Abstract
Ultrasound (US) is a non-thermal food processing method that can be used as a pre-treatment or integrated during drying to enhance mass transfer by inducing cavitation and forming microchannels in plant tissue. Thus, this study investigated the combined effect of ultrasound pre-treatment (21 kHz; 180 W; 10 min, 20 min, 30 min) and the subsequent hybrid drying process—ultrasound-assisted hot-air drying (temperature of 70 °C, frequency of 36 kHz; ultrasound power of 120 W, 160 W, 200 W)—on the drying kinetics and quality attributes of dried Gloster apples. The experimental design was optimized using the response surface methodology (RSM). The effects of ultrasound parameters on drying time, dry matter content, water activity, rehydration and hygroscopic properties, color change, textural properties, content of vitamin C, polyphenols and flavonoids, and antioxidant activity were evaluated. Among the analyzed variants, the most effective combinations were longer US duration (30 min) with lower US power (120 W) or shorter US duration (10 min) with higher US power (200 W). To obtain dried material with the most desirable rehydration and hygroscopic properties, a US power in the range of 120–160 W, preceded by a US pre-treatment lasting 20 min, should be selected. Conversely, optimizing the content of bioactive components would involve choosing the longest US treatment time and medium to high ultrasonic power during drying. These results provide actionable insights for the industry to tailor drying parameters based on the desired product attributes.
Keywords:
vitamin C; bioactive compounds; color; rehydration rate; hygroscopic properties; texture; structure 1. Introduction
Drying is an important process in the food industry as it enables the extension of the shelf life of food products as a consequence of the decrease in the material’s water activity. The most common drying method is the convective one. While convective drying offers numerous benefits, it can be a lengthy and energy-intensive method that often requires high-temperature conditions. The application of pre-treatments and additional drying techniques allows for a decrease in the negative impact of convective drying on plant tissue and enables the selection of optimal process parameters [1].
Ultrasound (US) is among the well-documented non-thermal pre-treatment methods employed in the food industry. Ultrasound waves are characterized by frequencies above 20 kHz. Depending on the wave frequency, intensity, and ultrasound application, the passive and active implementation of sonication are distinguished: (a) waves of low intensity at high frequencies reaching values above 100 kHz, which, due to their non-invasive impact, are used in food quality control and diagnostics; (b) waves of high intensity with frequencies ranging from 20 kHz to 100 kHz, which have been implemented in food processing to modify food properties. As a result of the formation of cavitation bubbles that penetrate the material at very high velocity (up to 200 m/s), microscopic tunnels appear, influencing the course of drying as the internal structure changes [1,2]. Most studies in the literature focus on US pre-treatment or US-assisted drying separately; this study uniquely integrates both and optimizes parameters holistically. Generally, US pre-treatment changes the biochemical, physical, and mechanical properties of the studied material; thus, as aforementioned, it directly impacts further processing. There are many examples in the literature of the application of ultrasound before drying and their positive influence on shortening the drying time and product quality. Abbaspour-Gilandeh et al. [3], Sledz et al. [4], and Tao et al. [5] reported reduced drying times of walnut kernel, basil, and mulberry leaves, respectively, after ultrasound pre-treatment. Moreover, ultrasound pre-treatment was utilized to enhance the extraction of bioactive compounds, resulting in compounds that exhibit antioxidant, antimicrobial, anti-inflammatory, or anticancer properties [6]. Additionally, it has been reported that sonication, in combination with other treatments, can lead to the inactivation of potentially health-threatening microorganisms [7]. Liao et al. [8] studied the influence of ultrasound combined with cold plasma on microbes and observed their synergistic effect, whereas Bi et al. [9] noted the inactivation of Salmonella typhimurium in liquid whole eggs after the application of ultrasound with lysosome. It should be noted that ultrasound pre-treatment is not the single application of sonication, as ultrasound is implemented during the drying process as well. Employing ultrasound technology during the drying process can greatly lower the drying temperature, minimize the drying duration, decrease energy usage, and enhance the quality of the dried product. This method helps maintain important nutrients (such as vitamins and minerals) and preserves the appearance (shape and color) of the materials. [10]. Szadzińska et al. [11] reported the shortening drying time and reduction in energy consumption during the convective drying of green peppers subjected to ultrasound with a power of 100 W and 200 W, by 32% and 37% and by 8.7% and 10.7%, respectively. Moreover, the use of sonication also had a positive effect on the final product’s quality. The sonicated samples contained approximately 18 and 25% more vitamin C, respectively, than the control ones did. The water activity decreased down to 0.32 and 0.29, respectively, which provided better protection against microbiological changes in the material, compared to the material not treated with ultrasound (water activity of 0.42). The application of sonication improved the rehydration properties of peppers, reduced the degree of degradation of natural pigments, and resulted in less deformation of the dried material [11].
