Randomized Controlled Clinical Trial to Evaluate Skeletal and Dental Treatment Effects of Rapid Maxillary Expansion in Children: Comparison Between Two-Band Expander and Bonded Palatal Expander
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
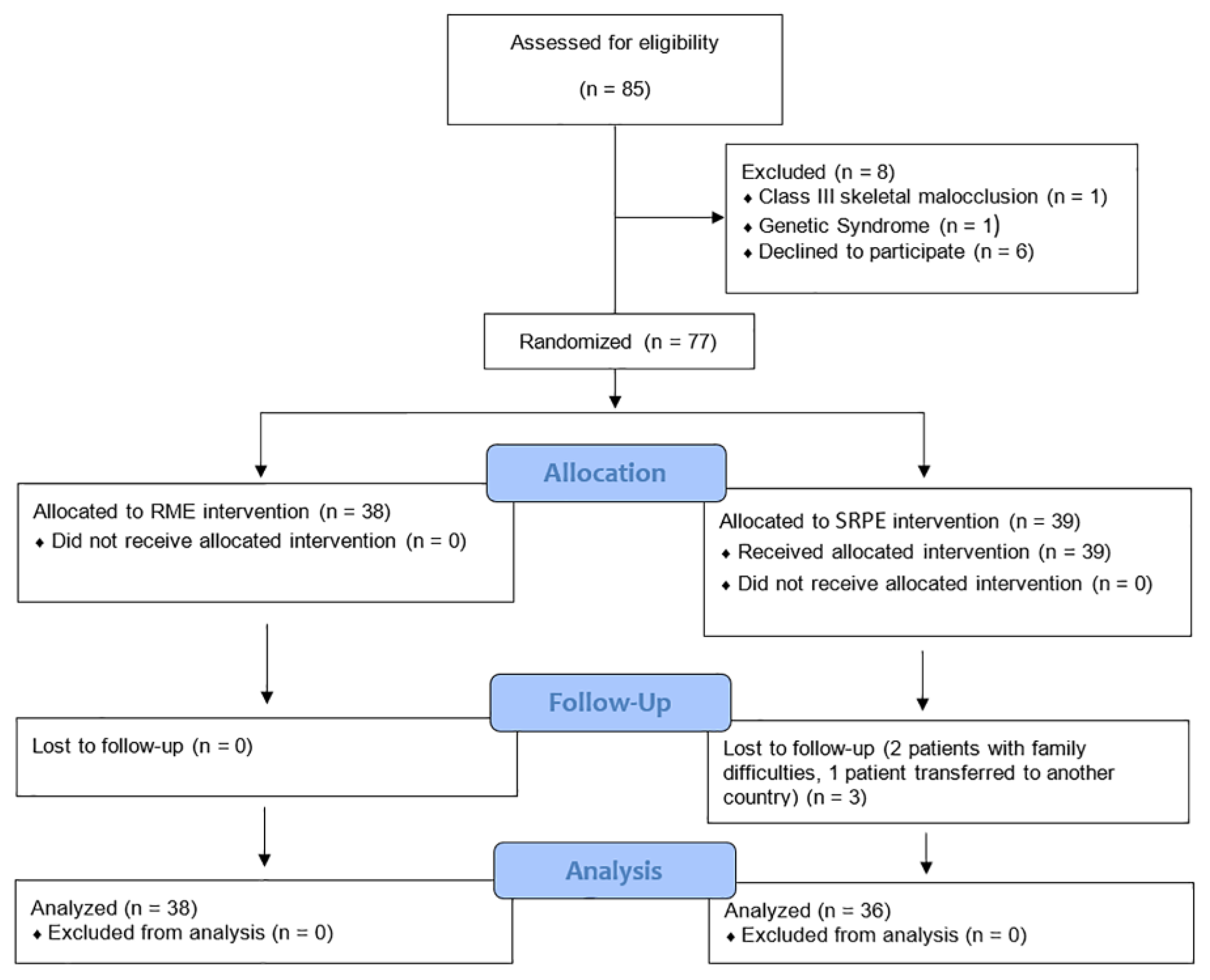
2.1. Study Design
2.2. Subjects and Methods
- Deciduous, early, or late mixed dentition;
- Unilateral or bilateral posterior crossbite affecting at least one cusp of the first permanent molar and/or second deciduous molar due to skeletal etiology.
- Birth defects or genetic syndromes (such as cleft lip or palate);
- Systemic diseases that can affect the normal growth pattern;
- Previous orthodontic treatment;
- Signs and/or symptoms of temporomandibular disorders;
- Severe periodontal problems.
