Abstract
The processing of peaches generates large quantities of by-products, including peels, pomace, and seeds. Despite containing high levels of bioactive compounds with antioxidant properties, these by-products are often discarded as waste, thereby contributing to increased food waste. The present paper aimed to evaluate the total bioactive compound content in peach pomace and biscuits fortified with various concentrations of peach pomace (5%, 10%, and 15%), with a view to utilizing this valuable by-product in functional foods. Compositional analysis revealed that peach pomace is a significant source of polyphenols (1771.64 mg GAE 100 g−1), flavonoids (478.99 mg RE 100 g−1), and anthocyanins (21.18 mg C3GE 100 g−1), and has a radical scavenging capacity of 40.41%. The FTIR analysis confirmed the presence of multiple functional groups in peach pomace that can be associated with polyphenols, polysaccharides, organic acids, esters, monosaccharides, and structurally bound water. Among the individual phenolic compounds, high concentrations of rutin (8.12 mg 100 g−1), chlorogenic acid (3.77 mg 100 g−1), and sinapic acid (2.70 mg 100 g−1) were recorded. Following the replacement of wheat flour with peach pomace, increases in the content of bioactive compounds were observed. At the maximum level of 15% pomace, the biscuits presented the highest concentrations of polyphenols (444.04 mg GAE 100 g−1), flavonoids (211.11 mg RE 100 g−1), anthocyanins (25.43 mg C3GE 100 g−1), sugars (46.48 g GluE 100 g−1), and radical scavenging activity (27.21%). Similar bands were found in the FTIR spectra of the biscuits, indicating the presence of phenolic compounds and glycosides. The 1366 cm−1 band, which is associated with C–O stretching and C–H and N–H deformation in peach pomace, appeared in the enriched biscuit samples at 1340–1374 cm−1 but not in the control sample. These results suggest that peach pomace represents an ingredient with significant potential for use in the food industry, having the ability to improve the nutritional value of biscuits.
Keywords:
peach pomace; functional biscuits; innovative foods; polyphenols; flavonoids; anthocyanins 1. Introduction
The bakery industry is in continuous development, with the products sold by this sector gaining notoriety among consumers [1]. Among the frequently consumed foods, also widespread globally, biscuits can be noted, which are usually prepared from wheat flour, sugar, and fat [2]. As demonstrated by Hu et al. (2022) [3], the annual per capita biscuit consumption exceeds 10 kg in the United States and other Western European countries, including the United Kingdom. In contrast, this quantity is lower in Southeast Asian countries such as Singapore, Hong Kong, Thailand, and Indonesia, though it still exceeds 4.25 kg per capita. Thanks to advancements in the manufacturing technology, an expanded product range, enhanced accessibility, a prolonged shelf life, and an appealing sensory profile [3,4], this versatile food can satisfy a wide range of tastes. This makes biscuits a viable food source in emergency situations [3].However, due to the high concentrations of carbohydrates and lipids and the relatively low level of biologically active substances [5], these products do not correspond to current consumption trends as they cannot be considered nutritionally rich foods [6]. The integration of fruit pomace into the manufacturing recipe of food products containing a high amount of white flour, such as biscuits, is a topic of great interest for the food industry [7]. Fruit pomace is highlighted by a high concentration of compounds with a protective role in health, such as polyphenols, flavonoids, anthocyanins, carotenoids, fibers, minerals, and vitamins, thus constituting an essential source of nutrients for the daily human diet [8]. Polyphenols exhibit multiple positive aspects for health, including neuroprotective, cardioprotective, antidiabetic effects, protection against oxidative stress and degenerative diseases, anti-inflammatory and anticancer properties, support of the gastrointestinal system health, improvement of endothelial functions, support of the immune system, antiallergic activity, and modulation of hormonal effects [9,10]. Flavonoids have beneficial effects on coronary heart disease, hypertension, glucose, and lipid metabolism [11], while anthocyanins stand out for their antioxidant, anti-inflammatory, antimicrobial, and anticancer action [12,13].
The peach (Prunus persica L. Batsch) is one of the world’s most valuable fruit crops, ranking just behind apples (Malus spp.) and pears (Pyrus spp.) [14]. Thanks to its high fiber, vitamin, and mineral content, the peel of this fruit has significant potential for use in developing functional food products. Using peach pomace as a fortifying ingredient is an innovative and sustainable way to enhance the nutritional value of food products [15]. However, peach pomace is currently considered waste and is often disposed of in landfills or fields, contributing to the accumulation of organic waste and the loss of valuable resources [16]. Wasting these natural resources has consequences for food security and the environment, increasing pollution in terrestrial and aquatic ecosystems [17]. Both the European Union and the United Nations emphasize the importance of reducing food waste. This is one of the targets set out in Sustainable Development Goal 12.3 of the UN’s 2030 Agenda for Sustainable Development. The aim is to minimize food losses and waste at every stage of the food chain. It is, therefore, crucial to adopt strategies that utilize residues generated by the food processing industry in order to address concerns about current waste management practices. It is estimated that reducing global food waste could cut greenhouse gas emissions—the main cause of global warming—by around 8% [18].
The aim of this paper is to characterize peach pomace by determining its chemical composition and verifying its potential as an ingredient in functional foods. In order to evaluate its impact on the chemical properties of biscuits and to create a sustainable, functional food product, peach pomace was incorporated into biscuits at concentrations of 5%, 10%, and 15%.
2. Materials and Methods
2.1. Vegetal Material
The peach pomace from the Victoria variety (Figure 1) was collected as a by-product of cold-pressing fruit to make juice at the Rodiana SRL processing plant in Sura Mare, Sibiu, Romania. After collection, the pomace was dehydrated at a temperature of 50–60 °C until it reached a constant moisture content. It was then ground into a fine powder using an electric grinder and stored in airtight glass containers at 4 ± 2 °C.

Figure 1.
Peach pomace at three stages: fresh, dehydrated, and ground.
