Investigation of Gluten Contamination in Commercial Hydrated Cassava Starch and Its Physicochemical Properties
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Partial Physicochemical Characterization of Hydrated Cassava Starch
2.2.1. pH
2.2.2. Moisture
2.2.3. Granulometry
2.3. Detection of Gluten in Solid Samples
2.4. Statistical Analysis
3. Results and Discussion
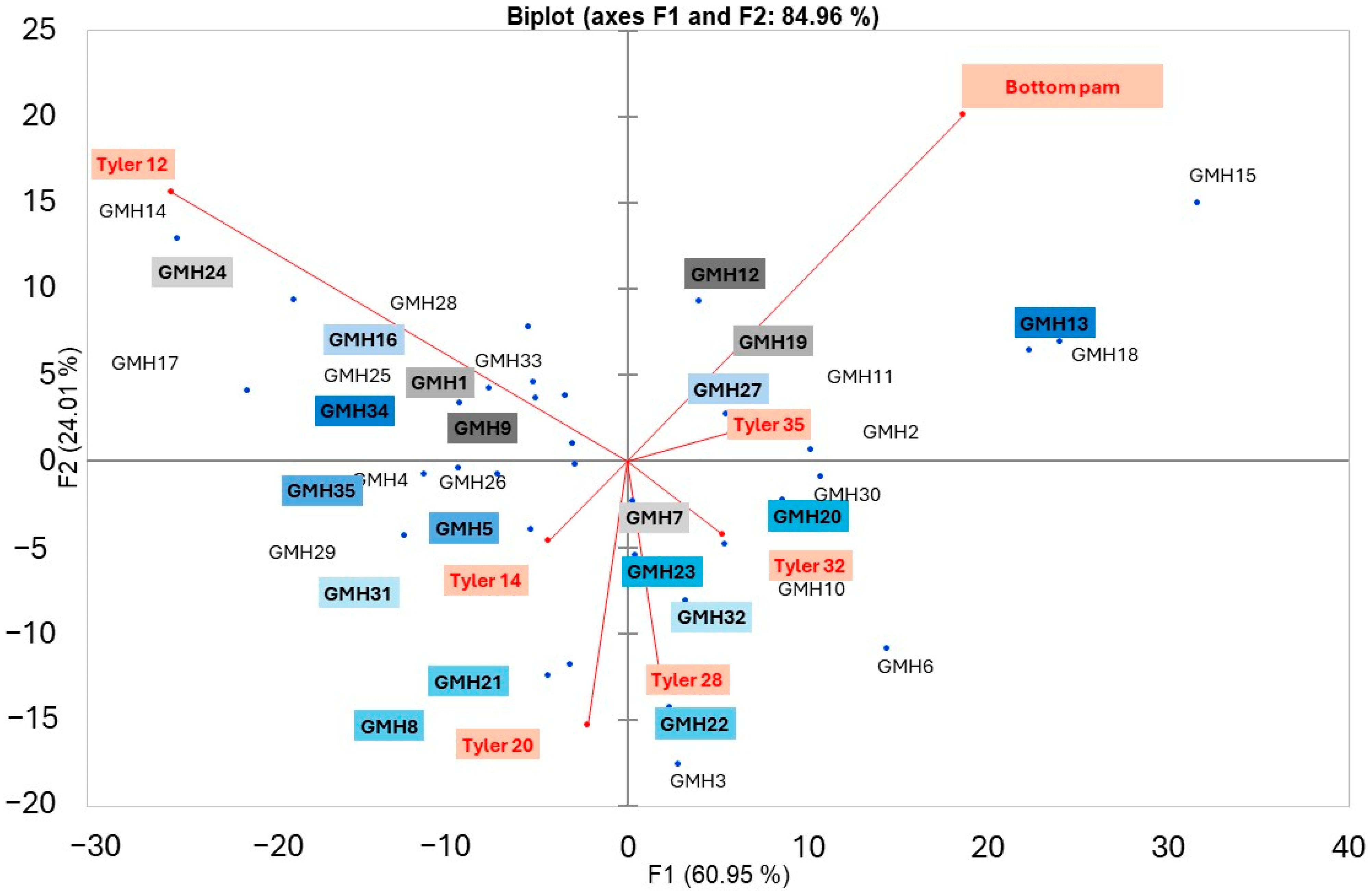
3.1. Particle Size Distribution
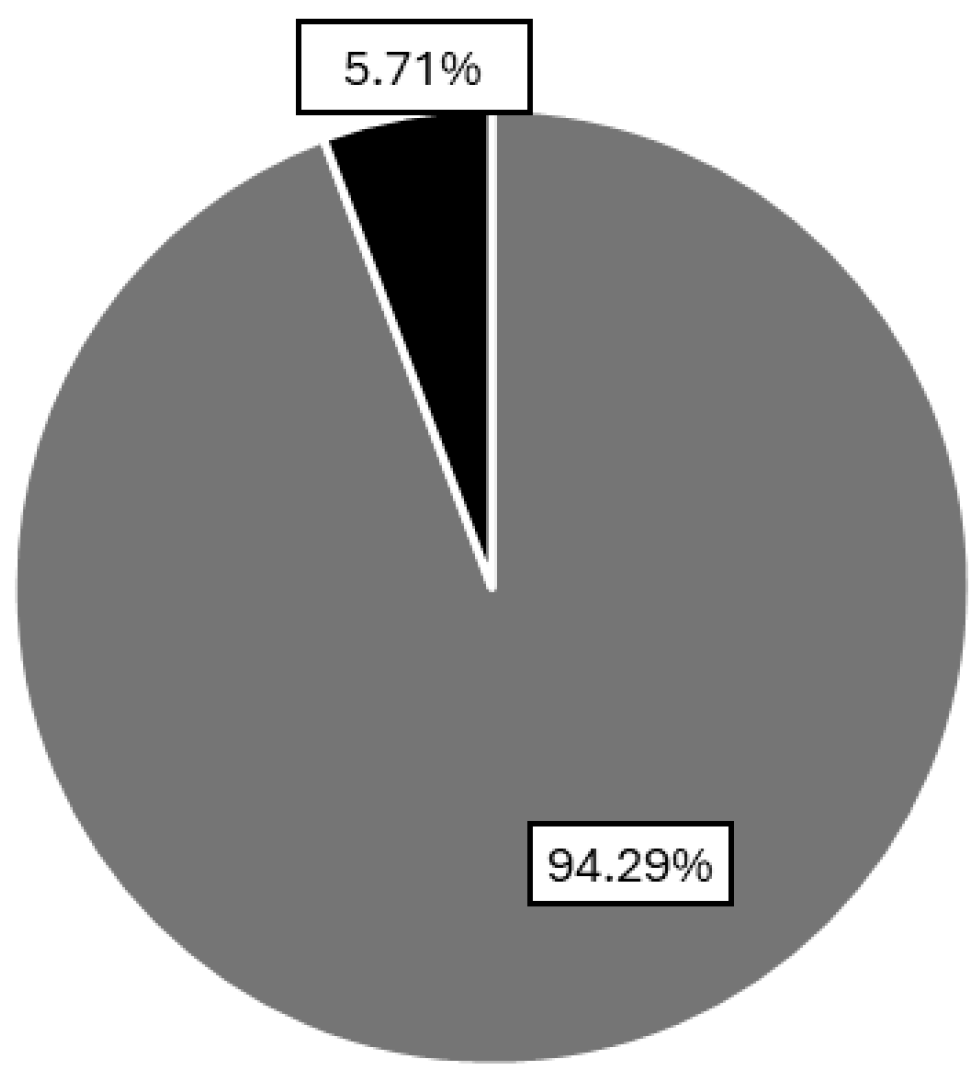
3.2. Detection of the Presence/Absence of Gluten in Commercial Hydrated Cassava Starch
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MRF. Cassava Market Research Report Information by Category (Conventional and Organic), by Application (Food & Beverages, Animal Feed, and Others), by Form (Solid and Liquid) and by Region (North America, Europe, Asia-Pacific, And Rest of the World)—Market Forecast Till 2034. Available online: https://www.marketresearchfuture.com/reports/cassava-market-4629?utm_term=&utm_campaign=&utm_source=adwords&utm_medium=ppc&hsa_acc=2893753364&hsa_cam=19912237177&hsa_grp=148712481999&hsa_ad=659589502539&hsa_src=g&hsa_tgt=dsa-2080758264400&hsa_kw=&hsa_mt=&hsa_net=adwords&hsa_ver=3&gad_source=1 (accessed on 2 May 2025).
- Conab. Mandioca—Análise Mensal—Fevereiro 2024. Available online: https://www.conab.gov.br/info-agro/analises-do-mercado-agropecuario-e-extrativista/analises-do-mercado/historico-mensal-de-mandioca/item/23156-mandioca-analise-mensal-fevereiro-2024 (accessed on 16 December 2024).
