Cellular Entry, Cytotoxicity, and Antifungal Activity of Newly Synthesized Dendrimers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
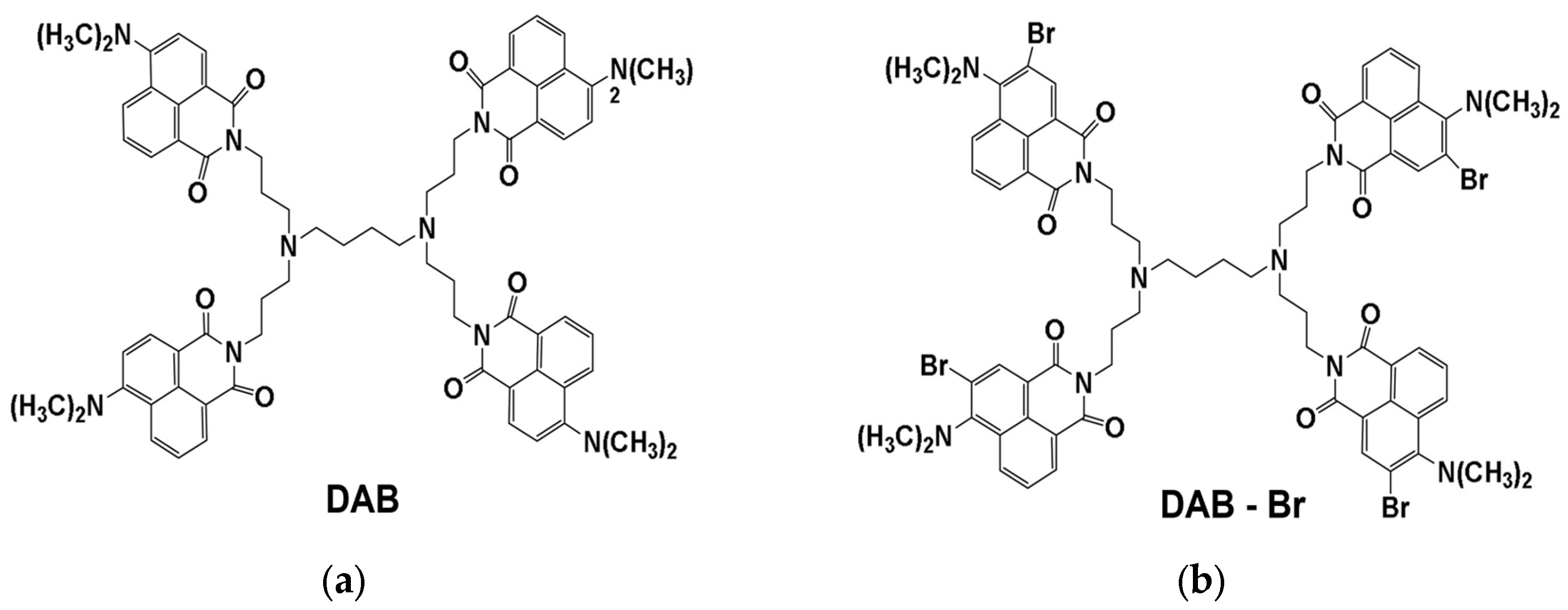
2.2. Synthesis of Dendrimers
2.3. Cell Models
2.3.1. Cell Culturing of Fibroblasts from HSF Cell Line
2.3.2. Fungal Cultures
2.4. Dynamic Light Scattering
2.5. Cell Cytotoxicity
2.5.1. MTT-Test
2.5.2. Antifungal Test
2.6. Evaluation of the Cell Morphology and Cell Membrane Permeabilization by TBE Test
2.7. Laurdan Fluorescence Spectroscopy
2.8. Statistics
3. Results
3.1. Size and Zeta (ζ) Potential of DAB and DAB-Br
3.2. Cell Cytotoxicity
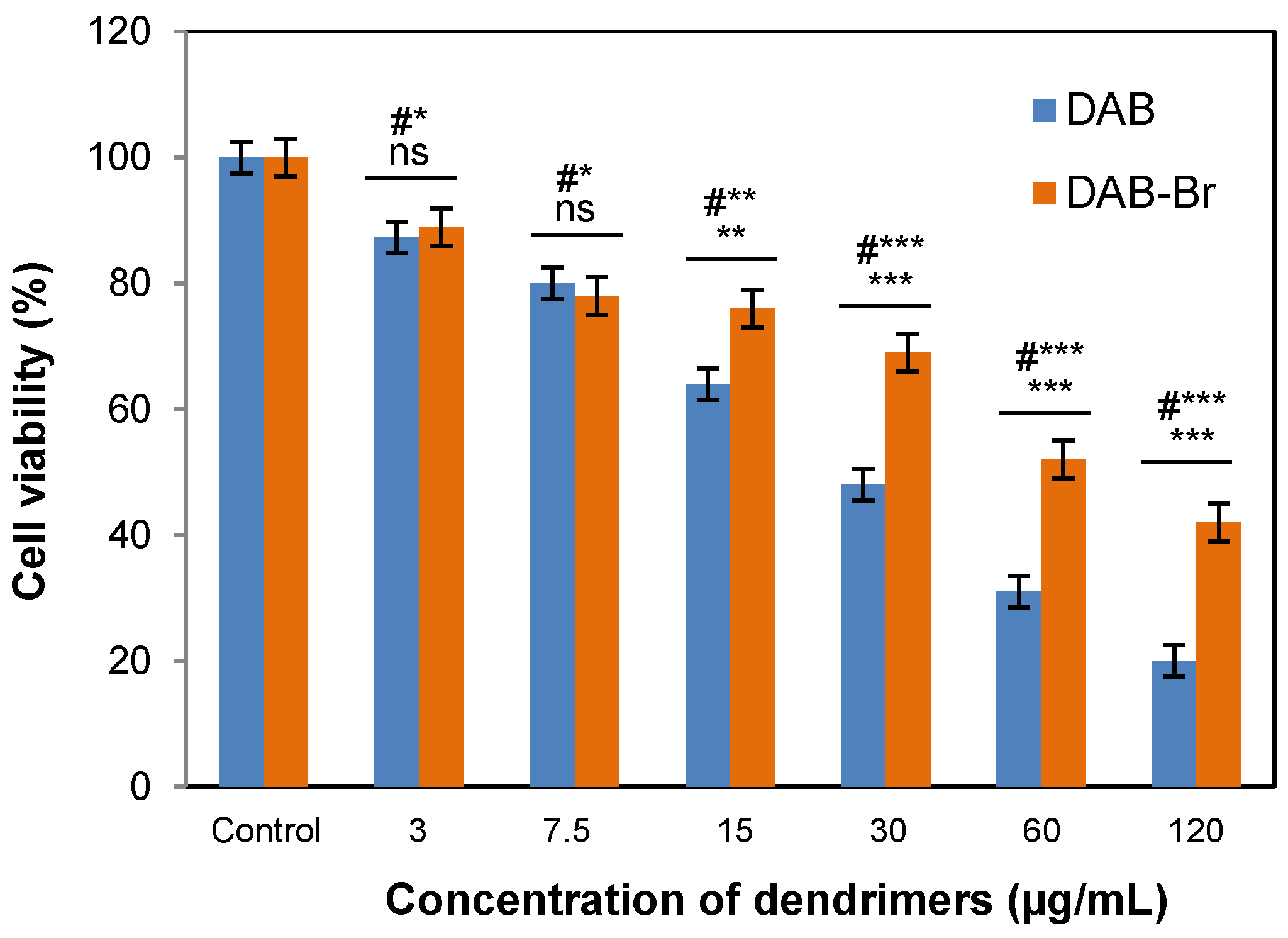
3.2.1. MTT Assay
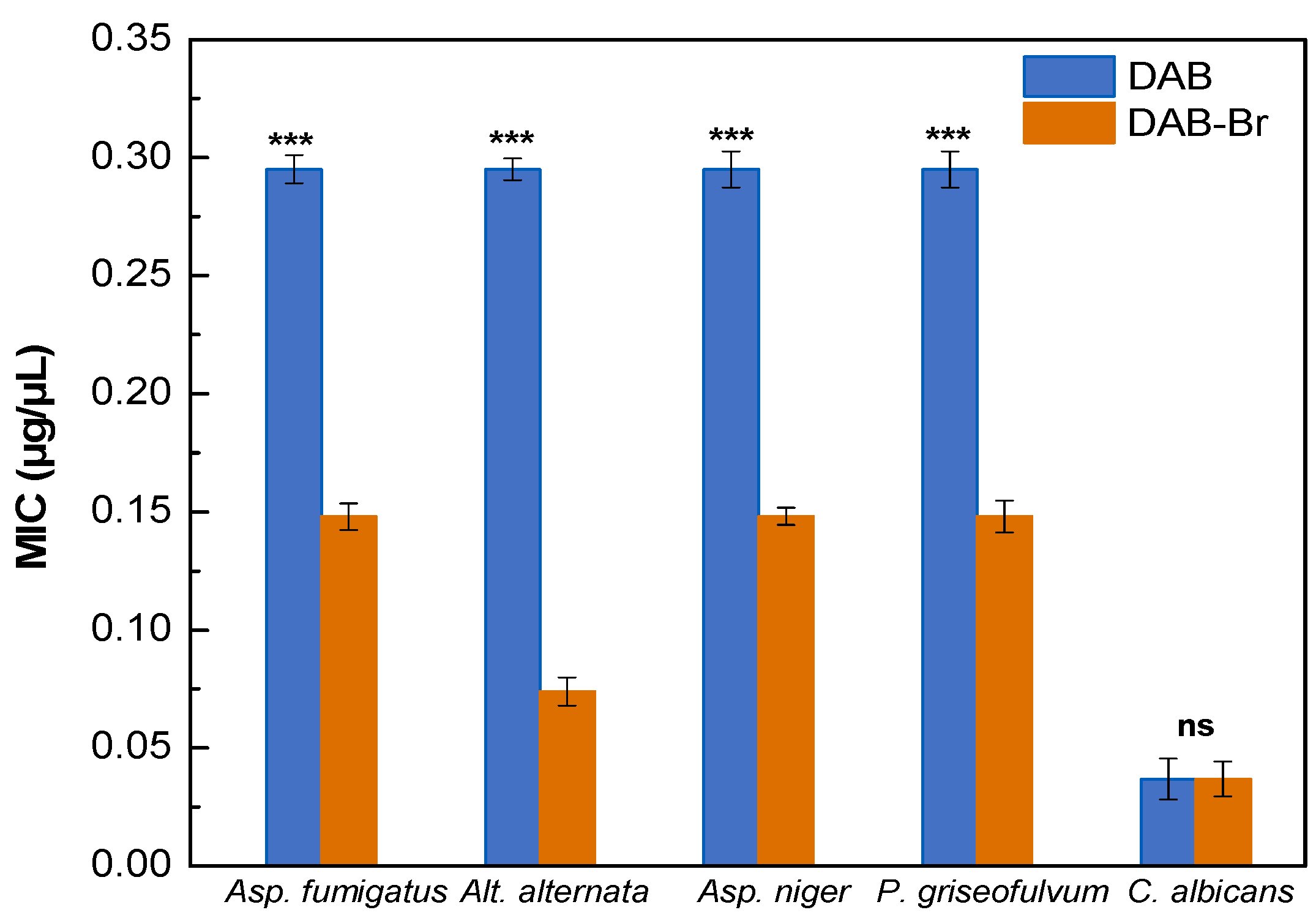
3.2.2. Antifungal Activity
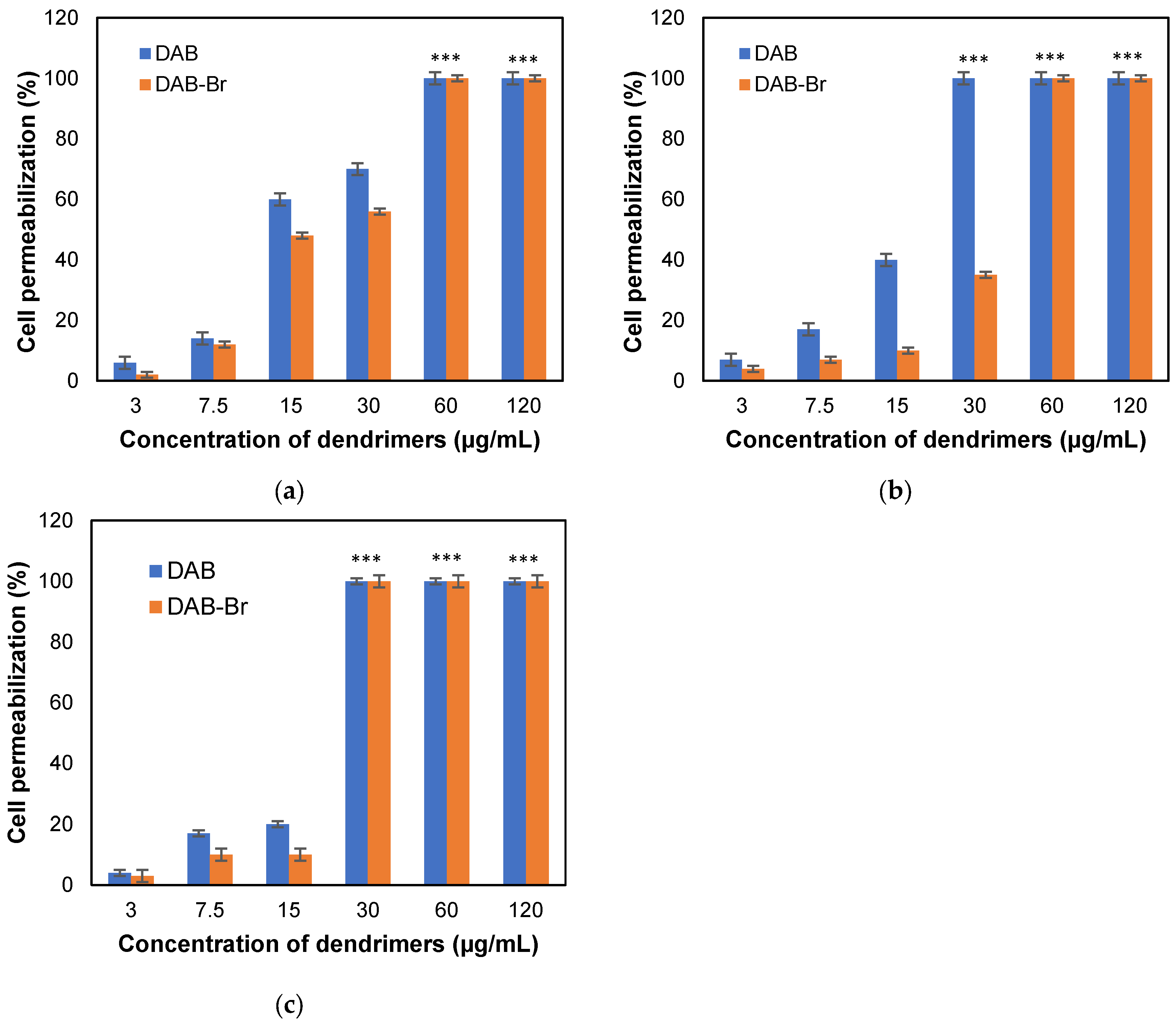
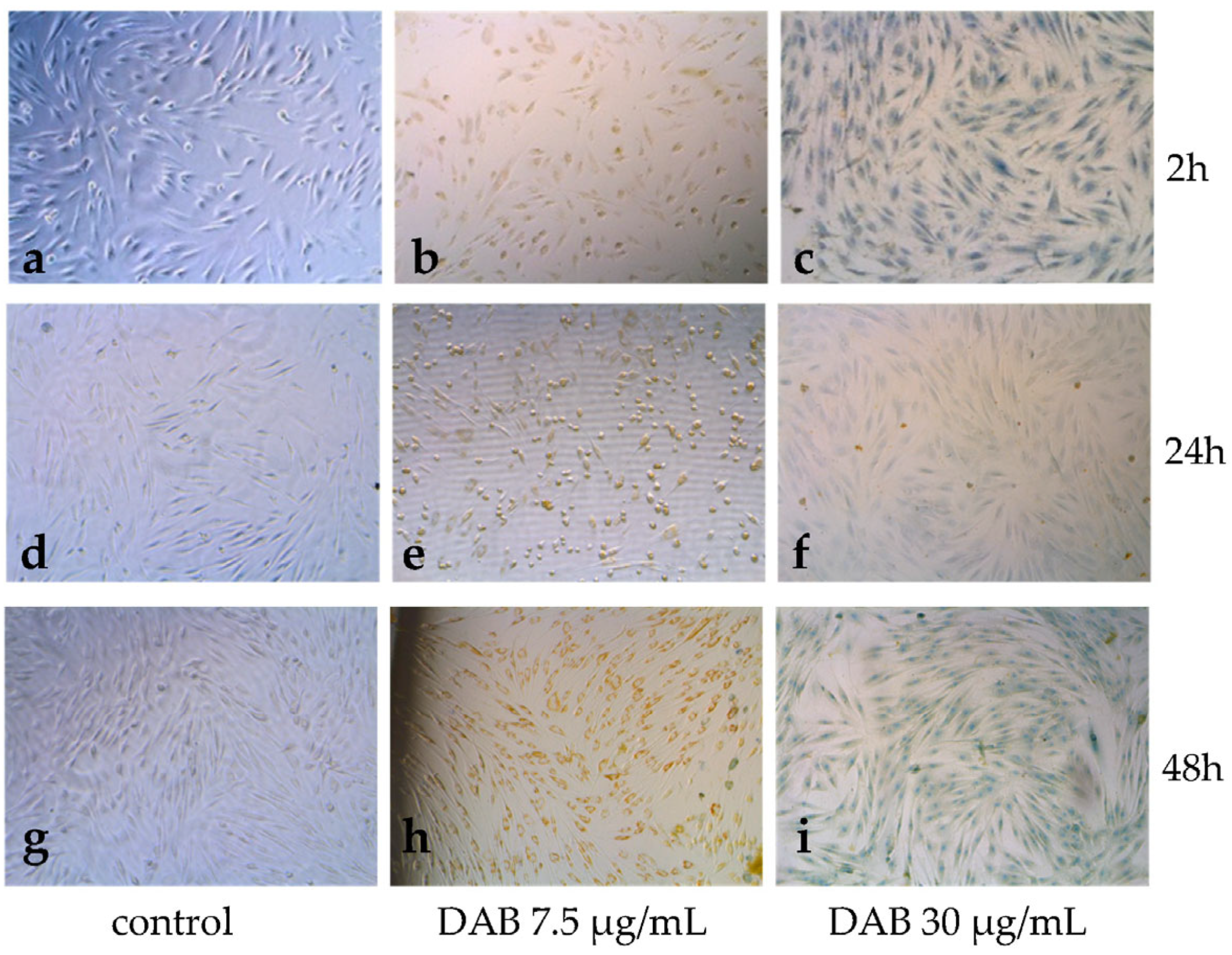
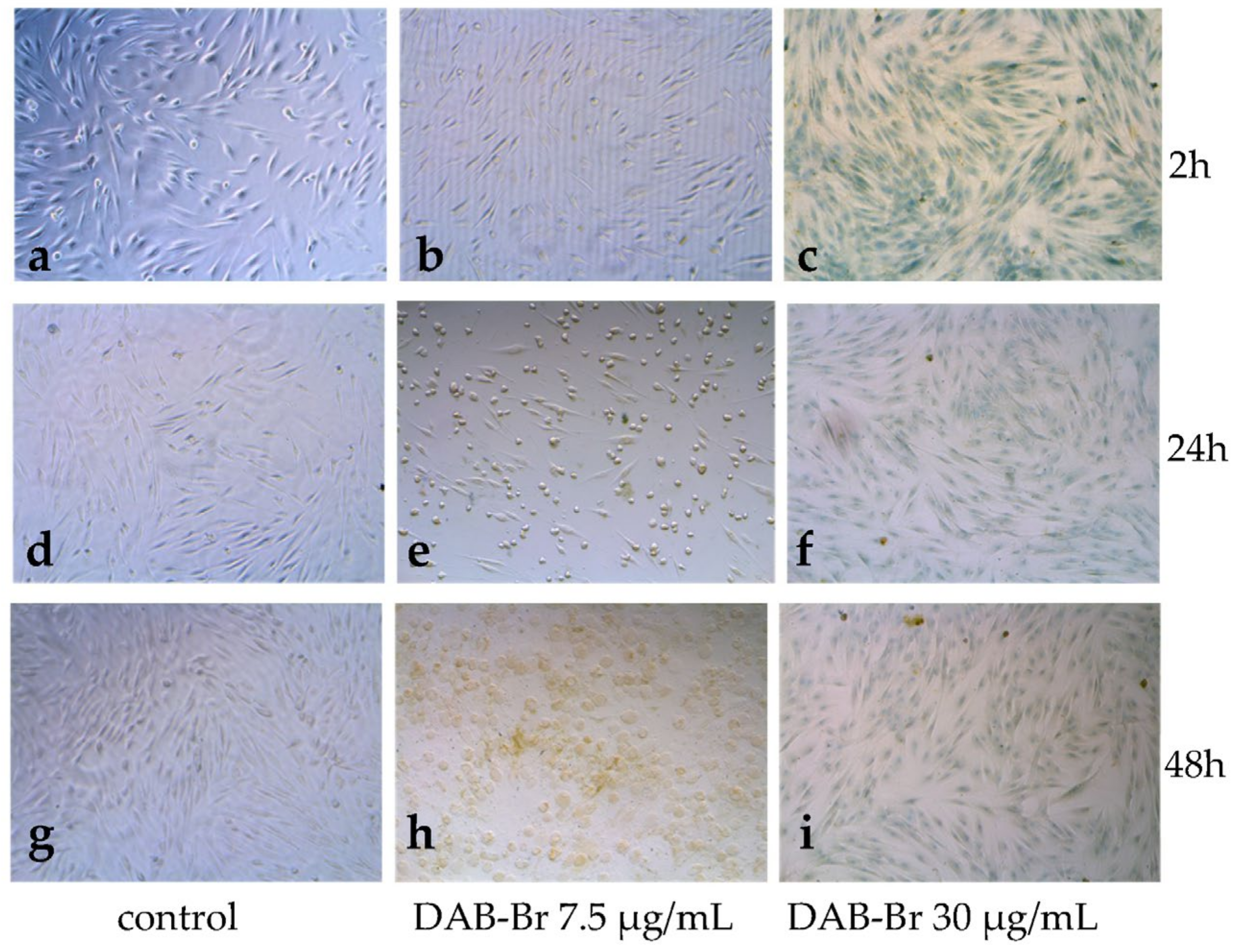
3.3. Permeabilization of Cell Membranes of HFF-1 Cells and Changes in Cell Morphology
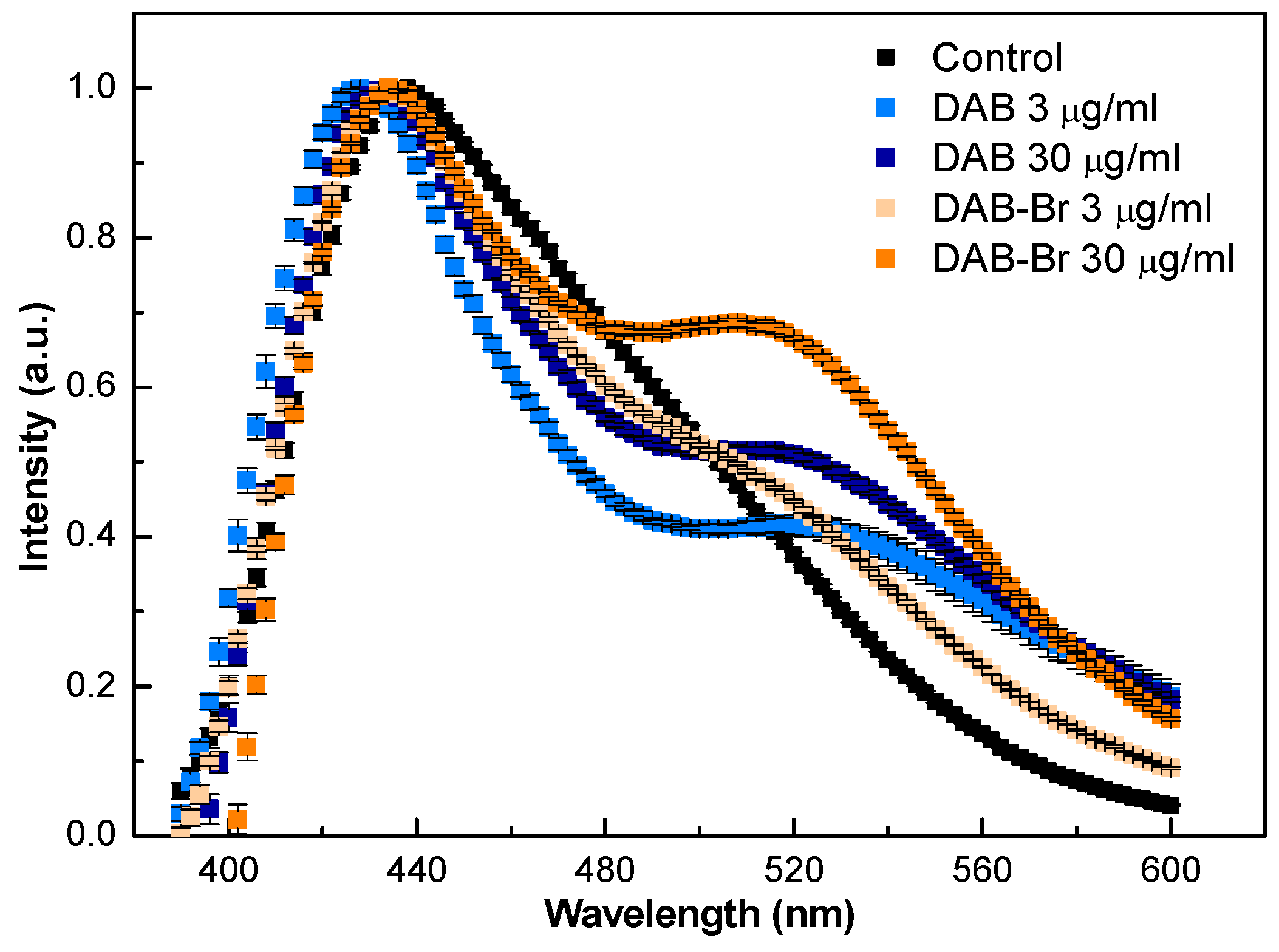
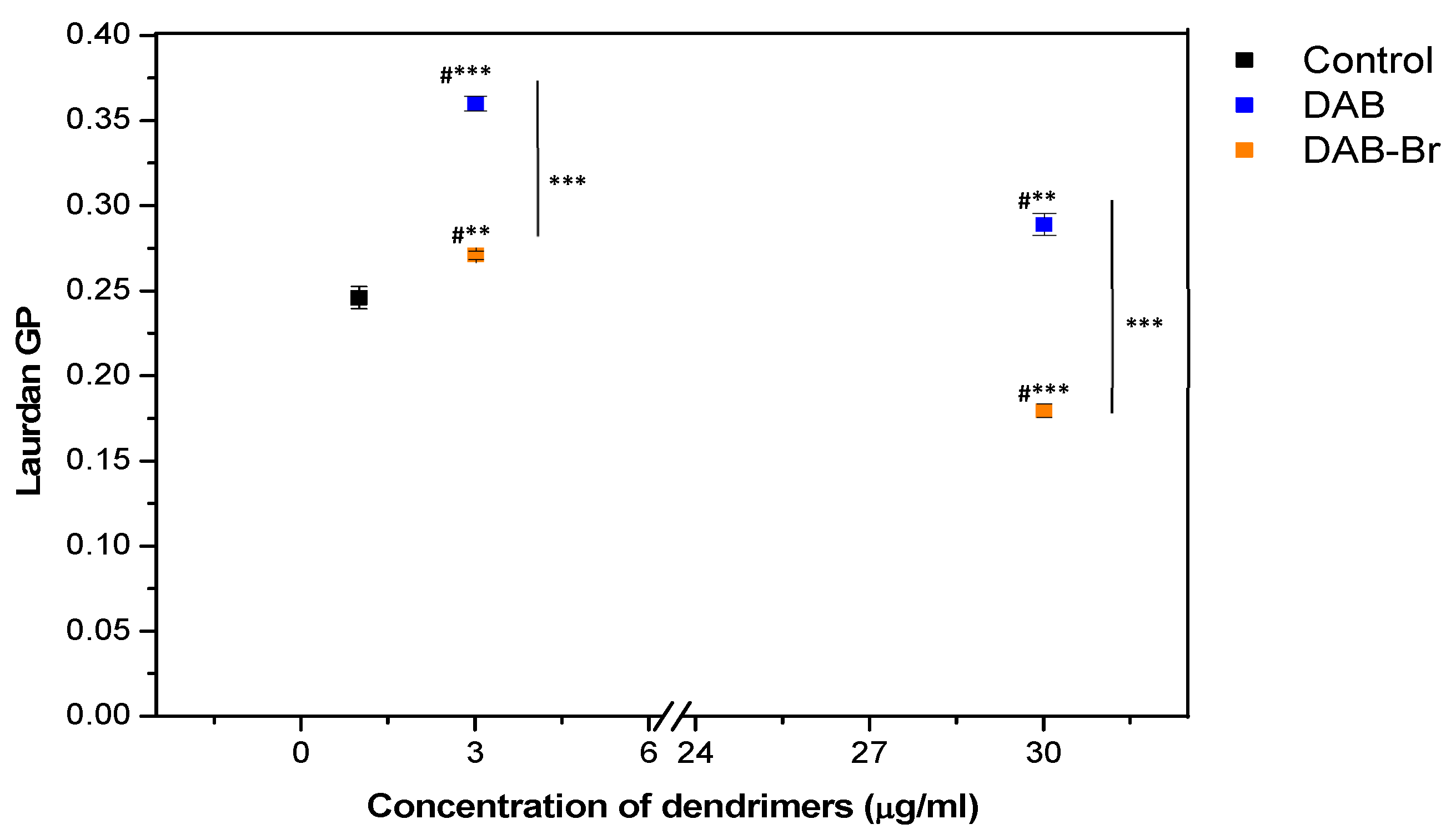
3.4. Laurdan Fluorescence Spectroscopy
4. Discussion
4.1. Cellular Entry, Cytotoxicity, and Interactions of DAB and DAB-Br with HFF-1 Cells
4.2. Antifungal Activities of DAB and DAB-Br
- (1)
- Electrostatic interaction between the cationic terminal groups of the dendrimer and the anionic cell membranes, leading to an increase in membrane permeability;
- (2)
- A “carpet” mechanism in which dendrimers form small pores in the cell membrane, compromising membrane integrity and membrane repair mechanisms;
- (3)
- Inhibition of 1,3-β-D-glucan synthase, which disrupts cell wall synthesis and leads to leakage of cellular components;
- (4)
- Chelation, by which microbial enzymes are inhibited.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Chaudhry, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Ameen, S.; Baskoutas, S. Nanomaterials-based biosensor and their applications: A review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef]
- Altemimi, A.B.; Farag, H.A.M.; Salih, T.H.; Awlqadr, F.H.; Al-Manhel, A.J.A.; Vieira, I.R.S.; Conte-Junior, C.A. Application of nanoparticles in human nutrition: A review. Nutrients 2024, 16, 636. [Google Scholar] [CrossRef] [PubMed]