2.2. The Technological Method Applied to Obtain Biscuits
The following raw and auxiliary materials were used to make the control sample: wheat flour type 000 (500 g), eggs (168 g), milk powder (50 g), sunflower oil (166 mL), sugar (213 g), salt (iodized sodium chloride) (2 g), and baking powder (2 g). To prepare the experimental biscuits (Figure 2), 25 g, 50 g, and 75 g of peach pomace were used to replace some of the wheat flour, representing 5%, 10%, and 15% of the total amount of flour in the recipe, respectively. The technological process of making the biscuits took place in the Vilceanu confectionery laboratory in Targu Jiu, Romania. This involved mixing all the raw and auxiliary materials together using a mixer. The resulting dough was processed by rolling and cutting with a stainless-steel mold. The biscuits were baked at 180 °C for 10 min and then cooled to 21–22 °C.

Figure 2.
Biscuits containing 5%, 10%, and 15% of added peach pomace.
2.3. The Procedure for the Extraction of Polyphenols, Flavonoids, Anthocyanins, and Sugars
The same extraction procedure was used to quantify the polyphenols, flavonoids, and anthocyanins in the peach pomace and biscuit samples. The amount of 1 g of each sample was mixed with 10 mL of absolute ethanol (Merck-Sigma-Aldrich, Darmstadt, Germany) for two minutes using a VX-200 Vortex Mixer (Corning-Labnet, Corning Life Sciences, Tewksbury, MA, USA). The samples were placed in a 40 kHz ultrasonic bath (ULTR-2L0-001, Labbox Labware, Migjorn, Spain) for 30 min. They were then centrifuged at 6000 rpm for a further 30 min using a Spectrafuge 6c centrifuge (Labnet International Inc., Edison, NJ, USA). The process was carried out again, involving an additional round of vortexing, ultrasonication, and centrifugation.
To quantify the sugars, 1 g of each sample was vortexed in 10 mL of distilled water for two minutes. The samples were then ultrasonicated at 99 °C for 30 min.
2.4. Determination of Total Polyphenol Content
The spectrophotometric method (UV-Vis Perkin Elmer Lambda25, Shelton, CT, USA) was employed to identify the total polyphenol content, following the methodology adapted from Cosmulescu et al. [19].Sample preparation involved mixing 0.5 mL of ethanolic extract of peach pomace and 2 mL of ethanolic extract of biscuits with 0.5 mL of Folin–Ciocalteu reagent. After shaking the samples, 2 mL of 10% sodium carbonate solution (Merck-Sigma-Aldrich, Darmstadt, Germany) was added to the test tubes, bringing the total volume to 10 mL with distilled water. The samples were incubated in the dark for two hours, after which the absorbance was measured at 765 nm. The results were calculated using the following calibration curve: y = 0.0358 + 0.2649x, R2 = 0.9992, where y represents the absorbance of the samples at 765 nm and x represents the concentration of gallic acid, which ranged from 0.1 to 1 mg L−1. The results are expressed as mg gallic acid equivalents (GAE) 100 g−1 sample.
2.5. Determination of Total Flavonoids Content
The total flavonoid content was assessed following the procedure outlined by Stamin et al. [20], with some modifications. The samples were obtained by mixing 0.5 mL of ethanolic extract of peach pomace and 2 mL of ethanolic extract of biscuits with 0.5 mL of 5% sodium nitrite. After stirring the mixture, 0.5 mL of 10% aluminum chloride solution and 2 mL of 1 M sodium hydroxide solution (Merck-Sigma-Aldrich, Darmstadt, Germany) were added one by one. After completing the samples with distilled water to a total volume of 10 mL, the absorbance was measured at 510 nm. The total flavonoid content was reported as mg rutin equivalents (RE) 100 g−1 sample, using the following calibration curve: y = −0.0473 + 2.9818x, R2 = 0.9998, where y represents the absorbance of the samples at 510 nm and x represents the concentration of rutin, with values ranging from 0.1 to 0.6 mg mL−1.
2.6. Determination of Total Anthocyanin Content
To determine the total anthocyanin content, the methodology described by Stamin et al. [20] was followed. For the analysis, 1 mL of ethanolic extract of each sample, 1 mL of absolute ethyl alcohol solution acidified with 0.1% pure HCl, 8 mL of pH 0.6 buffer solution (2% HCl solution), and pH 3.5 buffer solution (obtained by dissolving 21.7 g of disodium phosphate and 14.6 g of citric acid in distilled water to a total volume of 1 L) (Merck-Sigma-Aldrich, Darmstadt, Germany) were used. Absorbance (y) was measured at 520 nm, and the cyanidin 3-glucoside (C3G) concentrations (x), ranging from 0.1 to 0.7 mg L−1, were obtained using the following calibration curve: y = 0.0029 + 0.1699x, R2 = 0.9987. The anthocyanin contents are reported as mg equivalents of cyanidin 3-glucoside (C3GE) 100 g−1 sample.
2.7. Determination of Total Sugar Content
The spectrophotometric method was used to determine the sugar content. The samples were analyzed by mixing 0.2 mL of the aqueous extract with 0.8 mL of distilled water and 5 mL of concentrated sulfuric acid. After gentle stirring, 1 mL of 5% aqueous phenol solution (Merck-Sigma-Aldrich, Darmstadt, Germany) was quickly added, after which the mixture was shaken vigorously to develop the color. Absorbance (y) was measured at 490 nm, and the total sugar concentration (x) was calculated using the glucose calibration curve (y = 0.0281 + 0.1593x; R2 = 0.9989), which covers glucose concentrations ranging from 0.54 to 10.8 mg mL−1. The total sugar contents are reported as g glucose equivalents (GluE) 100 g−1 sample.