Apples represent a substantial proportion of global fruit production, with an increasing trend in their consumption worldwide [12]. For instance, according to the Eurostat data, apples accounted for around 43% of all fruit produced in Europe in 2024, while 10 years earlier, it was only around 26%. Moreover, Poland, followed by Italy and France, has been the largest producer of apples in Europe for more than 10 years [13]. Nowadays, apples are utilized in diverse forms, including fresh fruits, juice, dried derivatives, and various processed products. In dried form, they can be used as a valuable ingredient of ready-to-eat foods (snacks, breakfast cereals, other functional foods, etc.). However, being an important source of bioactive compounds with high antioxidant activity, apples can be particularly vulnerable to quality loss through processing [14]. Today’s consumers exhibit a growing demand for high-quality food products that retain fresh-like attributes, such as flavor, texture, and color, while prioritizing the preservation of nutritional value. Additionally, there is an increasing expectation for products with a stable or extended shelf life. Recently, there has been an increased focus on the health advantages of eating apples, prompting studies into how various processing methods influence the characteristics of apple products. The objective is to reduce quality loss and maintain their beneficial properties [15]. Specifically, there have been extensive investigations into the effects of ultrasound used before [16,17,18,19,20,21,22] or during drying [14,15,21,23,24,25,26,27] on the quality of dried apples.
The aim of this study was to investigate the effect of sonication used as a preliminary treatment and during the drying process (hybrid drying) on the course of the process and the properties of organic apples of the ‘Gloster’ variety. The novelty of this research is linked to the combined method where ultrasonic treatment with an ultrasound-assisted drying method was applied, and the response surface methodology (RSM) was used for the optimization of the whole processing (US pre-treatment with US-drying). Usually, the study is conducted separately for evaluation of the effect of ultrasonic treatment and then the effect of the chosen parameters of the sonication (based on the best obtained properties of plant material after US treatment) on the drying process conducted with different parameters. Such a procedure is not always correct. Some results show that after the drying process, the other US-pre-treated sample might obtain better results than at the beginning, which can be related to chemical reactions and changes occurring during the whole process of obtaining the final product. So, in this study, the whole processing was taken into account, and optimization was performed for the US pre-treatment together with the US-supported drying. The scope of this research included the analysis of the drying kinetics and selected chemical (dry matter content, vitamin C, total polyphenol content, total flavonoid content, antioxidant activity, sugar content, functional groups evaluated with FTIR analysis) and physical properties (water activity, total color change, rehydration properties, hygroscopic properties, texture, structure of the tissue evaluated using SEM and microtomography) of the dried material obtained as a result of the combination of the initial sonication time within 10, 20, or 30 min and the ultrasonic waves applied during drying with a power of 120, 160, or 200 W.
2. Materials and Methods
2.1. Materials
‘Gloster’ apples from an organic farm were used for this study. This variety was characterized by high polyphenol content, firm texture, and widespread industrial use in drying and processing applications. The apples used in the study had physiological/eating maturity. The skin of apples had a dark red color over most of the surface, with a slight greenish background under the red blush. The apple body flesh had the following color values: L* = 88.23 ± 0.88, a* = −3.20 ± 1.00, and b* = 25.75 ± 0.85, with 13.1 ± 0.6 °Brix and a total sugar content of 36.71 g/100 g dry matter—4.94 g of sucrose, 24.80 g of fructose, and 6.11 g of glucose per 100 g of d.m. The force needed to deform the material by 50% was equal to Fmax = 2.39 ± 0.53 N. The fruit was cut into slices 5 mm thick and then divided into 4 equal parts. Fresh material (uncut) was stored in a controlled atmosphere: 5 °C, 80% RH.
2.2. Technological Methods
The technological process is presented in Figure 1. The material was pre-treated using ultrasound (US) (ultrasonic bath, MKD Ultrasonic, Stary Konik, Poland) with a frequency of 21 kHz and a power of 180 W. The process was carried out for 10 min, 20 min, and 30 min in a glass beaker with distilled water in a ratio of 1:4. During the treatment, the temperature was kept at 25 °C ± 1 °C. The parameters were chosen based on our earlier study.
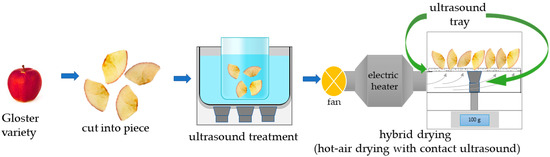
Figure 1.
The technological process of the ultrasound-treated apples that were dried with a hybrid method based on ultrasound-assisted hot-air drying.
After treatment, apples were dried on filter paper (slices were placed on the filter paper, once on each side), and 100 g of material was placed on a sieve of a prototype hybrid dryer (Promise Tech Inc., Wrocław, Poland) equipped with an ultrasound generator (contact ultrasound strainer). Drying was carried out at a drying air temperature of 70 °C. The air flowed perpendicular to the material at a speed of 1.5 m/s. The process was supported by sonication with an ultrasound power of 120 W, 160 W, or 200 W and a frequency of 36 kHz. Every 5 min, the process was automatically stopped, and the system weighed the screen with the material with an accuracy of ±0.1 g. The temporary interruption of the drying process was necessary to minimize the impact of airflow on the mass measurement. To ensure precise measurements, the drying process was briefly paused, only for the time required to take accurate mass readings. This brief interruption did not significantly affect the overall drying process or the dynamics of mass and energy transfer, as the drying conditions were consistently maintained between measurements. The experiment was designed using the response surface methodology (RSM-CCF), with one central point (C) with 3 repetitions and two variables on three levels (factor 1—time of initial sonication; factor 2—ultrasound power during drying), which is shown in Table 1.