- IBGE. Produção de Mandioca. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/mandioca/br (accessed on 16 December 2024).
- Verma, R.; Chauhan, N.; Singh, B.; Chandra, S.; Author, C.; Sengar, R. Cassava processing and its food application: A review. Pharma Innov. J. 2022, 11, 415–422. [Google Scholar]
- Krajang, M.; Malairuang, K.; Sukna, J.; Rattanapradit, K.; Chamsart, S. Single-step ethanol production from raw cassava starch using a combination of raw starch hydrolysis and fermentation, scale-up from 5-L laboratory and 200-L pilot plant to 3000-L industrial fermenters. Biotechnol. Biofuels 2021, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Ezeoha, S.L.; Ezenwanne, J.N. Production of Biodegradable Plastic Packaging Film from Cassava Starch. IOSR J. Eng. 2013, 3, 14–20. [Google Scholar] [CrossRef]
- Olivato, J.B. Chapter 11—Starch: A natural, safe, and multifunctional ingredient for cosmetic formulations. In Starch Industries: Processes and Innovative Products in Food and Non-Food Uses; Cereda, M.P., Vilpoux, O.F., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 255–269. [Google Scholar] [CrossRef]
- Sindipan, M.T. Tapioca se Populariza e Ganha Espaço no Mercado Nacional. Available online: https://www.marciorodrigues.com.br/mostraNoticia.php?codnoticia=900 (accessed on 19 December 2024).