2.8. Determination of Components with Anti-Radical Potential
The antioxidant activity of the ethanolic extracts was evaluated by the DPPH assay. This involved combining 3 mL of a methanolic DPPH solution (11.6 mmol L−1, Merck-Sigma-Aldrich,,) with 0.1 mL of the sample ethanolic extract, followed by agitation and incubation in the dark at room temperature for 20 min. Absorbance was recorded at 517 nm, and the DPPH radical scavenging activity (RSA%) was determined using the equation:
where control absorbance represents the absorbance of the stock solution of DPPH, at 517 nm [21].
y = [1 − (sample absorbance/control absorbance)] × 100
2.9. HPLC Analysis
For the determination of the individual phenolic profile, 1 g of plant material was subjected to an extraction process that included vortexing with 10 mL 50% ethanol and alkaline water for 3 min, followed by ultrasonication at 30°C for 30 min and centrifugation at 6000 rpm for 30 min. The samples were then refrigerated for 24 h at 4 ± 2 °C, and before HPLC analysis, the vortexing and ultrasonication procedures were repeated. The supernatant obtained was successively filtered through Whatman no. 4 filter paper and a 0.45 µm syringe filter and was subsequently introduced into vials for analysis. The analysis of phenolic compounds was performed using a high-performance liquid chromatograph (UHPLC UltiMate 3000 XRS, Thermo Scientific, Waltham, MA, USA), equipped with an autosampler, XRS pump, and UV–VIS diode array detector. The separation was performed on a Hypersil Gold column (150 × 4.6 mm, 5 µm particle size) at 25 °C, using a mobile phase consisting of 1% acetic acid (B) and 100% methanol (C), in gradient mode, with a flow rate of 0.8 mL min−1 and an injection volume of 5 µL. The compounds were detected at 278 nm, in the order of retention: gallic acid, neochlorogenic acid, (+)-catechin hydrate, chlorogenic acid, vanillic acid, caffeic acid, (-)-epicatechin, ferulic acid, sinapic acid, salicylic acid, ellagic acid, rutin and myricetin, according to the method proposed by Stoenescu et al. [22]. Each compound was identified by comparing its retention time with that of the standards analyzed under the same conditions. Quantification was then performed using the calibration curves generated from these standards. The results were expressed in mg 100 g−1 and represent the average of three successive determinations.
2.10. FTIR Analysis
Spectral measurements were performed using an FTIR Jasco 6300 spectrometer (Madison, WI, USA) equipped with a Pike Technologies ATR (Attenuated Total Reflection) diamond crystal accessory. Spectral data for peach pomace and experimental biscuits were recorded at a resolution of 4 cm−1 in the range 4000–400 cm−1 and with 100 scans. Data processing was performed with JASCO Spectra Manager II software. Background reference spectra were recorded using air after every sample to minimize interference from carbon dioxide and water vapor in the atmosphere. After each measurement, the ATR crystal was carefully cleaned with pure acetone (Sigma-Aldrich Co., Darmstadt, Germany) and dried with a soft cloth [23,24]. All measurements were taken at room temperature (22 ± 2 °C). To ensure spectral reproducibility and assess analytical precision, three replicate spectra were recorded for each sample, and the average spectrum was obtained.
2.11. Statistical Analysis
All measurements were conducted in triplicate, and the results are presented as mean ± standard deviation ( ± SD), using Microsoft Excel 2010 software. Statistical analysis was performed using IBM SPSS Statistics 26 software, including ANOVA and Duncan’s multiple range tests (p< 0.05).
3. Resultsand Discussions
3.1. Chemical Composition of Peach Pomace
The results obtained in the present study highlight the chemical composition of peach pomace, underlining the possibility of its valorization for nutritional purposes. Table 1 illustrates the total content of polyphenols, flavonoids, anthocyanins, and antioxidant activity of peach pomace. The concentration of polyphenols in peach pomace recorded an average of 1771.64 mg GAE 100 g−1, a result higher than the parameters identified in the specialized literature, which highlighted levels ranging from 204 to 416 mg GAE 100 g−1 [25], 0.47 mg GAE g−1 [26], 246 mg GAE 100 g−1 [27], but lower than the limit of 9.49–28.68 mg GAE g−1 [28]. The total flavonoid content identified in peach pomace was 478.99 mg RE 100 g−1, higher than the concentration of 156.84–299.86 mg RE 100 g−1 reported by Liu et al. [29]. Comparable levels were also observed in the studies conducted by Plazzotta et al. [25], Hong et al. [24], and Rudke et al. [28], where the flavonoid content in peach pomace was between 12.1 and 32 mg QE 100 g−1, 0.18 mg QE g−1, and, respectively, between 98 and 649 µg QE g−1. Regarding the anthocyanin content, a total content of 21.18 mg C3GE 100 g−1 was determined. In comparison, in the study conducted by Saidani et al. [30], the level of anthocyanins was lower, ranging between 0.24 and 17.6 mg C3GE 100 g−1. The differences between the values reported by different authors can be attributed to various factors, including the sample origin and variety, extraction techniques, the sensitivity and accuracy of the equipment used, and the purity of the reagents. All of these factors can influence analytical results [31]. Regarding the inhibition capacity, peach pomace presented a value of 40.41%, comparable to those indicated by Singh and Kulshrestha [32] and del Prado García-Aparicio et al. [31], who determined inhibition capacities of 81.05% and 14.4–67.3%, respectively. In the studies conducted by Plazzotta et al. [25] and Hong et al. [26], the antioxidant activity was 97–131 mg Trolox 100 g−1 and, respectively, 0.98 mg ascorbic acid g−1. The sugar content of peach pomace showed a level of 35.38 g GluE 100 g−1, a value close to that identified by del Prado García-Aparicio et al. [31], who indicated a sugar content of 36 g GluE 100 g−1. It, therefore, follows that peach pomace represents a valuable source of bioactive compounds, with a significant nutritional and antioxidant potential, thus supporting the possibility of its integration into functional food products or as an ingredient in the nutraceutical industry.

Table 1.
DPPH inhibitory activity and total polyphenol, flavonoid, and anthocyanin content of peach pomace.