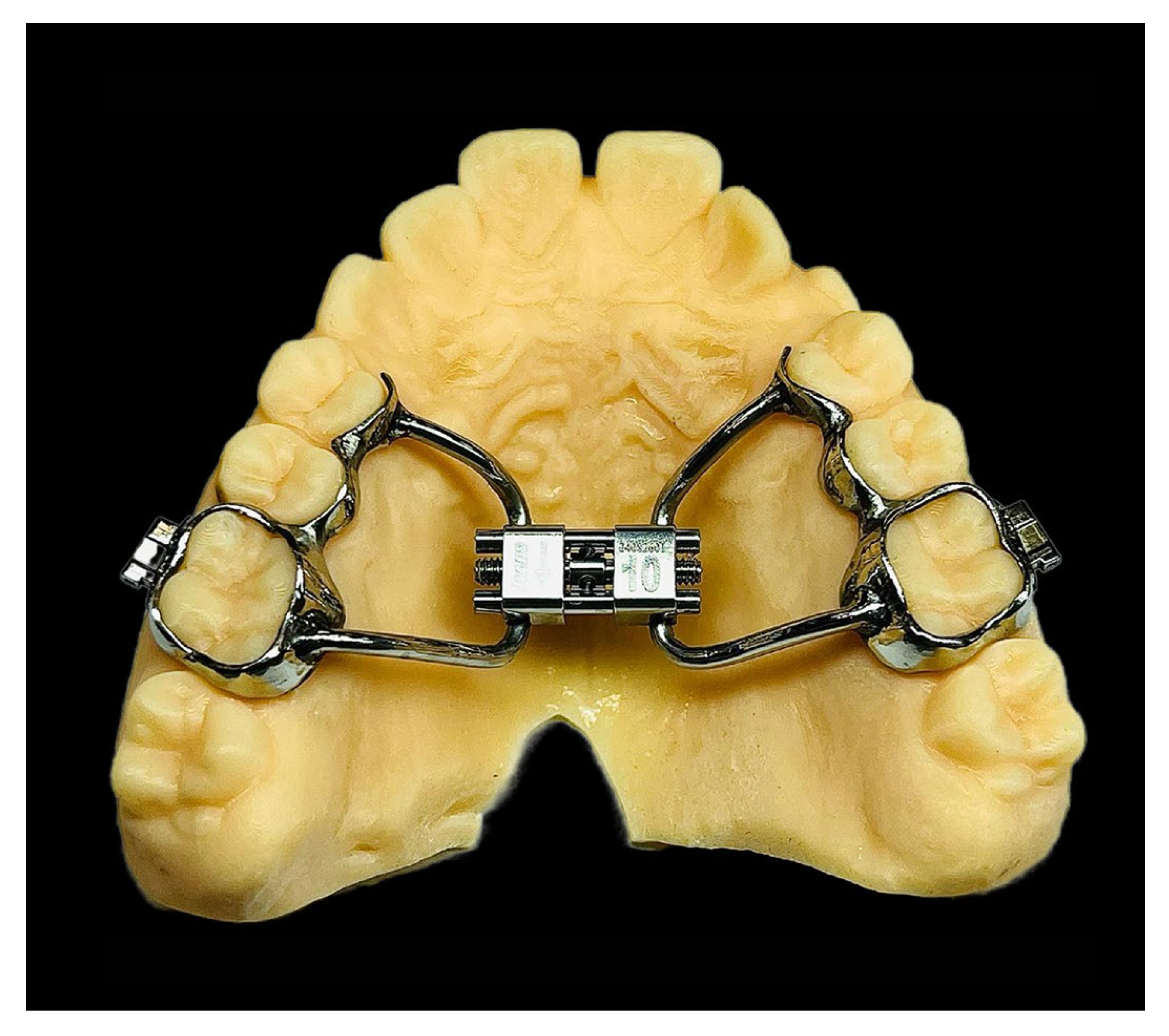
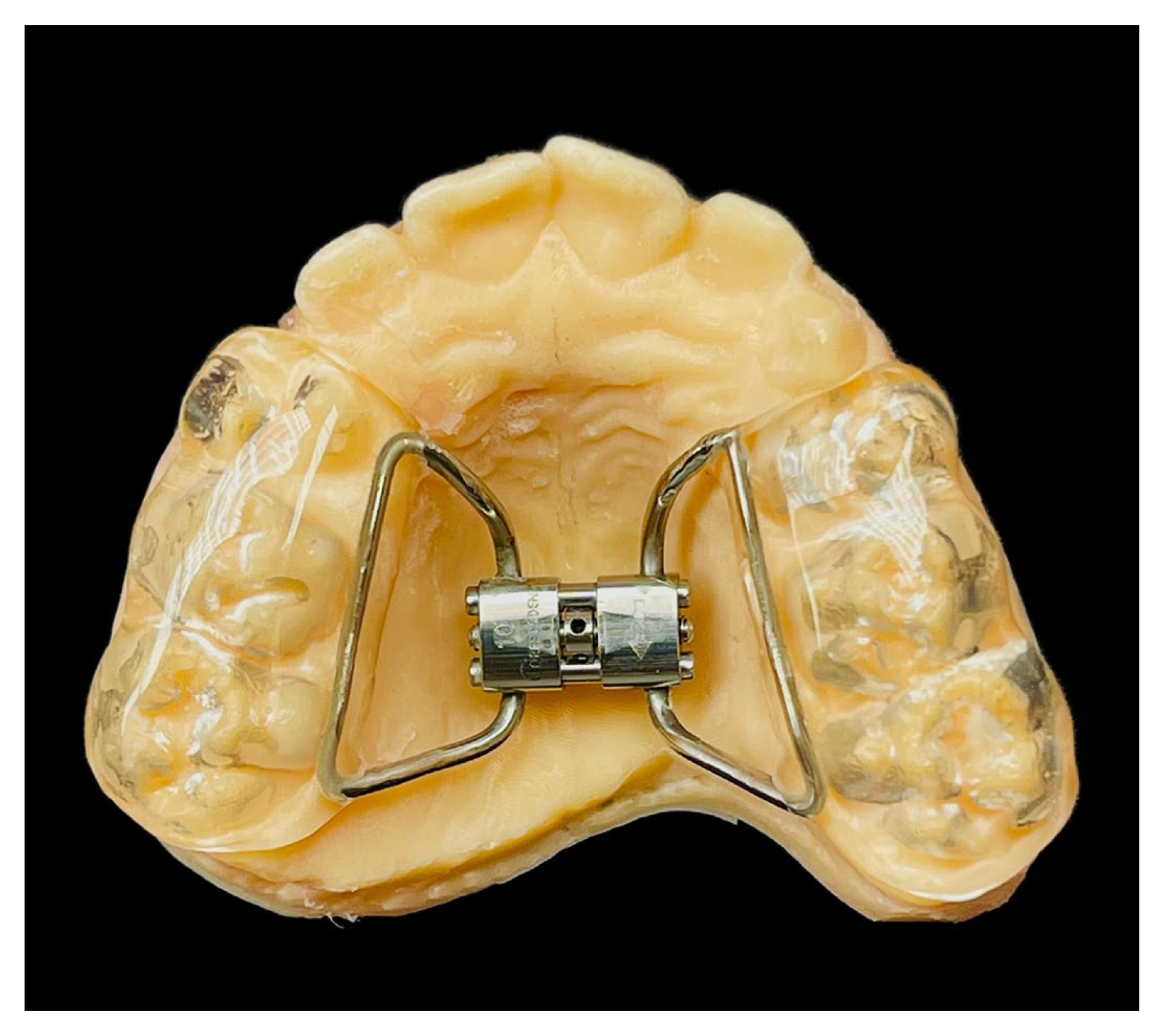
2.3. Treatments
2.4. Outcomes
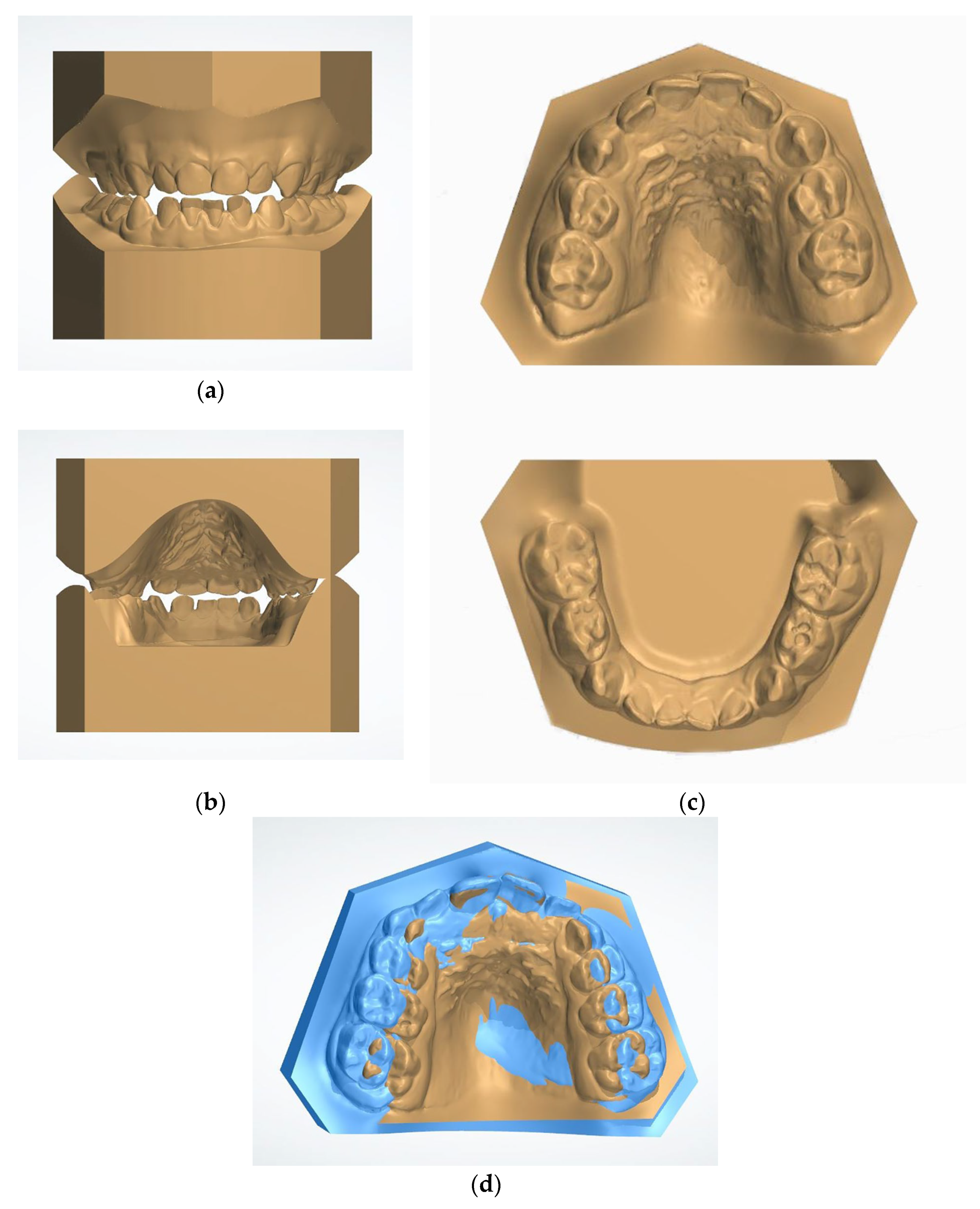
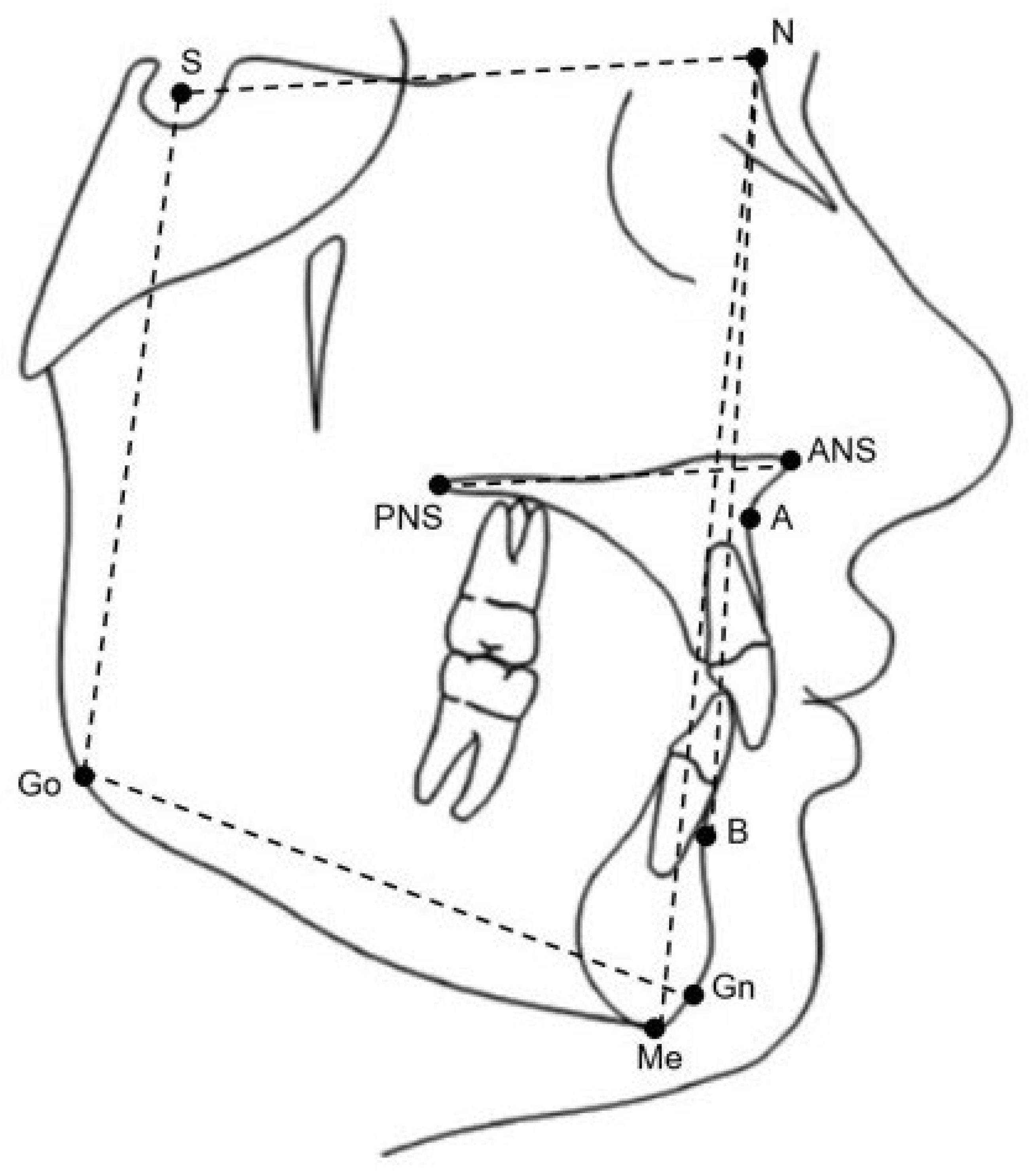
2.5. Cephalometric Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iodice, G.; Danzi, G.; Cimino, R.; Paduano, S.; Michelotti, A. Association between posterior crossbite, skeletal, and muscle asymmetry: A systematic review. Eur. J. Orthod. 2016, 38, 638–651. [Google Scholar] [CrossRef]
- Malandris, M.; Mahoney, E.K. Aetiology, diagnosis and treatment of posterior crossbites in the primary dentition. Int. J. Paediatr. Dent. 2004, 14, 155–166. [Google Scholar] [CrossRef]
- Petrèn, S.; Bondemark, L.; Soderfeldt, B. A systematic review concerning early orthodontic treatment of unilateral posterior cross bite. Angle Orthod. 2003, 73, 588–596. [Google Scholar] [PubMed]
- Thilander, B.; Bjerklin, K. Posterior crossbite and temporomandibular disorders (TMDs): Need for orthodontic treatment? Eur. Orthod. 2012, 34, 667–673. [Google Scholar] [CrossRef]
- Veli, I.; Uysal, T.; Ozer, T.; Ucar, F.I.; Eruz, M. Mandibular asymmetry in unilateral and bilateral posterior crossbite patients using cone-beam computed tomography. Angle Orthod. 2011, 81, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Lione, R.; Franchi, L.; Cozza, P. Does rapid maxillary expansion induce adverse effects in growing subjects? Angle Orthod. 2013, 83, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Bistaffa, A.G.I.; Belomo-Yamaguchi, L.; de Castro Ferreira Conti, A.C.; Oltramari, P.V.P.; de Almeida, M.R.; de Almeida-Pedrin, R.R.; Fernandes, T.M.F. Immediate skeletal effects of rapid maxillary expansion at midpalatal suture opening with Differential, Hyrax and Haas expanders. Dent. Press J. Orthod. 2022, 27, e2220525. [Google Scholar] [CrossRef]
