Abstract
A high-performance liquid chromatography with evaporative light scattering detection (HPLC-ELSD) method was developed and validated for analyzing fermentable and reducing sugars in brewing matrices. The method exhibited detection limits of 2.5–12.5 mg/L and quantification limits of 12.0–30.0 mg/L. Linearity was achieved for all sugars, fitted with a quadratic calibration model (R2 = 0.9998). Precision metrics revealed relative standard deviations (RSDs) below 2% for repeatability and below 6% for intermediate precision. Recovery rates between 86 and 119% confirmed robustness and minimal matrix interference. Application to brewing samples highlighted variability in sugar profiles, with sucrose concentrations in wort ranging from 3.5 to 22.0 g/L and maltose and maltotriose in finished beers between 0.80 and 1.50 g/L and 1.10–2.50 g/L, respectively. Batch variability analysis showed that brewing conditions had a greater impact on sugar concentrations than malt batch origin, with maltose variation reaching 34.6%. This HPLC-ELSD method provides a robust and reliable tool for sugar analysis in brewing, offering valuable insights into fermentation dynamics and batch consistency. Its application to industrial contexts underscores its potential for improving quality control and optimizing brewing processes.
1. Introduction
Sugars are fundamental to the beer production process, particularly during fermentation, and they impact several critical characteristics of the final product. The sweetness of beer, for instance, is directly influenced by the presence of simpler sugars that remain unfermented. These sugars, derived from malt or added as primings, contribute residual sweetness and, in the case of priming, help achieve specific CO2 levels by encouraging secondary fermentation [1]. Additionally, carbohydrates with more than four glycosidic units, while contributing little to sweetness, enhance beer’s body and texture by increasing its viscosity. These high-molecular-weight polysaccharides are less relevant to foam stability but do influence viscosity, which may, in turn, impact foam retention [2]. Residual components, such as undegraded starch and β-glucans, if present in the final product, can lead to turbidity due to increased resistance to enzymatic breakdown upon cooling [1].
Various analytical methods have been developed for determining sugars in beer and other fermented beverages. High-performance liquid chromatography (HPLC) with different detectors, such as ultraviolet (UV) and refractive index (RI), has been widely used for the individual quantification of sugars [3]. High-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) has shown effectiveness in quantifying oligosaccharides in beer without pre-treatment [4]. Spectrophotometric methods, including phenol sulfuric acid (PSA) and 3-methyl-2-benzo thiazoline hydrazone hydrochloride (MBTH), are commonly used for total sugar estimation [5]. Enzymatic biosensors have demonstrated potential for rapid and cost-effective sugar analysis during fermentation [6]. Novel approaches include the use of gold nanoparticles for localized surface plasmon resonance measurements [7] and capillary electrophoresis with laser-induced fluorescence [8] or indirect UV detection [9]. Gas chromatography–mass spectrometry has also been employed, utilizing solid-phase microextraction and on-fiber derivatization [10]. These methods have been applied to various stages of beer production, including wort analysis and final product testing. Additionally, HPLC-ELSD has been previously used to characterize sugar profiles in beer, as shown by Werrie et al. [11], demonstrating its potential for evaluating carbohydrates in dry-hopped beer. However, that work did not include full analytical validation or application to industrial batches under process control conditions.
Although refractive index (RI) detectors have historically been used for carbohydrate analysis, limitations in sensitivity and susceptibility to baseline drift make them less ideal for more precise applications [12]. ELSD is a quasi-universal detection method for compounds lacking chromophores, offering advantages over UV detection [13,14]. ELSD’s sensitivity is generally better than that of the RI, but the detector’s response is affected by analyte properties, including melting point and volatility [15]. Quantification can thus be challenging due to the lack of linear response and the impact of molecular structure on detection [16,17]. Despite its limitations, ELSD remains a valuable tool for various applications in pharmaceutical, food, and natural product analyses [14,18].
In this study, an HPLC-ELSD method was developed, optimized, and fully validated for the quantification of fermentable and reducing sugars in brewing matrices. The primary goal was to establish a reliable analytical tool suitable for routine application in industrial settings. The method was subsequently applied to real brewing samples to demonstrate its utility in monitoring fermentation progress and supporting process control decisions.