- Contin, M.; Garcia, C.; Dobrecky, C.; Lucangioli, S.; D’Accorso, N. Advances in drug delivery, gene delivery and therapeutic agents based on dendritic materials. Future Med. Chem. 2019, 11, 1791–1810. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Hong, X.Z.; Shen, J.; Geng, L.J.; Pan, Y.H.; Ling, W.; Zhao, H.L. Amyloids in site-specific autoimmune reactions and inflammatory responses. Front. Immunol. 2020, 10, 2980. [Google Scholar] [CrossRef] [PubMed]
- Fateh, S.T.; Aghaii, A.H.; Aminzade, Z.; Shahriari, E.; Roohpour, N.; Koosha, F.; Dezfuli, A.S. Inorganic nanoparticle-cored dendrimers for biomedical applications: A review. Heliyon 2024, 10, e29726. [Google Scholar] [CrossRef]
- Aminian, F.; Hemmati, A. Dendrimers as versatile platforms for advanced biosensor development: A review on chemistry, synthesis, and performance enhancements. JECE 2025, 13, 116365. [Google Scholar] [CrossRef]
- Jain, K.; Jain, N.K.; Kesharwani, P. Types of dendrimers. In Dendrimer-Based Nanotherapeutics, 1st ed.; Kesharwani, P., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 95–123. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: History, challenges, and latest developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef]
- Fox, L.J.; Richardson, R.M.; Briscoe, W.H. PAMAM dendrimer—Cell membrane interactions. Adv. Colloid Interface Sci. 2018, 257, 1–18. [Google Scholar] [CrossRef]
- Caminade, A.M.; Turrin, C.O. Dendrimers for drug delivery. J. Mater. Chem. B 2014, 2, 4055–4066. [Google Scholar] [CrossRef]
- Kesharwani, P.; Gothwal, A.; Iyer, A.K.; Jain, K.; Chourasia, M.K.; Gupta, U. Dendrimer nanohybrid carrier systems: An expanding horizon for targeted drug and gene delivery. Drug Discov. Today 2018, 23, 300–314. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and limitations of dendrimers in biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, P.; Perona, A.; Juanes, O.; Rumbero, Á.; Hernáiz, M. Synthesis of glycodendrimers with antiviral and antibacterial activity. Chem. A Eur. J. 2021, 27, 7593–7624. [Google Scholar] [CrossRef] [PubMed]
