Abstract
Rice is a vital component of the Ecuadorian diet and plays a significant role in global food security. Agricultural practices aimed at boosting production can, however, compromise grain quality. This study explores the effects of various pest control methods on the nutritional and biochemical quality of both white and brown rice. Compounds such as total phenolics (TPC), dietary fiber (TDF), gamma oryzanol, phytic acid (PA), antioxidant activity (AA), and the presence of heavy metals were analyzed. The research was carried out in Taura, Guayas Province, Ecuador, using a bifactorial experimental design with three replications. Statistical analysis included ANOVA and Tukey HDS tests (α = 0.05), complemented by PCA biplot analysis for comprehensive data exploration. The results highlight significant differences in all variables depending on the rice type (R), with brown rice exhibiting higher values. In terms of pest control type (C), only phytic acid showed significant variance, an effect also evident in the interaction (R × C). Cadmium (<0.30 mg/kg) and arsenic (<0.55 mg/kg) levels remained below national standards, with no significant differences across treatments. The biplot analysis revealed PA independence, with higher values in grains managed with biological control, whereas those under chemical control displayed slightly higher and varied values for other studied variables.
1. Introduction
Rice (Oryza sativa) is a crop of significant economic and dietary importance, essential for over half of the global population [1]. Predominantly cultivated in Asia and Africa, these two continents account for approximately 90% of its total production. Rice is an important source of vitamins B6 and C, carbohydrates, and other essential nutrients [2]. In Ecuador, over 340 hectares are dedicated to this crop, showing an annual production growth of 12.55% [3]. This expansion underscores the importance of researching the biological properties of rice in different geographic environments, especially considering that the composition of bioactive compounds varies significantly depending on factors such as locality, genotype, developmental stage, harvesting practices, applied treatments, and storage conditions [4,5].
The World Health Organization has reported that 200,000 people die annually worldwide due to the direct impact of pesticides. In addition, a large amount of synthetic pesticides persists in the environment, contaminating soil and water bodies and depleting the ozone layer [6]. These problems are exacerbated by climate change, whose extreme conditions have led to a significant proliferation of herbivorous insects, causing serious damage to the yield and quality of agricultural products. On the other hand, the growing concern for food security and the continuous improvements in living conditions have increased the demand and production of organic and ecological products, driving the need to develop adequate and sustainable plant protection [7,8].
Over time, plants have evolved a variety of complex defense mechanisms that enable them to resist pests and diseases [9]. However, the invasion of insect pests is inherent in the production of monocultures such as rice, especially over large areas of cultivation. To address this, farmers often resort to insecticides for the preventive and curative management of pests, which can lead to unnecessary problems [10]. In this context, integrated pest management (IPM) plays a crucial role in effectively combating pests, combining biological, cultural, physical, and chemical techniques to keep pest levels at an economically acceptable threshold [11]. The formalization of IPM offers significant benefits, including reduced damage to plant aerial systems, a more favorable economic response with higher yields, and the promotion of environmental sustainability. Nonetheless, poor agricultural practices can lead to pesticide resistance, trigger secondary pest outbreaks, and cause collateral environmental damage, such as water pollution, reduced biodiversity, and alterations in the nutritional properties of food [10,11,12].
In the management of pests in the agricultural sector, various control methods are employed, categorized by their specific techniques, such as the use of chemical pesticides, biological agents, cultural practices, application of physical barriers, or the use of hormonal traps that attract or repel pests [13]. However, most agricultural practices in Latin America utilize control methods applied before planting and during the crop cycle, which generally involve chemical and biological control methods [14,15]. Chemical control offers benefits such as rapid action, a broad spectrum of effectiveness against various types of pests, and an increase in crop yields, thereby contributing to food security [14,16]. In contrast, biological control effectively addresses the complexities of agricultural production systems, highlighting its positive impact on plant growth and protection, not only against pests but also synergistically against pathogens, through the production of natural chemicals, biofilm formation, and the production of hydrolytic enzymes [9,17].
Rice cultivation is influenced by various factors, including climatic conditions such as CO2 emissions, high temperatures, and precipitation [18], as well as non-climatic factors, which include production factors, cultivation area, labor force, type of fertilization, and the general use of agrochemicals [2,16,19]. Among these non-climatic factors, agricultural practices such as pest control emerge as underlying influences on the physiology and characteristics of rice plants. The implementation of pest management strategies, such as chemical and biological control, not only affects the health, growth, and yield of rice but can also alter its chemical and nutritional properties [20,21,22]. For example, the excessive use of chemical pesticides leads to the accumulation of residues in rice grains, affecting quality and safety for consumption [23,24]. Conversely, biological methods, with their apparent positive effect, natural environmental characteristics, and reduced toxicity, can enhance the accumulation of nutrients and bioactive compounds [17,25]. These responses from rice plants highlight the importance of selecting the best pest management method to protect, preserve, and enhance the nutritional quality of rice.