- Freitas, A.F.; Mattietto, R.A.; Carvalho, A.V. Composição Físico-Química de Farinha de Tapioca Comercializadas em Belém, Pará; Oriental, E.A., Ed.; Embrapa Amazônia Oriental: Belém, Brazil, 2022; p. 19. [Google Scholar]
- Vilpoux, O.F.; Cereda, M.P. Chapter 9—Traditional Brazilian foods based on partial gelatinization of cassava starch: Tapioca pearls (“sagu”), Brazilian tapioca (“tapioquinha”), broken tapioca pearls (“tapioca”), and popped tapioca pearls (“farinha de tapioca”). In Starch Industries: Processes and Innovative Products in Food and Non-Food Uses; Cereda, M.P., Vilpoux, O.F., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 211–232. [Google Scholar] [CrossRef]
- Landim Parente, G.D.; Almeida Macêdo, F.D.; Melo Diniz, N.C.; da Conceição, M.M.; Ubbink, J.; Mattos Braga, A.L. Observations on the formation and textural properties of “tapiocas”, a traditional cassava-based food from the Northeast of Brazil. Int. J. Gastron. Food Sci. 2021, 26, 100417. [Google Scholar] [CrossRef]
- Arslain, K.; Gustafson, C.R.; Baishya, P.; Rose, D.J. Determinants of gluten-free diet adoption among individuals without celiac disease or non-celiac gluten sensitivity. Appetite 2021, 156, 104958. [Google Scholar] [CrossRef]
- Guilherme, A.B. Avaliação Das Boas Práticas De Fabricação E Análises Microbiológicas Em Uma Indústria De Tapioca. Bachelor’s Thesis, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil, 2021. [Google Scholar]
- Wieser, H.; Segura, V.; Ruiz-Carnicer, Á.; Sousa, C.; Comino, I. Food Safety and Cross-Contamination of Gluten-Free Products: A Narrative Review. Nutrients 2021, 13, 2244. [Google Scholar] [CrossRef]
- Bianchi, D.M.; Maurella, C.; Gallina, S.; Gorrasi, I.S.R.; Caramelli, M.; Decastelli, L. Analysis of Gluten Content in Gluten-Free Pizza from Certified Take-Away Pizza Restaurants. Foods 2018, 7, 180. [Google Scholar] [CrossRef]
- Farage, P.; Zandonadi, R.P.; Gandolfi, L.; Pratesi, R.; Falcomer, A.L.; Araújo, L.S.; Nakano, E.Y.; Ginani, V.C. Accidental Gluten Contamination in Traditional Lunch Meals from Food Services in Brasilia, Brazil. Nutrients 2019, 11, e1924. [Google Scholar] [CrossRef]
- Gatti, S.; Rubio-Tapia, A.; Makharia, G.; Catassi, C. Patient and Community Health Global Burden in a World with More Celiac Disease. Gastroenterology 2024, 167, 23–33. [Google Scholar] [CrossRef]
- Jedrychowski, J.; Wichers, H.J. Chemical and Biological Properties of Food Allergens, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Sabença, C.; Ribeiro, M.; Sousa, T.D.; Poeta, P.; Bagulho, A.S.; Igrejas, G. Wheat/Gluten-Related Disorders and Gluten-Free Diet Misconceptions: A Review. Foods 2021, 10, 1765. [Google Scholar] [CrossRef]
- Cui, C.; Basen, T.; Philipp, A.T.; Yusin, J.; Krishnaswamy, G. Celiac disease and nonceliac gluten sensitivity. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2017, 118, 389–393. [Google Scholar] [CrossRef] [PubMed]
- McAllister, B.P.; Williams, E.; Clarke, K. A Comprehensive Review of Celiac Disease/Gluten-Sensitive Enteropathies. Clin. Rev. Allergy Immunol. 2019, 57, 226–243. [Google Scholar] [CrossRef]
- DeGeorge, K.C.; Frye, J.W.; Stein, K.M.; Rollins, L.K.; McCarter, D.F. Celiac Disease and Gluten Sensitivity. Prim. Care Clin. Off. Pract. 2017, 44, 693–707. [Google Scholar] [CrossRef]
- Therrien, A.; Kelly, C.P.; Silvester, J.A. Celiac Disease: Extraintestinal Manifestations and Associated Conditions. J. Clin. Gastroenterol. 2020, 54, 8–21. [Google Scholar] [CrossRef]
- Damasceno, R.P.B.; Zandonadi, R.P.; Mendes, M.; Cunha Junior, L.C.; Raposo, A.; Teixeira-Lemos, E.; Chaves, C.; Farage, P. Risk of Gluten Cross-Contamination Due to Food Handling Practices: A Mini-Review. Nutrients 2024, 16, e1198. [Google Scholar] [CrossRef]