2. Materials and Methods
2.1. Chemicals and Reagents
Ultrapure water was obtained from a Millipore Milli-Q purification system and used in all solution preparations. The reagents used included acetonitrile (ACN, ≥99.9%, Merck, Merck S.A., Algés, Portugal), maltose monohydrate (≥99.0%, Fluka, VWR International Lda, Carnaxide, Portugal), glucose (BDH Chemicals, VWR International Lda, Carnaxide, Portugal), maltotriose (≥96.0%, Fluka), sucrose (Merck), fructose (Merck), and nitrogen gas (≥99.9999%, Air Liquide, Algés, Portugal).
2.2. Equipment and Materials
All glassware used was class A, including 25.00, 50.00, and 100.00 mL volumetric flasks. Filtration was carried out using pleated filters (Sartorius, Sartorius Stedim Spain S.A.—Portuguese representation, Lisbon, Portugal) and 0.22 µm Millex PVDF syringe filters (Merck Millipore, Merck S.A., Algés, Portugal). Sample handling employed funnels, a glass syringe with a Luer lock (Socorex, Socorex Isba SA, Ecublens, Switzerland), and adjustable automatic pipettes (0.200 to 5.000 mL, Eppendorf Xplorer, Eppendorf AG, Lisbon, Portugal). A Mettler Toledo (Lisbon, Portugal) analytical balance (±0.1 mg) and a J.P. Selecta (TECNILAB Portugal, S.A., Lisbon, Portugal) ultrasonic bath were also used.
2.3. HPLC-ELSD Analysis
Chromatographic analysis was performed on a 1260 Infinity HPLC system (Agilent Technologies, Soquímica, Lisbon, Portugal), equipped with a quaternary pump (1260 Quat Pump), autosampler (1260 ALS), and a thermostat-controlled column compartment (1260 TCC). The analytical column used was a Spherisorb NH2 (250 × 4.6 mm, 5 µm; Waters, Waters Portugal, Porto, Portugal). Data acquisition and analysis were managed using Agilent OpenLab CDS ChemStation Edition C.01.04. Detection was carried out with a 380-ELSD detector (Agilent Technologies). The following detector parameters were optimized: gas flow (0.9–3.25 SLM), nebulizer temperature (25–90 °C), evaporator temperature (25–120 °C), light source (480 nm LED), gain (1–10), smoothing (0.1–5 s), and data rate (10, 40, or 80 Hz). Final operating conditions were set at a 1 mL/min flow rate, 30 °C column temperature, 10 µL injection volume, 60 °C nebulizer temperature, 85 °C evaporator temperature, and 1.1 SLM nitrogen flow. The ELSD detector was stabilized under active eluent flow for at least 1 h prior to each analysis.
2.4. Preparation of Standard Solutions
A mixed stock solution containing all target sugars was prepared in a 50 mL volumetric flask by accurately weighing the solids and correcting for purity and hydration. The final volume was completed with ultrapure water. Calibration standards were then prepared by pipetting appropriate aliquots of the stock solution into 25 mL volumetric flasks and diluting to the mark with ultrapure water. A control standard solution was also prepared and analyzed at least once per analytical session. If deviations exceeded ±5% of the theoretical concentration, the calibration curve was redone. All solutions were filtered through 0.22 µm Millex filters (Merck S.A., Algés, Portugal) using a glass syringe prior to injection.
2.5. Preparation of Samples
Beer, wort, and fermentation samples were filtered through pleated filters, with additional filtration if needed. When necessary, samples were decarbonated by transfer and agitation. Specific volumes were pipetted into volumetric flasks, and dilution factors were applied according to the matrix type to ensure that the analyte concentrations matched the midpoint of the calibration curve: 100× for wort, 10× for end-of-fermentation samples, and 5× for finished beer. All diluted samples were filtered again through 0.22 µm Millex filters before injection.
