P. Alternative feed ingredients for sustainable livestock production. In Futuristic Trends in Agriculture Engineering & Food Sciences Volume 3 Book 24; Iterative International Publishers, Selfypage Developers Pvt Ltd.: Karnataka, India, 2024; pp. 194–204. [Google Scholar]
- Popoola, Y.A.; Idowu, A.B.; Ajijola, S.; Popoola, K.A. Economic Importance of Kenaf Seed as Alternative Feed Resource in Micro Livestock Production: A Review. J. Agric. Ecol. Res. Int. 2024, 25, 45–51. [Google Scholar] [CrossRef]
- Akintan, O.; Gebremedhin, K.G.; Uyeh, D.D. Animal Feed Formulation—Connecting Technologies to Build a Resilient and Sustainable System. Animals 2024, 14, 1497. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Kumar, P.; Saxena, M.J. Feed Additives in Animal Health. In Nutraceuticals in Veterinary Medicine; Springer International Publishing: Cham, Switzerland, 2019; pp. 345–362. [Google Scholar]
- Alimi, N.; Assani, A.S.; Sanni Worogo, H.; Baco, N.M.; Traoré, I.A. Livestock Feed Resources Used as Alternatives during Feed Shortages and Their Impact on the Environment and Ruminant Performance in West Africa: A Systematic Review. Front. Vet. Sci. 2024, 11, 1352235. [Google Scholar] [CrossRef] [PubMed]
- Boudalia, S.; Smeti, S.; Dawit, M.; Senbeta, E.K.; Gueroui, Y.; Dotas, V.; Bousbia, A.; Symeon, G.K. Alternative Approaches to Feeding Small Ruminants and Their Potential Benefits. Animals 2024, 14, 904. [Google Scholar] [CrossRef] [PubMed]
- Fleurence, J. Feeding of Livestock. In Algae in Agrobiology; Wiley: Hoboken, NJ, USA, 2023; pp. 69–133. [Google Scholar]
- González-Meza, G.M.; Elizondo-Luevano, J.H.; Cuellar-Bermudez, S.P.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; Melchor-Martínez, E.M.; Parra-Saldívar, R. New Perspective for Macroalgae-Based Animal Feeding in the Context of Challenging Sustainable Food Production. Plants 2023, 12, 3609. [Google Scholar] [CrossRef]
- Michalak, I.; Tiwari, R.; Dhawan, M.; Alagawany, M.; Farag, M.R.; Sharun, K.; Emran, T.B.; Dhama, K. Antioxidant Effects of Seaweeds and Their Active Compounds on Animal Health and Production—A Review. Vet. Q. 2022, 42, 48–67. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Hayes, M. Potential of Seaweeds to Mitigate Production of Greenhouse Gases during Production of Ruminant Proteins. Glob. Chall. 2023, 7, 2200145. [Google Scholar] [CrossRef]
- Takolander, A. Seasonal Ecophysiology of Fucus vesiculosus (Phaeophyceae) in the Northern Baltic Sea. Eur. J. Phycol. 2023, 58, 300–314. [Google Scholar] [CrossRef]
- Circuncisão, A.R.; Ferreira, S.S.; Silva, A.M.S.; Coimbra, M.A.; Cardoso, S.M. Fucus vesiculosus-Rich Extracts as Potential Functional Food Ingredients: A Holistic Extraction Approach. Foods 2024, 13, 540. [Google Scholar] [CrossRef]
- André, R.; Pacheco, R.; Alves, A.C.; Santos, H.M.; Bourbon, M.; Serralheiro, M.L. The Hypocholesterolemic Potential of the Edible Algae Fucus vesiculosus: Proteomic and Quantitative PCR Analysis. Foods 2023, 12, 2758. [Google Scholar] [CrossRef]
- Yu, J.; Geng, Y.; Xia, H.; Ma, D.; Liu, C.; Wu, R.; Wu, J.; You, S.; Bi, Y. LAB Fermentation Improves Production of Bioactive Compounds and Antioxidant Activity of Withania somnifera Extract and Its Metabolic Signatures as Revealed by LC-MS/MS. J. Microbiol. Biotechnol. 2022, 32, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, A.; Przyborska, J.; McMahon, H. Differential Effects of Fucus vesiculosus Fucoidan on Fibroblast and Macrophage Cell Lines Inflammatory Activation. Results Chem. 2024, 7, 101443. [Google Scholar] [CrossRef]