- Chis, A.A.; Dobrea, C.M.; Rus, L.L.; Frum, A.; Morgovan, C.; Butuca, A.; Totan, M.; Juncan, A.M.; Gligor, F.G.; Arseniu, A.M. Dendrimers as non-viral vectors in gene-directed enzyme prodrug therapy. Molecules 2021, 26, 5976. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Mohammadi, R.; Arzanlou, M.; Akbari Dourbash, F.; Kouhsari, E.; Majidi, G.; Mohseni, S.M.; Nazari, S. In vitro antibacterial activity of poly (amidoamine)-G7 dendrimer. BMC Infect. Dis. 2017, 17, 395. [Google Scholar] [CrossRef]
- Falanga, A.; Del Genio, V.; Galdiero, S. Peptides and dendrimers: How to combat viral and bacterial infections. Pharmaceutics 2021, 13, 101. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Grozdanov, P.; Vilhelmova-Ilieva, N.; Nikolova, I.; Grabchev, I. Synthesis and photophysical characterisation of 3-bromo-4-dimethylamino-1, 8-naphthalimides and their evaluation as agents for antibacterial photodynamic therapy. J. Photochem. Photobiol. A Chem. 2020, 401, 112730. [Google Scholar] [CrossRef]
- Mlynarczyk, D.T.; Dlugaszewska, J.; Kaluzna-Mlynarczyk, A.; Goslinski, T. Dendrimers against fungi—A state of the art review. J. Control. Release 2021, 330, 599–617. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef]
- Khan, M.A.; Peng, R.; Liu, C.L.; Chen, Z. Synthesis, dynamics and applications (cytotoxicity and biocompatibility) of dendrimers: A mini-review. Eur. Polym. J. 2022, 181, 111708. [Google Scholar] [CrossRef]
- Baecker, D.; Kapp, T.; Schumacher, P.; Gust, R.; Kircher, B. Cell death-inducing properties of selected dendrimers against different breast cancer and leukemia cell lines. Arch. Pharm. 2020, 353, e2000209. [Google Scholar] [CrossRef]
- Žmudová, Z.; Šanderová, Z.; Liegertová, M.; Vinopal, S.; Herma, R.; Sušický, L.; Müllerová, M.; Strašák, T.; Malý, J. Biodistribution and toxicity assessment of methoxyphenyl phosphonium carbosilane dendrimers in 2D and 3D cell cultures of human cancer cells and zebrafish embryos. Sci. Rep. 2023, 13, 15477. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Deng, Y.; Zeng, Y.; Yan, B.; Deng, Y.; Zheng, Z.; Li, S.; Yang, Y.; Hao, J.; Xiao, X.; et al. The application advances of dendrimers in biomedical field. VIEW 2023, 4. [Google Scholar] [CrossRef]