- Raju, N.; Joshi, A.K.R.; Vahini, R.; Deepika, T.; Bhaskarachari, K.; Devindra, S. Gluten contamination in labelled and naturally gluten-free grain products in southern India. Food Addit. contaminants. Part A Chem. Anal. Control Expo. Risk Assess. 2020, 37, 531–538. [Google Scholar] [CrossRef]
- Vargas, F.M.D.; Cardoso, L.T.; Didoné, A.; Lima, J.P.M.; Venzke, J.G.; de Oliveira, V.R. Celiac Disease: Risks of Cross-Contamination and Strategies for Gluten Removal in Food Environments. Int. J. Environ. Res. Public Health 2024, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Lutz, I.A. Normas Analíticas do Instituto Adolfo Lutz, 4th ed.; Editora MS: Brasília, Brazil, 2005; p. 1020. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Sakhare, S.D.; Inamdar, A.A.; Soumya, C.; Indrani, D.; Rao, G.V. Effect of flour particle size on microstructural, rheological and physico-sensory characteristics of bread and south Indian parotta. J. Food Sci. Technol. 2014, 51, 4108–4113. [Google Scholar] [CrossRef] [PubMed]
- Felix, H.B.; Angela, M.F. Chapter 11—Acidified Foods: Food Safety Considerations for Food Processors. In Food Industry; Innocenzo, M., Ed.; IntechOpen: Rijeka, Croatia, 2013; pp. 231–239. [Google Scholar] [CrossRef]
- Dar, B.N.; Shah, M.A.; Mir, S.A. Shelf Life and Food Safety, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Pinto, U.M.; Landgraf, M.; Franco, B.D.G.d.M. Deterioração Microbiana dos Alimentos, 1st ed.; Rubio: Rio de Janeiro, Brazil, 2019; p. 272. [Google Scholar]
- Sar, T.; Marchlewicz, A.; Harirchi, S.; Mantzouridou, F.T.; Hosoglu, M.I.; Akbas, M.Y.; Hellwig, C.; Taherzadeh, M.J. Resource recovery and treatment of wastewaters using filamentous fungi. Sci. Total Environ. 2024, 951, 175752. [Google Scholar] [CrossRef]
- Gouveia, B.G.; Rijo, P.; Gonçalo, T.S.; Reis, C.P. Good manufacturing practices for medicinal products for human use. J. Pharm. Bioallied Sci. 2015, 7, 87–96. [Google Scholar] [CrossRef]
- Rymbai, H.; Meena, A. Futuristic Trends in Agriculture Engineering & Food Sciences; Iterative International Publishers (IIP), Selfypage Developers Pvt Ltd.: Chikmagalur, India, 2024; p. 297. [Google Scholar]
- Silva, A.R.A.D.; Santelli, R.E.; Braz, B.F.; Silva, M.M.N.; Melo, L.; Lemes, A.C.; Ribeiro, B.D. A Comparative Study of Dairy and Non-Dairy Milk Types: Development and Characterization of Customized Plant-Based Milk Options. Foods 2024, 13, 2169. [Google Scholar] [CrossRef]
- Aladhadh, M. A Review of Modern Methods for the Detection of Foodborne Pathogens. Microorganisms 2023, 11, 1111. [Google Scholar] [CrossRef]
- Rawat, S. Food Spoilage: Microorganisms and their prevention. Asian J. Plant Sci. Res. 2015, 5, 47–56. [Google Scholar]
- Sahu, M.; Bala, S. Food Processing, Food Spoilage and their Prevention: An Overview. Int. J. Life-Sci. Sci. Res. 2017, 3, 753–759. [Google Scholar] [CrossRef]
- Augusto, P.E.D.; Soares, B.M.C.; Castanha, N. Chapter 1—Conventional Technologies of Food Preservation. In Innovative Technologies for Food Preservation; Barba, F.J., Sant’Ana, A.S., Orlien, V., Koubaa, M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 3–23. [Google Scholar] [CrossRef]
- Singh, S.; Shalini, R. Effect of Hurdle Technology in Food Preservation: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Pavlić, B.; Šojić, B.; Zavadlav, S.; Žuntar, I.; Kao, L.; Kitonić, D.; Kovačević, D.B. Innovative Hurdle Technologies for the Preservation of Functional Fruit Juices. Foods 2020, 9, e699. [Google Scholar] [CrossRef]
- Tomaz, F.S.C. Profile of customers of a provider of cellular services company: Application of Ward’s clustering method. Multi-Sci. J. 2017, 1, 92–95. [Google Scholar] [CrossRef]
