Herbal Support for the Nervous System: The Impact of Adaptogens in Humans and Dogs
Abstract
1. Introduction
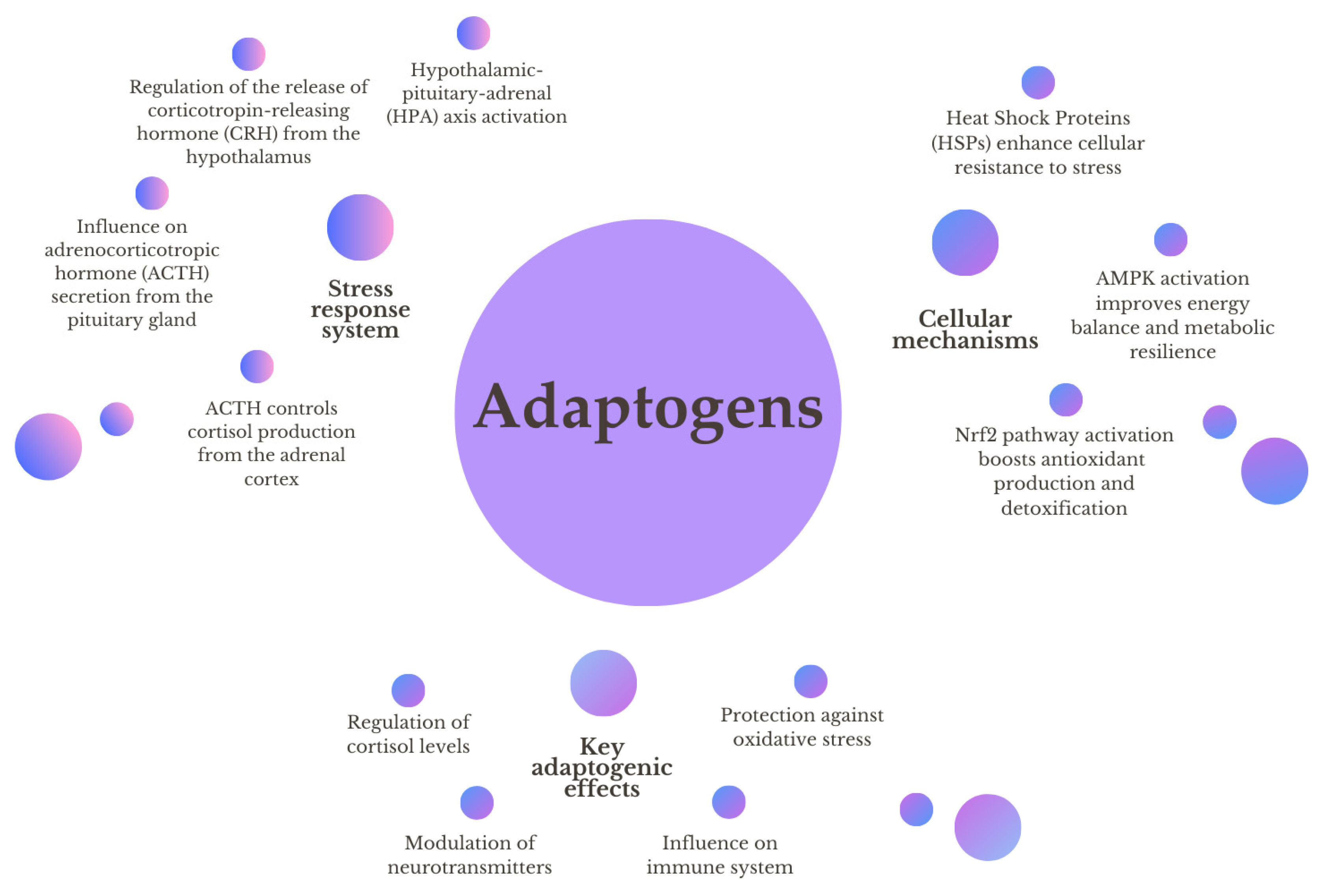
1.1. Stress in Humans and Dogs
1.2. Mechanism of Action of Adaptogens
2. Ginseng (Panax ginseng C.A. Mey)
2.1. Aspects and Composition
2.2. Studies Related to Therapeutic Effects on Humans
2.3. Studies Related to Therapeutic Effects on Dogs
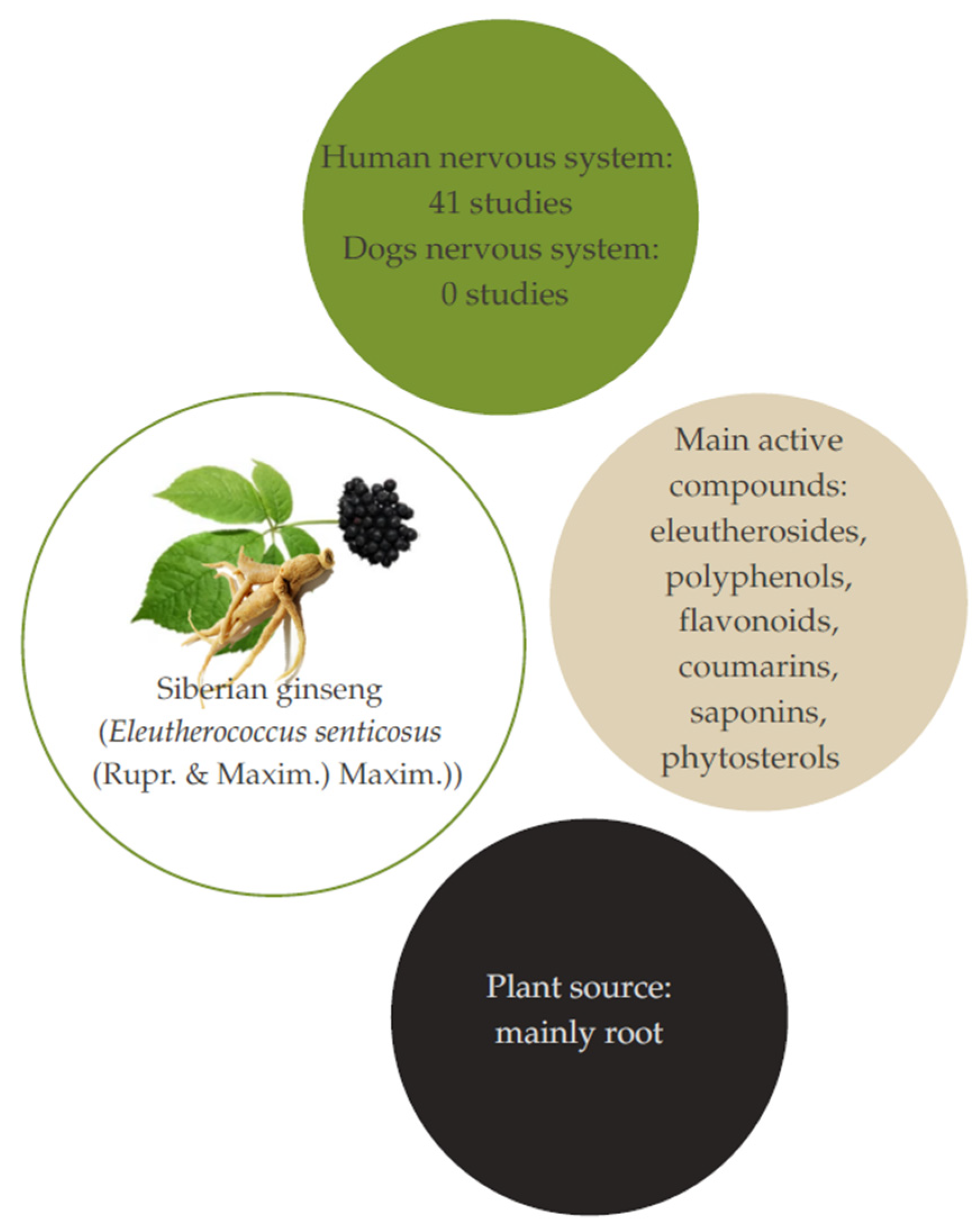
3. Siberian Ginseng (Eleutherococcus senticosus (Rupr. & Maxim.) Maxim.)
3.1. Aspects and Composition
3.2. Studies Related to Therapeutic Effects on Humans
3.3. Studies Related to Therapeutic Effects on Dogs
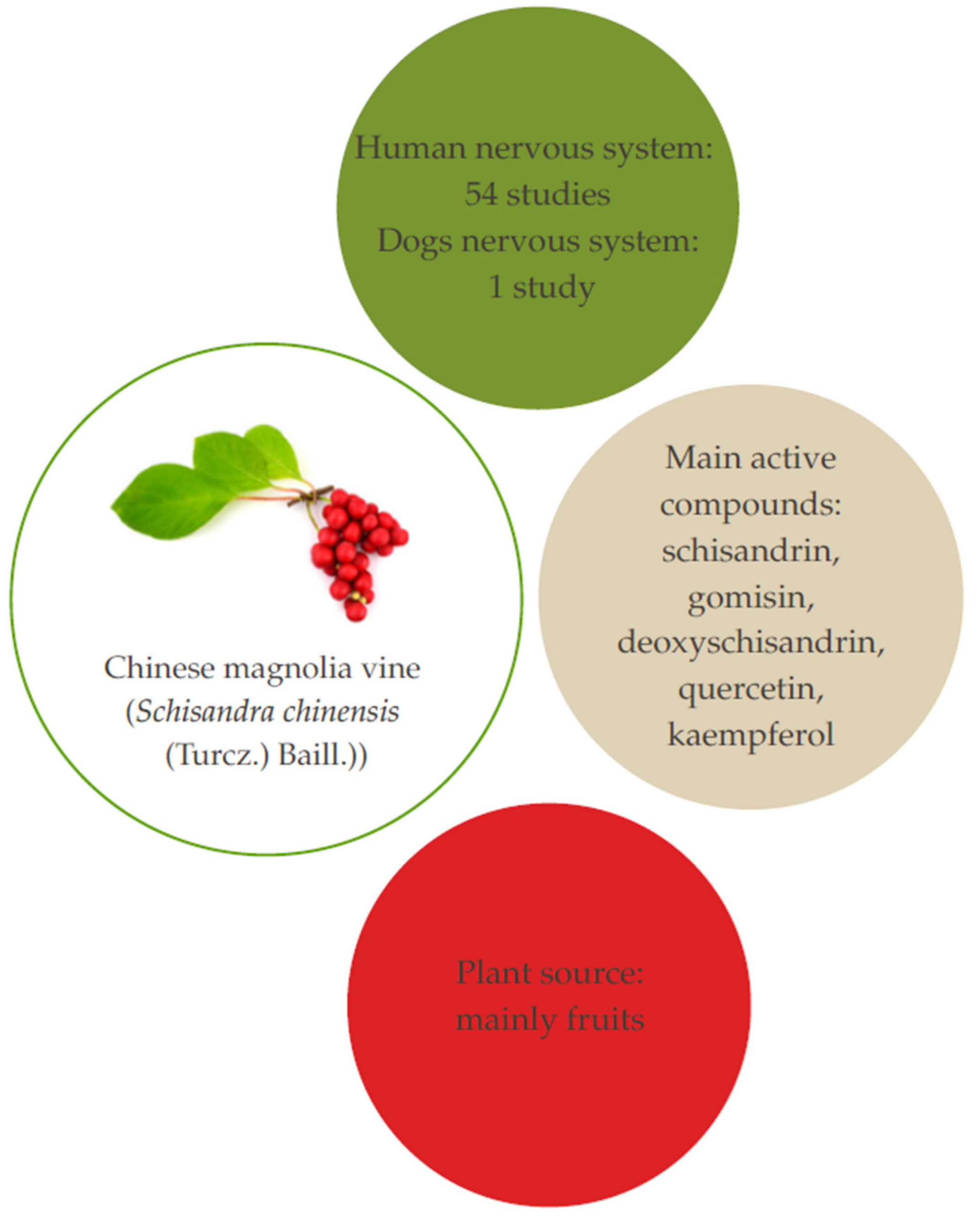
4. Chinese Magnolia Vine (Schisandra chinensis (Turcz.) Baill.
4.1. Aspects and Composition
4.2. Studies Related to Therapeutic Effects on Humans
4.3. Studies Related to Therapeutic Effects on Dogs
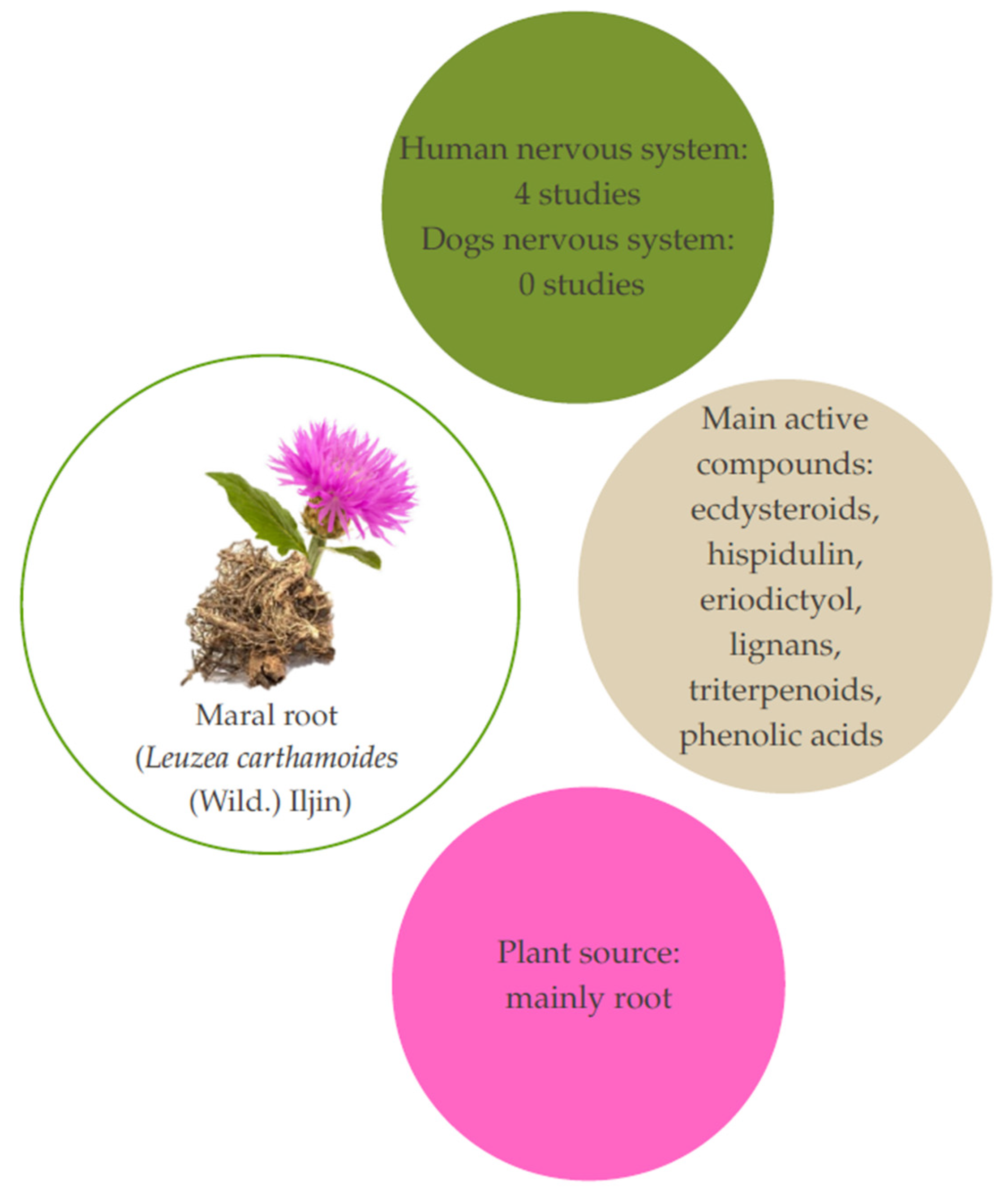
5. Maral Root (Leuzea carthamoides (Wild.) Iljin)
5.1. Aspects and Composition
5.2. Studies Related to Therapeutic Effects on Humans
5.3. Studies Related to Therapeutic Effects on Dogs
6. Golden Root (Rhodiola rosea)
6.1. Aspects and Composition
6.2. Studies Related to Therapeutic Effects on Humans
6.3. Studies Related to Therapeutic Effects on Dogs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPA | hypothalamic–pituitary–adrenal |
| BDNF | brain-derived neurotrophic factor |
| PPD | protopanaxadiol |
| PPT | protopanaxatriol |
| AD | Alzheimer’s disease |
| NFP | non-saponin fraction rich in polysaccharides |
| AHN | adult hippocampal neurogenesis |
| LPS | lipopolysaccharide |
| MC | micrandilactone C |
| HD | Huntington’s disease |
| 3-NPA | 3-nitropropionic acid |
References
- Zhao, R.; Zhang, J.; Gou, Q.; Gao, J. Popularity of traditional Chinese medicine use among breast cancer patients in North China: A cross-sectional study. Breast Cancer Targets Ther. 2023, 15, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Czigle, S.; Nagy, M.; Mladěnka, P.; Tóth, J. Pharmacokinetic and pharmacodynamic herb-drug interactions—Part I. Herbal medicines of the central nervous system. PeerJ 2023, 11, e16149. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, R.; Cáceres, A.; Velásquez, D.; Rodríguez, C.; Morales, D.; Castillo, A. Medicinal plants used in traditional Mayan medicine for the treatment of central nervous system disorders: An overview. J. Ethnopharmacol. 2022, 283, 114746. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Soleymani, A.; Cheng, Q. Traditional herbal medicines to overcome stress, anxiety and improve mental health in outbreaks of human coronaviruses. Phytother. Res. 2021, 35, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Todorova, V.; Ivanov, K.; Delattre, C.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S. Plant adaptogens-history and future perspectives. Nutrients 2021, 13, 2861. [Google Scholar] [CrossRef]
- Quintavalla, F. Phytotherapeutic approaches in canine pediatrics. Vet. Sci. 2024, 11, 133. [Google Scholar] [CrossRef]
- da Matta, E.C.; Takeda, M.; de Medeiros, N.S.S.; Hosomi, J.K.; Bonamin, L.V. Homeopathy, acupuncture and phytotherapy in the veterinary treatment or prophylaxis of diseases in animals: An overview of systematic reviews. Homeopathy 2025, 114, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cano, D.; Arnao, M.B. Beneficial effects of nutraceuticals, especially polyphenols on canine health. Pets 2024, 1, 228–254. [Google Scholar] [CrossRef]