- Ipsen, J.H.; Karlström, G.; Mouritsen, O.G.; Wennerström, H.; Zuckermann, M.J. Phase equilibria in the phosphatidylcholine-cholesterol system. BBA-Biomembr. 1987, 905, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Ammendolia, D.A.; Bement, W.M.; Brumell, J.H. Plasma membrane integrity: Implications for health and disease. BMC Biol. 2021, 19, 71. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2001, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Parasassi, T.; De Stasio, G.; d’Ubaldo, A.; Gratton, E. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys. J. 1990, 57, 1179–1186. [Google Scholar] [CrossRef]
- Gothwal, A.; Malik, S.; Gupta, U.; Jain, N.K.. Toxicity and biocompatibility aspects of dendrimers. In Pharmaceutical Applications of Dendrimers (Micro and Nano Technologies); Chauhan, A., Kulhari, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 251–274. [Google Scholar] [CrossRef]
- Lee, H.; Larson, D.R.; Lawrence, D.S. Illuminating the chemistry of life: Design, synthesis, and applications of ‘caged’ and related photoresponsive compounds. ACS Chem. Biol. 2008, 4, 409–427. [Google Scholar] [CrossRef]
- Choi, Y.; Thomas, T.; Kotlyar, A.; Islam, M.T.; Baker, J.R. Synthesis and functional evaluation of DNA-assembled polyamidoamine dendrimer clusters for cancer cell-specific targeting. Chem. Biol. 2006, 12, 35–43. [Google Scholar] [CrossRef]
- Kuo, J.H.; Jan, M.S.; Chiu, H.W. Mechanism of cell death induced by cationic dendrimers in RAW 264.1 murine macrophage-like cells. J. Pharm. Pharmacol. 2005, 57, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ziemba, B.; Halets, I.; Shcharbin, D.; Appelhans, D.; Voit, B.; Pieszynski, I.; Bryszewska, M.; Klajnert, B. Influence of fourth generation poly (propylene imine) dendrimers on blood cells. J. Biomed. Mater. Res. A 2012, 100A, 2870–2880. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sunoqrot, S.; Stowell, C.; Ji, J.; Lee, C.W.; Kim, J.W.; Khan, S.A.; Hong, S. Effect of size, surface charge, and hydrophobicity of poly(amidoamine) dendrimers on their skin penetration. Biomacromolecules 2012, 13, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Kheraldine, H.; Rachid, O.; Habib, A.M.; Moustafa, A.A.; Benter, I.F.; Akhtar, S. Emerging innate biological properties of nano-drug delivery systems: A focus on PAMAM dendrimers and their clinical potential. Adv. Drug Deliv. Rev. 2021, 178, 113908. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Zhang, M.; Sun, Y.; Zhang, X.; Guan, G.; Zhao, X.; Qiao, M.; Chen, D.; Hu, H. The cellular uptake mechanism, intracellular transportation, and exocytosis of polyamidoamine dendrimers in multidrug-resistant breast cancer cells. Int. J. Nanomed. 2016, 11, 3677–3690. [Google Scholar] [CrossRef]
- de Luca, S.; Seal, P.; Parekh, H.S.; Tupally, K.R.; Smith, S.C. Cell Membrane Penetration without Pore Formation: Chameleonic Properties of Dendrimers in Response to Hydrophobic and Hydrophilic Environments. Adv. Theory Simul. 2020, 3, 1900152. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, D.; Lin, X.; Shen, C.; Zhang, J.; Xu, J.; Zhao, Y.; Zhu, L.; Kong, H.; Zhang, M.; et al. Dendrimer-Derived Mimics of Host Defense Peptides Selectively Disrupt Cancer Cell Membranes for Melanoma Therapy. Pharmaceutics 2025, 17, 361. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Bellocco, E.; Laganà, G.; Barreca, D.; Magazù, S.; Wanderlingh, U.; Kiselev, M.A. Effect of anionic and cationic polyamidoamine (PAMAM) dendrimers on a model lipid membrane. BBA-Biomembr. 2016, 1858, 2769–2777. [Google Scholar] [CrossRef]
- Metwally, S.; Stachewicz, U. Surface potential and charges impact on cell responses on biomaterials interfaces for medical applications. Mater. Sci. Eng. C 2019, 104, 109883. [Google Scholar] [CrossRef]
- Jordanova, A.; Tsanova, A.; Stoimenova, E.; Minkov, I.; Kostadinova, A.; Hazarosova, R.; Angelova, R.; Antonova, K.; Vitkova, V.; Staneva, G.; et al. Molecular Mechanisms of Action of Dendrimers with Antibacterial Activities on Model Lipid Membranes. Polymers 2025, 17, 929. [Google Scholar] [CrossRef]
- Parasassi, T.; Krasnowska, E.; Bagatolli, L.; Gratton, E. Laurdan and Prodan as Polarity-Sensitive Fluorescent Membrane Probes. J. Fluoresc. 1998, 8, 365–373. [Google Scholar] [CrossRef]