- Gomes, E.A. Development of Colored Cassava Gum with Bioactives from Beetroot (Beta vulgaris). Bachelor’s Thesis, Federal University of Paraiba, João Pessoa, Brazil, 2017. [Google Scholar]
- Silva, P.A.; Cunha, R.L.; Lopes, A.S.; Pena, R.d.S. Caracterização de farinhas de tapioca produzidas no estado do Pará. Ciência Rural 2013, 43, 185–191. [Google Scholar] [CrossRef]
- Silva, P.A.; Oliveira, I.V.D.; Aguiar, R.O.; Cruz, W.P.D.; Martins, L.H.D.S.; Carvalho, F.I.M.; Lopes, A.S.; Pena, R.D.S. Development and characterization of tapioca flour obtained from cassava starches cultivated in the Northeast of Pará. Braz. J. Dev. 2020, 6, 42264–42281. [Google Scholar] [CrossRef]
- Mendes, M.L.B.; Torres, C.T.S.; Souza, I.T.; Souza, N.B.D.; Batista, N.B.; Wetmann, S.M.; Pires, C.R.F.; Zuniga, A.D.G.; Silva, C.A.S. Propriedades físico-químicas e tecnológicas de féculas de mandioca (Esculenta crantz) comercializada na região metropolitana de Palmas—TO. Tecnol. De Aliment. Tópicos Físicos Químicos E Biológicos 2020, 2, 531–544. [Google Scholar]
- Hernández-Cánovas, J.D.; Guillén-López, I.; Vizcaíno-Milla, P.; Andreo-López, M.I.; Sánchez-Rubio, M.; Taboada-Rodríguez, A.; Marín-Iniesta, F. Antimicrobial activity of Citrus spp. and Anethum graveolens components against Candida metapsilosis in ranch sauce. J. Food Sci. Technol. 2020, 57, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh-Aghdash, H.; Akbari, N.; Esazadeh, K.; Ezzati Nazhad Dolatabadi, J. Molecular and technical aspects on the interaction of serum albumin with multifunctional food preservatives. Food Chem. 2019, 293, 491–498. [Google Scholar] [CrossRef]
- Musyoka, J.N.; Abong′, G.O.; Mbogo, D.M.; Fuchs, R.; Low, J.; Heck, S.; Muzhingi, T. Effects of Acidification and Preservatives on Microbial Growth during Storage of Orange Fleshed Sweet Potato Puree. Int. J. Food Sci. 2018, 2018, 8410747. [Google Scholar] [CrossRef] [PubMed]
- McGuire, C.; Siliveru, K.; Chakraborty, S.; Ambrose, K.; Alavi, S. Flow Properties of Coarse Powders Used in Food Extrusion as a Function of Moisture Content. Processes 2024, 12, 1246. [Google Scholar] [CrossRef]
- Voelker, A.L.; Sommer, A.A.; Mauer, L.J. Moisture sorption behaviors, water activity-temperature relationships, and physical stability traits of spices, herbs, and seasoning blends containing crystalline and amorphous ingredients. Food Res. Int. 2020, 136, 109608. [Google Scholar] [CrossRef]
- Dawoodbhai, S.; Rhodes, C. The Effect of Moisture on Powder Flow and on Compaction and Physical Stability of Tablets. Drug Dev. Ind. Pharm. 2008, 15, 1577–1600. [Google Scholar] [CrossRef]
- Fitzpatrick, J.J. Characterization of the Caking Behaviour of Food Powders. In Food Powders Properties and Characterization; Ermiş, E., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 73–89. [Google Scholar] [CrossRef]
- Hischenhuber, C.; Crevel, R.; Jarry, B.; Mäki, M.; Moneret-Vautrin, D.A.; Romano, A.; Troncone, R.; Ward, R. Review article: Safe amounts of gluten for patients with wheat allergy or coeliac disease. Aliment. Pharmacol. Ther. 2006, 23, 559–575. [Google Scholar] [CrossRef]
- Calderón de la Barca, A.M.; Luna-Alcocer, V.; Valenzuela-Miranda, J.R.; Mejía-León, M.E. Gluten-Free Labeling Is Misused Frequently in Foods Marketed in Northwestern Mexico. Front. Nutr. 2021, 8, 687843. [Google Scholar] [CrossRef]
- Mehtab, W.; Sachdev, V.; Singh, A.; Agarwal, S.; Singh, N.; Malik, R.; Malhotra, A.; Ahuja, V.; Makharia, G. Gluten content in labeled and unlabeled gluten-free food products used by patients with celiac disease. Eur. J. Clin. Nutr. 2021, 75, 1245–1253. [Google Scholar] [CrossRef]
- Verma, A.K.; Gatti, S.; Galeazzi, T.; Monachesi, C.; Padella, L.; Baldo, G.D.; Annibali, R.; Lionetti, E.; Catassi, C. Gluten Contamination in Naturally or Labeled Gluten-Free Products Marketed in Italy. Nutrients 2017, 9, e115. [Google Scholar] [CrossRef]
