Influence of Nucleating Agents on the Crystallization, Thermal, and Mechanical Properties of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (P3HBHHx)
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Sample Characterization
2.4. Non-Isothermal Crystallization
2.5. Isothermal Crystallization
2.6. Self-Nucleation Experiments
3. Results and Discussion
3.1. Non-Isothermal Crystallization Behavior of P3HBHHx—Effect of Nucleating Agents
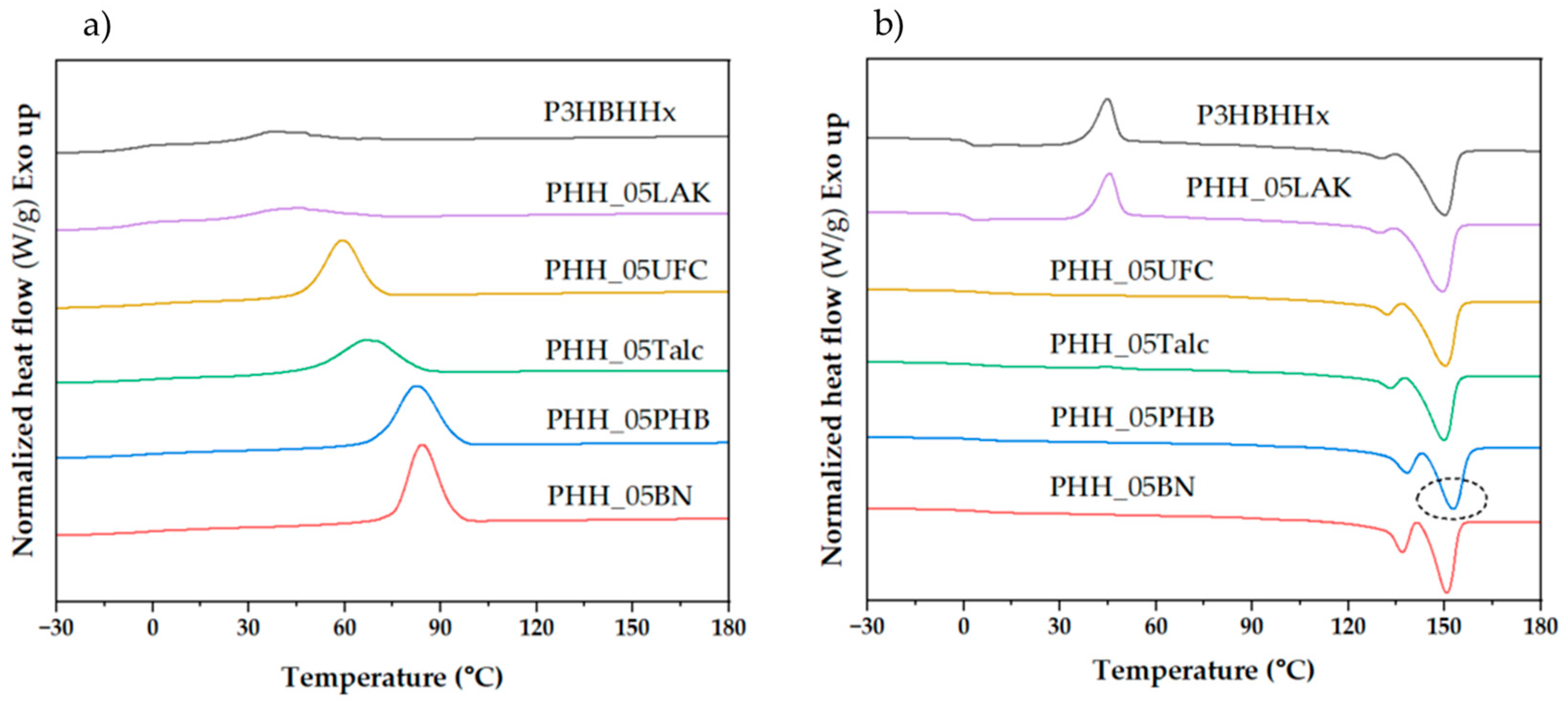
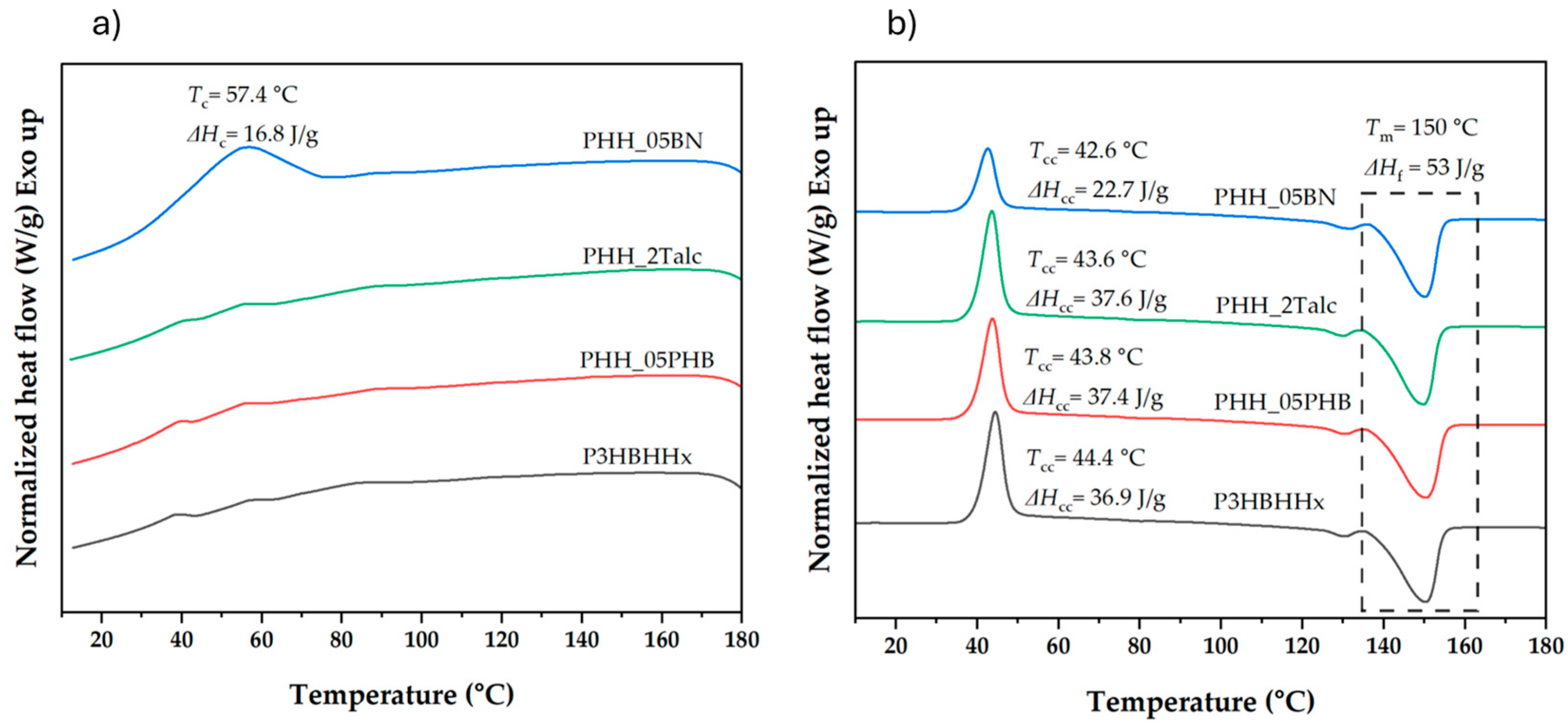
3.1.1. Thermal Behavior of Neat P3HBHHx
3.1.2. Effect of Nucleating Agents on Non-Isothermal Crystallization of P3HBHHx
3.1.3. Effect of Increased Nucleating Agent Concentration on the Crystallization Behavior of P3HBHHx
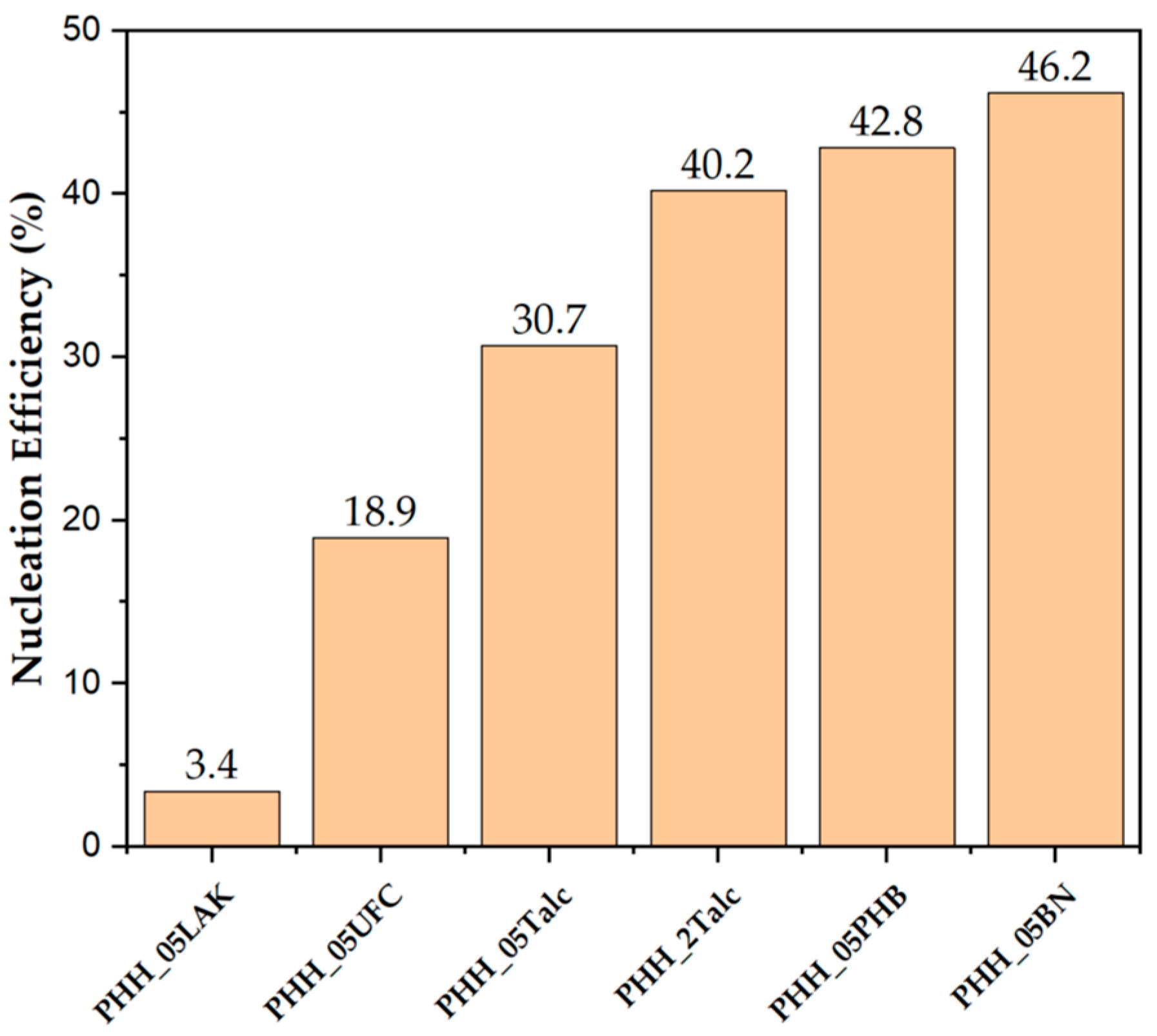
3.1.4. Nucleation Efficiency
3.2. Structural Comparison of P3HBHHx with Different Nucleating Agents
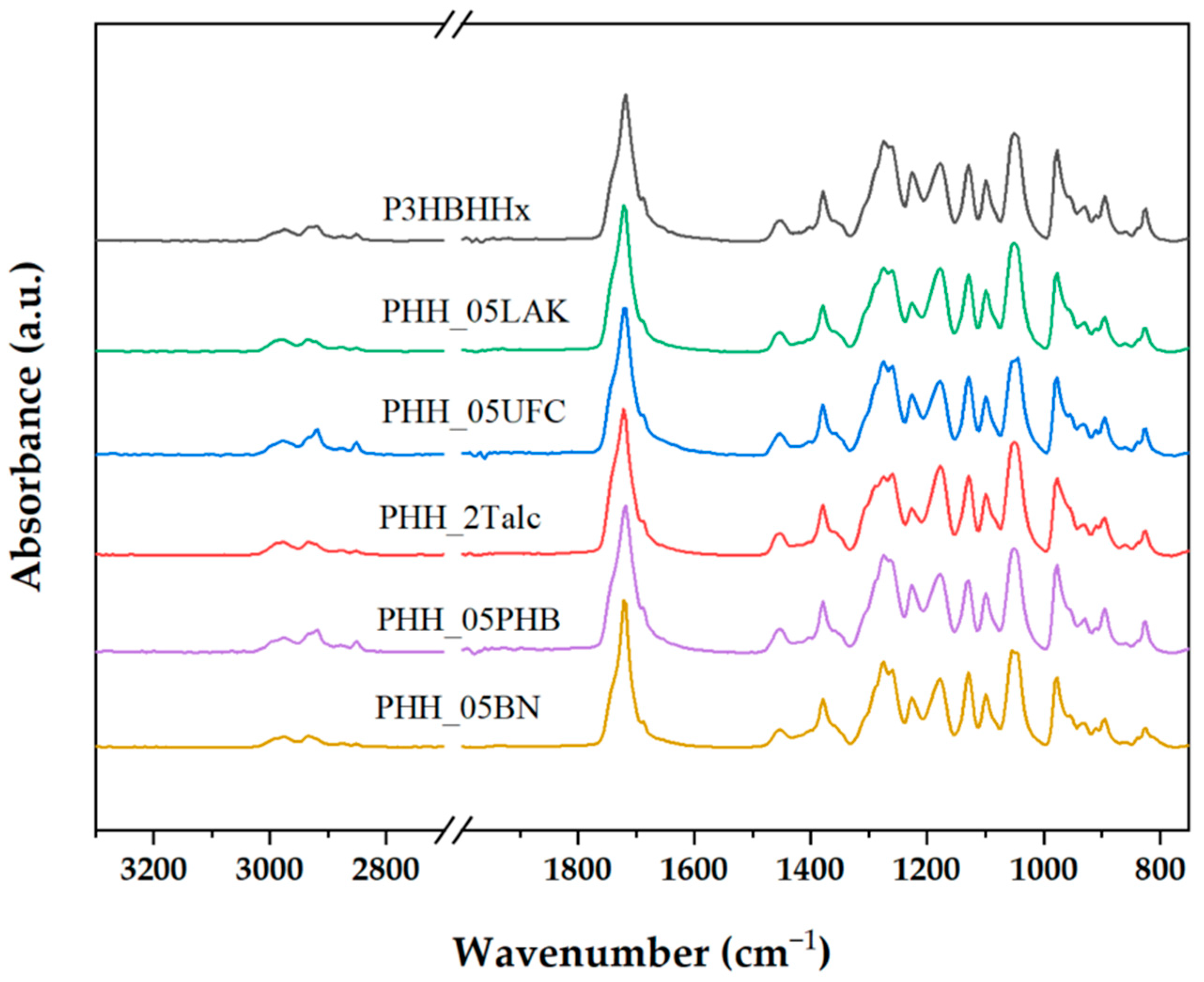
3.2.1. FTIR Analysis
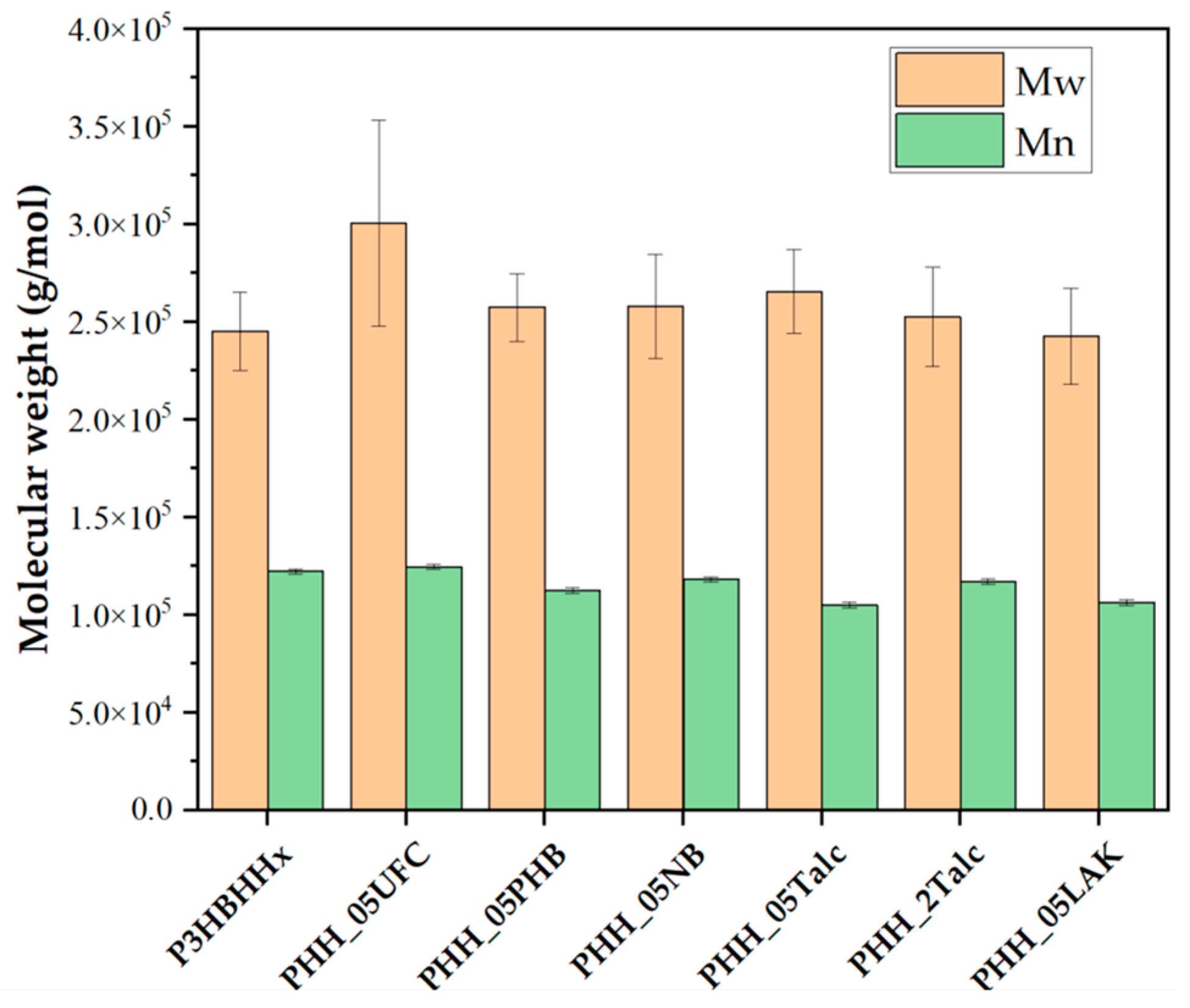
3.2.2. Degradation of Nucleated Samples During Processing
3.2.3. Influence of Nucleating Agents on the Thermal Stability of P3HBHHx
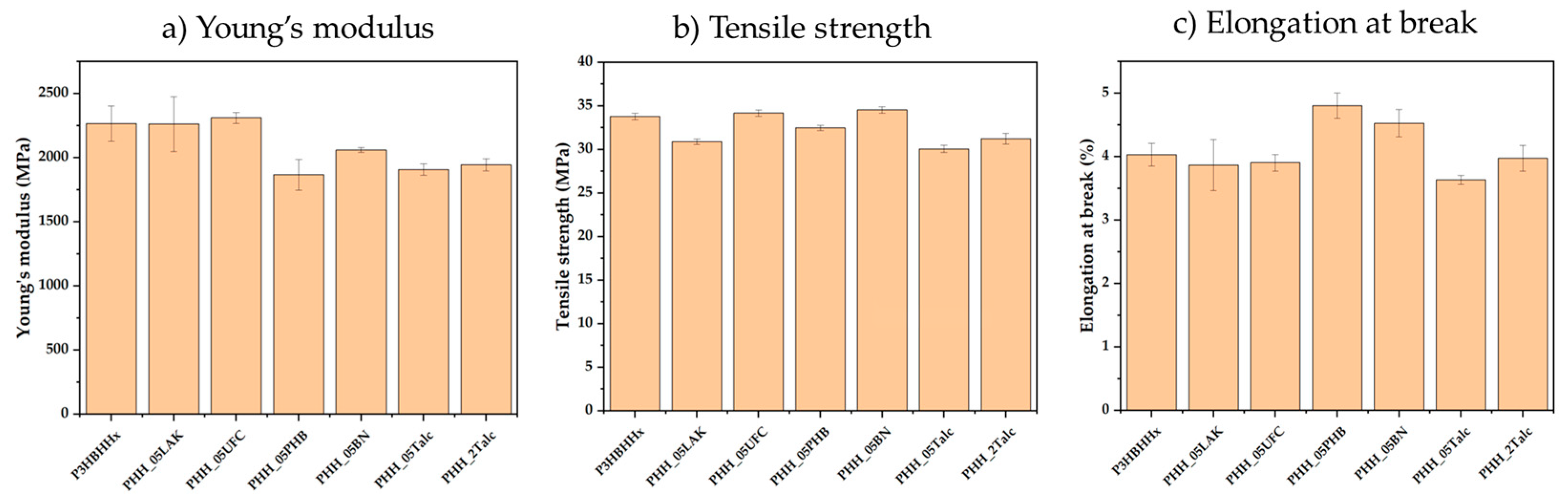
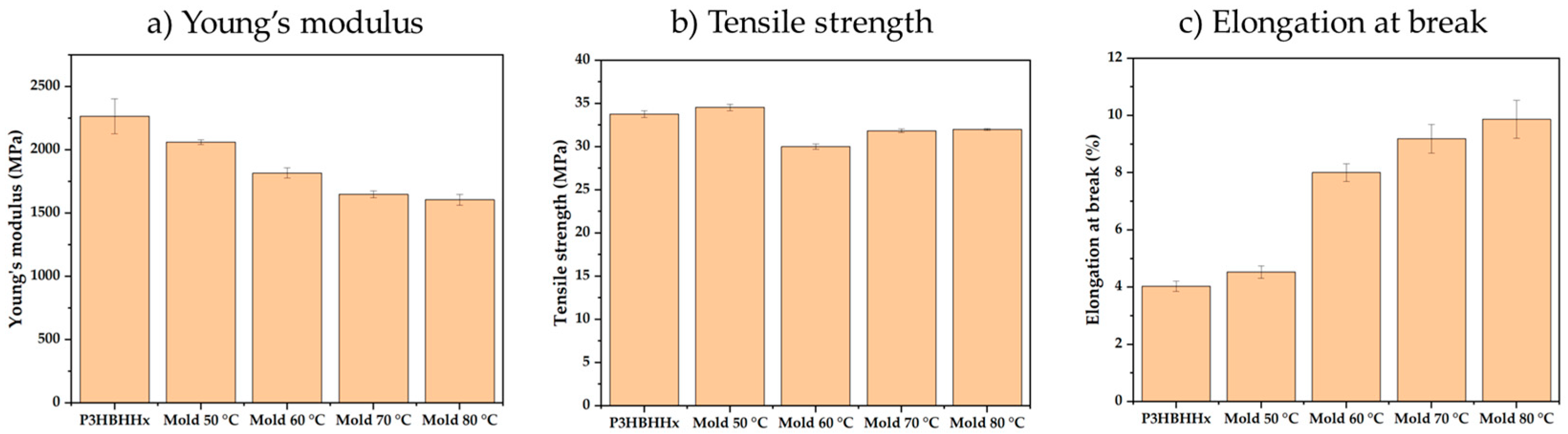
3.2.4. Mechanical Testing
3.3. Isothermal Crystallization and Kinetic Analysis
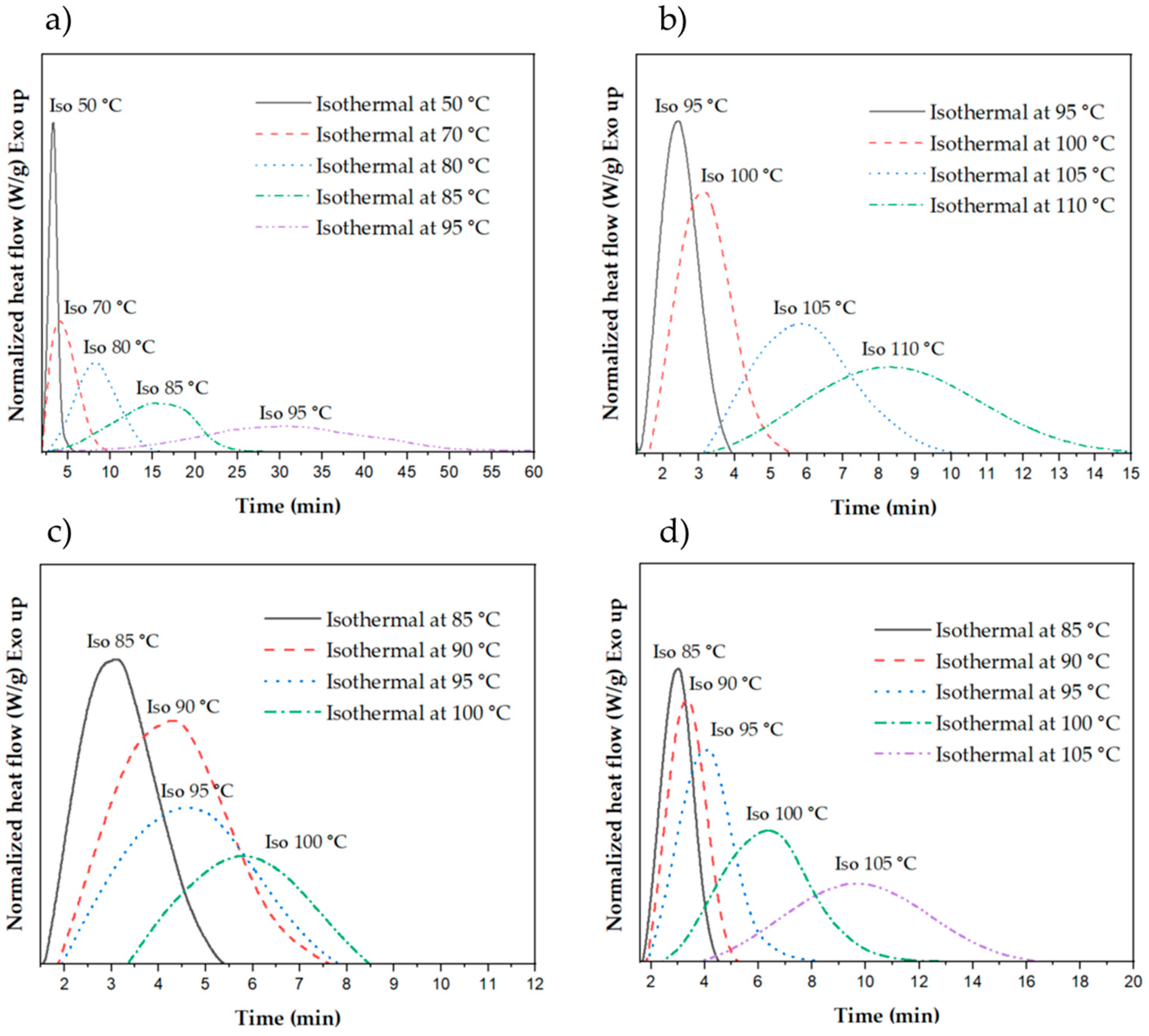
3.3.1. Crystallization of Neat and Nucleated P3HBHHx at Different Isothermal Temperatures
3.3.2. Evolution of the Relative Crystallinity over Time
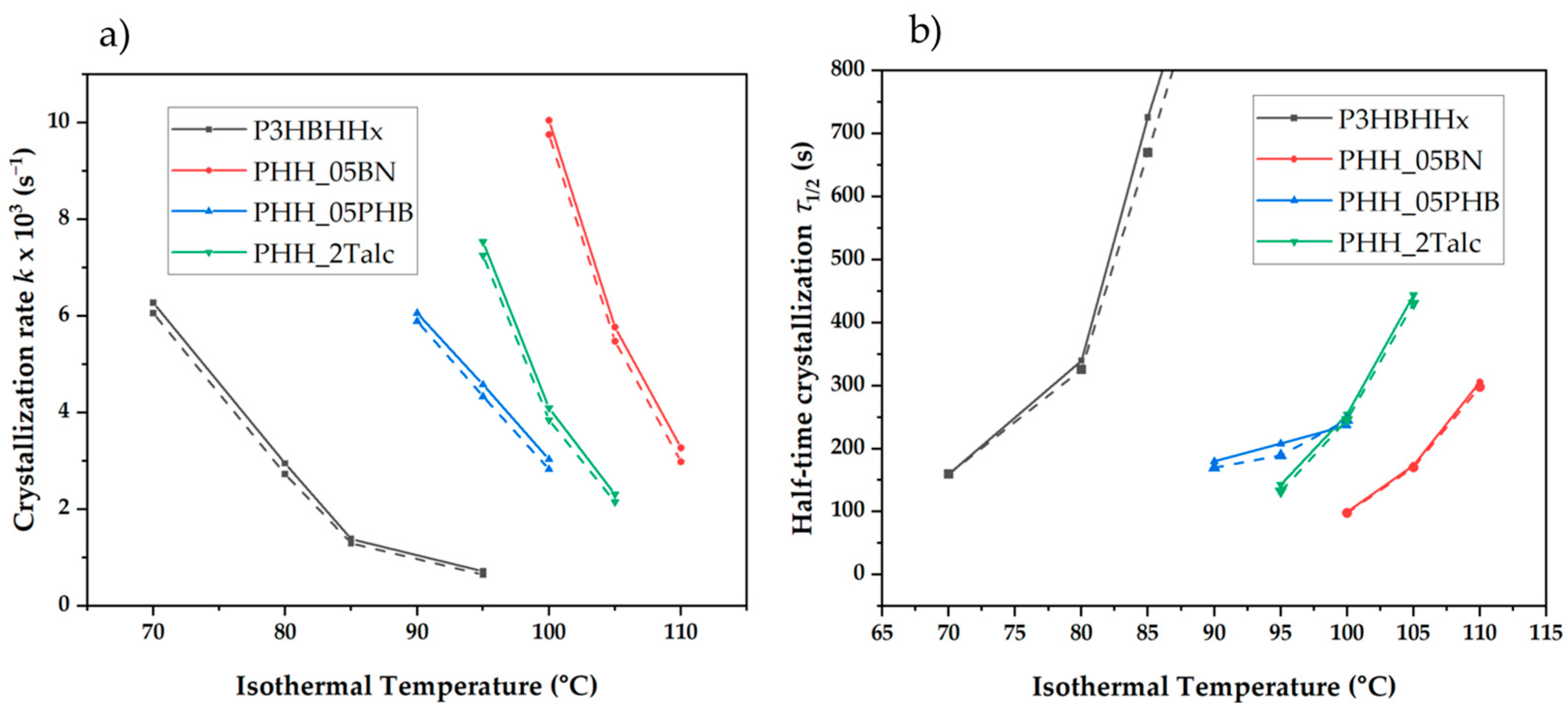
3.3.3. Avrami Modeling of Crystallization Kinetics
3.3.4. Analysis of Avrami Plots
3.4. Spherulitic Morphology and Crystal Growth of P3HBHHx Samples
3.4.1. Spherulitic Morphology
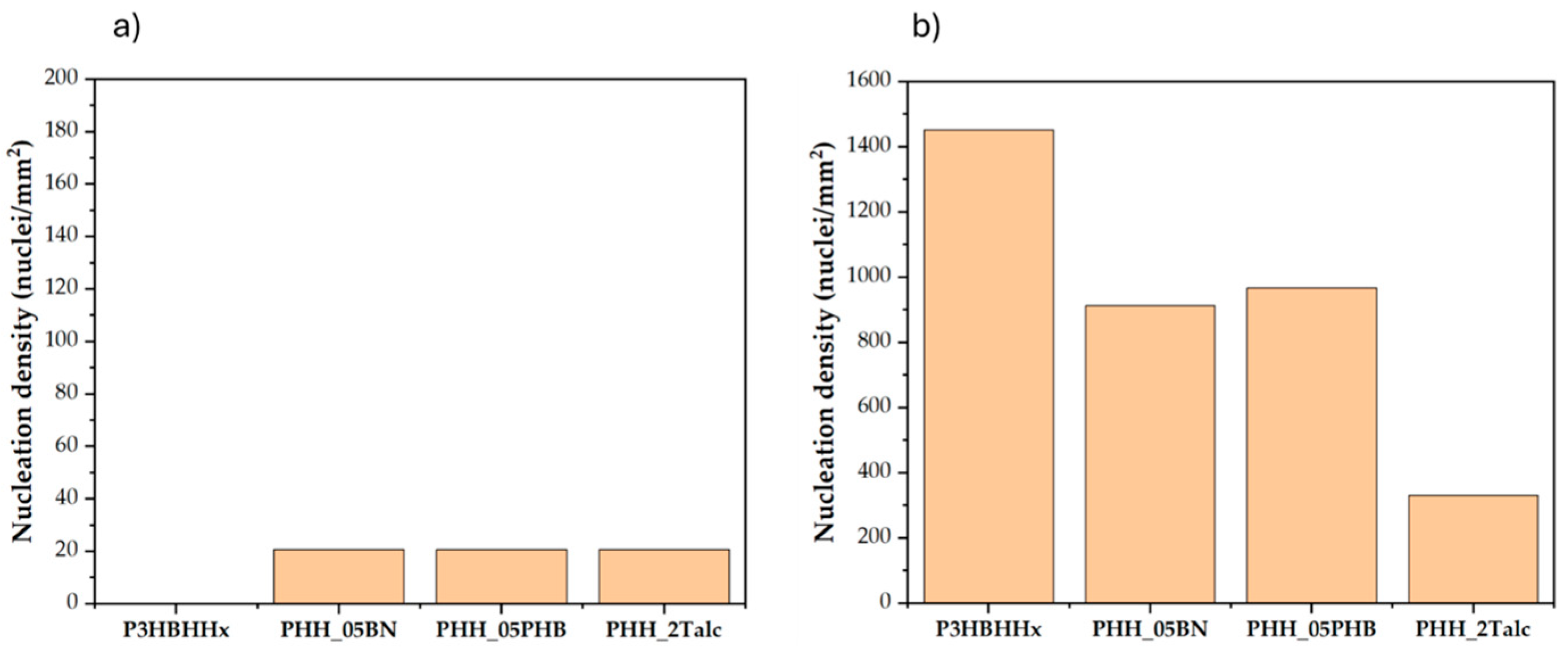
3.4.2. Nucleation Density
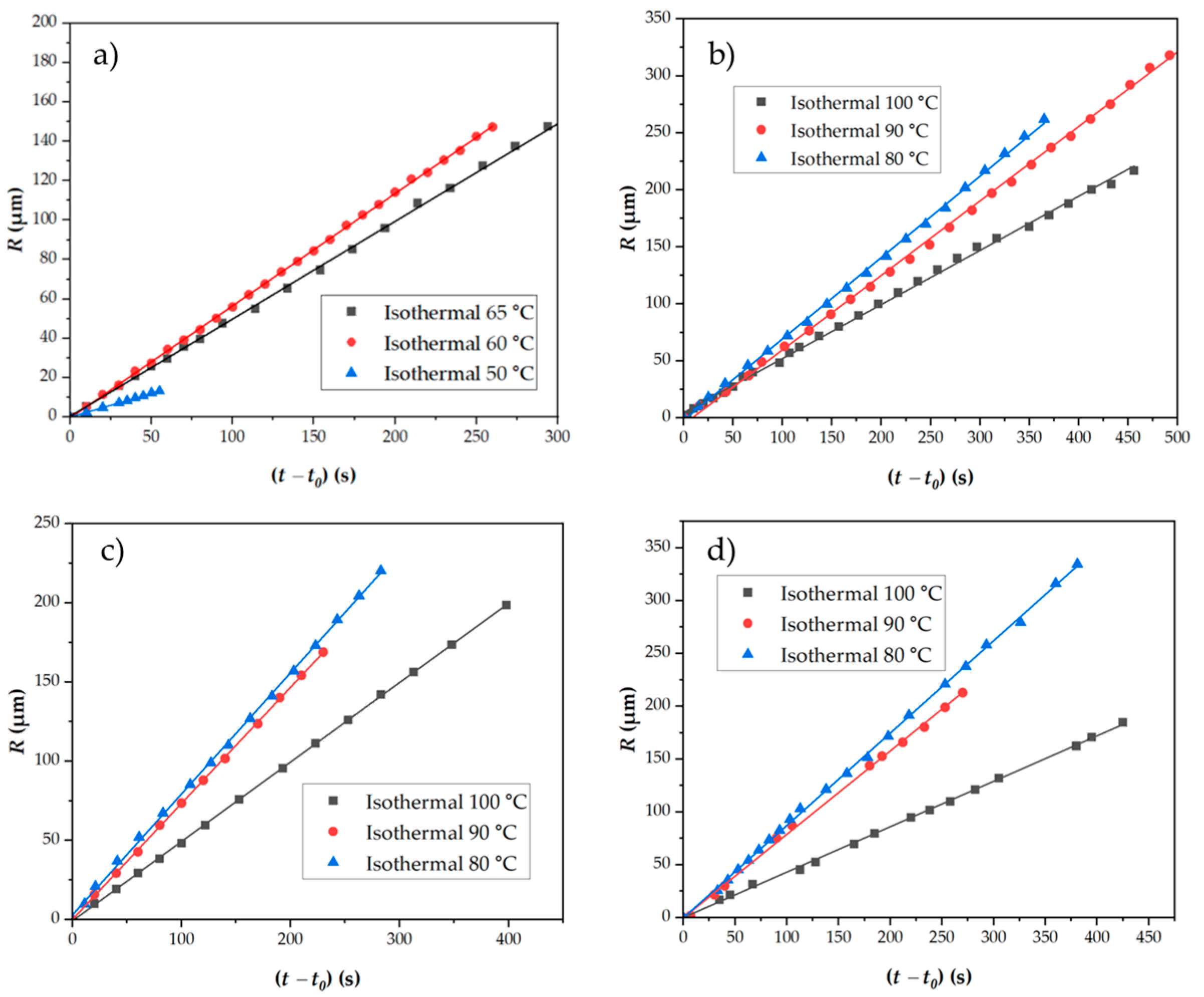
3.4.3. Crystal Growth Rates Determined from POM
3.4.4. Determination of Equilibrium Melting Temperature ()
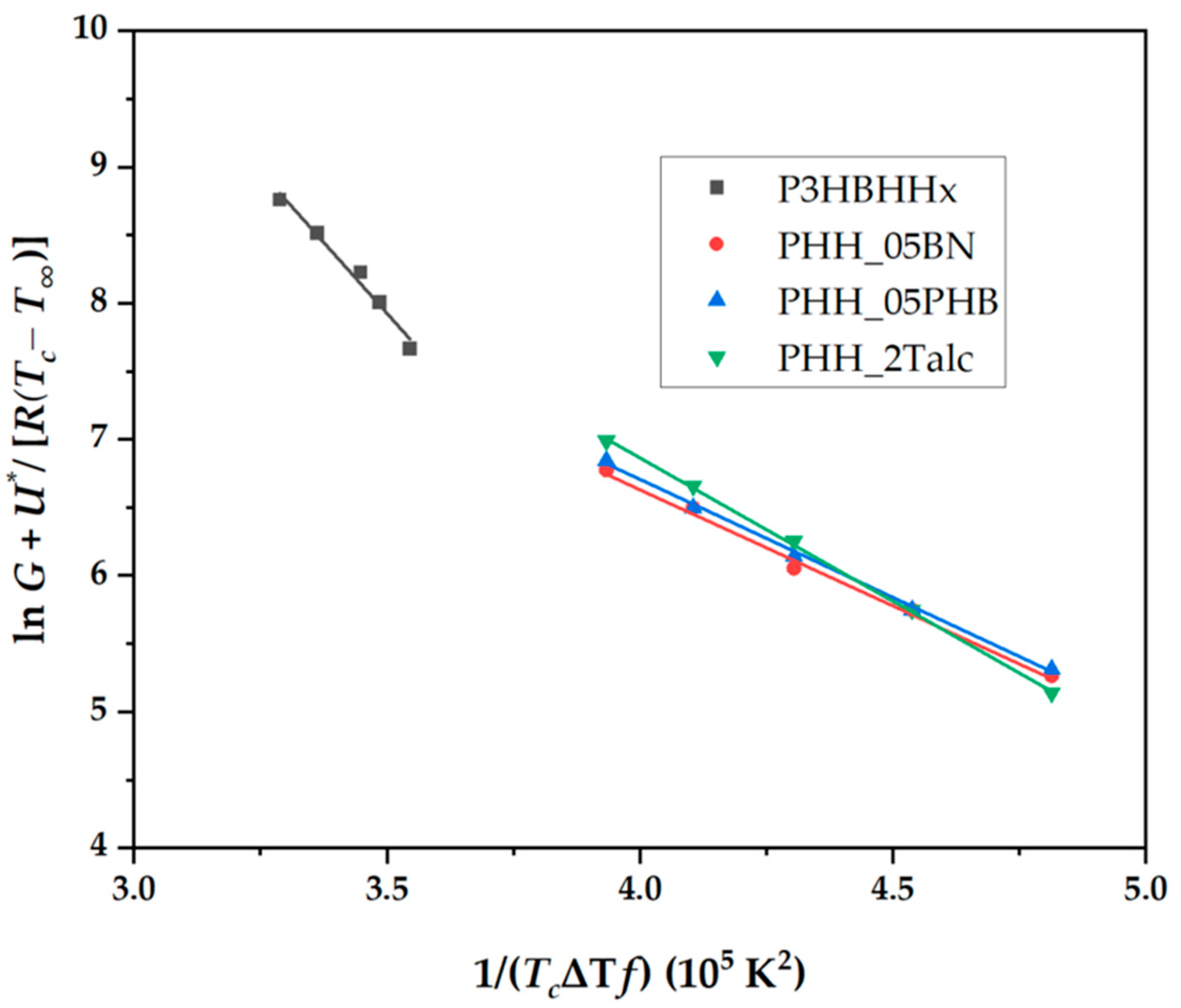
3.4.5. Kinetic Analysis Based on the Lauritzen−Hoffman Model
3.5. Fast Cooling Behavior of P3HBHHx and Nucleated Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| P3HBHHx | Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) |
| BN | Boron nitride |
| PHB | Poly(3-hydroxybutyrate) |
| DSC | Differential scanning calorimetry |
| POM | Polarized optical microscopy |
| TGA | Thermogravimetric analysis |
| PHAs | Polyhydroxyalkanoates |
| 3HHx | 3-hydroxyhexanoate |