2.6. Brewing Samples
Four types of commercial beer (Beer A, B, C, and D) were provided by a collaborating industrial brewery (Super Bock Group, Leça do Balio, Portugal). Each beer corresponds to a distinct production recipe and brewing protocol, differing in grist composition and adjunct usage—Beer A: standard lager brewed with malted barley and corn grits; Beer B: pale lager produced exclusively with malted barley (no adjuncts); Beer C: 100% malted barley recipe with increased kilning intensity; and Beer D: malted barley with the addition of brown sugar during mashing. All worts were prepared by infusion mashing, followed by lautering and boiling with hops. Fermentation was carried out in stainless steel cylindroconical fermenters using strain-specific bottom-fermenting yeasts. Fermentation temperatures ranged from 10 °C to 14 °C, and the primary fermentation phase lasted approximately 7 to 10 days. This was followed by cold maturation at ~0 °C for up to 14 days. Clarification and carbonation were performed prior to packaging. Representative samples from each production stage—wort, end-of-fermentation, and finished beer—were collected and analyzed.
3. Results and Discussion
3.1. Method Development and Optimization
Initial chromatographic conditions for HPLC-ELSD were guided by values from the literature, setting baseline parameters for analyzing a mixture of five sugars. Using these initial settings, the chromatographic run was optimized to improve peak resolution and S/N ratios. Optimization of ELSD parameters, specifically Gain, Smoothing, and Data Rate, led to an enhanced chromatogram, demonstrating significant improvements in S/N by adjusting the data smoothing and acquisition rates to 5.0 s and 10 Hz, respectively.
Following parameter adjustments, each sugar was injected individually to confirm elution order. The sugars eluted in the order of fructose, glucose, sucrose, maltose, and maltotriose. This elution sequence is attributed to the number of hydroxyl groups in each molecule, with fructose having fewer groups and thus weaker interactions with the stationary phase, and maltotriose, with three glucose residues, exhibiting stronger retention as a result of increased polarity and hydrogen bonding. Elution conditions were further optimized by adjusting the mobile phase composition (ACN:H2O ratio). Initial isocratic runs showed that lower water content decreased elution time but compromised separation of closely eluting peaks, such as fructose and glucose (Figure 1a).
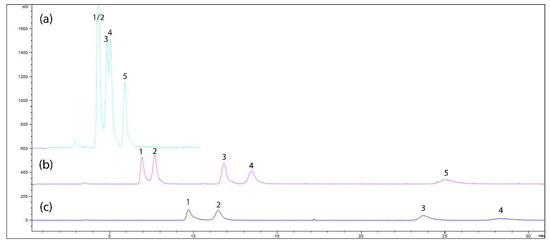
Figure 1.
Variation in ACN:H2O ratios: (a) 70:30; (b) 85:15; and (c) 90:10. The peaks correspond to 1—fructose, 2—glucose, 3—sucrose, 4—maltose, and 5—maltotriose (note that in (c), only the first four peaks are present, as maltotriose has yet to elute).
The study also demonstrated that the sensitivity of the ELSD response varied significantly with the composition of the mobile phase. For higher acetonitrile-to-water ratios (ACN:H2O), a substantial decrease in sensitivity was observed, while increased water content enhanced detection sensitivity (Figure 1c). This behavior is attributed to differences in viscosity between solvents: water (μ = 1.00 cP) exhibits higher viscosity than acetonitrile (μ = 0.37 cP). Considering that greater solvent viscosity results in larger mean particle diameters during nebulization, larger solute particles scatter more light, thereby improving detection sensitivity. This finding reveals that the composition of the mobile phase plays a crucial role in the sensitivity of the ELSD method, emphasizing the importance of carefully optimizing solvent ratios for improved analytical performance. A gradient starting with an ACN:H2O ratio of 85:15 followed by a shift to 75:25 improved separation of early peaks and shortened maltotriose elution time. Final elution conditions of 86:14 balanced peak separation and detection sensitivity, enabling a 20 min runtime with minimal baseline drift (Figure 2).
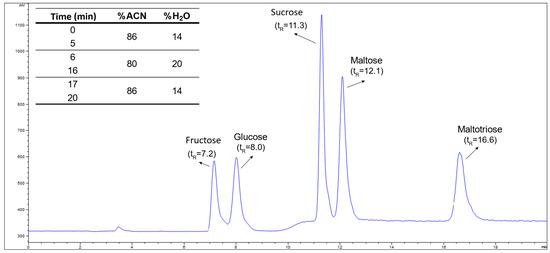
Figure 2.
Chromatogram showing the identification and retention time of the analytes, along with the optimized elution conditions.