- Guennouni, M.; Admou, B.; El khoudri, N.; Bourrhouat, A.; Zogaam, L.G.; Elmoumou, L.; Hilali, A. Gluten contamination in labelled gluten-free, naturally gluten-free and meals in food services in low-, middle- and high-income countries: A systematic review and meta-analysis. Br. J. Nutr. 2021, 127, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fritz, R.D.; Kock, L.; Garg, D.; Davis, R.M.; Kasturi, P. A stepwise, ‘test-all-positives’ methodology to assess gluten-kernel contamination at the serving-size level in gluten-free (GF) oat production. Food Chem. 2018, 240, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Khairuddin, M.A.N.; Lasekan, O. Gluten-Free Cereal Products and Beverages: A Review of Their Health Benefits in the Last Five Years. Foods 2021, 10, 2523. [Google Scholar] [CrossRef] [PubMed]
| Samples | Region | pH | Moisture (%) |
|---|---|---|---|
| GMH1 | South | 3.61 ± 0.16 h,i,j | 36.04 ± 2.53 g,h |
| GMH2 | Southeast | 4.36 ± 0.23 a,b,c,d | 38.42 ± 0.58 b,c,d,e,f,g,h |
| GMH3 | Southeast | 4.13 ± 0.06 c,d,e,f,g | 36.09 ± 0.48 g,h |
| GMH4 | Northeast | 3.83 ± 0.12 f,g,h,i,j | 39.72 ± 0.59 a,b,c,d |
| GMH5 | Southeast | 4.60 ± 0.26 a,b | 37.13 ± 1.45 d,e,f,g,h |
| GMH6 | South | 3.87 ± 0.15 f,g,h,i,j | 40.54 ± 0.35 a,b |
| GMH7 | Northeast | 3.70 ± 0.10 h,i,j | 39.08 ± 0.74 a,b,c,d,e,f |
| GMH8 | Southeast | 3.77 ± 0.06 g,h,i,j | 36.80 ± 0.23 f,g,h |
| GMH9 | Southeast | 3.67 ± 0.12 h,i,j | 38.07 ± 0.81 b,c,d,f,g,h |
| GMH10 | Southeast | 4.50 ± 0.17 a,b,c | 39.57 ± 0.63 a,b,c,d,e |
| GMH11 | Southeast | 3.83 ± 0.06 f,g,h,i,j | 37.28 ± 1.98 d,e,f,g,h |
| GMH12 | Southeast | 3.67 ± 0.23 h,i,j | 37.08 ± 0.45 d,e,f,g,h |
| GMH13 | Southeast | 4.40 ± 0.01 a,b,c,d | 38.51 ± 0.68 b,c,d,e,f,g,h |
| GMH14 | Northeast | 3.50 ± 0.01 i,j | 38.69 ± 1.06 b,c,d,e,f,g,h |
| GMH15 | Southeast | 4.43 ± 0.15 a,b,c | 38.65 ± 0.18 b,c,d,e,f,g,h |
| GMH16 | Central–West | 3.67 ± 0.23 h,i,j | 39.03 ± 1.24 a,b,c,d,e,f |
| GMH17 | Southeast | 4.17 ± 0.06 c,d,e,f,g | 36.00 ± 0.61 h |
| GMH18 | South | 3.63 ± 0.12 h,i,j | 40.55 ± 1.25 a,b |
| GMH19 | South | 3.83 ± 0.15 f,g,h,i,j | 38.68 ± 0.48 b,c,d,e,f,g,h |
| GMH20 | Southeast | 3.90 ± 0.10 e,f,g,h,i,j | 38.54 ± 0.47 b,c,d,e,f,g,h |
| GMH21 | Southeast | 3.87 ± 0.15 f,g,h,i,j | 37.15 ± 0.35 d,e,f,g,h |
| GMH22 | Southeast | 3.77 ± 0.06 g,h,i,j | 36.81 ± 0.29 e,f,g,h |
| GMH23 | Southeast | 3.90 ± 0.01 e,f,g,h,i | 39.45 ± 0.29 a,b,c,d,e,f |
| GMH24 | Northeast | 3.50 ± 0.10 i,j | 39.24 ± 0.22 a,b,c,d,e,f |
| GMH25 | Northeast | 3.63 ± 0.06 h,i,j | 39.56 ± 0.75 a,b,c,d,e |
| GMH26 | Northeast | 4.20 ± 0.10 b,c,d,e,f | 39.50 ± 0.53 a,b,c,d,e,f |
| GMH27 | Central–West | 3.60 ± 0.17 h,i,j | 41.47 ± 0.70 a |
| GMH28 | Southeast | 4.50 ± 0.01 a,b,c | 40.28 ± 0.49 a,b,c |
| GMH29 | Southeast | 3.47 ± 0.12 j | 40.56 ± 0.29 a,b |
| GMH30 | South | 4.17 ± 0.12 c,d,e,f,g | 40.31 ± 0.85 a,b,c |
| GMH31 | South | 4.63 ± 0.06 a | 39.30 ± 0.48 a,b,c |
| GMH32 | South | 4.40 ± 0.10 a,b,c,d | 37.66 ± 0.81 c,d,e,f,g,h |
| GMH33 | South | 4.00 ± 0.20 d,e,f,g,h | 38.78 ± 0.03 a,b,c,d,e,f,g |
| GMH34 | Southeast | 4.40 ± 0.10 a,b,c,d | 36.88 ± 0.71 e,f,g,h |
| GMH35 | Southeast | 4.30 ± 0.10 a,b,c,d,e | 37.58 ± 0.35 c,d,e,f,g,h |
| Group 1 (G1) | Group 2 (G2) | Group 3 (G3) | Group 4 (G4) |
|---|---|---|---|
| GMH1 a,A,P | GMH2 P | GMH3 A,P | GMH4 |
| GMH7 b | GMH5 e,P | GMH17 A,P | GMH6 A,P |
| GMH9 c,A,P | GMH10 P | GMH26 | GMH8 h |
| GMH12 c,A,P | GMH13 f,A,P | GMH30 P | GMH11 |
| GMH14 P | GMH15 A,P | GMH33 P | GMH19 a,A,P |
| GMH16 d | GMH28 A,P | GMH20 i | |
| GMH18 A | GMH31 g,P | GMH21 h | |
| GMH24 b | GMH32 g,P | GMH22 h | |
| GMH25 | GMH34 f,A,P | GMH23 i | |
| GMH27 d | GMH35 e,P | ||