- Santos, A.; Veiga, F.; Figueiras, A. New advances in general biomedical applications of PAMAM dendrimers. Molecules 2016, 23, 2849. [Google Scholar] [CrossRef]
- Hoque, J.; Akkapeddi, P.; Yadav, V.; Manjunath, G.B.; Uppu, D.S.; Konai, M.M.; Yarlagadda, V.; Sanyal, K.; Haldar, J. Broad spectrum antibacterial and antifungal polymeric paint materials: Synthesis, structure-activity relationship, and membrane-active mode of action. ACS Appl. Mater. Interfaces. 2015, 7, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.A.; Ren, D. Antimicrobial Peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. BBA-Biomembr. 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Powers, K.W.; Brown, S.C.; Krishna, V.B.; Wasdo, S.C.; Moudgil, B.M.; Roberts, S.M. Dendrimers as potential drug carriers; encapsulation of acidic hydrophobic drugs. Tetrahedron 2003, 59, 5285–5290. [Google Scholar] [CrossRef]
- Galanakou, C.; Dhumal, D.; Peng, L. Amphiphilic dendrimers against antibiotic resistance: Light at the end of the tunnel? Biomater. Sci. 2023, 11, 3379–3393. [Google Scholar] [CrossRef]
- Nouman; Rana, M.; Ahmedi, S.; Arif, R.; Manzoor, N.; Rahisuddin. 4-bromo-1, 8-naphthalimide derivatives as antifungal agents: Synthesis, characterization, DNA binding, molecular docking, antioxidant and ADMET studies. Polycycl. Aromat. Compd. 2024, 44, 748–772. [Google Scholar] [CrossRef]
- Pesee, S.; Angkananuwat, C.; Tancharoensukjit, S.; Muanmai, S.; Sirivan, P.; Bubphawas, M.; Tanarerkchai, N. In vitro activity of Caspofungin combined with Fluconazole on mixed Candida albicans and Candida glabrata biofilm. Med. Mycol. 2016, 54, 384–393. [Google Scholar] [CrossRef]
- Grela, E.; Zdybicka-Barabas, A.; Pawlikowska-Pawlega, B.; Cytrynska, M.; Wlodarczyk, M.; Grudzinski, W.; Luchowski, R.; Gruszecki, W.I. Modes of the antibiotic activity of amphotericin B against Candida albicans. Sci. Rep. 2019, 9, 17029. [Google Scholar] [CrossRef]
- Heredero-Bermejo, I.; Gómez-Casanova, N.; Quintana, S.; Soliveri, J.; de la Mata, F.J.; Pérez-Serrano, J.; Sánchez-Nieves, J.; Copa-Patiño, J.L. In Vitro Activity of Carbosilane Cationic Dendritic Molecules on Prevention and Treatment of Candida albicans Biofilms. Pharmaceutics 2020, 12, 918. [Google Scholar] [CrossRef] [PubMed]