The phenolic profile of peach pomace, determined by HPLC analysis (Figure S1), revealed the presence of compounds from the phenolic acid class, especially from the hydroxybenzoic acid subclass (Table 2). Among them, gallic acid and ellagic acid were identified. Ellagic acid was predominant, recording a concentration of 2.05 mg 100 g−1, while gallic acid had a value of 1.32 mg 100 g−1. The determined gallic acid content is lower than the value of 2.98 mg g−1 reported by Hong et al. [26], which can be attributed to differences in the origin of the raw material, the extraction conditions, or the analysis method. Within the hydroxycinnamic acid subclass (Table 2), four phenolic acids (chlorogenic, neochlorogenic, caffeic, and sinapic acid) were quantified. From this subclass, chlorogenic acid recorded the highest concentration (3.77 mg 100 g−1). Comparatively, in other studies, variations of chlorogenic acid are highlighted, ranging from 15.96 mg g−1 [26], 0.41–0.51 mg 100 g−1 [33], and 2944 μg g−1 [29]. Neochlorogenic acid and caffeic acid showed a level of 0.82 mg 100 g−1 and 0.11 mg 100 g−1, respectively. In other studies, the level of caffeic acid was higher, ranging from 0.21 to 0.41 mg 100 g−1 [33] and 0.98 mg g−1 [26]. Sinapic acid was noted in peach pomace, at a concentration of 2.70 mg 100 g−1.

Table 2.
Concentration of phenolic compounds in peach pomace.
Regarding the flavonoid class, myricetin emphasized a content of 1.49 mg 100 g−1, while rutin recorded the highest concentration (8.12 mg 100 g−1) among all the phenolic compounds analyzed. Rutin exhibits a diverse range of pharmacological properties that are utilized in medicine and human nutrition [34]. Although it is traditionally used as an antimicrobial, antifungal, and antiallergic agent, studies have also demonstrated its efficacy in treating chronic conditions such as cancer, diabetes, hypertension, and hypercholesterolemia [34,35]. In conclusion, the phenolic profile of peach pomace revealed the presence of valuable compounds, with the predominance of ellagic acid, chlorogenic acid, and rutin. These results confirm the potential of pomace as a source of bioactive compounds, usable in functional food products.
In further characterizing the phenolic compounds of peach pomace, in order to analyze the chemical structure and its biological potential, a determination was performed by FTIR spectroscopy. This method allowed the identification of functional groups and characteristic chemical bonds. FTIR measurements (Table 3, Figure 3) were performed in the range of 400–4000 cm−1, providing additional information about the chemical composition of the pomace. Several absorption bands are observed in different regions of the spectrum, each corresponding to specific vibrations of chemical bonds in a compound. The absorption band located at approximately 3400 cm−1 corresponds to –OH stretching vibrations, characteristic of water, alcohols, polyphenols, cellulose, and pectins [36]. The spectral band located approximately from ~2920 to ~2850 cm−1 is associated with the C–H stretching vibration and is characteristic of waxes, lipids, aliphatic chains in lignin [37]. The bands around the values of ~1740–1700 cm−1 are characteristic of C=O (carbonyl) groups in carboxylic acids, esters, and aldehydes [38]. The band at approximately 1600 cm−1 is characteristic of C=C vibrations in aromatic structures [39].

Table 3.
The correlation between FTIR bands and functional compounds contained in peach pomace.
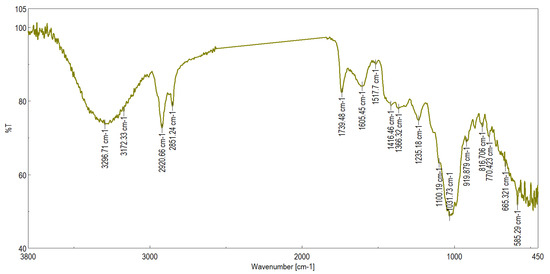
Figure 3.
FTIR spectrum of peach pomace.
The bands located between ~1510 and 1450 cm−1 are associated with the C–H and C=C stretching vibrations of phenolic compounds [40] and lignin, and the bands at approximately 1250–1000 cm−1 correspond to the C–O and C–O–C stretching vibrations of cellulose, hemicellulose, pectins, and sugars [41]. The FTIR spectrum, performed for the pomace, confirms the presence of hydroxyl and carbonyl groups—typical of plant fibers, sugars, and aromatic compounds—most likely from lignin and polyphenols, and C–O bonds—common in pectins, cellulose, and hemicellulose. In conclusion, the FTIR analysis of peach pomace revealed the presence of several functional groups and chemical bonds characteristic of polyphenols, polysaccharides, organic acids, esters, simple sugars, and bound water. This information confirms the chemical complexity of pomace and supports its bioactive potential, indicating opportunities for its use in various food and pharmaceutical applications.
3.2. Chemical Analysis of Biscuits with the Addition of Peach Pomace
The results presented in Table 4 highlight the impact of peach pomace integration on the concentrations of biologically active substances (polyphenols, flavonoids, anthocyanins, and sugars) in biscuits fortified with various proportions of pomace and provide a detailed insight into the potential of this raw material in improving the nutritional value of bakery products and in developing functional biscuits with health benefits.

Table 4.
The composition of polyphenols, flavonoids, anthocyanins, and sugars, and the radical scavenging activity of biscuits with different percentages of peach pomace.
The level of polyphenols registered a significant progressive increase (p < 0.05), dependent on the concentration of pomace applied. The lowest concentrations of polyphenols were identified in the control sample (390.21 mg GAE 100 g−1), followed by the biscuits in which minimal levels (5%) of pomace were integrated (425.25 mg GAE 100 g−1). Increasing the share of peach pomace from 10 to 15% also generated the highest levels of polyphenols, located between 434.54 and 444.04 mg GAE 100 g−1. The values identified in this work are superior to those revealed by Filipović et al. [42], who detected a polyphenol content ranging from 6.56 mg GAE 100 g−1 (0%) to 13.06 mg GAE 100 g−1 (15%) in biscuits with the addition of dehydrated peaches. The pomace integrated into the biscuit manufacturing recipe showed a significant influence in terms of increasing the phenolic content, providing significant increases even when used in low concentrations.