Further ELSD optimization included tuning the nebulizer gas flow rate and adjusting the evaporation temperature. Lowering the gas flow from the standard 1.6 SLM to 1.1 SLM enhanced detection sensitivity due to larger droplet formation, which improves light scattering (Figure S1, Supplementary Materials). The chosen evaporation temperature of 85 °C aligned with specifications for non-volatile compounds, balancing complete solvent evaporation and analyte stability. The nebulization temperature was set at 60 °C, a balance to prevent solvent boiling, which could lead to noise spikes in the baseline (Figure S2). The optimal conditions for all parameters were established, and these, along with the corresponding final chromatogram, are presented in Figure S3.
3.2. Method Validation
3.2.1. Selectivity
Selectivity was evaluated by calculating the resolution (Rs) between analyte peaks in a standard solution (Figure S4). Rs values exceeded the acceptable threshold of 1.5 for glucose–sucrose (Rs = 2.8) and maltose–maltotriose (Rs = 4.5), indicating good separation between these analyte pairs. However, the Rs values between fructose–glucose and sucrose–maltose pairs were below 1.5, suggesting less complete separation. Given the method’s objectives and the satisfactory balance between sensitivity and runtime, this level of resolution was deemed acceptable for accurate quantification.
3.2.2. Analytical Limits
Detection limits (LODs) and quantification limits (LOQs) were determined using the signal-to-noise (S/N) ratio method, focusing on practical concentrations that yield distinguishable signals. Noise was estimated by visually measuring the peak-to-peak height of baseline fluctuations in regions free of analyte peaks. This approach is in accordance with official analytical guidelines and is commonly used in ELSD applications, where detector response characteristics require empirical noise evaluation (Figure S5). Replicated measurements of standard solutions at these concentrations yielded consistent S/N ratios, with RSD values below 10%, confirming the reliability of these limits (Table S1, Supplementary Materials). Given that chromatographic or detector settings could affect signal response, recalibration of LODs and LOQs is recommended if significant changes occur in the analytical conditions.
3.2.3. Working Range and Linearity
A working range covering expected concentrations in the matrix was established for each sugar, with eight calibration points (Table S2). To ensure homogeneity of variances across the calibration range, tests were conducted at the extremes of the calibration range (A1 and A7). Results indicated no significant variance differences, as the PG test values were below critical F-values, validating the working range for all analytes (data available upon request). For linearity, peak area versus concentration was evaluated to determine the most suitable calibration curve fit. Quadratic regression provided the best correlation (R2 = 0.9998), as evidenced by the dispersion of residuals, which showed a random pattern only with the quadratic fit. Linear and logarithmic approaches did not yield comparable R2 values and showed non-random residual patterns (Figure 3). The Mandel test, which statistically compares the variances of the linear and quadratic fittings, was applied (data available upon request). After applying the test to all the sugars analyzed, it was found that quadratic approximation was the most appropriate. Thus, a quadratic model was selected for calibrations, ensuring an accurate representation of the ELSD response across the working range.
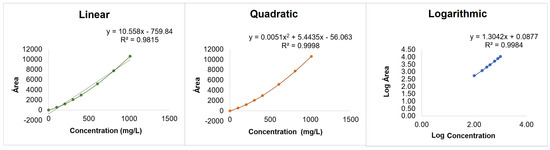
Figure 3.
Linear, quadratic, and logarithmic fittings, exemplified with the calibration curve of maltose.
3.2.4. Repeatability
Repeatability was assessed by analyzing ten replicates of four solutions: the lowest and highest calibration points, and samples of wort and beer. RSD values for each analyte across replicates were low, confirming high repeatability. A Grubbs’ test on calibration point 7 identified an outlier in one replicate, which was subsequently excluded from the RSD calculation (Table S3). Repeatability limits were also calculated at a 99% confidence level, providing thresholds for expected variability in repeated measurements.
3.2.5. Intermediate Precision
To experimentally estimate the intermediate precision of the analytical method, duplicate analyses were performed on a standard solution every three days over a three-week period. Intermediate precision yielded higher RSDs compared to repeatability, especially for lower concentration analytes like fructose (4.1%) and glucose (4.6%), likely due to greater variability at trace levels. However, RSDs for maltotriose (1.9%) and maltose (3.4%) were acceptably low, indicating good precision for higher concentrations (data available upon request).