| PLA | Polylactic acid |
| UFC | Ultrafine cellulose |
| GPC | Gel permeation chromatography |
| FTIR | Fourier-transform infrared |
References
- Chodak, I. Polyhydroxyalkanoates: Origin, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 451–477. [Google Scholar]
- Muthuraj, R.; Valerio, O.; Mekonnen, T.H. Recent Developments in Short- and Medium-Chain-Length Polyhydroxyalkanoates: Production, Properties, and Applications. Int. J. Biol. Macromol. 2021, 187, 422–440. [Google Scholar] [CrossRef] [PubMed]
- Raho, S.; Carofiglio, V.E.; Montemurro, M.; Miceli, V.; Centrone, D.; Stufano, P.; Schioppa, M.; Pontonio, E.; Rizzello, C.G. Production of the Polyhydroxyalkanoate PHBV from Ricotta Cheese Exhausted Whey by Haloferax Mediterranei Fermentation. Foods 2020, 9, 1459. [Google Scholar] [CrossRef]
- Shen, M.Y.; Chu, C.Y.; Sawatdeenarunat, C.; Bhuyar, P. Production, Downstream Processing, and Characterization of Polyhydroxyalkanoates (PHAs) Boosted by Pyruvate Supplement Using Mixed Microbial Culture (MMC) and Organic Wastewater. Biomass Convers. Biorefin. 2023, 13, 15861–15869. [Google Scholar] [CrossRef]
- Sabapathy, P.C.; Devaraj, S.; Meixner, K.; Anburajan, P.; Kathirvel, P.; Ravikumar, Y.; Zabed, H.M.; Qi, X. Recent Developments in Polyhydroxyalkanoates (PHAs) Production—A Review. Bioresour. Technol. 2020, 306, 123132. [Google Scholar] [CrossRef]
- Serafim, L.S.; Lemos, P.C.; Albuquerque, M.G.E.; Reis, M.A.M. Strategies for PHA Production by Mixed Cultures and Renewable Waste Materials. Appl. Microbiol. Biotechnol. 2008, 81, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Salehizadeh, H.; Van Loosdrecht, M.C.M. Production of Polyhydroxyalkanoates by Mixed Culture: Recent Trends and Biotechnological Importance. Biotechnol. Adv. 2004, 22, 261–279. [Google Scholar] [CrossRef]
- Sandhya, M.; Aravind, J.; Kanmani, P. Production of Polyhydroxyalkanoates from Ralstonia Eutropha Using Paddy Straw as Cheap Substrate. Int. J. Environ. Sci. Technol. 2013, 10, 47–54. [Google Scholar] [CrossRef]
- Palmeiro-Sánchez, T.; O’Flaherty, V.; Lens, P.N. Polyhydroxyalkanoate Bio-Production and Its Rise as Biomaterial of the Future. J. Biotechnol. 2022, 348, 10–25. [Google Scholar] [CrossRef]
- Poltronieri, P.; Kumar, P. Polyhydroxyalkanoates (PHAs) in Industrial Applications. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer: Cham, Switzerland, 2017; Volume 4, pp. 2843–2872. [Google Scholar]