Flavonoid concentrations varied significantly in all formulations with the addition of pomace. Both the minimum and maximum pomace weights applied (5–15%) guaranteed an amplification of the flavonoid content from 120.13 mg RE 100 g−1 (control sample) to 186.19 mg RE 100 g−1 (5%) and, subsequently, to 211.11 mg RE 100 g−1 (15%). Therefore, it can be emphasized that a higher concentration of peach pomace will directly contribute to the increase in the flavonoid content in the finished products.
The anthocyanin content showed an evolutionary trend in relation to the increase in the proportion of peach pomace. The lowest values of the anthocyanin content were detected in the control sample (18.78 mg C3GE 100 g−1), followed by the formulations that included 5% (23.37 mg C3GE 100 g−1) and 10% (24.38 mg C3GE 100 g−1) peach pomace. At maximum substitution percentages (15%), the anthocyanin level also highlighted the highest concentrations, which included 25.43 mg C3GE 100 g−1. The observed fluctuations between anthocyanin concentrations clearly indicate the effect of including fruit pomace on the chemical composition of the finished products; thus, increasing the content of pomace, rich in active substances, facilitates higher amounts of bioactive compounds in formulated products.
Anthocyanins are considered to be functional compounds. The European Food Safety Authority recommends a daily consumption of between 20 and 150 mg [43]. Regarding formulated products, the Code of Federal Regulations states that a 55 g portion of biscuits containing 15% pomace provides a maximum intake of 13.99 mg [44]. Therefore, to reach the recommended minimum intake of 20 mg, 110 g of biscuits (equivalent to two servings) would be necessary, providing 27.98 mg. For comparison, Antoniolli et al. [45] reported an anthocyanin content of 30.16 mg per 55 g serving of biscuits enriched with 10% grape marc. Anthocyanins are rapidly metabolized and eliminated from the body, and are considered safe and beneficial to health even at higher doses [46,47]. Clinical studies emphasize that most individuals tolerate the regular intake of 160 mg of anthocyanins twice daily for two months well, with only a small percentage (approximately 4%) reporting minor adverse effects such as gastrointestinal symptoms or eczema [48]. These findings support the idea that anthocyanins can be incorporated into a balanced daily diet without posing a significant risk to consumer health [48].
Regarding the level of sugars, a progression of values is observed, compared to the control sample (39.16 g GluE 100 g−1). Biscuits with an addition of 15% pomace register the highest value (46.48 g GluE 100 g−1) while biscuits with 5% pomace are distinguished by a content of 45.04 g GluE 100 g−1.
As expected, the control sample exhibited the lowest degree of inhibition (18.77%). Integrating peach pomace into the biscuit recipe at levels of 5–15% progressively increased antiradical activity, rising from 21.46% to 27.21%. A similar trend was observed in a study on bread enriched with peach pomace, with the greatest antiradical activity (42.48%) found in bread containing 15% pomace [49]. Imeneo et al. [50] found that biscuits containing lemon pomace exhibited significantly higher antioxidant activity than the control sample. Therefore, the presence of antioxidants can be used to determine or directly create antioxidant capacity during production.
Figure 4 shows the FTIR spectra of the experimental biscuits. Although there were slight variations in intensity, the biscuit samples exhibited similar bands, as shown in Table 5. Major bands were observed at 3000 and 2800 cm−1 in all samples, indicating stretching vibrations of the O–H, N–H, and C–H bonds. Bands found between 1744 and 1646 cm−1 were attributed to the C–O stretching vibration of an α,β-unsaturated compound. Tyagi et al. (2020) [51] obtained similar results with cookies enriched with powdered Tinospora cordifolia stems. Bands established at 1100–919 cm−1 could be attributed to the C–O bond and aliphatic C–N stretching. These bands were of higher intensity in biscuit sample B15 (containing 15% peach pomace) than in the control sample (CC), as it was enriched with peach pomace. These bands are related to the presence of phenolic compounds and glycosides [51], all of which are found in peach pomace. The band at 1366 cm−1 was also attributed to C–O stretching and C–H and N–H deformation from peach pomace. This was observed in biscuit samples enriched with peach at 1340–1374 cm−1, but not in the control sample. The FTIR results revealed the presence of many bioactive compounds in the formulated biscuits.
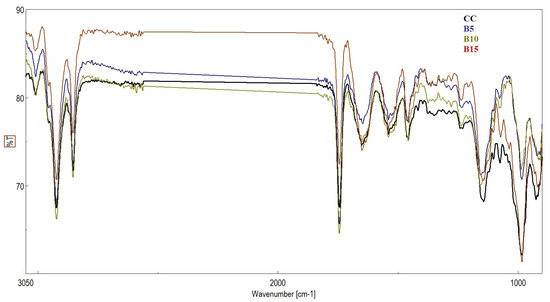
Figure 4.
FTIR spectra of biscuit samples. CC represents the control biscuits, while B5, B10, and B15 represent biscuit samples containing 5%, 10%, and 15% peach pomace, respectively.

Table 5.
Infrared bands of biscuits containing different percentages of peach pomace.
Considering the concentrations of identified phenolic compounds, peach pomace confirms its potential as a functional ingredient in the food industry. Although following the applied technological process, reductions in the concentrations of polyphenols and flavonoids occurred in the supplemented biscuits, it can be emphasized that even a minimum level of integrated pomace (5%) produces increases in bioactive compounds compared to non-supplemented samples. Czubaszeket al. [52] state that polyphenols show a high sensitivity to external factors, their level decreasing during food processing and preparation processes. They can also show instability under the conditions of pH changing [53], light [54], or contact with different environmental factors [55]. Also, the flavonoid content can be affected both by the phenolic degradation products generated during the thermal process and by the caloric action itself [56]. Ou et al. [57] state that anthocyanins are the most susceptible to degradation. The extent of loss depends on the duration and temperature of the thermal treatment, as well as on the presence of enzymes such as polyphenol oxidase. In contrast, Blanch et al. [58] state that certain non-enzymatic browning compounds have the ability to stabilize anthocyanins by interacting with the aglycone produced during the thermal degradation process. In the present study, the level of anthocyanins was lower in peach pomace compared to fortified biscuits, which attests that they did not suffer degradation following heat treatment.