- Harrison, J.E.; Ashby, D. Orthodontic treatment for posterior crossbites. Cochrane Database Syst. Rev. 2000, 2, CD000979. [Google Scholar]
- Caroccia, F.; Moscagiuri, F.; Falconio, L.; Festa, F.; D’Attilio, M. Early Orthodontic Treatments of Unilateral Posterior Crossbite: A Systematic Review. J. Clin. Med. 2020, 10, 33. [Google Scholar] [CrossRef]
- Basciftci, F.A.; Karaman, A.I. Effects of a modified acrylic bonded rapid maxillary expansion appliance and vertical chin cap on dentofacial structures. Angle Orthod. 2002, 72, 61–71. [Google Scholar]
- Lineberger, M.W.; McNamara, J.A.; Baccetti, T.; Herberger, T.; Franchi, L. Effects of rapid maxillary expansion in hyperdivergent patients. Am. J. Orthod. Dentofac. Orthop. 2012, 142, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Wendling, L.K.; McNamara, J.A., Jr.; Franchi, L.; Baccetti, T. A prospective study of the short-term treatment effects of the acrylic-splint rapid maxillary expander combined with the lower Schwarz appliance. Angle Orthod. 2005, 75, 7–14. [Google Scholar] [PubMed]
- Suzuki, H.; Moon, W.; Previdente, L.H.; Suzuki, S.S.; Garcez, A.S.; Consolaro, A. Miniscrew-assisted rapid palatal expander (BONE BORNE EXPANSION): The quest for pure orthopedic movement. Dent. Press J. Orthod. 2016, 21, 17–23. [Google Scholar] [CrossRef]
- Huang, X.; Han, Y.; Yang, S. Effect and stability of miniscrew-assisted rapid palatal expansion: A systematic review and meta-analysis. Korean J. Orthod. 2022, 52, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Bazargani, F.; Lund, H.; Magnuson, A.; Ludwig, B. Skeletal and dentoalveolar effects using tooth-borne and tooth-bone-borne RME appliances: A randomized controlled trial with 1-year follow-up. Eur. J. Orthod. 2021, 43, 245–253. [Google Scholar] [CrossRef]