- Yean, O.S.; Yee, C.J.; Kumar, S. Degradation of Polyhydroxyalkanoate (PHA): A Review. J. Sib. Fed. Univ. Biol. 2017, 10, 21–225. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Loh, X.J. Polyhydroxyalkanoates: Opening Doors for a Sustainable Future. NPG Asia Mater. 2016, 8, e265. [Google Scholar] [CrossRef]
- Tang, H.J.; Neoh, S.Z.; Sudesh, K. A Review on Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) [P(3HB-Co-3HHx)] and Genetic Modifications That Affect Its Production. Front. Bioeng. Biotechnol. 2022, 10, 1057067. [Google Scholar] [CrossRef]
- Cai, H.; Qiu, Z. Effect of Comonomer Content on the Crystallization Kinetics and Morphology of Biodegradable Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Phys. Chem. Chem. Phys. 2009, 11, 9569–9577. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Mori, T.; Aoyama, T.; Inoue, Y. Rapid Crystallization of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Copolymer Accelerated by Cyclodextrin-Complex as Nucleating Agent. Carbohydr. Polym. 2010, 80, 387–393. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Qin, Z.-Y.; Zhou, Z. Cellulose Nanocrystals as Green Fillers to Improve Crystallization and Hydrophilic Property of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Prog. Nat. Sci. Mater. Int. 2011, 21, 478–484. [Google Scholar] [CrossRef]
- Xu, P.; Cao, Y.; Lv, P.; Ma, P.; Dong, W.; Bai, H.; Wang, W.; Du, M.; Chen, M. Enhanced Crystallization Kinetics of Bacterially Synthesized Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) with Structural Optimization of Oxalamide Compounds as Nucleators. Polym. Degrad. Stab. 2018, 154, 170–176. [Google Scholar] [CrossRef]
- Jacquel, N.; Tajima, K.; Nakamura, N.; Kawachi, H.; Pan, P.; Inoue, Y. Nucleation Mechanism of Polyhydroxybutyrate and Poly(hydroxybutyrate-co-hydroxyhexanoate) Crystallized by Orotic Acid as a Nucleating Agent. J. Appl. Polym. Sci. 2010, 115, 709–715. [Google Scholar] [CrossRef]
- Qian, J.; Zhu, L.; Zhang, J.; Whitehouse, R.S. Comparison of Different Nucleating Agents on Crystallization of Poly(3-hydroxybutyrate-co-3-hydroxyvalerates). J. Polym. Sci. B Polym. Phys. 2007, 45, 1564–1577. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, P.; Xu, P.; Wang, R.; Dong, W.; Chen, M.; Joziasse, C. The Crystallization Behavior of Poly(lactic acid) with Different Types of Nucleating Agents. Int. J. Biol. Macromol. 2018, 106, 955–962. [Google Scholar] [CrossRef]