4. Conclusions
Peach pomace represents a valuable source of bioactive compounds that can supplement nutritional deficiencies in food products. It proves to be a significant source of polyphenols (neochlorogenic, chlorogenic, caffeic, sinapic, gallic, and ellagic acid), flavonoids (myricetin and rutin), and anthocyanins. Increasing the concentrations of pomace (5%, 10%, and 15%) led to a proportional increase in biochemicals in fortified biscuits. Biscuits containing 15% peach pomace exhibited the highest concentrations of polyphenols (444.04 mg GAE 100 g−1), flavonoids (211.11 mg RE 100 g−1), anthocyanins (25.43 mg C3GE 100 g−1), sugars (46.48 g GluE 100 g−1), and a radical scavenging activity (RSA) of 27.21% compared to the control sample, which had contents of 390.21 mg GAE 100 g−1, 120.13 mg RE 100 g−1, 18.78 mg C3GE 100 g−1, 39.16 g GluE 100 g−1 and an RSA of 18.77%.
Major absorption bands were observed at 3000 and 2800 cm−1 in all biscuit samples, indicating stretching vibrations of O–H, N–H, and C–H bonds. Other bands were observed between 1744 and 1646 cm−1, which are attributed to stretching vibrations of C–O bonds in α,β-unsaturated compounds. The bands observed between 1100 and 919 cm−1, which were more intense in the biscuit sample containing 15% peach pomace than in the control sample, are attributed to C–O bonds and aliphatic C–N stretching. The band observed at 1366 cm−1 was only present in the peach-enriched biscuit samples, not the control sample, and is attributed to C–O stretching, as well as C–H and N–H deformation from the peach pomace. Overall, the FTIR results revealed the presence of many bioactive compounds in the formulated biscuits.
Given the positive impact on the nutritional value, peach pomace, a residue of the juice processing industry, can be used for the development of new food products for consumers interested in functional and sustainable nutrition. In the future, the integration of pomace into food products will contribute not only to reducing food waste but also to improving consumer health, thus aligning with global sustainability and public health goals. Further studies are needed to investigate the influence of pomace on the technological, sensory, and nutritional properties of products, with the aim of optimizing formulations and ensuring high consumer acceptance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15136983/s1, Figure S1. The phenolic profile of peach pomace, determined by HPLC analysis, showing the following individual compounds: rutin (a), myricetin (b), sinapic acid (c), caffeic acid (d), chlorogenic acid (e), neochlorogenic acid (f), ellagic acid (g) and gallic acid (h).
Author Contributions
Conceptualization, S.C. and M.M.; methodology, C.M.T. and L.E.V.; software, M.M., C.M.T., and L.E.V.; validation, S.C., M.M., and L.E.V.; investigation, M.M.; writing—original draft preparation, S.C. and M.M.; writing—review and editing, S.C. and M.M.; supervision, S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jose, M.; Himashree, P.; Sengar, A.S.; Sunil, C.K. Valorization of Food Industry By-Product (Pineapple Pomace): A Study to Evaluate Its Effect on Physicochemical and Textural Properties of Developed Cookies. Meas. Food 2022, 6, 100031. [Google Scholar] [CrossRef]
- Lucini Mas, A.; Brigante, F.I.; Salvucci, E.; Ribotta, P.; Martinez, M.L.; Wunderlin, D.A.; Baroni, M.V. Novel cookie formulation with defatted sesame flour: Evaluation of its technological and sensory properties. Changes in phenolic profile, antioxidant activity, and gut microbiota after simulated gastrointestinal digestion. Food Chem. 2022, 389, 133122. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hu, L.; Zheng, J.; Rong, J. Classification, processing procedures, and market demand of Chinese biscuits and the breeding of special wheat for biscuit making. J. Food Qual. 2022, 2022, 6679776. [Google Scholar] [CrossRef]
- Pinto, D.; Moreira, M.M.; Vieira, E.F.; Svarc-Gajic, J.; Vallverdu-Queralt, A.; Brezo-Borjan, T.; Delerue-Matos, C.; Rodrigues, F. Development and Characterization of Functional Cookies Enriched with Chestnut Shells Extract as Source of Bioactive Phenolic Compounds. Foods 2023, 12, 640. [Google Scholar] [CrossRef]
- Šťastná, K.; Sumczynski, D.; Yalcin, E. Nutritional composition, in vitro antioxidant activity and phenolic profile of shortcrust cookies supplemented by edible flowers. Foods 2021, 10, 2531. [Google Scholar] [CrossRef]
- Naseem, Z.; Bhat, N.A.; Mir, S.A. Valorisation of apple pomace for the development of high-fibre and polyphenol-rich wheat flour cookies. Sci. Rep. 2024, 14, 25912. [Google Scholar] [CrossRef]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple pomace as a functional and healthy ingredient in food products: A review. Processes 2020, 8, 319. [Google Scholar] [CrossRef]
- Kruczek, M.; Drygaś, B.; Habryka, C. Pomace in fruit industry and their contemporary potential application. World Sci. News 2016, 48, 259–265. [Google Scholar]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Sun, S.; Liu, Z.; Lin, M.; Gao, N.; Wang, X. Polyphenols in Health and Food Processing: Antibacterial, Anti-Inflammatory, and Antioxidant Insights. Front. Nutr. 2024, 11, 1456730. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Khan, M.I.; Shang, X.; Kumar, V.; Kumari, V.; Kesarwani, A.; Ko, E.-Y. Dietary Sources, Stabilization, Health Benefits, and Industrial Application of Anthocyanins—A Review. Foods 2024, 13, 1227. [Google Scholar] [CrossRef] [PubMed]
- Szot, I.; Łysiak, G.P.; Sosnowska, B.; Chojdak-Łukasiewicz, J. Health-Promoting Properties of Anthocyanins from Cornelian Cherry (Cornus mas L.) Fruits. Molecules 2024, 29, 449. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAOSTAT. 2022. Available online: http://www.fao.org/faostat (accessed on 24 May 2025).