3.2.6. Accuracy
Accuracy was evaluated by comparing the results obtained with those from external proficiency testing rounds conducted under the Brewing Analyte Proficiency Scheme (BAPS). The measured concentrations for glucose, maltose, and maltotriose were compared against consensus reference values, and the resulting z-scores were 0.13, −0.05, and 0.12, respectively, demonstrating strong agreement. These values, all within the acceptable ±2 range, indicate that the method performs accurately in interlaboratory conditions and aligns with established reference methodologies. It is also worth noting that the official method for fermentable carbohydrate analysis in wort, as defined in Analytica-EBC Method 8.7, quantifies glucose, fructose, maltose, and maltotriose by HPLC. The method developed in this study follows the same chromatographic principles, with the key distinction being the use of evaporative light scattering detection (ELSD) instead of refractive index (RI) detection. This alignment further supports the validity of the proposed approach for routine application in brewing laboratories.
3.2.7. Recovery Studies
Recovery was assessed by spiking beer and wort samples with known concentrations of sugars at 50% and 150% of expected levels. Recovery rates for all analytes ranged between 86% (glucose) and 119% (sucrose), within the pre-established acceptable range. This confirms that the method is effective even in complex matrices and minimizes matrix interference, supporting the robustness of this approach for sugar quantification in various brewing matrices.
In summary, the developed HPLC-ELSD method demonstrated suitable selectivity, sensitivity, and precision for analyzing sugars in beer matrices. The method’s stability across validation metrics, including LOD, LOQ, linearity, and precision, makes it a reliable tool for routine applications in quality control and analytical research within brewing.
3.3. Application of the Method
3.3.1. Analysis of Brewing Matrices
The sugar profiles of key matrices—wort, end of fermentation, and finished beer—were analyzed for four different types of beer (Beer A, Beer B, Beer C, and Beer D). As depicted in Figure 4, significant differences in sugar concentrations were observed between wort and finished beer across beer types. Beer C showed lower maltose levels (57.5 g/L), a result of its production with 100% barley malt. The absence of adjuncts like corn grist, which provide additional starch, reduces the enzymatic production of maltose and maltotriose during mashing. By contrast, the Beer D wort exhibited a markedly higher sucrose concentration (22.0 g/L), attributed to the intentional addition of brown sugar during mashing to enhance the beer’s unique flavor profile.
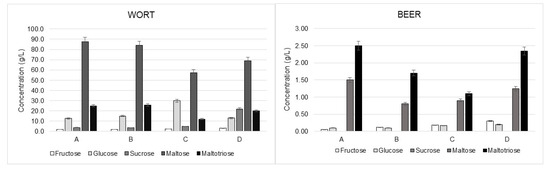
Figure 4.
Average sugar concentration values for wort and finished product across the four different beer types studied (A, B, C, and D).
In finished beers, maltose (0.80–1.50 g/L) and maltotriose (1.10–2.50 g/L) concentrations remained consistently high, as these disaccharides are metabolized at slower rates due to yeast preference for simpler sugars such as glucose and fructose. This aligns with fermentation dynamics reported in similar studies [19], which have emphasized the sequential consumption of sugars based on yeast enzymatic activity.
3.3.2. Fermentation Profile
The fermentation of wort corresponding to Beer A was monitored from initial inoculation with yeast (0.3 days after inoculation) to cold maturation (10.4 days). As shown in Figure 5, sucrose was rapidly hydrolyzed into glucose and fructose by yeast invertase enzymes. This enzymatic activity explains the complete depletion of sucrose within the first 0.5 days. Glucose was metabolized preferentially, serving as the primary carbon source for yeast during early fermentation, producing ethanol and carbon dioxide. Fructose consumption occurred later, consistent with yeast preference hierarchy. The structural complexity of nitrogen sources and oxygen availability influence glucose and fructose fermentation rates [20]. Maltose and maltotriose assimilation began when approximately 50% of glucose had been consumed, as observed at day 0.5, when glucose levels dropped to 7.1 g/L. Maltose and maltotriose uptake and metabolism by yeast during brewing fermentation affect the fermentation rate and beer flavor [21].