- Li, J.; Long, Q.; Ding, H.; Wang, Y.; Luo, D.; Li, Z.; Zhang, W. Progress in the treatment of central nervous system diseases based on nanosized traditional Chinese medicine. Adv. Sci. 2024, 11, 2308677. [Google Scholar] [CrossRef]
- Yelverton, A.P.; Perez, N.; Hall, B.; Broadway, M. Effectiveness of lavender aromatherapy in reducing canine stress in a veterinary setting. Dog Behav. 2024, 1, 13–22. [Google Scholar] [CrossRef]
- Nocera, F.P.; Mancini, S.; Najar, B.; Bertelloni, F.; Pistelli, L.; De Filippis, A.; Fiorito, F.; De Martino, L.; Fratini, F. Antimicrobial activity of some essential oils against methicillin-susceptible and methicillin-resistant Staphylococcus pseudintermedius-associated pyoderma in dogs. Animals 2020, 10, 1782. [Google Scholar] [CrossRef] [PubMed]
- Tárrega, A.; Salvador, A.; Meyer, M.; Feuillère, N.; Ibarra, A.; Roller, M.; Terroba, D.; Madera, C.; Iglesias, J.R.; Echevarría, J.; et al. Active compounds and distinctive sensory features provided by American ginseng (Panax quinquefolius L.) extract in a new functional milk beverage. J. Dairy Sci. 2012, 95, 4246–4255. [Google Scholar] [CrossRef]
- Byard, R.W.; Musgrave, I. The potential side effects of herbal preparations in domestic animals. Forensic. Sci. Med. Pathol. 2021, 17, 723–725. [Google Scholar] [CrossRef]
- Song, S.Y.; Chang, H.J.; Kim, S.D.; Kwag, E.B.; Park, S.J.; Yoo, H.S. Acute and sub-chronic toxicological evaluation of the herbal product HAD-B1 in Beagle dogs. Toxicol. Rep. 2021, 8, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Saba, E.; Sandhu, M.A.; Yousaf, A.; Rashid, U.; Rhee, M.H. Ginseng in veterinary practice: Benefits and consideration. In Complementary and Alternative Medicine: One Health Perspective; Sindhu, Z.D., Aslam, B., Uslu, U., Mohsin, M., Eds.; FahumSci: Lahore, Pakistan, 2023; pp. 244–250. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; Mayo, B.; et al. Scientific opinion on the safety and efficacy of a feed additive consisting of a tincture derived from the roots of Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (taiga root tincture) for use in dogs, cats and horses (FEFANA asbl). EFSA J. 2023, 21, e07876. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chang, Y.; Cao, F.; Yang, C.; Wang, Z.; Kuang, H. Simultaneous determination of six triterpenoid saponins in beagle dog plasma by UPLC-MS/MS and its application to a pharmacokinetic study after oral administration of the extract of the Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. leaves. Acta Chromatogr. 2023, 35, 88–98. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Durjava, M.; Kouba, M.; López-Alonso, M.; Puente, S.L.; Marcon, F.; Mayo, B.; et al. Safety and efficacy of a feed additive consisting of a tincture derived from the dried fruit of Schisandra chinensis (Turcz.) Baill. (omicha tincture) for poultry, horses, dogs and cats (FEFANA asbl). EFSA J. 2024, 22, e8731. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Jang, M.; Lee, M.J.; Choi, J.H.; Lee, S.J.; Kim, S.K.; Jang, D.S.; Cho, I.H. Schisandra chinensis stem ameliorates 3-nitropropionic acid-induced striatal toxicity via activation of the Nrf2 pathway and inhibition of the MAPKs and NF-κB pathways. Front. Pharmacol. 2017, 8, 673. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Losada, M.; Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Phytomelatonin content in valeriana officinalis l. and some related phytotherapeutic supplements. Int. J. Plant Based Pharm. 2022, 2, 176–181. [Google Scholar] [CrossRef]