Studies on the biological properties of rice often focus on analyzing variations between groups using one or two input factors such as moisture presence, germination, fermentation, or heat exposure [26,27,28]. This study employs PCA biplot techniques and analysis of variance to evaluate the chemical and biological properties of whole and polished rice grains, observing their response under chemical and biological pest control methods. The primary objective is to explore how these pest management practices affect the nutritional and biochemical characteristics of rice, including levels of phenolic compounds, dietary fiber, gamma oryzanol, phytic acid, antioxidant activity, and the accumulation of heavy metals. Figure 1 provides a comprehensive summary of the study.
Figure 1.
Graphical summary.
2. Materials and Methods
2.1. Raw Materials
Grains of brown rice and white rice obtained from the same brown rice from different experimental plots located in the “Asociación de Productores de Arroz del Río Culebra” were used. This association is situated in the Taura–Guayas region, Ecuador, at an altitude of 72 m, with an average annual temperature ranging from 24 to 28 °C and an annual relative humidity of 85%.
2.2. Experimental Design
To ensure the reliability of the results, an experimental design was implemented in triplicate for two types of rice treated by biological and chemical methods for pest control, respectively; in total, four groups of samples were obtained, distributed in two plots.
- WRBC: White Rice—Biological Control.
- WRCC: White Rice—Chemical Control.
- BRBC: Brown Rice—Biological Control.
- BRCC: Brown Rice—Chemical Control.
Each sample underwent quantification of bioactive compounds: phenolic compounds, dietary fiber, γ-oryzanol, phytic acid, antioxidant activity, and heavy metals (cadmium and arsenic).
The chemical method consisted of the application of the insecticide Lorsban, whose active ingredient is chlorpyrifos, an organophosphate compound widely used for pest control in rice crops. This product was selected because of its proven efficacy in the management of relevant pests in the agricultural context of rice. In addition, Diazinon was applied at doses of 0.50–0.75 L ha−1, only when the estimated economic damage exceeded 30%, following technical recommendations to maximize control effectiveness and minimize environmental impacts.
The biological method used the endophytic fungus Beauveria bassiana, recognized for its ability to significantly reduce the attack of Spodoptera frugiperda, one of the main rice pests. This fungus, by establishing itself asymptomatically within healthy plant tissue, strengthens the plant’s natural defenses and offers sustainable and environmentally responsible control.
2.3. Sample Preparation
The rice samples were frozen at −21 °C for 24 h, then dehydrated in a lyophilizer (LABCONCO FreeZone 6, Kansas City, MO, USA) at vacuum pressures of 0.01 Mbar/−85 °C for 3 days. The samples were then ground using a mill and finally sieved through a No. 100 mesh.
2.4. Total Phenolic Compounds (TPC)
A sample of 0.5 g of sieved rice was extracted in 10 mL of an 80% (v/v) methanol–HCl solution (1000/1) in distilled water, using continuous magnetic stirring at room temperature for 16 h. The extracts were centrifuged at 5000 rpm at 5 °C for 5 min, as described by [29,30] Cáceres.
The determination of total phenolic compounds (TPC) in rice samples was conducted using the Folin–Ciocalteu phenolic reagent, as described by Singleton et al. in 1999 and previously modified by Torino et al. in 2013 [29]. Briefly, a 100 µL aliquot of each extract was mixed with 625 µL of ultrapure water, 250 µL of 7.5% (w/v) Na2CO3, and 25 µL of Folin reagent in 1.5 mL Eppendorf tubes for each group. The tubes were vortexed for 8 s and incubated for 2 h at room temperature in the dark. The TPC was quantified using a calibration curve with gallic acid as the standard at various concentrations (4.5, 9, 22.5, 45, 67.5, 90, and 112.5 µg/mL). Finally, absorbance was measured at 739 nm using a spectrophotometer (BioTek Instruments, Winooski, VT, USA), and results were expressed as milligrams of gallic acid equivalent (GAE) per gram of extract (mg GAE/g).
2.5. Antioxidant Activity (AA)
The antioxidant activity was evaluated using the oxygen radical absorbance capacity (ORAC-FL) method, following the procedure described by Torino et al. [29] and previously modified by Cáceres et al. in 2014 [30]. The assays were conducted at 37 °C using 75 mM sodium phosphate buffer (pH 7.4) over 165 min under dark conditions. Serial dilutions of the samples were prepared in ratios of 1:10 and 1:20. Mixtures containing 100 µL of the sample or Trolox standard at different concentrations (0, 8, 24, 48, 60, 100, 120, and 160 µM) and 600 µL of fluorescein solution were placed in Eppendorf tubes. Absorbance readings were taken at 485 nm and 538 nm using a spectrophotometer (BioTek Instruments), and the results were expressed as milligrams of Trolox equivalent (TE) per 100 g of sample.