- Wang, L.; Oliveira, C.; Li, Q.; Ferreira, A.S.; Nunes, C.; Coimbra, M.A.; Reis, R.L.; Martins, A.; Wang, C.; Silva, T.H.; et al. Fucoidan from Fucus vesiculosus Inhibits Inflammatory Response, Both In Vitro and In Vivo. Mar. Drugs 2023, 21, 302. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zheng, Y.; Jia, R.-B.; Luo, D.; Chen, C.; Zhao, M. Fucus vesiculosus Polysaccharide Alleviates Type 2 Diabetes in Rats via Remodeling Gut Microbiota and Regulating Glycolipid Metabolism-Related Gene Expression. Int. J. Biol. Macromol. 2023, 248, 126504. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Smekhova, I.E.; Shikov, A.N. The Biochemical Composition and Antioxidant Properties of Fucus vesiculosus from the Arctic Region. Mar. Drugs 2022, 20, 193. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 22nd ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2023. [Google Scholar]
- Frazzini, S.; Reggi, S.; Dell’Anno, M.; Fifi, A.P.; Scaglia, E.; Ferri, I.; Rossi, L. Chemical-Functional Characterization of Ascophyllum nodosum and Phymatolithon calcareum and Dietary Supplementation in Post-Weaning Pigs. Front. Vet. Sci. 2024, 11, 1431091. [Google Scholar] [CrossRef]
- Tierney, M.S.; Smyth, T.J.; Rai, D.K.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Enrichment of Polyphenol Contents and Antioxidant Activities of Irish Brown Macroalgae Using Food-Friendly Techniques Based on Polarity and Molecular Size. Food Chem. 2013, 139, 753–761. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Attard, E. A Rapid Microtitre Plate Folin-Ciocalteu Method for the Assessment of Polyphenols. Open Life Sci. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput Micro Plate Assays for Screening Flavonoid Content and DPPH-scavenging Activity in Sorghum Bran and Flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can Phlorotannins Purified Extracts Constitute a Novel Pharmacological Alternative for Microbial Infections with Associated Inflammatory Conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef]
- Frazzini, S.; Zuorro, A.; Panseri, S.; Pavlovic, R.; Sgoifo Rossi, C.A.; Rossi, L. Repurposing Hazelnut Waste Products for a Sustainable Economy: A Metabolomic Analysis of Cuticles and Shells to Highlight Their Antioxidant Potential and Inhibitory Activity against Verocytotoxic Escherichia Coli. Sustainability 2023, 15, 3268. [Google Scholar] [CrossRef]
- Frazzini, S.; Torresani, M.C.; Hejna, M.; Di Dio, M.; Rossi, L. Ascophillum nodosum and Lithothamnium calcareum and Their Prebiotic Potential on Lactobacillus Strains. J. Funct. Foods 2024, 118, 106257. [Google Scholar] [CrossRef]
- Rossi, L.; Canala, B.; Fifi, A.P.; Frazzini, S. In Vitro Evaluation of Functional Properties of Extracts of Fucus vesiculosus Obtained with Different Conventional Solvents. Algal Res. 2024, 84, 103787. [Google Scholar] [CrossRef]
- Pepe, G.; Braga, D.; Renzi, T.A.; Villa, A.; Bolego, C.; D’Avila, F.; Barlassina, C.; Maggi, A.; Locati, M.; Vegeto, E. Self-Renewal and Phenotypic Conversion Are the Main Physiological Responses of Macrophages to the Endogenous Estrogen Surge. Sci. Rep. 2017, 7, 44270. [Google Scholar] [CrossRef]
- Bao, Z.; Huang, Y.; Chen, J.; Wang, Z.; Qian, J.; Xu, J.; Zhao, Y. Validation of Reference Genes for Gene Expression Normalization in RAW264.7 Cells under Different Conditions. Biomed. Res. Int. 2019, 2019, 6131879. [Google Scholar] [CrossRef]