| GMH29 |
| Parameter | G1 | G2 | G3 | G4 |
|---|---|---|---|---|
| pH | 3.47–3.70 | 4.30–4.63 | 4.00–4.20 | 3.77–3.91 |
| pH average * | 3.60 ± 0.05 d | 4.45 ± 0.08 a | 4.13 ± 0.09 b | 3.84 ± 0.07 c |
| Moisture (%) | 36.04–41.47 | 36.88–40.28 | 36.00–40.31 | 36.8–40.54 |
| Moisture (%) average * | 39.03 ± 1.50 a | 38.4 ± 1.05 a | 38.14 ± 1.77 a | 38.33 ± 1.31 a |
| Samples | Tyler | ||||||
|---|---|---|---|---|---|---|---|
| 12 | 14 | 20 | 28 | 32 | 35 | Bottom | |
| GMH1 | 30.44 ± 0.6 d,e,f,g | 10.84 ± 0.3 d,e,f,g | 16.62 ± 0.2 e,f,g,h | 13.76 ± 1.7 b,c,d,e | 10.49 ± 3.0 c,d,e,f,g,h | 8.17 ± 0.5 a,b,c,d,e,f | 9.10 ± 4.2 c,d,e,f,g,h,i,j,k |
| GMH2 | 17.10 ± 2.3 k,l,m,n,o,p | 9.87 ± 0.5 e,f,g,h,i,j | 15.31 ± 0.2 f,g,h | 15.11 ± 1.3 b,c,d,e | 14.44 ± 1.3 b,c,d,e,f,g | 13.42 ± 0.7 a | 14.55 ± 4.7 c,d,e,f,g |
| GMH3 | 15.02 ± 8.4 m,n,o,p | 10.87 ± 3.2 d,e,f,g | 24.58 ± 5.2 b,c | 28.71 ± 8.7 a | 11.45 ± 8.7 c,d,e,f,g,h | 5.91 ± 6.9 b,c,d,e,f | 2.14 ± 1.8 j,k |
| GMH4 | 31.85 ± 9.6 d,e,f | 11.45 ± 0.9 d,e,f | 18.14 ± 2.6 d,e,f | 18.47 ± 9.2 b,d,c,e | 7.07 ± 4.1 e,f,g,h | 6.28 ± 3.1 a,b,c,d,e,f | 5.00 ± 2.5 f,g,h,i,j,k |
| GMH5 | 27.88 ± 0.9 d,e,f,g,h,i | 9.63 ± 0.5 e,f,g,h,i,j | 16.52 ± 0.6 e,f,h,g | 23.52 ± 4.0 a,b | 11.56 ± 1.4 c,d,e,f,g,h | 4.29 ± 1.0 b,d,c,e,f | 4.90 ± 0.5 g,h,i,j,k |
| GMH6 | 9.41 ± 2.0 op | 6.80 ± 1.4 i,j,k | 16.36 ± 1.2 e,f,g,h | 22.36 ± 5.6 a,b,c | 23.60 ± 4.6 a | 11.21 ± 4.7 a,b | 8.35 ± 5.1 d,e,f,g,h,i,j,k |
| GMH7 | 23.46 ± 1.36 d,e,f,g,h,i,j,k,l,m | 9.50 ± 0.6 e,f,g,h,i,j | 16.10 ± 0.1 e,f,g,h | 15.55 ± 0.5 b,d,c,e | 16.38 ± 1.5 a,b,c | 10.56 ± 0.4 a,b | 6.17 ± 1.0 e,f,g,h,i,j,k |
| GMH8 | 21.13 ± 3.0 g,h,i,j,k,l,m | 16.06 ± 0.6 a,b | 31.45 ± 0.5 a | 16.29 ± 1.5 b,d,c,e | 6.12 ± 1.1 g,h | 2.93 ± 0.7 c,d,e,f | 3.53 ± 0.3 i,j,k |
| GMH9 | 27.44 ± 0.2 d,e,f,g,h,i,j | 9.43 ± 0.1 e,f,g,h,i,j | 16.83 ± 0.6 d,e,f,g | 13.23 ± 0.7 c,d,e | 13.49 ± 3.2 b,d,c,e,f,g | 9.42 ± 1.1 a,b,d,c,e | 7.13 ± 5.0 d,e,f,g,h,i,j,k |
| GMH10 | 17.94 ± 3.8 j,k,l,m,n,o | 10.88 ± 1.5 d,e,f,g | 18.06 ± 1.5 d,e,f,g | 15.80 ± 1.3 b,d,c,e | 15.91 ± 2.7 a,b,c,d | 11.05 ± 0.8 a,b | 8.18 ± 1.9 d,e,f,g,h,i,j,k |
| GMH11 | 19.84 ± 4.0 h,i,j,k,l,m,n | 10.17 ± 0.6 e,f,g,h,i | 17.20 ± 1.6 d,e,f,g | 14.63 ± 0.5 b,d,c,e | 11.19 ± 0.7 c,d,e,f,g,h | 9.63 ± 0.8 a,b,c,d | 15.17 ± 1.4 c,d,e,f |
| GMH12 | 25.90 ± 0.7 d,e,f,g,h,i,j,k,l | 8.75 ± 0.1 f,g,h,i,j | 15.12 ± 0.3 f,g,h | 12.21 ± 0.0 d,e | 9.22 ± 0.3 c,d,e,f,g,h | 7.79 ± 0.3 a,b,c,d,e,f | 18.94 ± 0.5 b,c |
| GMH13 | 10.39 ± 2.8 n,o,p | 7.34 ± 1.2 h,i,j,k | 16.60 ± 0.7 e,f,g,h | 14.31 ± 0.9 b,d,c,e | 11.16 ± 0.8 c,d,e,f,g,h | 9.48 ± 0.4 a,b,c,d | 28.70 ± 3.2 b |
| GMH14 | 50.48 ± 2.4 a | 10.32 ± 0.4 d,e,f,g,h | 13.28 ± 0.5 g,h | 10.99 ± 1.2 e | 6.84 ± 1.2 f,g,h | 2.24 ± 0.4 e,f | 4.03 ± 1.5 h,i,j,k |
| GMH15 | 7.50 ± 1.3 p | 4.62 ± 1.2 k | 11.91 ± 1.8 h | 13.47 ± 0.4 c,d,e | 10.35 ± 0.2 f,g,h | 10.97 ± 1.5 a,b | 39.92 ± 5.0 a |
| GMH16 | 32.29 ± 1.2 c,d,e | 12.02 ± 0.5 c,d,e,f | 18.29 ± 0.6 d,e,f | 12.44 ± 0.4 d,e | 8.29 ± 2.0 f,g,h | 6.17 ± 0.4 b,d,c,e,f | 9.03 ± 0.7 c,d,e,f,g,h,i,j,k |
| GMH17 | 42.33 ± 2.4 a,b,c | 15.50 ± 1.1 a,b | 17.52 ± 0.4 d,e,f,g | 12.61 ± 2.2 c,d,e | 7.03 ± 1.9 f,g,h | 2.00 ± 0.8 f | 1.39 ± 0.6 k |
| GMH18 | 9.46 ± 1.4 o,p | 6.74 ± 1.0 j,k | 14.48 ± 0.4 f,g,h | 14.84 ± 0.3 b,d,c,e | 12.84 ± 0.3 b,d,c,e,f,g | 11.16 ± 0.6 a,b | 29.06 ± 2.5 b |
| GMH19 | 21.68 ± 0.7 f,g,h,i,j,k,l,m | 9.32 ± 0.9 e,f,g,h,i,j | 16.50 ± 0.9 e,f,g,h | 13.47 ± 0.5 c,d,e | 11.26 ± 1.0 c,d,e,f,g,h | 8.95 ± 1.1 a,b,c,d,e,f | 16.88 ± 3.9 c,d |
| GMH20 | 17.09 ± 4.7 l,m,n,o,p | 9.74 ± 0.3 e,f,g,h,i,j | 17.12 ± 0.7 d,e,f,g | 16.50 ± 0.8 b,d,c,e | 15.35 ± 1.8 a,b,c,d,e,f | 10.79 ± 1.8 a,b | 12.43 ± 6.6 c,d,e,f,g,h,i |