- Rudke, C.R.M.; Zielinski, A.A.F.; Ferreira, S.R.S. From Biorefinery to Food Product Design: Peach (Prunus persica) By-Products Deserve Attention. Food Bioprocess Technol. 2023, 16, 1197–1215. [Google Scholar] [CrossRef]
- Solomakou, N.; Drosaki, A.M.; Kaderides, K.; Mourtzinos, I.; Goula, A.M. Valorization of Peach By-Products: Utilizing Them as Valuable Resources in a Circular Economy Model. Sustainability 2024, 16, 1289. [Google Scholar] [CrossRef]
- Plakantonaki, S.; Roussis, I.; Bilalis, D.; Priniotakis, G. Dietary Fiber from Plant-Based Food Wastes: A Comprehensive Approach to Cereal, Fruit, and Vegetable Waste Valorization. Processes 2023, 11, 1580. [Google Scholar] [CrossRef]
- Parfitt, J.; Barthel, M.; Macnaughton, S. Food Waste within Food Supply Chains: Quantification and Potential for Change to 2050. Phil. Trans. R. Soc. B 2010, 365, 3065–3081. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Trandafir, I.; Nour, V.; Botu, M. Total phenolic, flavonoid distribution and antioxidant capacity in skin, pulp and fruit extracts of plum cultivars. J. Food Biochem. 2015, 39, 64–69. [Google Scholar] [CrossRef]
- Stamin, F.D.; Vijan, L.E.; Topală, C.M.; Cosmulescu, S.N. The Influence of Genotype, Environmental Factors, and Location on the Nutraceutical Profile of Rosa canina L. Fruits. Agronomy 2024, 14, 2847. [Google Scholar] [CrossRef]
- Cosmulescu, S.N.; Trandafir, I.; Cornescu, F. Antioxidant capacity, total phenols, total flavonoids and colour component of cornelian cherry (Cornus mas L.) wild genotypes. Not. Bot. Horti Agrobot. 2019, 47, 390–394. [Google Scholar] [CrossRef]
- Stoenescu, A.-M.; Trandafir, I.; Cosmulescu, S. Determination of Phenolic Compounds Using HPLC-UV Method in Wild Fruit Species. Horticulturae 2022, 8, 84. [Google Scholar] [CrossRef]
- Vîjan, L.E.; Mazilu, I.C.; Enache, C.; Enache, S.; Topală, C.M. Botanical Origin Influence on Some Honey Physicochemical Characteristics and Antioxidant Properties. Foods 2023, 12, 2134. [Google Scholar] [CrossRef] [PubMed]
- Topală, C.M.; Vîjan, L.E.; Giura, S.; Botu, M. Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR): A Method for the Biochemical Study of Walnut Leaves. Curr. Trends Nat. Sci. 2020, 9, 266–272. [Google Scholar] [CrossRef]
- Plazzotta, S.; Ibarz, R.; Manzocco, L.; Martín-Belloso, O. Modelling the Recovery of Biocompounds from Peach Waste Assisted by Pulsed Electric Fields or Thermal Treatment. J. Food Eng. 2021, 290, 110196. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. High-Throughput Screening and Characterization of Phenolic Compounds in Stone Fruits Waste by LC-ESI-QTOF-MS/MS and Their Potential Antioxidant Activities. Antioxidants 2021, 10, 234. [Google Scholar] [CrossRef]
- Ciccoritti, R.; Ciorba, R.; Ceccarelli, D.; Amoriello, M.; Amoriello, T. Phytochemical and Functional Properties of Fruit and Vegetable Processing By-Products. Appl. Sci. 2024, 14, 9172. [Google Scholar] [CrossRef]
- Rudke, C.R.M.; Rudke, A.R.; Germano, A.T.; Vitali, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Phenolic compounds and pectin-rich extracts recovered from peach pomace by sequential pressurized liquid extractions. Food Bioprocess Technol. 2025, 1, 16. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Jiang, W. Evaluation and comparison of vitamin C, phenolic compounds, antioxidant properties and metal chelating activity of pulp and peel from selected peach cultivars. LWT-Food Sci. Technol. 2015, 63, 1042–1048. [Google Scholar] [CrossRef]
- Saidani, F.; Gimenez, R.; Aubert, C.; Challot, G. Phenolic, sugar and acid profiles and the antioxidant composition in the peel and pulp of peach fruits. J. Food Comp. Anal. 2017, 62, 126–133. [Google Scholar] [CrossRef]
- Del Prado García-Aparicio, M.; Castro-Rubio, F.; Marina, M.L. Unlocking peach juice byproduct potential in food waste biorefineries: Phenolic compounds profile, antioxidant capacity and fermentable sugars. Bioresour. Technol. 2024, 396, 130441. [Google Scholar] [CrossRef]
- Singh, S.; Kulshrestha, K. Peach juice and pomace powder; nutritive value and use of pomace powder in biscuits. Int. J. Food Sci. Technol (IJFST) 2016, 6, 5–16. [Google Scholar]
- Baltacioğlu, C.; Baltacioğlu, H.; Okur, İ.; Yetişen, M.; Alpas, H. Recovery of phenolic compounds from peach pomace using conventional solvent extraction and different emerging techniques. J. Food Sci. 2024, 89, 1672–1683. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabi, N.A.; Arasu, M.V.; Park, C.H.; Park, S.U. An up-to-date review of rutin and its biological and pharmacological activities. EXCLI J. 2015, 14, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic Potential and Recent Advances in Drug Delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Thummajitsakul, S.; Samaikam, S.; Tacha, S.; Silprasit, K. Study on FTIR spectroscopy, total phenolic content, antioxidant activity and anti-amylase activity of extracts and different tea forms of Garcinia schomburgkiana leaves. LWT 2020, 134, 110005. [Google Scholar] [CrossRef]