- Khatua, S.; Simal-Gandara, J.; Acharya, K. Understanding Immune-Modulatory Efficacy in Vitro. Chem. Biol. Interact. 2022, 352, 109776. [Google Scholar] [CrossRef]
- Ulett, G.C.; Ketheesan, N.; Hirst, R.G. Cytokine Gene Expression in Innately Susceptible BALB/c Mice and Relatively Resistant C57BL/6 Mice during Infection with Virulent Burkholderia pseudomallei. Infect. Immun. 2000, 68, 2034–2042. [Google Scholar] [CrossRef]
- da Silva, J.B.; Carvalho, E.; Covarrubias, A.E.; Ching, A.T.C.; Mattaraia, V.G.M.; Paiva, D.; de Franco, M.; Fávaro, R.D.; Pereira, M.M.; Vasconcellos, S.; et al. Induction of TNF-Alfa and CXCL-2 MRNAs in Different Organs of Mice Infected with Pathogenic Leptospira. Microb. Pathog. 2012, 52, 206–216. [Google Scholar] [CrossRef]
- Mandal, M.; Rakib, A.; Kiran, S.; Al Mamun, M.A.; Raghavan, S.; Kumar, S.; Singla, B.; Park, F.; Leo, M.D.; Singh, U.P. Inhibition of MicroRNA-34c Reduces Detrusor ROCK2 Expression and Urinary Bladder Inflammation in Experimental Cystitis. Life Sci. 2024, 336, 122317. [Google Scholar] [CrossRef]
- Ruiz-Villalba, A.; Mattiotti, A.; Gunst, Q.D.; Cano-Ballesteros, S.; van den Hoff, M.J.B.; Ruijter, J.M. Reference Genes for Gene Expression Studies in the Mouse Heart. Sci. Rep. 2017, 7, 24. [Google Scholar] [CrossRef]
- Díaz-Rubio, M.E.; Pérez-Jiménez, J.; Saura-Calixto, F. Dietary Fiber and Antioxidant Capacity in Fucus vesiculosus Products. Int. J. Food Sci. Nutr. 2009, 60, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.; Agregán, R.; Munekata, P.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F. Proximate Composition and Nutritional Value of Three Macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef] [PubMed]
- Drygaś, B.; Piechowiak, T.; Kreczko, J.; Matłok, N.; Saletnik, B.; Balawejder, M. The Utilisation of Fucus vesiculosus Algae Extracts in the Production of Microgreens Hordeum vulgare L. with an Increased Content of Selected Bioactive Compounds. Plants 2024, 13, 2871. [Google Scholar] [CrossRef] [PubMed]
- Nova, P.; Pimenta-Martins, A.; Maricato, É.; Nunes, C.; Abreu, H.; Coimbra, M.A.; Freitas, A.C.; Gomes, A.M. Chemical Composition and Antioxidant Potential of Five Algae Cultivated in Fully Controlled Closed Systems. Molecules 2023, 28, 4588. [Google Scholar] [CrossRef]
- Neto, R.T.; Marçal, C.; Queirós, A.S.; Abreu, H.; Silva, A.M.S.; Cardoso, S.M. Screening of Ulva rigida, Gracilaria Sp., Fucus vesiculosus and Saccharina latissima as Functional Ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef]
- Frazzini, S.; Torresani, M.C.; Roda, G.; Dell’Anno, M.; Ruffo, G.; Rossi, L. Chemical and Functional Characterization of the Main Bioactive Molecules Contained in Hulled Cannabis sativa L. Seeds for Use as Functional Ingredients. J. Agric. Food Res. 2024, 16, 101084. [Google Scholar] [CrossRef]
- Cavia, M.M.; Arlanzón, N.; Busto, N.; Carrillo, C.; Alonso-Torre, S.R. The Impact of In Vitro Digestion on the Polyphenol Content and Antioxidant Activity of Spanish Ciders. Foods 2023, 12, 1861. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Nogueira, D.P.; Esparza, I.; Vaz, A.A.; Jiménez-Moreno, N.; Martín-Belloso, O.; Ancín-Azpilicueta, C. Stability and Bioaccessibility of Phenolic Compounds in Rosehip Extracts during In Vitro Digestion. Antioxidants 2023, 12, 1035. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Wang, Y.; Wu, Z.-F.; Mei, J.; Zhang, W.-Q.; Shang, Y.-F.; Thakur, K.; Wei, Z.-J. Exploring the Effect of in Vitro Digestion on the Phenolics and Antioxidant Activity of Lycium barbarum Fruit Extract. Food Biosci. 2023, 51, 102255. [Google Scholar] [CrossRef]