2.6. Gamma-Oryzanol (γ-Oryzanol)
The extraction of γ-oryzanol was performed according to the methodology described by Srisaipet and Nuddagul in 2014 [31], with certain modifications. In a test tube, 1 g of the prepared sample was added along with 4 mL of solvent (absolute isopropanol), and the mixture was subjected to orbital shaking overnight at 200 rpm. Subsequently, the mixture was centrifuged at 3000 rpm for 5 min, and the supernatant containing γ-oryzanol was collected. A standard curve (0–50 mg/L) was prepared from a γ-oryzanol solution of 0.1 mg/mL using absolute isopropanol, and absorbance readings were taken with a spectrophotometer (BioTek Instruments).
2.7. Phytic Acid (PA)
The photometric determination was conducted following the methodology described by Reichwald and Hatzack in 2008 [32]. A volume of 400 µL of the sample (extracted with 1M HCl), standard (phytic acid solution in 0.2M HCl), and blank (0.2M HCl) was used. Each compound was mixed with 800 µL of ferric solution (0.05 g of FeCl3 in 500 mL of 0.2M HCl). The mixtures were then heated at 90 °C for 45 min at 10× g-force. The samples were cooled in an ice bath for 15 min to allow the formation of iron phytate precipitate and centrifuged at 10× g for 5 min at room temperature. Aliquots of 600 µL of supernatants were transferred to 800 µL of complexing reagent (0.5 g of 2,2′-bipyridine and 65 µL of thioglycolic acid in 50 mL of 0.2 M HCl), and the absorbance was measured at 540 nm.
2.8. Total Dietary Fiber (TDF)
The analysis of soluble, insoluble, and total fiber was conducted following the Megazyme© Total Dietary Fiber procedure, based on AOAC 991.43 (AOAC Official Method 991.43; Total, Soluble, and Insoluble Dietary Fiber in Foods. AOAC INTERNATIONAL: Rockville, MD, USA, 1994.) “Total, Soluble, and Insoluble Dietary Fibre in Foods” (First Action 1991) and AACC 32-07 (AACC Method 32-07.01; Determination of Soluble, Insoluble, and Total Dietary Fiber in Foods and Food Products. Cereals & Grains Association: St. Paul, MN, USA, 1991.) “Determination of Soluble, Insoluble, and Total Dietary Fiber in Foods and Food Products” (Final Approval 10-16-91).
where
- R1 = Weight of residue 1 from m1;
- R2 = Weight of residue 2 from m2;
- m1 = Weight of sample 1;
- m2 = Weight of sample 2;
- A = Weight of ash from R1;
- p = Protein weight from R2.
- BR = Blank residue;
- BA = Blank ash from BR2;
- BP = Blank protein from BR1.
2.9. Heavy Metals
The concentration levels of cadmium and arsenic in the analyzed samples were determined using the AOAC 2006.03 (AOAC Official Method 2006.03; Arsenic, Cadmium, Cobalt, Chromium, Lead, Molybdenum, Nickel, and Selenium in Fertilizers. AOAC INTERNATIONAL: Rockville, MD, USA, 2006.) methodology for cadmium and AOAC 986.15 (AOAC Official Method 986.15; Mercury in Fish. AOAC INTERNATIONAL: Rockville, MD, USA, 1990.) for arsenic (atomic absorption).
2.10. Statistical Analysis
The primary biological characteristics of polished and whole rice grains, such as total phenolic compounds, dietary fiber, γ-oryzanol, phytic acid, antioxidant activity, and heavy metals (cadmium and arsenic), were measured in triplicate. The results of all analyses were examined using two-factor analysis of variance (ANOVA) at a significance level of p < 0.05 to determine significant differences between quantified parameters based on grain group and treatment. Tukey’s multiple range test was applied with a 95% confidence level (α = 0.05). Additionally, the data were subjected to multivariate statistical analysis using PCA biplot techniques with R software version 4.3.2, excluding the heavy metals data.
PCA Biplot Analysis
The PCA biplot enables the standardization of a dataset to extract the main patterns of potential groups and investigate the correlations between objective indicators. The PCA biplot reduces the data volume and displays the relative position of each indicator within a quadrant of the plane axes. The PCA biplot method approximates the matrix through suitable factorization, resulting in the following expression [33,34]:
where
- is the original data matrix that we want to reduce in dimensionality.
- represents the singular value decomposition (SVD) of the matrix X.
- is the eigenvector matrix (or principal components) of the rows of X.
- is a diagonal matrix with the singular values.
- is the eigenvector matrix of the columns of X.
These are full-rank matrices, defined as follows:
depending on the selected value for p (0 ≤ p ≤ 1), where
- is a submatrix of U, containing only the first q eigenvectors.
- is a weighted version of the diagonal matrix of singular values , adjusted by a parameter p. This parameter p allows control over how the singular values in the matrix are weighted.
- = is a submatrix of V, which also contains the first q eigenvectors.
- is the singular value matrix adjusted by the complementary parameter 1 − p. This is used to balance the weighting of the singular values in the matrix
Parameter p
The parameter p (where 0 ≤ p ≤ 1) provides flexibility in how the approximation is performed:
When p = 0, the matrix is unweighted, while retains all the weighting of
When p = 1, the weighting is reversed, giving more importance to .