- Zou, G.X.; Jiao, Q.W.; Zhang, X.; Zhao, C.X.; Li, J.C. Crystallization Behavior and Morphology of Poly(lactic acid) with a Novel Nucleating Agent. J. Appl. Polym. Sci. 2015, 132, 41367. [Google Scholar] [CrossRef]
- Kai, W.; He, Y.; Inoue, Y. Fast Crystallization of Poly(3-hydroxybutyrate) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with Talc and Boron Nitride as Nucleating Agents. Polym. Int. 2005, 54, 780–789. [Google Scholar] [CrossRef]
- Xu, P.; Wang, Q.; Yu, M.; Yang, W.; Weng, Y.; Dong, W.; Chen, M.; Wang, Y.; Ma, P. Enhanced Crystallization and Storage Stability of Mechanical Properties of Biosynthesized Poly(3-hydroxybutyrate-co-3-hydroxyhexanate) Induced by Self-Nucleation. Int. J. Biol. Macromol. 2021, 184, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Aliotta, L.; Cinelli, P.; Coltelli, M.B.; Righetti, M.C.; Gazzano, M.; Lazzeri, A. Effect of Nucleating Agents on Crystallinity and Properties of Poly (lactic acid) (PLA). Eur. Polym. J. 2017, 93, 822–832. [Google Scholar] [CrossRef]
- Shazleen, S.S.; Yasim-Anuar, T.A.T.; Ibrahim, N.A.; Hassan, M.A.; Ariffin, H. Functionality of Cellulose Nanofiber as Bio-Based Nucleating Agent and Nano-Reinforcement Material to Enhance Crystallization and Mechanical Properties of Polylactic Acid Nanocomposite. Polymers 2021, 13, 389. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ji, C.; Sun, J.; Yao, Q.; Liu, J.; Saeed, R.M.Y.; Zhu, Q. Kinetic Thermal Behavior of Nanocellulose Filled Polylactic Acid Filament for Fused Filament Fabrication 3D Printing. J. Appl. Polym. Sci. 2020, 137, 48374. [Google Scholar] [CrossRef]
- Li, S.D.; Yu, P.H.; Cheung, M.K. Thermogravimetric analysis of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J. Appl. Polym. Sci. 2001, 80, 2237–2244. [Google Scholar] [CrossRef]
- Chan, C.M.; Johansson, P.; Magnusson, P.; Vandi, L.J.; Arcos-Hernandez, M.; Halley, P.; Laycock, B.; Pratt, S.; Werker, A. Mixed Culture Polyhydroxyalkanoate-Rich Biomass Assessment and Quality Control Using Thermogravimetric Measurement Methods. Polym. Degrad. Stab. 2017, 144, 110–120. [Google Scholar] [CrossRef]
- Volova, T.G.; Uspenskaya, M.V.; Kiselev, E.G.; Sukovatyi, A.G.; Zhila, N.O.; Vasiliev, A.D.; Shishatskaya, E.I. Effect of Monomers of 3-Hydroxyhexanoate on Properties of Copolymers Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Polymers 2023, 15, 2890. [Google Scholar] [CrossRef]