- Logvinov, S.V.; Pugachenko, N.V.; Potapov, A.V.; Krasnov, E.A.; Plotnikov, M.B.; Maslov, M. Ischemia-induced changes in synaptoarchitectonics of brain cortex and their correction with ascovertin and leuzea extract. Bull. Exp. Biol. Med. 2001, 132, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Zomborszki, Z.P.; Kúsz, N.; Hohmann, J.; Csupor, D. Three novel constituents from the roots of Rhaponticum carthamoides. Acta Pharm. Hung. 2023, 93, 9–14. [Google Scholar] [CrossRef]
- Elgendy, S.A.; Soliman, M.M.; Ghamry, H.I.; Shukry, M.; Mohammed, L.A.; Nasr, H.E.; Alotaibi, B.S.; Jafri, I.; Sayed, S.; Osman, A.; et al. Exploration of tilmicosin cardiotoxicity in rats and the protecting role of the Rhodiola rosea extract: Potential roles of cytokines, antioxidant, apoptotic, and anti-fibrotic pathways. Toxics 2023, 11, 857. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Shan, X.; Zhao, X.; Cao, Y.; Zhou, T.; Fan, G. Absolute bioavailability of salidroside in Beagle dog. J. Pharm. Pract. Serv. 2021, 39, 62–67. [Google Scholar] [CrossRef]
- Stojcheva, E.I.; Quintela, J.C. The effectiveness of Rhodiola rosea L. preparations in alleviating various aspects of life-stress symptoms and stress-induced conditions—Encouraging clinical evidence. Molecules 2022, 27, 3902. [Google Scholar] [CrossRef] [PubMed]
- Juszczyk, G.; Mikulska, J.; Kasperek, K.; Pietrzak, D.; Mrozek, W.; Herbet, M. Chronic stress and oxidative stress as common factors of the pathogenesis of depression and Alzheimer’s disease: The role of antioxidants in prevention and treatment. Antioxidants 2021, 10, 1439. [Google Scholar] [CrossRef] [PubMed]
- Agorastos, A.; Chrousos, G.P. The neuroendocrinology of stress: The stress-related continuum of chronic disease development. Mol. Psychiatry 2022, 27, 502–513. [Google Scholar] [CrossRef]
- Kujala, M.V. Canine emotions as seen through human social cognition. Anim. Sentienc. 2017, 14, 1–34. [Google Scholar] [CrossRef]
- Katayama, M.; Kubo, T.; Yamakawa, T.; Fujiwara, K.; Nomoto, K.; Ikeda, K.; Mogi, K.; Nagasawa, M.; Kikusui, T. Emotional contagion from humans to dogs is facilitated by duration of ownership. Front. Psychol. 2019, 10, 1678. [Google Scholar] [CrossRef]
- Weinmann, T.; Wibowo, R.; Forster, F.; Gerlich, J.; Wengenroth, L.; Weinmayr, G.; Genuneit, J.; Nowak, D.; Vogelberg, C.; Radon, K.; et al. Association of chronic stress during studies with depressive symptoms 10 years later. Sci. Rep. 2025, 15, 2379. [Google Scholar] [CrossRef] [PubMed]
- Hussenoeder, F.S.; Conrad, I.; Pabst, A.; Engel, C.; Zachariae, S.; Zeynalova, S.; Riedel-Heller, S.G. Connecting chronic stress and anxiety: A multi-dimensional perspective. Psychol. Health Med. 2024, 29, 427–441. [Google Scholar] [CrossRef]