3. Results and Discussion
This research aimed to identify the biological characteristics in which samples of brown and white rice are grouped when they originate from two different types of pest control treatments.
Chemical control: This method employs specific synthetic pesticides that act directly on pests to eliminate them or inhibit their growth. Although effective for crop protection, the use of pesticides can influence the biochemical composition of rice, affecting the concentration of antioxidant compounds and other nutrients due to chemical exposure.
Biological control: In this method, natural organisms such as bacteria, fungi, or beneficial insects are used to reduce pest populations. This type of control is less invasive to the crop and the environment and is considered to have a less disruptive impact on the bioactive compounds and nutrients in rice, which could lead to more stable levels of antioxidants and other nutritional properties.
3.1. Analysis of the Chemical and Biological Characteristics of the Grains
A two-factor analysis of variance (ANOVA) evaluated the nutritional differences between rice types cultivated in plots subjected to different treatments. Additionally, the Tukey HSD test (α = 0.05) was used to analyze and compare these sources of variation, presenting the mean values of triplicate trials for total phenolic compounds, dietary fiber, γ-oryzanol, phytic acid, and antioxidant activity (Table 1).

Table 1.
Evaluation of the nutritional value of two groups of rice treated by chemical and biological methods.
The results demonstrate that among the treatments differentiating the study groups, the type of rice (white and brown) shows a highly significant response variation across all variables studied. Additionally, the high values in the sum of squares suggest that this factor can explain a large portion of the data variability (between 80 and 95%). Similarly, the low residual values indicate that factors such as rice type (R), control (C), and their interaction (R × C) are sufficient to explain the total data variability. Furthermore, among all the nutritional characteristics of the rice types by treatment, the analysis found that phytic acid levels were the only ones that changed concerning the type of control. This is evidenced both by the independent effect of the factors R and C and by their interactive effect (R × C) on the variable studied.
The correlation matrix presented in Table 2 shows that all the study variables are highly correlated, with Pearson coefficients greater than 0.8 in all cases, as well as their levels of significance. Notably, total phenolic compounds (TPC) showed highly significant correlations with almost all variables, particularly highlighting their strong association with antioxidant activity (AA). These results are consistent with those obtained by Tuncel and Yilmaz in 2011 [26], who found a high relationship between these two characteristics, emphasizing that total phenolic levels largely depend on the extraction method, which is limited when classical solvents are used.

Table 2.
Correlation matrix.
Another highly significant correlation is evident in the levels of gamma-oryzanol. Overall, the study variables demonstrate a linear dependence on one another. However, the presented results are specific to the context of evaluating the biochemical differences in white and brown rice cultivated under chemical and biological control methods, making them uniquely intrinsic to these conditions. Characteristics such as TPC or AA explicitly depend on factors like the type of cultivar, the degree of germination, the presence of spontaneous fermentation [4], moisture percentage, and temperature exposure before and during the assay [29,30].
The disparity in the values of all variables between white and brown rice groups can be attributed to various causes. However, the most influential factor is the distinct structure of the grains. Unlike white rice, brown rice retains its embryo and bran (representing 8 to 10% of the total weight) [35]. The bran layer is an external structure of the grain, rich in cellulose and capable of storing different organic compounds, thus providing higher nutritional properties in certain aspects [23,26,36]. Therefore, the presence of bran increases certain characteristics, such as total phenolic compounds by up to 2.5 times compared to white rice samples, as well as antioxidant activity [26]. Studies by Saleh et al. in 2019, Srisaipet and Nuddagul, and Biswas et al. in 2011 [23,31,37] attribute this difference to the same cause, demonstrating that γ-oryzanol, dietary fiber, and phytic acid are dependent or correlated, thus their levels also increase in brown rice samples.
3.2. Heavy Metals
Table 3 shows the concentration of heavy metals, especially cadmium (Cd) and arsenic (As), according to the type of sample and treatment. The results indicate that Cd concentrations in all samples analyzed, including brown and white rice grains from chemically and biologically controlled plots, remained below 0.30 mg/kg; similarly, arsenic concentrations in all samples were also found to be below 0.55 mg/kg. This demonstrates that both cadmium and arsenic do not exceed the maximum limits recommended for human consumption and that, in the context of our study, there were no significant differences between the two sources of variation.

Table 3.
Heavy metals.
Rice, like other crops, can absorb heavy metals such as cadmium, lead, arsenic, and mercury from the soil and irrigation water, with levels varying based on location, planting conditions, and processing methods [5]. Brown rice typically contains higher levels of contaminants compared to white rice due to the outer layers of the grain, which retain most of the heavy metals and are removed during processing [23,38]. However, the contaminant load significantly depends on environmental and anthropogenic factors, such as the pollution of cultivation areas and the techniques used for rice processing and storage [5,39].