- Asrar, J.; Valentin, H.E.; Berger, P.A.; Tran, M.; Padgette, S.R.; Garbow, J.R. Biosynthesis and Properties of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Polymers. Biomacromolecules 2002, 3, 1006–1012. [Google Scholar] [CrossRef]
- Qu, X.H.; Wu, Q.; Liang, J.; Zou, B.; Chen, G.Q. Effect of 3-Hydroxyhexanoate Content in Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) on In Vitro Growth and Differentiation of Smooth Muscle Cells. Biomaterials 2006, 27, 2944–2950. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Sato, H.; Noda, I.; Ozaki, Y. Multiple Melting Behavior of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Investigated by Differential Scanning Calorimetry and Infrared Spectroscopy. Polymer 2007, 48, 4777–4785. [Google Scholar] [CrossRef]
- Fillon, B.; Wittmann, J.C.; Lotz, B.; Thierry, A. Self-nucleation and recrystallization of isotactic polypropylene (α phase) investigated by differential scanning calorimetry. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 1383–1393. [Google Scholar] [CrossRef]
- Fillon, B.; Lotz, B.; Thierry, A.; Wittmann, J.C. Self-nucleation and enhanced nucleation of polymers. Definition of a convenient calorimetric “efficiency scale” and evaluation of nucleating additives in isotactic polypropylene (α phase). J. Polym. Sci. Part B Polym. Phys. 1993, 31, 1395–1405. [Google Scholar] [CrossRef]
- Liu, C.; Jia, M.; Qu, J.; Noda, I.; Chase, D.B.; Street, R.; Rabolt, J.F. Intermolecular hydrogen bonding between poly[(R)-3-hydroxybutyrate] (PHB) and pseudoboehmite and its effect on crystallization of PHB. ACS Appl. Polym. Mater. 2020, 2, 4762–4769. [Google Scholar] [CrossRef]
- Zhang, L.; Hsieh, Y. Lo Ultrafine Cellulose Acetate Fibers with Nanoscale Structural Features. J. Nanosci. Nanotechnol. 2008, 8, 4461–4469. [Google Scholar] [CrossRef]
- Chen, J.; Xu, C.; Wu, D.; Pan, K.; Qian, A.; Sha, Y.; Wang, L.; Tong, W. Insights into the Nucleation Role of Cellulose Crystals during Crystallization of Poly(β-hydroxybutyrate). Carbohydr. Polym. 2015, 134, 508–515. [Google Scholar] [CrossRef]
- Yang, H.X.; Sun, M.; Zhou, P. Investigation of Water Diffusion in Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by Generalized Two-Dimensional Correlation ATR-FTIR Spectroscopy. Polymer 2009, 50, 1533–1540. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. Cellulose Fibers Hydrophobization via a Hybrid Chemical Modification. Polymers 2019, 11, 1174. [Google Scholar] [CrossRef]