- Molchanova, N.; Nielsen, J.E.; Sørensen, K.B.; Prabhala, B.K.; Hansen, P.R.; Lund, R.; Barron, A.E.; Jenssen, H. Halogenation as a tool to tune antimicrobial activity of peptoids. Sci. Rep. 2020, 10, 14805. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, M.T.; Moretti, M.; Cioffi, M.; Giordano, C.; Boschetti, F.; Lagana, K.; Pietrabissa, R. The effect of hydrodynamic shear on 3D engineered chondrocyte systems subject to direct perfusion. Biorheology 2006, 43, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Benedetto Tiz, D.; Bagnoli, L.; Rosati, O.; Marini, F.; Santi, C.; Sancineto, L. FDA-Approved Small Molecules in 2022: Clinical Uses and Their Synthesis. Pharmaceutics 2022, 14, 2538. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, Z.; Liu, Y.; Lu, Y.; Chen, K.; Zhu, W. Halogen bond: Its role beyond drug-target binding affinity for drug discovery and development. J. Chem. Inf. Model. 2014, 54, 69–78. [Google Scholar] [CrossRef]
- Zhou, P.; Lv, J.; Zou, J.; Tian, F.; Shang, Z. Halogen–water–hydrogen bridges in biomolecules. J. Struct. Biol. 2010, 169, 172–182. [Google Scholar] [CrossRef]
- Cochereau, R.; Maffeis, V.; dos Santos, E.C.; Lörtscher, E.; Palivan, C.G. Polymeric giant unilamellar vesicles with integrated DNA-origami nanopores: An efficient platform for tuning bioreaction dynamics through controlled molecular diffusion. Adv. Funct. Mater. 2023, 33, 2304782. [Google Scholar] [CrossRef]
- Duo, M.; Zhang, M.; Luk, Y.Y.; Ren, D. Inhibition of Candida albicans growth by brominated furanone. Appl. Microbiol. Biotechnol. 2010, 85, 1551–1563. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhou, C.H. Synthesis and activities of naphthalimide azoles as a new type of antibacterial and antifungal agents. Bioorg. Med. Chem. Lett. 2011, 21, 4349–4352. [Google Scholar] [CrossRef]
| Dendrimer Dispersions (40 µg/mL) | Average Size (nm) | Apparent ζ-Potential (mV) |
|---|---|---|
| DAB (1st day) | 118 ± 66 ** | 28.7 ± 0.4 ** |
| DAB (3rd day) | 123 ± 45 ** | 26.5 ± 1.6 ** |
| DAB (7th day) | 123 ± 54 ** | 26.5 ± 2.7 ** |
| DAB-Br (1st day) | 222 ± 39 ** | 35.2 ± 1.9 ** |
| DAB-Br (3rd day) | 234 ± 82 ** | 31.6 ± 2.4 ** |