3.3. PCA Analysis
To better visualize the biological characteristics of the rice grains, a PCA biplot is presented along with the explored classification of the data using the Euclidean distance method (Figure 2). The PCA shows the formation of four groups using the variability of five biochemical characteristics of the samples: total phenolic compounds (TPC), antioxidant activity (AA), γ-oryzanol, phytic acid (PA), and total dietary fiber (TDF). The total accumulated inertia is 97.8%, indicating that the first two dimensions are highly significant in explaining the majority of the data variability and precisely evaluating the most important sample locations in the factorial plane.
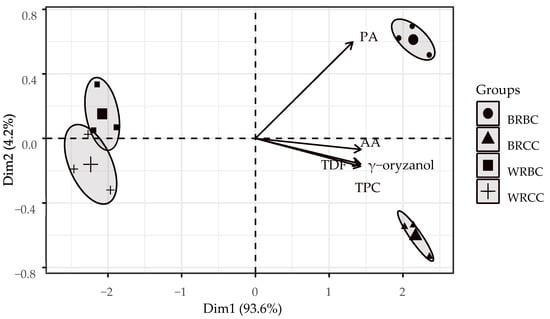
Figure 2.
Principal component analysis.
The replicates forming the groups of white rice with biological control (WRBC) and chemical control (WRCC) are the furthest from the represented variables, suggesting that they share similarly low values across the factorial plane. However, they are slightly distant from each other, indicating that they are influenced by the nature of the pest control treatment. On the right side of the plane are the groups of brown rice with biological control (BRBC) and chemical control (BRCC), with the highest values in the entire data distribution, particularly highlighting the levels of PA for the BRBC group.
Although whole rice samples exhibit the highest levels of phytic acid, it is observed that within the same type of rice, samples from grains grown under biological control show higher values. However, for the rest of the nutritional and biochemical properties, chemical control displays slightly higher values (this is also reflected in the Tukey test of Table 1).
Biological control elevates PA levels due to the properties of phytic acid as a natural antioxidant and its role as an intermediate in inositol phosphate metabolism, with derivatives involved in various signal transduction pathways, including the biological stress response, as reported by Pramitha et al. in 2021 [36]. In addition, the use of biological agents for pest control triggers a basal hypersensitivity response in rice plants, activating genes in the penetration zones that result in the encoding of specialized enzymes [12], increasing the phytic acid content in rice grains, as it depends on the level of expression of genes associated with limiting enzymes [22].
Figure 1 also shows that the variables TPC, γ-oryzanol, and TDF are grouped together, indicating correlations between these variables, i.e., the increase in the values of these variables follows a similar proportion, as shown in the correlation matrix (Table 2). However, reducing the dimensionality of the data in a biplot shows a different pattern, where a slight independence in the behavior of phytic acid levels is indicated for brown rice samples coming from plots that employed a biological control; this is also reflected with white rice samples. This is due to the specific and complex role of rice biochemistry involving factors such as control, type, species, or source environment, making some components, such as phytic acid, not necessarily match the metabolic pathway of other components [25,27].
Finally, the AA levels show a slight separation from the classification group and are located closer to the horizontal plane, indicating a stronger relationship with the first component. In addition, the medium vector length could indicate a lower variability with respect to the other characteristics; generally, antioxidant activity levels are high [4], with slight but significant differences for rice samples, white, brown, or pure bran. This due to the presence of tocopherols and tocotrienols; moreover, especially the antioxidant activity varies depending on the extraction method, the content of bioactive compounds, whether they are bound or free, and the pre-treatments used to recover the test extracts [4,23].
3.4. Evaluation of Key Rice Traits and Their Relationship to Pest Control Methods
The characteristics selected for this study stand out for their importance in the evaluation of nutritional quality, safety, and response of rice (Oryza sativa) to different pest control methods. The criteria that support the choice of each one are detailed below:
- Phenolic compounds: Chosen for their role in the plant’s defense against stress caused by pests and for their antioxidant capacity. In addition, they are relevant indicators of the nutritional quality of rice in agricultural contexts [29].
- Dietary fiber: Analysis of dietary fiber allows the identification of possible effects of control methods on the structure and composition of this macronutrient essential for digestive health [30].
- Gamma oryzanol: Recognized for its antioxidant properties and potential health benefits, such as cholesterol reduction, this compound is sensitive to agricultural practices and environmental conditions [31].
- Phytic acid: Important for its function as phosphorus storage and its role as an anti-nutrient, its concentration varies according to the treatments used [29].
- Dietary fiber: Its analysis allows the identification of possible effects of control methods on the structure and composition of this macronutrient essential for digestive health [30].
- Gamma oryzanol: Recognized for its antioxidant properties and potential health benefits, such as cholesterol reduction, this compound is sensitive to agricultural practices and environmental conditions [31].
- Phytic acid: Important for its function as phosphorus storage and its role as an anti-nutrient, its concentration varies according to the treatments used [29].
- Antioxidant activity: Indicator of how control methods affect the ability of rice to counteract oxidative damage, an essential factor in the preservation of its nutritional quality [29].