- Leigh, S.J.; Uhlig, F.; Wilmes, L.; Sanchez-Diaz, P.; Gheorghe, C.E.; Goodson, M.S.; Kelley-Loughnane, N.; Hyland, N.P.; Cryan, J.F.; Clarke, G. The impact of acute and chronic stress on gastrointestinal physiology and function: A microbiota–gut–brain axis perspective. J. Physiol. 2023, 601, 4491–4538. [Google Scholar] [CrossRef] [PubMed]
- Tafet, G.E.; Nemeroff, C.B. The links between stress and depression: Psychoneuroendocrinological, genetic, and environmental interactions. J. Neuropsychiatry Clin. Neurosci. 2015, 28, 77–88. [Google Scholar] [CrossRef] [PubMed]
- von Majewski, K.; Kraus, O.; Rhein, C.; Lieb, M.; Erim, Y.; Rohleder, N. Acute stress responses of autonomous nervous system, HPA axis, and inflammatory system in posttraumatic stress disorder. Transl. Psychiatry 2023, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Wulsin, A.C.; Wick-Carlson, D.; Packard, B.A.; Morano, R.; Herman, J.P. Adolescent chronic stress causes hypothalamo–pituitary–adrenocortical hypo-responsiveness and depression-like behavior in adult female rats. Psychoneuroendocrinology 2016, 65, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Magnavita, N.; Soave, P.M.; Antonelli, M. Prolonged stress causes depression in frontline workers facing the COVID-19 pandemic—A repeated cross-sectional study in a COVID-19 hub-hospital in central Italy. Int. J. Environ. Res. Public Health 2021, 18, 7316. [Google Scholar] [CrossRef]
- FEDIAF. Facts & Figures; The European Pet Food Industry: Bruxelles, Belgium, 2024. [Google Scholar]
- Custance, D.; Mayer, J. Empathic-like responding by domestic dogs (Canis familiaris) to distress in humans: An exploratory study. Anim. Cogn. 2012, 15, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Helsly, M.; Priymenko, N.; Girault, C.; Duranton, C.; Gaunet, F. Dog behaviours in veterinary consultations: Part II. The relationship between the behaviours of dogs and their owners. Vet. J. 2022, 281, 105789. [Google Scholar] [CrossRef]
- Grigg, E.K.; Chou, J.; Parker, E.; Gatesy-Davis, A.; Clarkson, S.T.; Hart, L.A. Stress-related behaviors in companion dogs exposed to common household noises, and owners ‘interpretations of their dogs’ behaviors. Front. Vet. Sci. 2021, 8, 760845. [Google Scholar] [CrossRef]
- Wilson, C.; Campbell, K.; Petzel, Z.; Reeve, C. Dogs can discriminate between human baseline and psychological stress condition odours. PLoS ONE 2022, 17, e0274143. [Google Scholar] [CrossRef]
- Malkani, R.; Paramasivam, S.; Wolfensohn, S.A. Multidimensional evaluation of the factors in the Animal Welfare Assessment Grid (AWAG) that are associated with, and predictive of, behaviour disorders in dogs. Animals 2024, 14, 528. [Google Scholar] [CrossRef]
- Nagasawa, M.; Mogi, K.; Kikusui, T. Attachment between humans and dogs. Jpn. Psychol. Res. 2009, 51, 209–221. [Google Scholar] [CrossRef]
- Hiai, S.; Yokoyama, H.; Oura, H.; Yano, S. Stimulation of pituitary-adrenocortical system by ginseng saponin. Endocrinol. Jpn. 1979, 26, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G. Effects of adaptogens on the central nervous system and the molecular mechanisms associated with their stress—Protective activity. Pharmaceuticals 2010, 3, 188–224. [Google Scholar] [CrossRef] [PubMed]