- Wu, X.; Han, Q. Thermal Conductivity of Monolayer Hexagonal Boron Nitride: From Defective to Amorphous. Comput. Mater. Sci. 2020, 184, 109938. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. I: General Theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Crist, B.; Schultz, J.M. Polymer Spherulites: A Critical Review. Prog. Polym. Sci. 2016, 56, 1–63. [Google Scholar] [CrossRef]
- Xu, J.; Ye, H.; Zhang, S.; Guo, B. Organization of Twisting Lamellar Crystals in Birefringent Banded Polymer Spherulites: A Mini-Review. Crystals 2017, 7, 241. [Google Scholar] [CrossRef]
- Nagarajan, S.; Widyantari, R.; Chuang, W.T.; Lin, J.M.; Woo, E.M. Grating Harmony of Orderly-Banded Spherulites of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with Tunable Structures. Polymer 2025, 317, 127892. [Google Scholar] [CrossRef]
- Lotz, B.; Cheng, S.Z.D. A Critical Assessment of Unbalanced Surface Stresses as the Mechanical Origin of Twisting and Scrolling of Polymer Crystals. Polymer 2005, 46, 577–610. [Google Scholar] [CrossRef]
- Owen, A.J.; Heinzel, J.; Škrbić, Ž.; Divjaković, V. Crystallization and melting behaviour of PHB and PHB/HV copolymer. Polymer 1992, 33, 1563–1567. [Google Scholar] [CrossRef]
- Keridou, I.; Del Valle, L.J.; Funk, L.; Turon, P.; Franco, L.; Puiggalí, J. Non-Isothermal Crystallization Kinetics of Poly(4-hydroxybutyrate) Biopolymer. Molecules 2019, 24, 2840. [Google Scholar] [CrossRef]
- Lauritzen, J.I.; Hoffman, J.D. Extension of Theory of Growth of Chain-Folded Polymer Crystals to Large Undercoolings. J. Appl. Phys. 1973, 44, 4340–4352. [Google Scholar] [CrossRef]
- Chen, C.; Cheung, M.K.; Yu, P.H.F. Crystallization Kinetics and Melting Behaviour of Microbial Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Polym. Int. 2005, 54, 1055–1064. [Google Scholar] [CrossRef]
- Suzuki, T.; Kovacs, A.J. Temperature dependence of spherulitic growth rate of isotactic polystyrene. A critical comparison with the kinetic theory of surface nucleation. Polym. J. 1970, 1, 82–100. [Google Scholar] [CrossRef]









| Sample Code | Nucleating Agent | Concentration (wt%) |
|---|---|---|
| P3HBHHx | None | 0 |
| PHH_05BN/PHH_2BN | Boron nitride | 0.5/2 |
| PHH_05PHB/PHH_2PHB | P3HB | 0.5/2 |
| PHH_05Talc/PHH_2Talc | Talc | 0.5/2 |
| PHH_05LAK/PHH_2LAK | Organic potassium salt | 0.5/2 |
| PHH_05UFC/PHH_2UFC | Ultrafine cellulose | 0.5/2 |