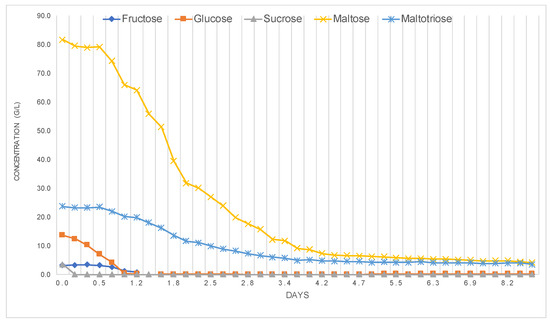
Figure 5.
Profile of various sugars throughout fermentation for Beer A.
The observed trends are in agreement with studies by Briggs et al. [22], which described the sequential fermentation of sugars in brewing. This process can be influenced by various factors, including wort composition, yeast strain, and fermentation conditions [23]. Understanding these sugar consumption patterns is crucial for optimizing fermentation processes and selecting appropriate yeast strains.
The total sugar profile obtained via HPLC-ELSD aligned closely with extract levels determined using the Alcolyzer (Figure S6, Supplementary Material). The discrepancy between the two methods, with extract values consistently higher than HPLC sugars, reflects the inclusion of dextrins and β-glucans in the extract measurement, which are not quantified by HPLC. Total β-glucan content in malt is directly associated with achievable extract in the brewhouse, making it a key determinant of malt quality [24]. β-Glucans increase viscosity and decrease filterability of beer solutions, with molecular weight playing a crucial role [25]. The final attenuation of 68% indicated that 68% of sugars present in the original wort were converted into ethanol, consistent with the expected range (67–73%) for Beer A. Figure 5 and Figure S6 also illustrate a reduction in yeast activity after day 4, likely due to nutrient depletion and ethanol toxicity, as reported in classic fermentation studies [26,27].
3.3.3. Batch Variability
Variability in sugar profiles was assessed across nine consecutive brews using malt from two distinct batches (Batch 1 and Batch 2). Control charts for Batch 1 (Figure 6) showed significant variations in sugar concentrations among brews, particularly for maltose and maltotriose. Coefficients of variation (CVs) confirmed this trend, with maltose exhibiting the highest variability (29.3% for Batch 1 and 34.6% for Batch 2). Fisher’s variance ratio test confirmed statistically significant differences in variances, suggesting that brewing conditions, rather than malt batch origin, were the primary source of variability (data available upon request).
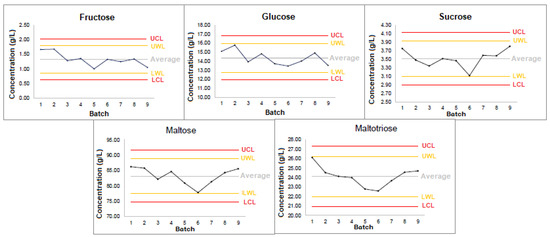
Figure 6.
Control charts for sugar concentrations across various brews from Malt Batch 1. UCL = upper control limit, UWL = upper warning limit, LWL = lower warning limit, LCL = Lower control limit.
To deepen the investigation, the mean sugar concentrations across nine brews for each malt batch were compared using t-tests (data available upon request). The analysis revealed that for all sugars, except sucrose and maltose, no significant differences were observed in their concentrations between different malt batches. These results indicate that, although variability between malt batches contributes to differences in sugar profiles, the variability between individual brews exerts a more pronounced effect. This underscores the critical importance of maintaining strict process control during wort production to minimize inconsistencies in the final product.
4. Conclusions
This study aimed to develop and validate a high-performance liquid chromatography method with evaporative light scattering detection (HPLC-ELSD) for the quantification of fermentable and reducing sugars in brewing matrices. The method demonstrated high sensitivity, with detection and quantification limits ranging from 2.5 to 12.5 mg/L and 12.0 to 30.0 mg/L, respectively. Linearity was confirmed for all analytes using a quadratic calibration model (R2 = 0.9998), and both repeatability and intermediate precision fell within acceptable ranges. Recovery rates between 86% and 119% confirmed the method’s robustness, even in complex matrices. The application of this method to real brewing samples revealed substantial variability in sugar concentrations across batches, underscoring its potential as a reliable tool for process monitoring and quality control in the brewing industry. By providing accurate and reproducible sugar profiles, this method can support brewers in optimizing mashing conditions, fermentation dynamics, and product consistency. Future work will also focus on correlating the analytical results with sensory perception, in order to explore the impact of individual sugars on beer flavor and mouthfeel.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15126412/s1, Figure S1: Effect of nebulizer gas flow variation on the ELSD response for different analytes; Figure S2: Chromatogram at a nebulization temperature of 80 °C; Figure S3: Chromatogram after optimization and selected conditions for the analysis; Figure S4: Chromatogram of the standard solution, showing the analytes of interest and their corresponding resolution; Figure S5: Chromatograms corresponding to the limits of (a) detection (S/N ≈ 3) and (b) quantification (S/N ≈ 10); Figure S6: Profile of other parameters related to sugars throughout the fermentation of wort/beer; Table S1: Concentrations corresponding to the analytical limits and replicas of the S/N determination; Table S2: Working range used for calibrations; Table S3: Trials for the highest calibration point under repeatability conditions, and the corresponding statistical parameters.