- Hovhannisyan, A.; Nylander, M.; Wikman, G.; Panossian, A. Efficacy of adaptogenic supplements on adapting to stress: A randomized, controlled trial. J. Athl. Enhanc. 2015, 4, 4. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Di Simone, S.C.; Acquaviva, A.; Nilofar, N.; Libero, M.N.; Brunetti, L.; Recinella, L.; Leone, S.; Orlando, G.; Zengin, G.; et al. Neuromodulatory effects induced by the association of Moringa oleifera Lam., Tribulus terrestris L., Rhodiola rosea Lam., and Undaria pinnatidifida extracts in the hypothalamus. Chem. Biodivers. 2024, 21, e202302075. [Google Scholar] [CrossRef] [PubMed]
- Lukoyanova, L.; Kriyachko, O.; Gaponova, V.; Anisimova, K.; Shafiev, A. Study of adaptogenic properties of the drug klim pet under stress of dogs in a Megalopolis. FASEB J. 2021, 35, 02469. [Google Scholar] [CrossRef]
- He, J.; Xu, L.; Yang, L.; Wang, X. Epigallocatechin gallate is the most effective catechin against antioxidant stress via hydrogen peroxide and radical scavenging activity. Med. Sci. Monit. 2018, 24, 8198–8206. [Google Scholar] [CrossRef]
- Bharani, K.K.; Devarasetti, A.K.; Carey, L.; Khurana, A.; Kollipaka, R.; Hanuman, D.D.V.; Chetla, V.S.; Banothu, A.K. Effects of ashwagandha (Withania somnifera) root extract on aging-related changes in healthy geriatric dogs: A randomized, double-blinded placebo-controlled study. Vet. Med. Sci. 2024, 10, e1556. [Google Scholar] [CrossRef]
- Dimpfel, W.; Schombert, L.; Keplinger-Dimpfel, I.K.; Panossian, A. Effects of an adaptogenic extract on electrical activity of the brain in elderly subjects with mild cognitive impairment: A randomized, double-blind, placebo-controlled, two-armed cross-over study. Pharmaceuticals 2020, 13, 45. [Google Scholar] [CrossRef]
- Oni, J.O.; Oyenekan, D.O.; Olayemi, Y.O.; Irabor, O.C.; Ogbuji, P.; Tova, M.O.; Adeleke, P.A.; Abbas, G.A.; Umukoro, S. Ginseng exhibits adaptogenic-like activity in mice exposed to hypoxic-anoxic stress through activation of antioxidant/BDNF protective mechanisms and inhibition of pro-inflammatory cytokines/NF-KB signaling pathways. Pharmacol. Res. Mod. Chin. Med. 2025, 14, 100578. [Google Scholar] [CrossRef]
- Panossian, A. Understanding adaptogenic activity: Specificity of the pharmacological action of adaptogens and other phytochemicals. Ann. N. Y. Acad. Sci. 2017, 1401, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Panax ginseng 人参 (Renshen, Ginseng). In Dietary Chinese Herbs; Liu, Y., Wang, Z., Zhang, J., Eds.; Springer: Vienna, Austria, 2015. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, Y.H.; Kim, Y.J. Bioactive Compounds and Biological Activities of Korean Ginseng (Panax ginseng Meyer). In Bioactive Compounds in the Storage Organs of Plants; Murthy, H.N., Paek, K.Y., Park, S.Y., Eds.; (Reference Series in Phytochemistry); Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Lee, S.H.; Jung, B.H.; Kim, S.Y.; Lee, E.H.; Chung, B.C. The antistress effect of ginseng total saponin and ginsenoside Rg3 and Rb1 evaluated by brain polyamine level under immobilization stress. Pharmacol. Res. 2006, 54, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, C.F.; Pei, G.; Guo, Y.Y.; Li, X. Antagonistic effect of pseudoginsenoside-F11 on the behavioral actions of morphine in mice. Pharmacol. Biochem. Behav. 2000, 66, 595–601. [Google Scholar] [CrossRef]
- Scopus. Available online: https://www.scopus.com/ (accessed on 26 March 2025).