| Samples | Tc (°C) | ΔHc (J/g) | Tg (°C) | Tcc (°C) | ΔHcc (°C) | Tm1 (°C) | Tm2 (°C) | ΔHf (J/g) |
|---|---|---|---|---|---|---|---|---|
| P3HBHHx | 36.4 | 8.9 | 1.4 | 44.7 | 44.6 | 130.0 | 150.2 | 55.6 |
| PHH_05LAK | 45.6 | 18.3 | 1.4 | 45.5 | 38.4 | 129.8 | 149.5 | 58.0 |
| PHH_05UFC | 58.9 | 48.5 | 4.8 | - | - | 132.1 | 150.2 | 56.5 |
| PHH_05Talc | 66.5 | 46.3 | 3.7 | 45.3 | 0.8 | 133.2 | 149.9 | 49.7 |
| PHH_05PHB | 81.9 | 59.5 | 4.4 | - | - | 138.1 | 152.7 | 60.0 |
| PHH_05BN | 84.1 | 56.5 | 3.3 | - | - | 136.6 | 150.7 | 56.0 |
| Tc (°C) a | NE (%) b | |
|---|---|---|
| P3HBHHx | 54.5 | - |
| PHH_05LAK | 57.0 | 3.4 |
| PHH_05UFC | 68.3 | 18.9 |
| PHH_05Talc | 76.9 | 30.7 |
| PHH_2Talc | 83.9 | 40.2 |
| PHH_05PHB | 85.8 | 42.8 |
| PHH_05BN | 88.3 | 46.2 |
| Tmax (°C) | T5% (°C) | T50% (°C) | T90% (°C) | Residue at 450 °C (wt%) | |
|---|---|---|---|---|---|
| P3HBHHx | 275.7 | 246.9 | 269.1 | 276.7 | 0 |
| PHH_05LAK | 273.5 | 246.8 | 267.3 | 275.1 | 0.57 |
| PHH_05UFC | 261.1 | 236.3 | 255.4 | 263.6 | 0.50 |
| PHH_05PHB | 273.6 | 247.1 | 267.8 | 275.3 | 0.52 |
| PHH_05BN | 258.3 | 236.1 | 255.2 | 263.8 | 0.58 |
| PHH_05Talc | 271.4 | 246.7 | 266.9 | 274.6 | 0.53 |
| PHH_2Talc | 279.5 | 247.9 | 264.3 | 281.7 | 1.76 |
| Sample | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| P3HBHHx | 2264 ± 138 | 33.7 ± 0.4 | 4.0 ± 0.2 |
| PHH_05LAK | 2260 ± 213 | 30.8 ± 0.2 | 3.8 ± 0.1 |
| PHH_05UFC | 2309 ± 42 | 34.1 ± 0.4 | 3.9 ± 0.1 |
| PHH_05PHB | 1866 ± 119 | 32.4 ± 0.3 | 4.8 ± 0.2 |
| PHH_05BN | 2060 ± 19 | 34.5 ± 0.4 | 4.5 ± 0.2 |
| PHH_05Talc | 1906 ± 43 | 30.0 ± 0.4 | 3.6 ± 0.1 |
| PHH_2Talc | 1943 ± 46 | 31.2 ± 0.6 | 3.9 ± 0.2 |
| Sample | Isothermal Temperature (°C) | Enthalpy of Crystallization (J/g) | Half-Time of Crystallization τ(1/2) (s) | Crystallization Rate k × 103 (s−1) |
|---|---|---|---|---|
| P3HBHHx | 50 | 41.5 | 77 | 13.06 |
| 70 | 55.1 | 159 | 6.27 | |
| 80 | 60.3 | 340 | 2.94 | |
| 85 | 60.1 | 726 | 1.38 | |
| 95 | 60.8 | 1404 | 0.71 | |
| PHH_05BN | 95 | 48.1 | 68 | 14.67 |
| 100 | 64.3 | 99 | 10.05 | |
| 105 | 66.5 | 173 | 5.77 | |
| 110 | 66.7 | 306 | 3.27 | |
| PHH_05PHB | 85 | 46.9 | 104 | 9.66 |
| 90 | 59.4 | 165 | 6.06 | |
| 95 | 60.2 | 193 | 5.18 | |
| 100 | 60.5 | 242 | 4.13 | |
| PHH_2Talc | 85 | 44.5 | 84 | 11.84 |
| 90 | 55.6 | 106 | 9.47 | |
| 95 | 58.0 | 133 | 7.53 | |
| 100 | 58.4 | 244 | 4.09 | |
| 105 | 60.3 | 434 | 2.31 |