- Accumulation of heavy metals: This aspect is closely linked to food safety, since certain agricultural strategies can influence the concentration of heavy metals in the grain, with direct implications for human health [23,38].
3.5. Metabolic Changes Associated with Pest Control Methods
The impact of pest control methods on phenolic content, phytic acid, and antioxidant activity of rice reflects the interactions of these strategies with plant metabolic and physiological processes.
- Phenolic compounds: Biological control promotes phenol synthesis by activating the shikimic acid pathway, strengthening plant defenses and antioxidant capacity. In contrast, chemical methods tend to inhibit these responses by reducing the natural stimuli that activate these metabolic pathways [29].
- Phytic acid: Biological treatments enhance the interaction with soil microorganisms, optimizing nutrient uptake and favoring the accumulation of this compound. On the other hand, chemical pesticides can alter its synthesis by interfering with enzymatic processes related to phosphorus metabolism [32].
- Antioxidant activity: Biological controls stimulate systemic acquired defense responses (SARs), which enhances the presence of antioxidant compounds such as phenols and flavonoids. However, chemical control may limit these responses by reducing plant interaction with environmental factors that activate these natural mechanisms [29].
4. Limitations and Future Perspectives
- Limitations:
- The sample size and number of field sites may be somewhat limited; however, the study employs a robust bifactorial design with replications to ensure reliability.
- The focus of the study does not include nutritional markers, such as vitamins (B and E), essential minerals (iron and zinc), and amino acid profiles; however, it prioritized key bioactive compounds, such as total phenolic compounds and gamma-oryzanol.
- The analyses focused on plots located in one province of Ecuador, providing a valuable local perspective.
- Future Perspectives:
- The sample and field sites should be expanded, incorporating different growing areas and environmental conditions. This would allow analysis of variability associated with external factors and improve the representativeness of the findings.
- Analysis of vitamins, minerals, and amino acid profiles should be included in future studies to provide a more complete assessment of the impact of pest control methods on the functional and nutritional quality of rice.
- The geographical scope of the study should be expanded to rice cropping systems in different regions of the country, evaluating variations in the biochemical and nutritional composition of rice according to specific edaphic and climatic characteristics.
5. Conclusions
This research reveals that rice grain types from plots with a biological and chemical IPM reflected in the significant independent (rice and control) and interactive (R × C) effects on phytic acid. The differentiation in PA levels indicates that the type of pest control induces different responses regardless of the type of rice, probably due to a hypersensitive immune response caused by the organisms used for pest management. In our study, this distinction affects rice grains with biological control, increasing the phytic acid concentration, expressed in milligrams per gram of rice, by 32.5% on average. Correlation tests also show that all nutritional and biochemical properties are linearly related and with high significance in total phenolic compounds and antioxidant activity. On the other hand, the evaluation on the concentration of heavy metals such as cadmium and arsenic showed no difference between one group and the other; however, we argue that, although brown rice grains might concentrate higher contaminants, these depend mostly on environmental and anthropogenic factors. Finally, analysis of the trials shows that each replicate is within its respective experimental group, suggesting the possibility of a bias in the distribution or assignment of treatments. Furthermore, the biplot patterns were useful to find visual differences in both correlations and mean differences, indicating that phytic acid has higher values when grains from biological control are tested and that the rest of the nutritional properties show a slight but not significant increase with the chemical control.
Author Contributions
Conceptualization, M.d.R.V.-A. and C.V.V.-A.; formal analysis, J.D.O.-M.; investigation, J.D.V.-C.; methodology, M.d.R.V.-A., C.V.V.-A. and J.D.V.-C.; supervision, J.D.O.-M.; writing—original draft, M.d.R.V.-A., C.V.V.-A., J.D.V.-C. and J.D.O.-M.; writing—review and editing, M.d.R.V.-A. and C.V.V.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad Estatal de Milagro (UNEMI) Scholarship.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to the Universidad Estatal de Milagro (UNEMI).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAOSTAT. FAO Statistics Database on the World Wide Web. FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 12 June 2024).
- Al-hashimi, A.M. A Review: Growing Rice in the Controlled Environments. Biosci. Biotechnol. Res. Asia 2023, 20, 13–28. [Google Scholar] [CrossRef]
- Orbe, D.; Cuichán, M. Encuesta de Superficie y Producción Agropecuaria Continua; INEC (Instituto Nacional de Estadística y Censos): Quito, Ecuador, 2022; pp. 2–14. [Google Scholar]
- Jung, T.-D.; Shin, G.-H.; Kim, J.-M.; Choi, S.-I.; Lee, J.-H.; Lee, S.J.; Park, S.J.; Woo, K.S.; Oh, S.K.; Lee, O.-H. Comparative analysis of γ-oryzanol, β-glucan, total phenolic content and antioxidant activity in fermented rice bran of different varieties. Nutrients 2017, 9, 571. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Song, Y.; Chen, L.; Ji, J.; Li, J.; Yuan, X.; Yang, Z.; Ayoko, G.A.; Frost, R.L.; Theiss, F. Human health risks of heavy metals in paddy rice based on transfer characteristics of heavy metals from soil to rice. Catena 2019, 175, 339–348. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of Botanical Pesticides in Agriculture as an Alternative to Synthetic Pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- El Cambio Climático Favorece la Propagación de Plagas Que Destruyen Los Cultivos. Available online: https://news.trust.org/item/20210602151218-6e6zv (accessed on 18 November 2022).