Author Contributions
Conceptualization, L.F.G. and F.T.O.; methodology, P.F.L.; validation, P.F.L.; investigation, P.F.L.; writing—original draft preparation, P.F.L.; writing—review and editing, P.F.L., F.T.O. and L.F.G.; supervision, L.F.G. and F.T.O.; project administration, L.F.G. and F.T.O.; funding acquisition, L.F.G. and F.T.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received financial support from PT national funds (FCT/MCTES, Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) through the project UIDB/50006/2020|UIDP/50006/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed at the corresponding author.
Acknowledgments
During the preparation of this manuscript, the authors used ChatGPT (OpenAI, GPT-4, 2024) for the purposes of improving the English language and style. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
Author Fábio B. Oliveira was employed by the company Super Bock Group. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HPLC | High-performance liquid chromatography |
| ELSD | Evaporative light scattering detection |
References
- Baxter, E.D.; Hughes, P.S. Beer: Quality, Safety and Nutritional Aspects; The Royal Society of Chemistry: London, UK, 2001. [Google Scholar]
- Chen, X.; Wang, J.J.; Li, Q. Simultaneous Determination of Maltooligosaccharides in Beer Using HPLC-ELSD and Their Influence on Beer Foam Stability. J. Am. Soc. Brew. Chem. 2015, 73, 78–83. [Google Scholar] [CrossRef]
- Debebe, A.; Temesgen, S.; Redi-Abshiro, M.; Chandravanshi, B.S.; Ele, E. Improvement in Analytical Methods for Determination of Sugars in Fermented Alcoholic Beverages. J. Anal. Methods Chem. 2018, 2018, 4010298. [Google Scholar] [CrossRef] [PubMed]
- Arfelli, G.; Sartini, E. Characterisation of brewpub beer carbohydrates using high performance anion exchange chromatography coupled with pulsed amperometric detection. Food Chem. 2014, 142, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Panagiotopoulos, C.; Sempéré, R. Analytical methods for the determination of sugars in marine samples: A historical perspective and future directions. Limnol. Oceanogr. Methods 2005, 3, 419–454. [Google Scholar] [CrossRef]
- Monošík, R.; Magdolen, P.; Streďanský, M.; Šturdík, E. Monitoring of monosaccharides, oligosaccharides, ethanol and glycerol during wort fermentation by biosensors, HPLC and spectrophotometry. Food Chem. 2013, 138, 220–226. [Google Scholar] [CrossRef]
- Scarano, S.; Pascale, E.; Palladino, P.; Fratini, E.; Minunni, M. Determination of fermentable sugars in beer wort by gold nanoparticles @polydopamine: A layer-by-layer approach for Localized Surface Plasmon Resonance measurements at fixed wavelength. Talanta 2018, 183, 24–32. [Google Scholar] [CrossRef]
- Szilágyi, T.G.; Vecseri, B.H.; Kiss, Z.; Hajba, L.; Guttman, A. Analysis of the oligosaccharide composition in wort samples by capillary electrophoresis with laser induced fluorescence detection. Food Chem. 2018, 256, 129–132. [Google Scholar] [CrossRef]
- Aredes, R.S.; Peixoto, F.C.; Sphaier, L.A.; Silva, V.N.H.; Duarte, L.M.; Marques, F.F.d.C. Determination of carbohydrates in brewer’s wort by capillary electrophoresis with indirect UV detection. J. Food Compos. Anal. 2023, 120, 105321. [Google Scholar] [CrossRef]
- D’Auria, M.; Emanuele, L.; Racioppi, R.; Stefanizzi, N. Determination of simple carbohydrates in beer using SPME, on fiber derivatization and gas chromatography mass spectrometry. Food Res. 2020, 4, 1156–1161. [Google Scholar] [CrossRef]