| Sample | Tc (°C) | n | Z × 106 (s−n) | k × 103 (s−1) Avrami | τ(1/2) (s) Avrami |
|---|---|---|---|---|---|
| P3HBHHx | 70 | 2.10 | 16.11 | 5.26 | 160 |
| 80 | 2.34 | 0.890 | 2.63 | 326 | |
| 85 | 2.54 | 0.046 | 1.29 | 670 | |
| 95 | 2.75 | 0.002 | 0.64 | 1366 | |
| PHH_05BN | 100 | 2.39 | 11.98 | 8.76 | 98 |
| 105 | 2.20 | 8.38 | 4.98 | 170 | |
| 110 | 2.39 | 0.87 | 2.88 | 298 | |
| PHH_05PHB | 90 | 2.29 | 5.76 | 5.15 | 165 |
| 95 | 2.10 | 11.23 | 4.43 | 189 | |
| 100 | 2.12 | 5.79 | 3.43 | 246 | |
| PHH_2Talc | 95 | 2.32 | 8.42 | 6.45 | 132 |
| 100 | 2.68 | 0.27 | 3.54 | 246 | |
| 105 | 2.88 | 0.02 | 2.04 | 431 |
| Sample | P3HBHHx | G (µm/s) | Induction Time (s) |
|---|---|---|---|
| P3HBHHx | 50 | 0.23 | 1 |
| 60 | 0.57 | 45 | |
| 65 | 0.49 | 50 | |
| PHH_05BN | 80 | 0.71 | 1 |
| 90 | 0.65 | 71 | |
| 100 | 0.48 | 150 | |
| PHH_05PHB | 80 | 0.76 | 1 |
| 90 | 0.73 | 22 | |
| 100 | 0.50 | 80 | |
| PHH_2Talc | 80 | 0.87 | 1 |
| 90 | 0.79 | 75 | |
| 100 | 0.43 | 100 |
| Sample | Linear Plot | R2 | Kg 10−5 (K2) |
|---|---|---|---|
| P3HBHHx | y = −4.097x + 22.295 | 0.9946 | 4.10 |
| PHH_05BN | y = −1.707x + 13.463 | 0.9924 | 1.71 |
| PHH_05PHB | y = −1.734x + 13.644 | 0.9957 | 1.73 |
| PHH_2Talc | y = −2.107x + 15.293 | 0.9996 | 2.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, A.; Pérez, G.; del Valle, L.J.; Puiggalí, J. Influence of Nucleating Agents on the Crystallization, Thermal, and Mechanical Properties of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (P3HBHHx). Appl. Sci. 2025, 15, 6120. https://doi.org/10.3390/app15116120
Jin A, Pérez G, del Valle LJ, Puiggalí J. Influence of Nucleating Agents on the Crystallization, Thermal, and Mechanical Properties of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (P3HBHHx). Applied Sciences. 2025; 15(11):6120. https://doi.org/10.3390/app15116120
Chicago/Turabian StyleJin, Anyi, Germán Pérez, Luis J. del Valle, and Jordi Puiggalí. 2025. "Influence of Nucleating Agents on the Crystallization, Thermal, and Mechanical Properties of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (P3HBHHx)" Applied Sciences 15, no. 11: 6120. https://doi.org/10.3390/app15116120
APA StyleJin, A., Pérez, G., del Valle, L. J., & Puiggalí, J. (2025). Influence of Nucleating Agents on the Crystallization, Thermal, and Mechanical Properties of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (P3HBHHx). Applied Sciences, 15(11), 6120. https://doi.org/10.3390/app15116120