- Krüsi, M.; Eliades, T.; Papageorgiou, S.N. Are there benefits from using bone-borne maxillary expansion instead of tooth-borne maxillary expansion? A systematic review with meta-analysis. Prog. Orthod. 2019, 20, 9. [Google Scholar] [CrossRef]
- Vincenzo, G.; Fabrizia, D.; Abdolreza, J.; Felice, F.; Lorenzo, F.; Letizia, P. Comparison between rapid and mixed maxillary expansion through an assessment of arch changes on dental casts. Prog. Orthod. 2015, 16, 20. [Google Scholar] [CrossRef]
- Sampson, A.; Figueiredo, D.S.F.; Jeremiah, H.G.; Oliveira, D.D.; Freitas, L.R.P.; Chahoud, M.; Soares, R.V.; Cobourne, M.T. The effect of social media on patient acceptance of temporary anchorage devices. Angle Orthod. 2021, 91, 363–370. [Google Scholar] [CrossRef]
- Mehta, S.; Arqub, S.A.; Vishwanath, M.; Upadhyay, M.; Yadav, S. Biomechanics of conventional and miniscrew-assisted rapid palatal expansion. J. World Fed. Orthod. 2024, 13, 105–112. [Google Scholar] [CrossRef]
- Cheng, J.H.-C.; Chen, D.D.-S.; Tan, Y.; Hu, H.T. Factors associated with usage frequency and pricing of temporary anchorage devices among orthodontists. J. Dent. Sci. 2024, 19, 404–410. [Google Scholar] [CrossRef]
- Putrino, A.; Caputo, M.; Galeotti, A.; Marinelli, E.; Zaami, S. Type I Dentin Dysplasia: The Literature Review and Case Report of a Family Affected by Misrecognition and Late Diagnosis. Medicina 2023, 59, 1477. [Google Scholar] [CrossRef] [PubMed]
- Lione, R.; Huanca Ghislanzoni, L.T.; Defraia, E.; Franchi, L.; Cozza, P. Bonded versus banded rapid palatal expander followed by facial mask therapy: Analysis on digital dental casts. Eur. J. Orthod. 2016, 38, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Laganà, G.; Paoloni, V.; Pavoni, C.; Palmacci, D.; Malara, A. Tridimensional Changes in Mandibular Arch after Rapid Maxillary Expansion Therapy: A Clinical Study. Children 2023, 10, 775. [Google Scholar] [CrossRef] [PubMed]