- Shang, H.; He, D.; Li, B.; Chen, X.; Luo, K.; Li, G. Environmentally Friendly and Effective Alternative Approaches to Pest Management: Recent Advances and Challenges. Agronomy 2024, 14, 1807. [Google Scholar] [CrossRef]
- Liang, X.; Liao, G.; Li, J.; Fan, W.; Liu, Y.; Wang, S.; Chen, L.; Wang, Y.; Liu, J. Exogenous ABA promotes resistance to Sitobion avenae (Fabricius) in rice seedlings. Pest. Manag. Sci. 2024, 80, 3389–3400. [Google Scholar] [CrossRef] [PubMed]
- Longkumer, I.Y.; Ahmad, M.A.; Choudhary, S.; Laichattiwar, M.A.; Bajia, R. Validation of Integrated Pest Management Modules against Piercing and Sucking Insect Pest of Rice. Agric. Sci. Dig. 2024, 44, 351–354. [Google Scholar] [CrossRef]
- Alam, M.Z.; Haque, M.M.; Islam, M.S.; Hossain, E.; Hasan, S.B.; Hasan, S.B.; Hossain, M.S. Comparative Study of Integrated Pest Management and Farmers Practices on Sustainable Environment in the Rice Ecosystem. Int. J. Zool. 2016, 2016, 7286040. [Google Scholar] [CrossRef]
- Afzal, F.; Khurshid, R.; Ashraf, M.; Gul Kazi, A. Reactive Oxygen Species and Antioxidants in Response to Pathogens and Wounding. In Oxidative Damage to Plants: Antioxidant Networks and Signaling; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 397–424. [Google Scholar]
- Khandayataray, P.; Murthy, M.K.; Samal, D.; Gurusubramanian, G. Sustainable Integrated Pest Management using Pheromones: Types, Synthesis, Mechanism of Action and Applications. Research Journal of Biotechnology. World Res. Assoc. 2024, 19, 140–157. [Google Scholar]
- Abubakar, Y.; Tijjani, H.; Egbuna, C.; Adetunji, C.O.; Kala, S.; Kryeziu, T.L.; Ifemeje, J.C.; Patrick-Iwuanyanwu, K.C. Pesticides, history, and classification. In Natural Remedies for Pest, Disease and Weed Control; Elsevier: Amsterdam, The Netherlands, 2019; pp. 29–42. [Google Scholar]
- Zimmerer, K.S.; Vanek, S.J.; Baumann, M.D.; van Etten, J. Global modeling of the socioeconomic, political, and environmental relations of farmer seed systems (FSS): Spatial analysis and insights for sustainable Development. Elementa 2023, 11, 00069. [Google Scholar] [CrossRef]
- Adeyi, A.A.; Babalola, B.; Akpotu, S.O. Occurrence, distribution, and risk of organochlorine pesticides in food and greenness assessment of method. Environ. Sci. Pollut. Res. 2021, 28, 33433–33444. [Google Scholar] [CrossRef] [PubMed]
- Varatharaju, G.; Nithya, K.; Suresh, P.; Rekha, M.; Balasubramanian, N.; Gomathinayagam, S.; Manoharan, P.T.; Shanmugaiah, V. Biocontrol properties and functional characterization of rice rhizobacterium Pseudomonas sp. VsMKU4036. J. Pure Appl. Microbiol. 2020, 14, 1545–1556. [Google Scholar] [CrossRef]
- Chandio, A.A.; Gokmenoglu, K.K.; Ahmad, M.; Jiang, Y. Towards Sustainable Rice Production in Asia: The Role of Climatic Factors. Earth Syst. Environ. 2022, 6, 1–14. [Google Scholar] [CrossRef]
- Didawat, R.K.; Sharma, V.K.; Nath, D.J.; Patra, A.; Kumar, S.; Biswas, D.R.; Chobhe, K.A.; Bandyopadhyay, K.K.; Trivedi, A.; Chopra, I.; et al. Soil biochemical properties and nutritional quality of rice cultivated in acidic inceptisols using long-term organic farming practices. Arch. Agron. Soil Sci. 2023, 69, 1282–1297. [Google Scholar] [CrossRef]
- Gao, H.; Peng, X.; Dai, L.; Li, J.; Yang, Q.; Dou, Z.; Xu, Q. Determination of Soil Cadmium Safety Thresholds for Food Production in a Rice-Crayfish Coculture System. Foods 2022, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Jan, S.S.; Shahid, M.; Asaf, S.; Khan, A.L.; Lubna; Al-Rawahi, A.; Lee, I.-J.; Al-Harrasi, A. Novel Insights into Exogenous Phytohormones: Central Regulators in the Modulation of Physiological, Biochemical, and Molecular Responses in Rice Under Metal(loid) Stress. Metabolites 2023, 13, 1036. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Lal, M.K.; Sahoo, U.; Sahoo, S.K.; Sah, R.P.; Tiwari, R.K.; Kumar, R.; Sharma, S. Combinatorial effect of heat processing and phytic acid on mineral bioavailability in rice grain. Food Chem. Adv. 2023, 2, 100232. [Google Scholar] [CrossRef]