- Werrie, P.Y.; Deckers, S.; Fauconnier, M.L. Brief Insight into the Underestimated Role of Hop Amylases on Beer Aroma Profiles. J. Am. Soc. Brew. Chem. 2021, 80, 66–74. [Google Scholar] [CrossRef]
- Kraiczek, K.G.; Rozing, G.P.; Zengerle, R. G-index: A new metric to describe dynamic refractive index effects in HPLC absorbance detection. Talanta 2018, 187, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Dvořáčková, E.; Šnóblová, M.; Hrdlička, P. Carbohydrate analysis: From sample preparation to HPLC on different stationary phases coupled with evaporative light-scattering detection. J. Sep. Sci. 2014, 37, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Megoulas, N.C.; Koupparis, M.A. Twenty Years of Evaporative Light Scattering Detection. Crit. Rev. Anal. Chem. 2005, 35, 301–316. [Google Scholar] [CrossRef]
- Webster, G.K.; Jensen, J.S.; Diaz, A.R. An investigation into detector limitations using evaporative light-scattering detectors for pharmaceutical applications. J. Chromatogr. Sci. 2004, 42, 484–490. [Google Scholar] [CrossRef]
- Melis, S.; Foubert, I.; Delcour, J.A. Normal-Phase HPLC-ELSD to Compare Lipid Profiles of Different Wheat Flours. Foods 2021, 10, 428. [Google Scholar] [CrossRef]
- Aruda, W.O.; Walfish, S.; Krull, I.S. Review and Optimization of Linearity and Precision in Quantitative HPLC-ELSD with Chemometrics. Lc Gc N. Am. 2008, 26, 1032. [Google Scholar]
- Ganzera, M.; Stuppner, H. Evaporative light scattering detection (ELSD) for the analysis of natural products. Curr. Pharm. Anal. 2005, 1, 135–144. [Google Scholar] [CrossRef]
- Stewart, G.G.; Russell, I.; Anstruther, A. Handbook of Brewing; Taylor & Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Miranda, M.; Batistote, M.; Ernandes, J.R. Glucose and Fructose Fermentation by Wine Yeasts in Media Containing Structurally Complex Nitrogen Sources. J. Inst. Brew. 2008, 114, 199–204. [Google Scholar]
- Stewart, G. Studies on the uptake and metabolism of wort sugars during brewing fermentations. MBAA Tech. Q. 2006, 43, 265–269. [Google Scholar] [CrossRef]
- Briggs, D.E.; Brookes, P.A.; Stevens, R.B.; Boulton, C.A. Brewing: Science and Practice; Woodhead Publishing: New Delhi, India, 2004. [Google Scholar]
- Boulton, C. Fermentation of Beer, in Brewing: New Technologies; Bamforth, C.W., Ed.; Woodhead Publishing: New Delhi, India, 2006; pp. 228–253. [Google Scholar]
- Marconi, O.; Tomasi, I.; Dionisio, L.; Perretti, G.; Fantozzi, P. Effects of malting on molecular weight distribution and content of water-extractable β-glucans in barley. Food Res. Int. 2014, 64, 677–682. [Google Scholar] [CrossRef]
- Sadosky, P.; Schwarz, P.B.; Horsley, R.D. Effect of arabinoxylans, β-glucans, and dextrins on the viscosity and membrane filterability of a beer model solution. J. Am. Soc. Brew. Chem. 2002, 60, 153–162. [Google Scholar] [CrossRef]
- Guido, L.F.; Rodrigues, P.G.; Rodrigues, J.A.; Gonçalves, C.R.; Barros, A.A. The impact of the physiological condition of the pitching yeast on beer flavour stability: An industrial approach. Food Chem. 2004, 87, 187–193. [Google Scholar] [CrossRef]
- Walker, G.M. Pichia anomala: Cell physiology and biotechnology relative to other yeasts. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2011, 99, 25–34. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).