- Corona, G.; Coman, M.M.; Guo, Y.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Effect of Simulated Gastrointestinal Digestion and Fermentation on Polyphenolic Content and Bioactivity of Brown Seaweed Phlorotannin-rich Extracts. Mol. Nutr. Food Res. 2017, 61, 1700223. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Andrés-Lacueva, C. Polyphenols and Health: Current State and Progress. J. Agric. Food Chem. 2012, 60, 8773–8775. [Google Scholar] [CrossRef]
- Verzelloni, E.; Tagliazucchi, D.; Conte, A. Relationship between the Antioxidant Properties and the Phenolic and Flavonoid Content in Traditional Balsamic Vinegar. Food Chem. 2007, 105, 564–571. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In Vitro Bio-Accessibility and Antioxidant Activity of Grape Polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- McDougall, G.J.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Assessing Potential Bioavailability of Raspberry Anthocyanins Using an in Vitro Digestion System. J. Agric. Food Chem. 2005, 53, 5896–5904. [Google Scholar] [CrossRef] [PubMed]
- de Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The Pharmacokinetics of Anthocyanins and Their Metabolites in Humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef]
- Bohn, T.; McDougall, G.J.; Alegría, A.; Alminger, M.; Arrigoni, E.; Aura, A.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the Gap—Deficits in Our Knowledge of Aspects Impacting the Bioavailability of Phytochemicals and Their Metabolites—A Position Paper Focusing on Carotenoids and Polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Mateo Anson, N.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Bioavailability of Ferulic Acid Is Determined by Its Bioaccessibility. J. Cereal Sci. 2009, 49, 296–300. [Google Scholar] [CrossRef]
- Rupérez, P.; Ahrazem, O.; Leal, J.A. Potential Antioxidant Capacity of Sulfated Polysaccharides from the Edible Marine Brown Seaweed Fucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef]
- Zaragozá, M.C.; López, D.; Sáiz, M.P.; Poquet, M.; Pérez, J.; Puig-Parellada, P.; Màrmol, F.; Simonetti, P.; Gardana, C.; Lerat, Y.; et al. Toxicity and Antioxidant Activity in Vitro and in Vivo of Two Fucus vesiculosus Extracts. J. Agric. Food Chem. 2008, 56, 7773–7780. [Google Scholar] [CrossRef]
- Dzah, C.S.; Zhang, H.; Gobe, V.; Asante-Donyinah, D.; Duan, Y. Anti- and pro-Oxidant Properties of Polyphenols and Their Role in Modulating Glutathione Synthesis, Activity and Cellular Redox Potential: Potential Synergies for Disease Management. Adv. Redox Res. 2024, 11, 100099. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Telles, C.B.S.; Dantas-Santos, N.; Camara, R.B.G.; Cordeiro, S.L.; Costa, M.S.S.P.; Almeida-Lima, J.; Melo-Silveira, R.F.; Oliveira, R.M.; et al. Antioxidant and Antiproliferative Activities of Heterofucans from the Seaweed Sargassum filipendula. Mar. Drugs 2011, 9, 952–966. [Google Scholar] [CrossRef] [PubMed]
- Veena, C.K.; Josephine, A.; Preetha, S.P.; Varalakshmi, P. Beneficial Role of Sulfated Polysaccharides from Edible Seaweed Fucus vesiculosus in Experimental Hyperoxaluria. Food Chem. 2007, 100, 1552–1559. [Google Scholar] [CrossRef]