- Saleh, A.S.M.; Wang, P.; Wang, N.; Yang, L.; Xiao, Z. Brown Rice Versus White Rice: Nutritional Quality, Potential Health Benefits, Development of Food Products, and Preservation Technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1070–1096. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, F.; Gao, S.; Zhang, L.; Fu, Q.; Cui, S. Neonicotinoid insecticides in paddy fields: Dissipation dynamics, migration, and dietary risk. Chemosphere 2024, 359, 142371. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Lee, S.; Oh, S.; Cho, H.; Park, S.; Lee, H.; Kim, H.; Yeo, Y. Multivariate analysis for the safety assessment of genetically modified rices in the anti-nutrients and phenolic compounds. Int. J. Food Sci. Technol. 2016, 51, 765–776. [Google Scholar] [CrossRef]
- Tuncel, N.B.; Yilmaz, N. Gamma-oryzanol content, phenolic acid profiles and antioxidant activity of rice milling fractions. Eur. Food Res. Technol. 2011, 233, 577–585. [Google Scholar] [CrossRef]
- Cornejo, F.; Caceres, P.J.; Martínez-Villaluenga, C.; Rosell, C.M.; Frias, J. Effects of germination on the nutritive value and bioactive compounds of brown rice breads. Food Chem. 2015, 173, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, T.B.; Chattopadhyay, K.; Sivashankari, M.; Roy, S.; Kumar, A.; Biswas, T.; Pal, S. Effect of different processing technologies on phenolic acids, flavonoids and other antioxidants content in pigmented rice. J. Cereal Sci. 2021, 100, 103263. [Google Scholar] [CrossRef]
- Torino, M.I.; Limón, R.I.; Martínez-Villaluenga, C.; Mäkinen, S.; Pihlanto, A.; Vidal-Valverde, C.; Frias, J. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chem. 2013, 136, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, P.J.; Martínez-Villaluenga, C.; Amigo, L.; Frias, J. Maximising the phytochemical content and antioxidant activity of Ecuadorian brown rice sprouts through optimal germination conditions. Food Chem. 2014, 152, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Srisaipet, A.; Nuddagul, M. Influence of Temperature on Gamma-Oryzanol Stability of Edible Rice Bran Oil During Heating. Int. J. Chem. Eng. Appl. 2014, 5, 303–306. [Google Scholar] [CrossRef]
- Reichwald, K.; Hatzack, F. Application of a modified Haug and Lantzsch method for the rapid and accurate photometrical phytate determination in soybean, wheat, and maize meals. J. Agric. Food Chem. 2008, 56, 2888–2891. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, K.R. The biplot graphic display of matrices with application to principal component analysis. Biometrika 1971, 58, 453–467. [Google Scholar] [CrossRef]
- Galindo-Villardón, P. Una Alternativa de Representación Simultánea: HJ-Biplot. Qüestiió Quad. D’estadística I Investig. Oper. 1986, 10, 13–23. [Google Scholar]
- Lupotto, E.; Valè, G. Value of Wholegrain Rice in a Healthy Human Nutrition. Agriculture 2021, 11, 720. [Google Scholar] [CrossRef]
- Pramitha, J.L.; Rana, S.; Aggarwal, P.R.; Ravikesavan, R.; Joel, A.J.; Muthamilarasan, M. Diverse role of phytic acid in plants and approaches to develop low-phytate grains to enhance bioavailability of micronutrients. Adv. Genet. 2021, 107, 89–120. [Google Scholar] [PubMed]
- Biswas, S.; Sircar, D.; Mitra, A.; De, B. Phenolic constituents and antioxidant properties of some varieties of Indian rice. Nutr. Food Sci. 2011, 41, 123–135. [Google Scholar] [CrossRef]
- Naseri, M.; Vazirzadeh, A.; Kazemi, R.; Zaheri, F. Concentration of some heavy metals in rice types available in Shiraz market and human health risk assessment. Food Chem. 2015, 175, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, M.; Fakhri, Y.; Oliveri Conti, G.; Keramati, H.; Zandsalimi, Y.; Bahmani, Z.; Pouya, R.H.; Sarkhosh, M.; Moradi, B.; Amanidaz, N.; et al. Heavy metals (As, Cr, Pb, Cd and Ni) concentrations in rice (Oryza sativa) from Iran and associated risk assessment: A systematic review. Toxin Rev. 2017, 36, 331–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).