- Conroy-Piskai, C.; Galang-Boquiren, M.T.S.; Obrez, A.; Costa Viana, M.G.; Oppermann, N.; Sanchez, F.; Edgren, B.; Kusnoto, B. Assessment of vertical changes during maxillary expansion using quad helix or bonded rapid maxillary expander. Angle Orthod. 2016, 86, 925–933. [Google Scholar] [CrossRef]
- Solow, B.; Kreiborg, S. Soft-tissue stretching: A possible control factor in craniofacial morphogenesis. Scand J. Dent. Res. 1977, 85, 505–507. [Google Scholar] [CrossRef]
- Galeotti, A.; Viarani, V.; Franchi, L.; Martina, S.; Rongo, R.; D’Antò, V.; Uomo, R.; Aristei, F.; Festa, P. Cephalometric changes of pushing splints 3 compared to rapid maxillary expansion and facemask therapy on the airway space in class III growing patients: A randomized clinical trial. Orthod. Craniofac. Res. 2024, 27, 552–559. [Google Scholar] [CrossRef]
- Chang, J.Y.; McNamara, J.A., Jr.; Herberger, T.A. A longitudinal study of skeletal side effects induced by rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 1997, 112, 330–337. [Google Scholar] [CrossRef]
- O’Grady, P.W.; McNamara, J.A., Jr.; Baccetti, T.; Franchi, L. A long-term evaluation of the mandibular Schwarz appliance and the acrylic splint expander in early mixed dentition patients. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 202–213. [Google Scholar] [CrossRef]
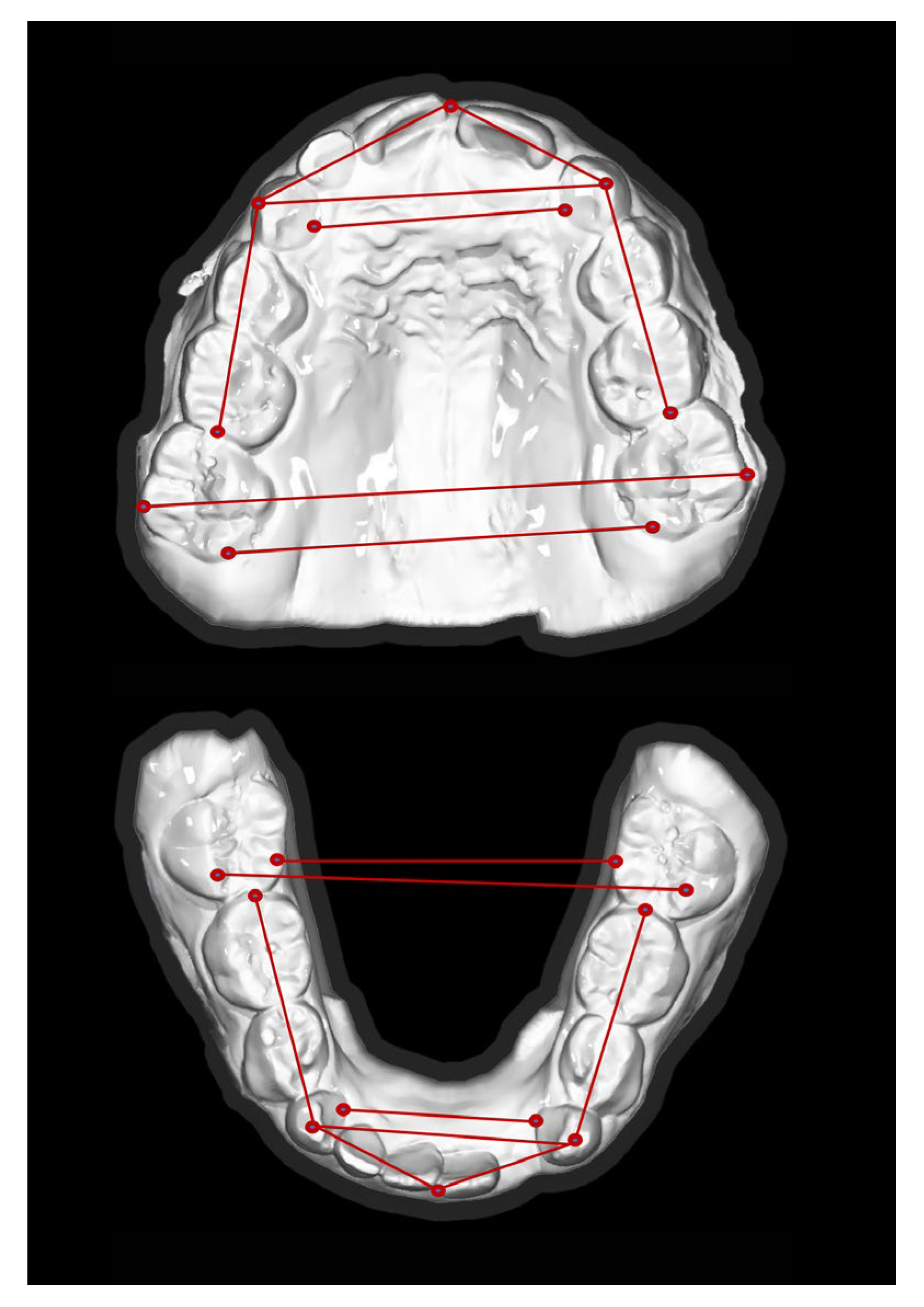
| Group A (n = 38) | Group B (n = 36) | |||||
|---|---|---|---|---|---|---|
| Cephalometric Measurements | Mean | SD | Mean | SD | 95% CI for Mean | p Value (t Test) |
| ANB (degrees) | 4.6 | 2.4 | 4 | 2.1 | 0.57 | 0.1 |