- Dutra, R.L.T.; Dantas, A.M.; Marques, D.d.A.; Batista, J.D.F.; Meireles, B.R.L.d.A.; de Magalhães Cordeiro, Â.M.T.; Magnani, M.; Borges, G.d.S.C. Bioaccessibility and Antioxidant Activity of Phenolic Compounds in Frozen Pulps of Brazilian Exotic Fruits Exposed to Simulated Gastrointestinal Conditions. Food Res. Int. 2017, 100, 650–657. [Google Scholar] [CrossRef]
- Dantas, A.M.; Mafaldo, I.M.; Oliveira, P.M.d.L.; Lima, M.d.S.; Magnani, M.; Borges, G.d.S.C. Bioaccessibility of Phenolic Compounds in Native and Exotic Frozen Pulps Explored in Brazil Using a Digestion Model Coupled with a Simulated Intestinal Barrier. Food Chem. 2019, 274, 202–214. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Gawlik-Dziki, U.; Świeca, M. Influence of Phenolic-Food Matrix Interactions on In Vitro Bioaccessibility of Selected Phenolic Compounds and Nutrients Digestibility in Fortified White Bean Paste. Antioxidants 2021, 10, 1825. [Google Scholar] [CrossRef]
- Wang, K.; Hu, S. The Synergistic Effects of Polyphenols and Intestinal Microbiota on Osteoporosis. Front. Immunol. 2023, 14, 1285621. [Google Scholar] [CrossRef]
- Sandsdalen, E.; Haug, T.; Stensvåg, K.; Styrvold, O.B. The Antibacterial Effect of a Polyhydroxylated Fucophlorethol from the Marine Brown Alga, Fucus vesiculosus. World J. Microbiol. Biotechnol. 2003, 19, 777–782. [Google Scholar] [CrossRef]
- Buedenbender, L.; Astone, F.A.; Tasdemir, D. Bioactive Molecular Networking for Mapping the Antimicrobial Constituents of the Baltic Brown Alga Fucus vesiculosus. Mar. Drugs 2020, 18, 311. [Google Scholar] [CrossRef]
- Ayrapetyan, O.N.; Obluchinskaya, E.D.; Zhurishkina, E.V.; Skorik, Y.A.; Lebedev, D.V.; Kulminskaya, A.A.; Lapina, I.M. Antibacterial Properties of Fucoidans from the Brown Algae Fucus vesiculosus L. of the Barents Sea. Biology 2021, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Capuano, E.; Pellegrini, N. An Integrated Look at the Effect of Structure on Nutrient Bioavailability in Plant Foods. J. Sci. Food Agric. 2019, 99, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of Bioactive Food Compounds: A Challenging Journey to Bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Ellouali, M.; Boisson-Vidal, C.; Jozefonvicz, J. Antiproliferative Effect and Interaction of Fucans with Cells. Colloids Surf. B Biointerfaces 1994, 2, 305–314. [Google Scholar] [CrossRef]
- Yoo, Y.-C.; Kim, W.-J.; Kim, S.-Y.; Kim, S.-M.; Chung, M.-K.; Park, J.-W.; Suh, H.-H.; Lee, K.-B.; Park, Y.-I. Immunomodulating Activity of a Fucoidan Isolated from Korean Undaria Pinnatifida Sporophyll. Algae 2007, 22, 333–338. [Google Scholar] [CrossRef]
- Nishiguchi, T.; Jiang, Z.; Ueno, M.; Takeshita, S.; Cho, K.; Roh, S.W.; Kang, K.-H.; Yamaguchi, K.; Kim, D.; Oda, T. Reevaluation of Bactericidal, Cytotoxic, and Macrophage-Stimulating Activities of Commercially Available Fucus vesiculosus Fucoidan. Algae 2014, 29, 237–247. [Google Scholar] [CrossRef][Green Version]
- Catarino, M.D.; Silva, A.; Cruz, M.T.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Phlorotannins from Fucus vesiculosus: Modulation of Inflammatory Response by Blocking NF-ΚB Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 6897. [Google Scholar] [CrossRef]