- Bolade, O.P.; Akinsiku, A.A.; Adeyemi, A.O.; Jolayemi, G.E.; Williams, A.B.; Benson, N.U. Qualitative analysis, total phenolic content, FT-IR and GC-MS characterisation of Canna indica: Bioreducing agent for nanoparticles synthesis. J. Phys. Conf. Ser. 2019, 1299, 012135. [Google Scholar] [CrossRef]
- Okur, I.; Baltacıoğlu, C.; Ağçam, E.; Baltacıoğlu, H.; Alpas, H. Evaluation of the effect of different extraction techniques on sour cherry pomace phenolic content and antioxidant activity and determination of phenolic compounds by FTIR and HPLC. Waste Biomass Valorization 2019, 10, 3545–3555. [Google Scholar] [CrossRef]
- Aziz, A.H.A.; Engliman, N.S.; Mansor, M.F.; Nasaruddin, R.R. Extraction of Phenolic Compound Using Natural Deep Eutectic Solvent from Biomass Waste. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1192, 012001. [Google Scholar] [CrossRef]
- Patle, T.K.; Shrivas, K.; Kurrey, R.; Upadhyay, S.; Jangde, R.; Chauhan, R. Phytochemical screening and determination of phenolics and flavonoids in Dilleniapentagyna using UV–vis and FTIR spectroscopy. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2020, 242, 118717. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Gegechkori, V.; Petrovich, D.S.; Ilinichna, K.T.; Morton, D.W. HPTLC and FTIR Fingerprinting of Olive Leaves Extracts and ATR-FTIR Characterisation of Major Flavonoids and Polyphenolics. Molecules 2021, 26, 6892. [Google Scholar] [CrossRef]
- Filipović, V.; Lončar, B.; Filipović, J.; Nićetin, M.; Knežević, V.; Šeregelj, V.; Košutić, M.; Solarov, M.B. Addition of combinedly dehydrated peach to the cookies—Technological quality testing and optimization. Foods 2022, 11, 1258. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Various Food(s)/Food Constituent(s) and Protection of Cells from Premature Aging, Antioxidant Activity, Antioxidant Content and Antioxidant Properties, and Protection of DNA, Proteins and Lipids from Oxidative Damage Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1489. [Google Scholar] [CrossRef]
- USA Food and Drug Administration. Code of Federal Regulations (Annual Edition); Office of the Federal Register, National Archives and Records Administration: College Park, MD, USA, 2022. [Google Scholar]
- Antoniolli, A.; Becerra, L.; Piccoli, P.; Fontana, A. Phenolic, Nutritional and Sensory Characteristics of Bakery Foods Formulated with Grape Pomace. Plants 2024, 13, 590. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS). Scientific Opinion on the Re-Evaluation of Anthocyanins (E 163) as a Food Additive. EFSA J. 2013, 11, 3145. [Google Scholar]
- Matsumoto, H.; Inaba, H.; Kishi, M.; Tominaga, S.; Hirayama, M.; Tsuda, T. Orally Administered Delphinidin 3-Rutinoside and Cyanidin 3-Rutinoside Are Directly Absorbed in Rats and Humans and Appear in the Blood as the Intact Forms. J. Agric. Food Chem. 2001, 49, 1546–1551. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Efecteledietetice ale antocianilorînsănătateaumană: O revizuirecuprinzătoare. Farmaceutice 2021, 14, 690. [Google Scholar] [CrossRef]
- Mandache, M.B.; Vijan, L.E.; Cosmulescu, S. Insight into Bioactive Compounds and Antioxidant Activity of Bakery Products Fortified with Fruit Pomace. Foods 2025, 14, 806. [Google Scholar] [CrossRef]
- Imeneo, V.; Romeo, R.; Gattuso, A.; De Bruno, A.; Piscopo, A. Functionalized Biscuits with Bioactive Ingredients Obtained by Citrus Lemon Pomace. Foods 2021, 10, 2460. [Google Scholar] [CrossRef]
- Tyagi, P.; Chauhan, A.K.; Aparna. Optimization and characterization of functional cookies with addition of Tinospora cordifolia as a source of bioactive phenolic antioxidants. LWT 2020, 130, 109639. [Google Scholar] [CrossRef]
- Czubaszek, A.; Czaja, A.; Sokół-Łętowska, A.; Kolniak-Ostek, J.; Kucharska, A.Z. Changes in Antioxidant Properties and Amounts of Bioactive Compounds during Simulated In Vitro Digestion of Wheat Bread Enriched with Plant Extracts. Molecules 2021, 26, 6292. [Google Scholar] [CrossRef]
- Chethan, S.; Malleshi, N.G. Finger millet polyphenols: Optimization of extraction and the effect of pH on their stability. Food Chem. 2007, 105, 862–870. [Google Scholar] [CrossRef]
- Pérez-Gregorio, M.R.; Garcia-Falcon, M.S.; Simal-Gandara, J. Flavonoids changes in fresh-cut onions during storage in different packaging systems. Food Chem. 2011, 124, 652–658. [Google Scholar] [CrossRef]
- Ioannou, I.; Hafsa, I.; Hamdi, S.; Charbonnel, C.; Ghoul, M. Review of the effects of food processing and formulation on flavonol and anthocyanin behaviour. J. Food Eng. 2012, 111, 208–217. [Google Scholar] [CrossRef]
- Reis, S.F.; Rai, D.K.; Abu-Ghannam, N. Apple pomace as a potential ingredient for the development of new functional foods. Int. J. Food Sci. Technol. 2014, 49, 1743–1750. [Google Scholar] [CrossRef]
- Ou, J.; Wang, M.; Zheng, J.; Ou, S. Positive and negative effects of polyphenol incorporation in baked foods. Food Chem. 2019, 284, 90–99. [Google Scholar] [CrossRef]
- Blanch, G.P.; Ruiz del Castillo, M.L. Effect of Baking Temperature on the Phenolic Content and Antioxidant Activity of Black Corn (Zea mays L.) Bread. Foods 2021, 10, 1202. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).