- Liu, S.; Liu, F.; Wang, T.; Liu, J.; Hu, C.; Sun, L.; Wang, G. Polysaccharides extracted from Panax ginseng C.A. Mey enhance complement component 4 biosynthesis in human hepatocytes. Front. Pharmacol. 2021, 12, 734394. [Google Scholar] [CrossRef]
- Sun, L.; Wu, D.; Ning, X.; Yang, G.; Lin, Z.; Tian, M.; Zhou, Y. α-Amylase-assisted extraction of polysaccharides from Panax ginseng. Int. J. Biol. Macromol. 2015, 75, 152–157. [Google Scholar] [CrossRef]
- Lian, Y.; Zhu, M.; Yang, B. Characterization of a novel polysaccharide from red ginseng and its ameliorative effect on oxidative stress injury in myocardial ischemia. Chin. Med. 2022, 17, 111. [Google Scholar] [CrossRef]
- Kang, M.; Park, S.; Son, S.-R.; Noh, Y.; Jang, D.S.; Lee, S. Anti-aging and anti-inflammatory effects of compounds from fresh panax ginseng roots: A study on TNF-α/IFN-γ-induced skin cell damage. Molecules 2024, 29, 5479. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Xu, M.H.; Li, Y. Bioactive oligopeptides from ginseng (Panax ginseng Meyer) suppress oxidative stress-induced senescence in fibroblasts via NAD+/SIRT1/PGC-1α signaling pathway. Nutrients 2022, 14, 5289. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhao, N.; Yu, X.; Han, X.; Gao, H.; Zhang, X. Extensive characterization of peptides from Panax ginseng C. A. Meyer using mass spectrometric approach. Proteomics 2016, 16, 2788–2791. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, T.; Kim, S.W.; Hwang, S.Y.; Sohn, S.H.; Yoo, S.K.; Kim, S.K. Panax ginseng reduces oxidative stress and restores antioxidant capacity in aged rats. Nutr. Res. 2012, 32, 718–726. [Google Scholar] [CrossRef]
- Cho, K.M.; Lee, H.Y.; Cho, D.Y.; Jung, J.G.; Kim, M.J.; Jeong, J.B.; Jang, S.-N.; Lee, G.O.; Sim, H.-S.; Kang, M.J. Comprehensive comparison of chemical composition and antioxidant activity of Panax ginseng sprouts by different cultivation systems in a plant factory. Plants 2022, 11, 1818. [Google Scholar] [CrossRef]
- Shin, S.J.; Nam, Y.; Park, Y.P.; Kim, M.J.; Lee, E.; Jeon, S.G.; Bae, B.S.; Seo, J.; Shim, S.L.; Kim, J.S.; et al. Therapeutic effects of non-saponin fraction with rich polysaccharide from Korean red ginseng on aging and Alzheimer’s disease. Free Radic. Biol. Med. 2021, 164, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Bach, H.V.; Kim, J.; Myung, S.K.; Cho, Y.A. Efficacy of ginseng supplements on fatigue and physical performance: A meta-analysis. J. Korean Med. Sci. 2016, 31, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Flagg, A.J. Traditional and current use of ginseng. Nurs. Clin. N. Am. 2021, 56, 109–121. [Google Scholar] [CrossRef]
- Wu, M.; Li, K.; Wu, J.; Ding, X.; Ma, X.; Wang, W.; Xiao, W. Ginsenoside Rg1: A bioactive therapeutic agent for diverse liver diseases. Pharmacol. Res. 2025, 212, 107571. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.B.; Limroh, B.; White, R.P. Cardiovascular actions of panax ginseng in dogs. Jpn. J. Pharmacol. 1964, 14, 284–294. [Google Scholar] [CrossRef]
- Takagi, K.; Saito, H.; Nabata, H. Pharmacological studies of panax ginseng root: Estimation of pharmacological actions of Panax ginseng root. Jpn. J. Pharmacol. 1972, 22, 245–259. [Google Scholar] [CrossRef]
- Hielm-Björkman, A.; Reunanen, V.; Meri, P.; Tulamo, R.M. Panax ginseng in combination with brewers yeast (Gerivet®) as a stimulant for geriatric dogs: A controlled-randomized blinded study. J. Vet. Pharmacol. Ther. 2007, 30, 295–304. [Google Scholar] [CrossRef]
- Opuwari, C.S. Chapter 5.4.3—Herbal medicines (Eleutherococcus senticosus, Astragalus membranaceus) used to treat andrological problems: Asia and Indian Subcontinent. In Herbal Medicine in Andrology; Henkel, R., Agarwal, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 113–121. [Google Scholar] [CrossRef]
- Murray, M.T. Eleutherococcus senticosus (Siberian Ginseng). In Textbook of Natural Medicine, 5th ed.; Pizzorno, J.E., Murray, M.T., Eds.; Churchill Livingstone: London, UK, 2020; pp. 574–577. [Google Scholar] [CrossRef]
- Jin, L.; Schmiech, M.; El Gaafary, M.; Zhang, X.; Syrovets, T.; Simmet, T. A comparative study on root and bark extracts of Eleutherococcus senticosus and their effects on human macrophages. Phytomedicine 2020, 68, 153181. [Google Scholar] [CrossRef] [PubMed]
- Załuski, D.; Olech, M.; Galanty, A.; Verpoorte, R.; Kuźniewski, R.; Nowak, R.; Bogucka-Kocka, A. Phytochemical Content and Pharma-Nutrition Study on Eleutherococcus senticosus Fruits Intractum. Oxidative Med. Cell. Longev. 2016, 1, 9270691. [Google Scholar] [CrossRef]
- Ahmed, S.; Moni, D.A.; Sonawane, K.D.; Paek, K.Y.; Shohael, A.M. A comprehensive in silico exploration of pharmacological properties, bioactivities and COX-2 inhibitory potential of eleutheroside B from Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. J. Biomol. Struct. Dyn. 2020, 39, 6553–6566. [Google Scholar] [CrossRef]
- Graczyk, F.; Gębalski, J.; Sulejczak, D.; Małkowska, M.; Wójciak, M.; Gawenda-Kempczyńska, D.; Piskorska, E.; Krolik, K.; Markiewicz, M.; Kondrzycka-Dąda, A.; et al. UHPLC-DAD/ESI-TOF-MS phytochemical characterization and evaluation of the impact of Eleutherococcus senticosus fruit intractum on biochemical, hepatological, and blood parameters in balb/c mice. Int. J. Mol. Sci. 2024, 25, 9295. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.H.; Bang, S.I.; Shin, H.; Cho, E.J.; Lee, S. Antioxidant activity of edible sprouts and phytosterol contents by HPLC/UV analysis. Hortic. Environ. Biotechnol. 2022, 63, 769–778. [Google Scholar] [CrossRef]
- Graczyk, F.; Gębalski, J.; Makuch-Kocka, A.; Gawenda-Kempczyńska, D.; Ptaszyńska, A.A.; Grzyb, S.; Bogucka-Kocka, A.; Załuski, D. Phenolic profile, antioxidant, anti-enzymatic and cytotoxic activity of the fruits and roots of Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Molecules 2022, 27, 5579. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.E.; Yang, J.M.; Jeong, C.W.; Park, S.H.; Jo, H.D.; Cho, J.H. Assessment of the functional components and antioxidant activity of Eleutherococcus senticosus leaf extracts based on the cultivation regions and extraction solvents. J. Korean Soc. Food Sci. Nutr. 2025, 54, 228–245. [Google Scholar] [CrossRef]