- Pinteus, S.; Silva, J.; Alves, C.; Horta, A.; Thomas, O.; Pedrosa, R. Antioxidant and Cytoprotective Activities of Fucus spiralis Seaweed on a Human Cell in Vitro Model. Int. J. Mol. Sci. 2017, 18, 292. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Grady, M.N.; Queguineur, B.; Hanniffy, D.; Troy, D.J.; Kerry, J.P.; O’Brien, N.M. Assessment of the Ability of Seaweed Extracts to Protect against Hydrogen Peroxide and Tert-Butyl Hydroperoxide Induced Cellular Damage in Caco-2 Cells. Food Chem. 2012, 134, 1137–1140. [Google Scholar] [CrossRef]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Vo, T.-S.; Kim, S.-K. Fucoidans as a Natural Bioactive Ingredient for Functional Foods. J. Funct. Foods 2013, 5, 16–27. [Google Scholar] [CrossRef]
- Golshany, H.; Siddiquy, M.; Elbarbary, A.; Seddiek, A.S.; Kamal, A.; Yu, Q.; Fan, L. Exploring Fucus vesiculosus Phlorotannins: Insights into Chemistry, Extraction, Purification, Identification and Bioactivity. Food Biosci. 2024, 61, 104769. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. An Overview of Potential Seaweed-Derived Bioactive Compounds for Pharmaceutical Applications. Mar. Drugs 2022, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, E.-A.; Ahn, G.; Jee, Y.; Jeon, Y.-J. Anti-Inflammatory Activity of a Sulfated Polysaccharide Isolated from an Enzymatic Digest of Brown Seaweed Sargassum horneri in RAW 264.7 Cells. Nutr. Res. Pract. 2017, 11, 3. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in Inflammation, Autoimmunity and Cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Tomaszek, Ł. The Biological Role of IL-1, IL-6 and CRP and Their Application in the Diagnosis of the Inflammatory Process. Diagn. Lab. 2022, 58, 66–73. [Google Scholar] [CrossRef]
- Latifynia, A.; Khamesipour, A.; Khansari, N. Proinflammatory Cytokines (TNF-a, IL-1B, IL-6) and Antioxidant in Different Stages of Patients with Cutaneous Leishmaniasis. World J. Adv. Res. Rev. 2023, 17, 825–835. [Google Scholar] [CrossRef]
- Jeong, J.-W.; Hwang, S.J.; Han, M.H.; Lee, D.-S.; Yoo, J.S.; Choi, I.-W.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Kim, G.-Y.; et al. Fucoidan Inhibits Lipopolysaccharide-Induced Inflammatory Responses in RAW 264.7 Macrophages and Zebrafish Larvae. Mol. Cell Toxicol. 2017, 13, 405–417. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ko, C.-I.; Ahn, G.; You, S.; Kim, J.-S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.-J. Molecular Characteristics and Anti-Inflammatory Activity of the Fucoidan Extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef]
- Ni, L.; Wang, L.; Fu, X.; Duan, D.; Jeon, Y.-J.; Xu, J.; Gao, X. In Vitro and in Vivo Anti-Inflammatory Activities of a Fucose-Rich Fucoidan Isolated from Saccharina japonica. Int. J. Biol. Macromol. 2020, 156, 717–729. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, J.; Jing, Y.; Liu, W.; Yang, X.; Hou, X.; Gao, L.; Wei, L. The Concentration of Tumor Necrosis Factor-α Determines Its Protective or Damaging Effect on Liver Injury by Regulating Yap Activity. Cell Death Dis. 2020, 11, 70. [Google Scholar] [CrossRef]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The Interplay between Cytokines, Inflammation, and Antioxidants: Mechanistic Insights and Therapeutic Potentials of Various Antioxidants and Anti-Cytokine Compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.-S.; Kang, N.; Ranasinghe, P.; Lee, H.-S.; Jeon, Y.-J. A Fucoidan Fraction Purified from Chnoospora minima; a Potential Inhibitor of LPS-Induced Inflammatory Responses. Int. J. Biol. Macromol. 2017, 104, 1185–1193. [Google Scholar] [CrossRef]