| GMH21 | 20.71 ± 1.2 g,h,i,j,k,l,m | 15.30 ± 0.5 a,b,c | 30.49 ± 0.5 a | 16.35 ± 0.3 b,d,c,e | 6.90 ± 0.8 f,g,h | 4.39 ± 0.7 b,d,c,e,f | 4.05 ± 0.5 h,i,j,k |
| GMH22 | 16.23 ± 1.4 l,m,n,o,p | 12.03 ± 1.2 c,d,e,f | 29.16 ± 2.2 a,b | 20.98 ± 0.4 a,b,c,d | 9.74 ± 1.2 c,d,e,f,g,h | 5.26 ± 1.8 b,d,c,e,f | 5.20 ± 0.9 f,g,h,i,j,k |
| GMH23 | 22.49 ± 1.4 e,f,g,h,i,j,k,l,m | 8.82 ± 0.4 f,g,h,i,j | 6.37 ± 0.8 e,f,g,h | 17.98 ± 4.7 b,d,c,e | 21.14 ± 1.8 a,b | 6.89 ± 4.2 a,b,c,d,e,f | 4.66 ± 2.3 g,h,i,j,k |
| GMH24 | 43.84 ± 1.7 a,b | 10.21 ± 0.7 e,f,g,h,i | 15.40 ± 0.5 f,g,h | 11.98 ± 1.4 d,e | 7.60 ± 0.5 d,e,f,g,h | 4.86 ± 1.3 b,d,c,e,f | 5.49 ± 2.3 f,g,h,i,j,k |
| GMH25 | 33.70 ± 4.0 b,c,d | 9.57 ± 0.3 e,f,g,h,i,j | 14.82 ± 1.1 f,g,h | 14.11 ± 3.4 b,d,c,e | 13.56 ± 3.0 b,d,c,e,f,g | 5.73 ± 2.0 b,d,c,e,f | 5.55 ± 1.9 f,g,h,i,j,k |
| GMH26 | 27.37 ± 1.0 d,e,f,g,h,i,j,k | 9.31 ± 0.9 e,f,g,h,i,j | 16.03 ± 0.5 e,f,g,h | 16.34 ± 3.8 b,d,c,e | 15.64 ± 4.28 a,b,d,c,e | 6.68 ± 3.1 a,b,c,d,e,f | 7.16 ± 5.4 d,e,f,g,h,i,j,k |
| GMH27 | 21.82 ± 0.8 f,g,h,i,j,k,l,m | 8.94 ± 0.2 f,g,h,i,j | 15.77 ± 0.4 e,f,g,h | 14.46 ± 0.6 b,d,c,e | 13.41 ± 0.6 b,d,c,e,f,g | 9.94 ± 0.7 a,b,c | 14.04 ± 0.2 c,d,e,f,g,h |
| GMH28 | 32.68 ± 3.9 c,d,e | 9.69 ± 0.4 e,f,g,h,i,j | 15.95 ± 0.5 e,f,g,h | 12.44 ± 0.6 d,e | 8.89 ± 0.6 c,d,e,f,g,h | 6.64 ± 0.4 a,b,c,d,e,f | 12.25 ± 2.9 c,d,e,f,g,h,i,j |
| GMH29 | 30.77 ± 0.9 d,e,f,g | 17.08 ± 0.6 a | 24.81 ± 3.0 b,c | 14.21 ± 1.7 b,d,c,e | 4.18 ± 2.5 h | 2.50 ± 1.7 d,e,f | 2.88 ± 3.0 i,j,k |
| GMH30 | 14.48 ± 0.5 m,n,o,p | 12.69 ± 0.8 b,d,c,e | 18.54 ± 2.2 d,e,f | 14.35 ± 1.6 b,d,c,e | 10.37 ± 0.9 c,d,e,f,g,h | 7.62 ± 1.1 a,b,c,d,e,f | 16.24 ± 2.2 c,d,e |
| GMH31 | 29.97 ± 2.3 d,e,f,g,h | 10.90 ± 0.5 d,e,f,g | 20.41 ± 0.5 c,d,e | 16.43 ± 0.5 b,d,c,e | 8.54 ± 2.1 c,d,e,f,g,h | 5.00 ± 0.8 b,d,c,ef | 6.63 ± 3.7 e,f,g,h,i,j,k |
| GMH32 | 18.68 ± 3.9 i,j,k,l,m,n,o | 7.74 ± 2.0 g,h,i,j,k | 18.57 ± 0.7 d,e,f | 20.91 ± 4.1 a,b,c,d | 15.40 ± 4.5 a,b,c,d,e,f | 8.07 ± 5.1 a,b,c,d,e,f | 5.27 ± 1.7 f,g,h,i,j,k |
| GMH33 | 28.84 ± 1.5 d,e,f,g,h,i | 8.77 ± 1.1 f,g,h,i,j | 15.68 ± 1.3 e,f,g,h | 13.68 ± 0.5 b,d,c,e | 10.58 ± 2.0 c,d,e,f,g,h | 7.97 ± 0.8 a,b,c,d,e,f | 9.30 ± 4.2 c,d,e,f,g,h,i,j,k |
| GMH34 | 30.92 ± 2.6 d,e,f,g | 9.05 ± 1.5 f,g,h,i,j | 14.91 ± 0.7 f,h,g | 15.33 ± 3.2 b,d,c,e | 9.55 ± 0.3 c,d,e,f,g,h | 7.38 ± 1.8 a,b,c,d,e,f | 9.33 ± 4.1 c,d,e,f,g,h,i,j,k |
| GMH35 | 32.58 ± 2.7 c,d,e | 13.64 ± 0.6 b,c,d | 21.46 ± 0.4 c,d | 14.41 ± 2.6 b,d,c,e | 7.66 ± 2.2 d,e,f,g,h | 3.09 ± 0.5 c,d,e,f | 4.80 ± 1.8 g,h,i,j,k |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malta, M.M.C.; Pinto, G.M.T.; Guimarães, I.C.C.; Melo, L.; Lemes, A.C.; Pereira, K.S. Investigation of Gluten Contamination in Commercial Hydrated Cassava Starch and Its Physicochemical Properties. Appl. Sci. 2025, 15, 7510. https://doi.org/10.3390/app15137510
Malta MMC, Pinto GMT, Guimarães ICC, Melo L, Lemes AC, Pereira KS. Investigation of Gluten Contamination in Commercial Hydrated Cassava Starch and Its Physicochemical Properties. Applied Sciences. 2025; 15(13):7510. https://doi.org/10.3390/app15137510
Chicago/Turabian StyleMalta, Marina Magalhães Cardoso, Giovanna Musco Twardowski Pinto, Isabela Caldas Castañon Guimarães, Lauro Melo, Ailton Cesar Lemes, and Karen Signori Pereira. 2025. "Investigation of Gluten Contamination in Commercial Hydrated Cassava Starch and Its Physicochemical Properties" Applied Sciences 15, no. 13: 7510. https://doi.org/10.3390/app15137510
APA StyleMalta, M. M. C., Pinto, G. M. T., Guimarães, I. C. C., Melo, L., Lemes, A. C., & Pereira, K. S. (2025). Investigation of Gluten Contamination in Commercial Hydrated Cassava Starch and Its Physicochemical Properties. Applied Sciences, 15(13), 7510. https://doi.org/10.3390/app15137510