- Yang, J.M.; Park, S.H.; Choi, Y.E.; Jeong, C.W.; Jo, H.D.; Cho, J.H. Study on changes in components and antioxidant activity of the stems of Eleutherococcus senticosus according to harvest time and region. J. Food Hyg. Saf. 2025, 40, 53–67. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Z.; Shenren, X.T.; Zhao, X.; Jiang, L.; Teng, L. Quantitative proteomics analysis for effect of Acanthopanax senticosus extract on neuroinflammation. Pak. J. Pharm. Sci. 2015, 28, 313–318. [Google Scholar] [PubMed]
- Huang, Y.H.; Ding, W.L.; Li, X.T.; Cai, M.T.; Li, H.L.; Yang, Z.Y.; Piao, X.H.; Zhu, S.; Tohda, C.; Komatsu, K.; et al. Memory enhancement effect of saponins from Eleutherococcus senticosus leaves and blood-brain barrier-permeated saponins profiling using a pseudo targeted monitoring strategy. Food Funct. 2022, 13, 3603–3620. [Google Scholar] [CrossRef]
- Liu, S.-M.; Li, X.-Z.; Zhang, S.-N.; Yang, Z.-M.; Wang, K.-X.; Lu, F.; Wang, C.-Z.; Yuan, C.-S. Acanthopanax senticosus protects structure and function of mesencephalic mitochondria in a mouse model of Parkinson’s disease. Chin. J. Integr. Med. 2018, 24, 835–843. [Google Scholar] [CrossRef]
- Tan, J.; Luo, J.; Meng, C.; Jiang, N.; Cao, J.; Zhao, J. Syringin exerts neuroprotective effects in a rat model of cerebral ischemia through the FOXO3a/NF-κB pathway. Int. Immunopharmacol. 2021, 90, 107268. [Google Scholar] [CrossRef] [PubMed]
- Sadykova, Y.R.; Rodimova, E.V.; Kornilova, E.A.; Pastukhova, L.A. Homeostasis-preserving paths in dogs of service breeds at the critical periods of postnatal ontogenesis. IOP Conf. Ser. Earth Environ. Sci. 2020, 548, 022045. [Google Scholar] [CrossRef]
- Skalski, B.; Kuźniak, E.; Kowalska, I.; Sikora, M.; Olas, B. A review of the biological activity and structure–property relationships of the main compounds from Schisandra chinensis. Nutrients 2025, 17, 436. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef]
- Jafernik, K.; Motyka, S.; Calina, D. Comprehensive review of dibenzocyclooctadiene lignans from the Schisandra genus: Anticancer potential, mechanistic insights and future prospects in oncology. Chin. Med. 2024, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Bernatoniene, J. Antioxidant effects of Schisandra chinensis fruits and their active constituents. Antioxidants 2021, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Zagórska-Dziok, M.; Wójciak, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Hoian, U.; Klimczak, K.; Szczepanek, D.; Sowa, I. Evaluation of the antioxidant, cytoprotective and antityrosinase effects of Schisandra chinensis extracts and their applicability in skin care product. Molecules 2022, 27, 8877. [Google Scholar] [CrossRef]
- Yan, T.; Shang, L.; Wang, M.; Zhang, C.; Zhao, X.; Bi, K.; Jia, Y. Lignans from Schisandra chinensis ameliorate cognition deficits and attenuate brain oxidative damage induced by D-galactose in rats. Metab. Brain Dis. 2016, 31, 653–661. [Google Scholar] [CrossRef] [PubMed]
- John, T.M. Chapter 21—Chronic Hepatitis. In Integrative Medicine, Fourth Edition; David, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 198–210. [Google Scholar] [CrossRef]
- Jang, M.; Choi, J.H.; Jang, D.S.; Cho, I.-H. Micrandilactone C, a nortriterpenoid isolated from roots of Schisandra chinensis, ameliorates Huntington’s disease by inhibiting microglial STAT3 pathways. Cells 2023, 12, 786. [Google Scholar] [CrossRef]
- Yang, B.-Y.; Han, W.; Han, H.; Liu, Y.; Guan, W.; Li, X.-M.; Kuang, H.-X. Effects of lignans from Schisandra chinensis rattan stems against Aβ1-42-induced memory impairment in rats and neurotoxicity in primary neuronal cells. Molecules 2018, 23, 870. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Mao, Q.; Zhang, X.; Wu, B.; Bi, K.; He, B.; Jia, Y. Schisandra chinensis protects against dopaminergic neuronal oxidative stress, neuroinflammation and apoptosis via the BDNF/Nrf2/NF-κB pathway in 6-OHDA-induced Parkinson’s disease mice. Food Funct. 2021, 12, 4079–4091. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Zakłos-Szyda, M.; Błasiak, J.; Nowak, A.; Zhang, Z.; Zhang, B. Potential of Schisandra chinensis (Turcz.) Baill. in human health and nutrition: A review of current knowledge and therapeutic perspectives. Nutrients 2019, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Myrzagaliyeva, A.; Irsaliyev, S.; Tustubayeva, S.; Samarkhanov, T.; Orazov, A.; Alemseitova, Z. Natural resources of Rhaponticum carthamoides in the Tarbagatai State National Nature Park. Diversity 2024, 16, 676. [Google Scholar] [CrossRef]
- Skała, E.; Grąbkowska, R.; Sitarek, P.; Kuźma, Ł.; Błauż, A.; Wysokińska, H. Rhaponticum carthamoides regeneration through direct and indirect organogenesis, molecular profiles and secondary metabolite production. Plant Cell Tiss. Organ. Cult. 2015, 123, 83–98. [Google Scholar] [CrossRef]
- Roumanille, R.; Vernus, B.; Brioche, T.; Descossy, V.; Van Ba, C.T.; Campredon, S.; Philippe, A.G.; Delobel, P.; Bertrand-Gaday, C.; Chopard, A.; et al. Acute and chronic effects of Rhaponticum carthamoides and Rhodiola rosea extracts supplementation coupled to resistance exercise on muscle protein synthesis and mechanical power in rats. J. Int. Soc. Sports Nutr. 2020, 17, 58. [Google Scholar] [CrossRef]
- Todorova, V.; Todorova, M.N.; Savova, M.S.; Ivanov, K.; Georgiev, M.I.; Ivanova, S. Maral root extract and its main constituent 20-hydroxyecdysone enhance stress resilience in Caenorhabditis elegans. Int. J. Mol. Sci. 2025, 26, 3739. [Google Scholar] [CrossRef]
- Koleckar, V.; Opletal, L.; Brojerova, E.; Rehakova, Z.; Cervenka, F.; Kubikova, K.; Kuca, K.; Jun, D.; Polasek, M.; Kunes, J.; et al. Evaluation of natural antioxidants of Leuzea carthamoides as a result of a screening study of 88 plant extracts from the European Asteraceae and Cichoriaceae. J. Enzyme Inhib. Med. Chem. 2008, 23, 218–224. [Google Scholar] [CrossRef]