- Jung, D.H.; Kim, K.-H.; Byeon, H.E.; Park, H.J.; Park, B.; Rhee, D.-K.; Um, S.H.; Pyo, S. Involvement of ATF3 in the Negative Regulation of INOS Expression and NO Production in Activated Macrophages. Immunol. Res. 2015, 62, 35–45. [Google Scholar] [CrossRef]
- Prestes-Carneiro, L.; Shio, M.; Fernandes, P.; Jancar, S. Cross-Regulation of INOS and COX-2 by Its Products in Murine Macrophages Under Stress Conditions. Cell. Physiol. Biochem. 2007, 20, 283–292. [Google Scholar] [CrossRef] [PubMed]

| Gene 1 | Nucleotide Sequence | Accession Number | Reference |
|---|---|---|---|
| IL-1β | F: TCATGGGATGATGATGATAACCTGCT | NM_008361 | [31] |
| R: CCCATACTTTAGGAAGACACGGATT | |||
| IL-6 | F: CGTGGAAATGAGAAAAGAGTTGTGC | NM_001314054 | [32] |
| R: ATGCTTAGGCATAACGCACTAGGT | |||
| TNF-α | F: CACAAGATGCTGGGACAGTGA | NM_013693 | [33] |
| R: TCCTTGATGGTGGTGCATGA | |||
| iNOS | F: GAGACAGGGAAGTCTGAAGCAC | NM_010927 | [34] |
| R: CCAGCAGTAGTTGCTCCTCTTC | |||
| GAPDH | F: CTCCCACTCTTCCACCTTCG | AC166162.6 | [35] |
| R: GCCTCTCTTGCTCAGTGTCC |
| Atomic Mass (u) | Concentration (mg/kg) | |
|---|---|---|
| Be | 9.0121831 | n.d. |
| B | 10.81 | 163.27 ± 4.15 |
| Al | 26.9815384 | 380.38 ± 73.36 |
| Ti | 46.95176 | 5.47 ± 1.17 |
| Ti | 47.947 | 23.42 ± 3.29 |
| V | 50.9961 | 2.93 ± 0.07 |
| Cr | 51.9961 | 4.87 ± 0.41 |
| Mn | 54.938045 | 265.28 ± 20.13 |
| Fe | 55.845 | 761.82 ± 12.88 |
| Co | 58.933195 | 1.28 ± 0.05 |
| Ni | 58.6934 | 5.51 ± 0.38 |
| Cu | 63.546 | 3.05 ± 0.02 |
| Zn | 65.38 | 15.11 ± 0.52 |
| As | 74.91595 | 39.83 ± 0.26 |
| Se | 78.971 | 0.52 ± 0.08 |
| Sr | 87.62 | 823.84 ± 24.33 |
| Mo | 95.95 | 0.93 ± 0.07 |
| Ag | 107.87 | n.d. |
| Cd | 112.441 | 0.84 ± 0.02 |
| Sb | 121.76 | n.d. |
| Ba | 137.327 | 10.24 ± 0.23 |
| Tl | 204.3833 | n.d. |
| Pb | 205.974465 | n.d. |
| Pb | 206.9758969 | n.d. |
| U | 238.03 | 1.36 ± 0.01 |
| Extract Concentration (mg/mL) | Inhibition Rate (%) | |
|---|---|---|
| Extract | 11.5 | 86.15 |
| SSF | 13.5 | 83.98 |
| SGF | 10 | 87.94 |
| SIF | 12 | 85.63 |
| Total digestion | 9.5 | 88.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frazzini, S.; Rizzi, N.; Fifi, A.P.; Fusi, E.; Pilu, S.R.; Rossi, L. Functional Evaluation of Fucus vesiculosus Extract: Bioactivity Retention After In Vitro Digestion and Anti-Inflammatory Effects on Murine Peritoneal Macrophages. Appl. Sci. 2025, 15, 7911. https://doi.org/10.3390/app15147911
Frazzini S, Rizzi N, Fifi AP, Fusi E, Pilu SR, Rossi L. Functional Evaluation of Fucus vesiculosus Extract: Bioactivity Retention After In Vitro Digestion and Anti-Inflammatory Effects on Murine Peritoneal Macrophages. Applied Sciences. 2025; 15(14):7911. https://doi.org/10.3390/app15147911
Chicago/Turabian StyleFrazzini, Sara, Nicoletta Rizzi, Anna Paola Fifi, Eleonora Fusi, Salvatore Roberto Pilu, and Luciana Rossi. 2025. "Functional Evaluation of Fucus vesiculosus Extract: Bioactivity Retention After In Vitro Digestion and Anti-Inflammatory Effects on Murine Peritoneal Macrophages" Applied Sciences 15, no. 14: 7911. https://doi.org/10.3390/app15147911
APA StyleFrazzini, S., Rizzi, N., Fifi, A. P., Fusi, E., Pilu, S. R., & Rossi, L. (2025). Functional Evaluation of Fucus vesiculosus Extract: Bioactivity Retention After In Vitro Digestion and Anti-Inflammatory Effects on Murine Peritoneal Macrophages. Applied Sciences, 15(14), 7911. https://doi.org/10.3390/app15147911