| SNB (degrees) | 76.3 | 3.1 | 75.7 | 3.1 | 0.53 | 0.3 |
| SNA (degrees) | 80.8 | 3.4 | 79.7 | 3 | 1.1 | 0.2 |
| SN-GoGn (degrees) | 32.8 | 4.3 | 33.8 | 5.2 | −1 | 0.5 |
| SN_ANS_PNS (degrees) | 9.3 | 3.2 | 9.3 | 3.6 | −0.1 | 0.9 |
| ANS_PNS_GoGn (degrees) | 26.2 | 4.2 | 27.1 | 4.8 | −0.8 | 0.8 |
| P-A face height (percentage) | 62.9 | 3.8 | 62.2 | 4.1 | 0.7 | 0.5 |
| Group A (n = 38) | Group B (n = 36) | |||||
|---|---|---|---|---|---|---|
| Baseline Data | Mean | SD | Mean | SD | 95% CI for Mean | p Value (t Test) |
| Maxilla | ||||||
| Intermolar distance (mesiobuccal cusp tips) | 45.1 | 3.9 | 44.7 | 5.6 | 0.4 | 0.87 |
| Intercanine distance (mesiobuccal cusp tips) | 29.6 | 3.7 | 28.4 | 5 | 1.2 | 0.51 |
| Mandible | ||||||
| Intermolar distance (mesiobuccal cusp tips) | 45.3 | 4.2 | 45.3 | 3.2 | −0.1 | 0.65 |
| Intercanine distance (mesiobuccal cusp tips) | 25.9 | 2.4 | 25.9 | 3 | 0 | 0.92 |
| Overjet | 2.3 | 2.1 | 2.4 | 2 | −0.1 | 0.96 |
| Overbite | 1.9 | 1.8 | 1.5 | 1.6 | 0.45 | 0.14 |
| Group A (n = 38) | Group B (n = 36) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cephalometric Measurements | Mean | SD | Difference | p Value (t Test) | Mean | SD | Difference | p Value (t Test) |
| ANB (degrees) | 4.3 | 2.1 | −0.3 | 0.264 | 3.8 | 1.8 | −0.2 | 0.253 |
| SNB (degrees) | 76 | 2.9 | 0 | 0.414 | 75.4 | 3.1 | −0.3 | 0.321 |
| SNA (degrees) | 80.3 | 3.5 | −0.5 | 0.099 | 79.1 | 3.3 | −0.7 | 0.137 |
| SN-GoGn (degrees) | 32.9 | 4.1 | 0.1 | 0.82 | 34.7 | 5.8 | −0.9 | 0.009 * |
| SN_ANS_PNS (degrees) | 9.4 | 3 | −0.1 | 0.787 | 10 | 3.7 | 0.7 | 0.084 |
| ANS_PNS_GoGn (degrees) | 26.1 | 3.6 | −0.2 | 0.767 | 27.3 | 4.6 | 0.2 | 0.564 |
| P-A face height (percentage) | 62.7 | 3.2 | 0.2 | 0.667 | 61.1 | 4.8 | 1.1 | 0.008 * |