- Kosović, E.; Lino, K.; Kuchař, M. HPLC-MS methodology for R. carthamoides extract quality evaluation: A simultaneous determination of eight bioactive compounds. Diversity 2022, 14, 880. [Google Scholar] [CrossRef]
- Kokoska, L.; Janovska, D.; Rada, V.; Nepovim, A.; Vanek, T. In vitro antibacterial activity of four Leuzea species. Pharm. Biol. 2005, 43, 8–11. [Google Scholar] [CrossRef]
- Todorova, V.; Ivanov, K.; Ivanova, S. Comparison between the biological active compounds in plants with adaptogenic properties (Rhaponticum carthamoides, Lepidium meyenii, Eleutherococcus senticosus and Panax ginseng). Plants 2022, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Nosal, R.; Perečko, T.; Jančinová, V. Naturally appearing N-feruloylserotonin isomers suppress oxidative burst of human neutrophils at the protein kinase C level. Pharmacol. Rep. 2011, 63, 790–798. [Google Scholar] [CrossRef]
- Dinan, L.; Dioh, W.; Veillet, S.; Lafont, R. 20-hydroxyecdysone, from plant extracts to clinical use: Therapeutic potential for the treatment of neuromuscular, cardio-metabolic and respiratory diseases. Biomedicines 2021, 9, 492. [Google Scholar] [CrossRef] [PubMed]
- Todorov, I.N.; Mitrokhin, Y.I.; Efremova, O.I. Effect of extract from Rhaponticum carthamoides on RNA and protein biosynthesis in mice. Pharm. Chem. J. 2000, 34, 479–481. [Google Scholar] [CrossRef]
- Erst, A.A.; Petruk, A.A.; Zibareva, L.N. Morphological, histochemical and biochemical features of cultivated Rhodiola rosea (Altai Mountains Ecotype). Contemp. Probl. Ecol. 2021, 14, 701–710. [Google Scholar] [CrossRef]
- Węglarz, Z.; Przybył, J.; Geszprych, A. Roseroot (Rhodiola rosea L.): Effect of Internal and External Factors on Accumulation of Biologically Active Compounds. In Bioactive Molecules and Medicinal Plants; Ramawat, K., Merillon, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Guo, N.; Zhu, M.; Han, X.; Sui, D.; Wang, Y.; Yang, Q. The metabolism of salidroside to its aglycone p-tyrosol in rats following the administration of salidroside. PLoS ONE 2014, 9, e103648. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, W.; Kang, X.; Yang, R.; Li, R.; Huang, L.; Chen, J.; Yang, Q.; Sun, X. Salidroside protects dopaminergic neurons by regulating the mitochondrial MEF2D-ND6 pathway in the MPTP/MPP(+) -induced model of Parkinson’s disease. J. Neurochem. 2020, 153, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.; Kumar, P.; Kaur, E.; Sood, A.; Acharya, V.; Warghat, A.R. Comparative transcriptome and tissue-specific expression analysis of genes reveal tissue-cultured plants as an alternative source for phenylethanoids and phenylpropanoids in Rhodiola imbricata (Edgew.). Gene 2022, 836, 146672. [Google Scholar] [CrossRef]
- Guo, L.; Cai, C.; Zhang, F.; Ma, R.; Jenis, J. Quantitative analysis of phenylpropanoids in Rhodiola rosea from different producing areas. J. Food Bioact. 2023, 23, 68–73. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Nazhand, A. Rhodiola rosea: Main features and its beneficial properties. Rend. Fis. Acc. Lincei 2022, 33, 71–82. [Google Scholar] [CrossRef]
- Zhumagul, M.; Kurmanbayeva, M.; Kubentayev, S.; Kurmantayeva, A.; Turgumbayeva, A.; Nurpeissova, I.; Cherepkova, N.; Moldakaryzova, A. Studies on the biological activity of different populations of the medicinal plant Rhodiola rosea L. (golden root). Pak. J. Bot. 2023, 55, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Perfumi, M.; Mattioli, L. Adaptogenic and central nervous system effects of single doses of 3% rosavin and 1% salidroside Rhodiola rosea L. extract in mice. Phytother. Res. 2007, 21, 37–43. [Google Scholar] [CrossRef]
- Darbinyan, V.; Aslanyan, G.; Amroyan, E.; Gabrielyan, E.; Malmström, C.; Panossian, A. Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderate depression. Nord. J. Psychiatry 2007, 61, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.J.; Xie, S.X.; Zee, J.; Soeller, I.; Li, Q.S.; Rockwell, K.; Amsterdam, J.D. Rhodiola rosea versus sertraline for major depressive disorder: A randomized placebo-controlled trial. Phytomedicine 2015, 22, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Sanobar, S.; Ahuja, D. Effect of Rhodiola rosea root on spatial learning memory, brain antioxidant enzymes activity against Morris water maze model in mice. Nat. Vol. Essent. Oils 2021, 8, 15840–15851. [Google Scholar]
- Kim, K.J.; Jung, Y.S.; You, D.M.; Lee, S.H.; Lee, G.; Kwon, K.B.; Kim, D.O. Neuroprotective effects of ethanolic extract from dry Rhodiola rosea L. rhizomes. Food Sci. Biotechnol. 2021, 30, 287–297. [Google Scholar] [CrossRef]
- van Diermen, D.; Marston, A.; Bravo, J.; Reist, M.; Carrupt, P.A.; Hostettmann, K. Monoamine oxidase inhibition by Rhodiola rosea L. roots. J. Ethnopharmacol. 2009, 122, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, G.; Wang, D.; Yan, Y.; Yang, Q. Effects of nano-Rhodiola rosea combined with treadmill exercise on anti-exercise fatigue in rats. Front. Nutr. 2024, 11, 1446944. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Hu, X.; Chu, X.; Li, X.; Han, F. Neuroprotective effects of a Rhodiola crenulata extract on amyloid-β peptides (Aβ1-42) -induced cognitive deficits in rat models of Alzheimer’s disease. Phytomedicine 2019, 57, 331–338. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kępińska-Pacelik, J.; Biel, W. Herbal Support for the Nervous System: The Impact of Adaptogens in Humans and Dogs. Appl. Sci. 2025, 15, 5402. https://doi.org/10.3390/app15105402
Kępińska-Pacelik J, Biel W. Herbal Support for the Nervous System: The Impact of Adaptogens in Humans and Dogs. Applied Sciences. 2025; 15(10):5402. https://doi.org/10.3390/app15105402
Chicago/Turabian StyleKępińska-Pacelik, Jagoda, and Wioletta Biel. 2025. "Herbal Support for the Nervous System: The Impact of Adaptogens in Humans and Dogs" Applied Sciences 15, no. 10: 5402. https://doi.org/10.3390/app15105402
APA StyleKępińska-Pacelik, J., & Biel, W. (2025). Herbal Support for the Nervous System: The Impact of Adaptogens in Humans and Dogs. Applied Sciences, 15(10), 5402. https://doi.org/10.3390/app15105402