| DAB-Br (7th day) | 265 ± 72 ** | 34.5 ± 1.7 ** |
| DAB | 1.179 µg/µL | 0.589 µg/µL | 0.295 µg/µL | 0.148 µg/µL | 0.074 µg/µL | 0.0369 µg/µL |
|---|---|---|---|---|---|---|
| Asp. fumigatus | 96 h | 96 h | 24 h | |||
| Alt. alternata | 96 h | 96 h | 72 h | |||
| Asp. niger | 96 h | 96 h | 24 h | |||
| P. griseofulvum | 96 h | 96 h | 24 h | |||
| C. albicans | 96 h | 96 h | 24 h | 24 h | 24 h | 24 h |
| DAB-Br | 1.179 µg/µL | 0.589 µg/µL | 0.295 µg/µL | 0.148 µg/µL | 0.074 µg/µL | 0.0369 µg/µL |
|---|---|---|---|---|---|---|
| Asp. fumigatus | 96 h | 96 h | 96 h | 24 h | ||
| Alt. alternata | 96 h | 96 h | 96 h | 96 h | 24 h | |
| Asp. niger | 96 h | 96 h | 96 h | 24 h | ||
| P. griseofulvum | 96 h | 96 h | 96 h | 24 h | ||
| C. albicans | 96 h | 96 h | 96 h | 48 h | 24 h | 24 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostadinova, A.; Gaydarska, E.; Topouzova-Hristova, T.; Benkova, D.; Staneva, G.; Krumova, E.; Hazarosova, R.; Marinov, M.; Tsanova, A.; Jordanova, A.; et al. Cellular Entry, Cytotoxicity, and Antifungal Activity of Newly Synthesized Dendrimers. Appl. Sci. 2025, 15, 7764. https://doi.org/10.3390/app15147764
Kostadinova A, Gaydarska E, Topouzova-Hristova T, Benkova D, Staneva G, Krumova E, Hazarosova R, Marinov M, Tsanova A, Jordanova A, et al. Cellular Entry, Cytotoxicity, and Antifungal Activity of Newly Synthesized Dendrimers. Applied Sciences. 2025; 15(14):7764. https://doi.org/10.3390/app15147764
Chicago/Turabian StyleKostadinova, Aneliya, Ema Gaydarska, Tanya Topouzova-Hristova, Dayana Benkova, Galya Staneva, Ekaterina Krumova, Rusina Hazarosova, Miroslav Marinov, Asya Tsanova, Albena Jordanova, and et al. 2025. "Cellular Entry, Cytotoxicity, and Antifungal Activity of Newly Synthesized Dendrimers" Applied Sciences 15, no. 14: 7764. https://doi.org/10.3390/app15147764
APA StyleKostadinova, A., Gaydarska, E., Topouzova-Hristova, T., Benkova, D., Staneva, G., Krumova, E., Hazarosova, R., Marinov, M., Tsanova, A., Jordanova, A., & Grabchev, I. (2025). Cellular Entry, Cytotoxicity, and Antifungal Activity of Newly Synthesized Dendrimers. Applied Sciences, 15(14), 7764. https://doi.org/10.3390/app15147764