| Group A (n = 38) | Group B (n = 36) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline Data | Mean | SD | Difference | p Value (t Test) | Mean | SD | Difference | p Value (t Test) |
| Maxilla | ||||||||
| Intermolar distance (mesiobuccal cusp tips) | 52.4 | 3.3 | −7.3 | 0.000 * | 52.3 | 2.8 | −7.6 | 0.000 * |
| Intercanine distance (mesiobuccal cusp tips) | 32.7 | 2.8 | −3.1 | 0.000 * | 34.4 | 2.8 | −5.9 | 0.000 * |
| Mandible | ||||||||
| Intermolar distance (mesiobuccal cusp tips) | 45.7 | 2.7 | −0.4 | 0.409 | 45.9 | 2.9 | −0.6 | 0.175 |
| Intercanine distance (mesiobuccal cusp tips) | 25.9 | 2.2 | 0 | 0.994 | 27 | 2.2 | −1.1 | 0.028 ‡ |
| Overjet | 2.9 | 2.2 | −0.6 | 0.011 ‡ | 2.6 | 1.6 | −0.2 | 0.47 |
| Overbite | 2.4 | 1.6 | −0.5 | 0.05 | 2.1 | 1.6 | −0.6 | 0.010 ‡ |
| Group A (n = 38) | Group B (n = 36) | |||||
|---|---|---|---|---|---|---|
| Cephalometric Measurements | Mean | SD | Mean | SD | Difference | p Value (t Test) |
| ANB (degrees) | −0.3 | 1.6 | −0.2 | 1.2 | −0.1 | 0.67 |
| SNB (degrees) | −0.2 | 1.6 | −0.3 | 2 | 0.1 | 0.78 |
| SNA (degrees) | −0.5 | 1.8 | −0.7 | 2.7 | 0.2 | 0.62 |
| SN-GoGn (degrees) | 0.1 | 2.3 | 0.9 | 2 | −0.8 | 0.06 |
| SN_ANS_PNS (degrees) | 0.1 | 2.6 | 0.7 | 2.3 | −0.5 | 0.56 |
| ANS_PNS_GoGn (degrees) | −0.2 | 3.4 | 0.2 | 2.4 | −0.4 | 0.43 |
| P-A face height (percentage) | −0.2 | 2.5 | 1.1 | 2.3 | −1.3 | 0.1 |
| Group A (n = 38) | Group B (n = 36) | |||||
|---|---|---|---|---|---|---|
| Baseline Data | Mean | SD | Mean | SD | Difference | p Value (t Test) |
| Maxilla | ||||||
| Intermolar distance (mesiobuccal cusp tips) | 7.3 | 3.4 | 7.6 | 5.1 | −0.3 | 0.54 |
| Intercanine distance (mesiobuccal cusp tips) | 3.1 | 2.9 | 6 | 4.8 | −2.8 | 0.000 * |
| Mandible | ||||||
| Intermolar distance (mesiobuccal cusp tips) | 0.4 | 3 | 0.6 | 2.5 | −0.2 | 0.27 |
| Intercanine distance (mesiobuccal cusp tips) | 0 | 1.4 | 1.1 | 2.9 | −1.1 * | 0.1 |
| Overjet | 0.6 | 1.4 | 0.2 | 1.7 | 0.4 | 0.76 |
| Overbite | 0.5 | 1.5 | 0.6 | 1.4 | −0.1 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viarani, V.; Festa, P.; Galasso, G.; D’Antò, V.; Putrino, A.; Mariani, A.; Bompiani, G.; Galeotti, A. Randomized Controlled Clinical Trial to Evaluate Skeletal and Dental Treatment Effects of Rapid Maxillary Expansion in Children: Comparison Between Two-Band Expander and Bonded Palatal Expander. Appl. Sci. 2025, 15, 7187. https://doi.org/10.3390/app15137187
Viarani V, Festa P, Galasso G, D’Antò V, Putrino A, Mariani A, Bompiani G, Galeotti A. Randomized Controlled Clinical Trial to Evaluate Skeletal and Dental Treatment Effects of Rapid Maxillary Expansion in Children: Comparison Between Two-Band Expander and Bonded Palatal Expander. Applied Sciences. 2025; 15(13):7187. https://doi.org/10.3390/app15137187
Chicago/Turabian StyleViarani, Valeria, Paola Festa, Giorgia Galasso, Vincenzo D’Antò, Alessandra Putrino, Andrea Mariani, Gaia Bompiani, and Angela Galeotti. 2025. "Randomized Controlled Clinical Trial to Evaluate Skeletal and Dental Treatment Effects of Rapid Maxillary Expansion in Children: Comparison Between Two-Band Expander and Bonded Palatal Expander" Applied Sciences 15, no. 13: 7187. https://doi.org/10.3390/app15137187
APA StyleViarani, V., Festa, P., Galasso, G., D’Antò, V., Putrino, A., Mariani, A., Bompiani, G., & Galeotti, A. (2025). Randomized Controlled Clinical Trial to Evaluate Skeletal and Dental Treatment Effects of Rapid Maxillary Expansion in Children: Comparison Between Two-Band Expander and Bonded Palatal Expander. Applied Sciences, 15(13), 7187. https://doi.org/10.3390/app15137187