Table 1.
The scheme of a two-factorial design.
Drying kinetics were plotted as dimensionless water content (MR) as a function of time. The relative moisture ratio was calculated based on the following equation [28]:
where uτ is the moisture content during a given moment of the drying process (g water/g d.m.), and u0 is the initial moisture content (g water/g d.m.).
2.3. Analytical Methods
2.3.1. Dry Matter and Water Activity
The gravimetric method was used to determine the dry matter content in both fresh and dried apples according to the AOAC method [29]. The crushed apples were dried in a laboratory dryer (SLW 115 STD, Pol-Eko-Aparatura, Wodzisław-Śląski, Poland) at 70 °C for 24 h.
For the determination of the water activity of the apple tissue, an AquaLab Series3TE device was used (Decagon Devices, Inc., Pullman, Washington, DC, USA) with an accuracy of ±0.001 at a temperature of 25 °C ± 1 °C [28]. Both measurements were conducted in triplicate for each sample.
2.3.2. Color
The color was measured using a colorimeter (Chroma Meter Konica-Minolta CR-5, Osaka, Japan) working in the CIE L* a* b* system. In this mode, color chromaticity coordinates are defined as L*: brightness, a*: greenness-redness, and b*: blueness-yellowness. The device was initially calibrated with black and white standards. For the measurement, a standard light source D65 and a standard observer 2° were used. The color analysis consisted of six repetitions for each sample. Based on the parameters measured, the total color differences (ΔE) between a fresh apple and each material were calculated according to the following equation [30]:
where
- L*control, a*control, and b*control—color parameters of fresh apples;
- L*sample, a*sample, and b*sample—color parameters of US-treated and/or dried apples.
2.3.3. Rehydration Properties
The hydration process was carried out in beakers to determine the rehydration capacity of dried apples. About half of the dried apple slices were weighed on an analytical balance (model AE 240S, METTLER, Warsaw, Poland) with an accuracy of ±0.0001 g, and then placed in 100 mL beakers of distilled water [31]. After 60 min, the material was filtered on a sieve and dried using filter paper. The material was then weighed again on a scale, and the weight gain was calculated. The determination was made in three repetitions for each dried material.
After rehydration, the apples were crushed and placed in aluminum weighing vessels, which were weighed on an analytical balance with an accuracy of ±0.0001 g. The material was then dried for 24 h at 70 °C. After this time, the vials were removed from the dryer and placed in a desiccator until room temperature was reached, after which the vials were weighed again and the dry substance content was calculated, based on which the loss of soluble dry matter components during rehydration was determined. The test was performed in three replicates for each sample.
2.3.4. Hygroscopic Properties
Dried apple slices were weighed on an analytical balance with an accuracy of ±0.0001 g. The material was placed in a desiccator over a NaCl solution in an environment with a water activity of 0.75 to simulate the water vapor absorption similar to the environment. Then, the weight was measured after 1 h, 24 h, 48 h, and 72 h [32]. The measurement was carried out in two repetitions for each dried material.
2.3.5. Textural Properties
The textural properties of dried apples were evaluated by measuring the force needed to deform the material by 50%. The texture was measured using the TA.HD.plus Texture Analyzer (Stable Micro Systems, Godalming, UK) and the Texture Exponent computer program [33]. A head with a force of 5 N was used to carry out the experiment, which was set at a height of 6 mm above the stage. Three apple slices were selected for each of the dried variants. The material was placed on a metal table, and the measurement was started. During the measurement, the head moved at a speed of 1 mm/s, and at the moment of contact with the material, it reduced its speed by half. Then, from the obtained results, the maximum force recorded during the experiment was analyzed. The 12 repetitions for each dried material were carried out.
2.3.6. X-Ray Micro-Computed Tomography (XRCT) and Scanning Electron Microscopy (SEM)
Skyscan 1272 (Bruker, Kontich, Belgium) X-ray computed microtomography (XRCT) was used for the visualization of the internal structure [34]. A piece of dried apple was excised using a scalpel and mounted with its shorter edge onto a metal table with a 25 mm diameter. Imaging was performed at a source voltage of 40 kV, a current of 193 μA, a resolution of 15 μm, and a rotation step of 0.3°. The raw scan data were reconstructed with Recon software version 1.6.10.5 (Bruker, Kontich, Belgium) and subsequently binarized within the 25–925 range using CTAn software version 1.23.0.2 (Bruker, Kontich, Belgium).
The material was placed in the EM-Tec PS4 pin vise clamp and covered with a 5 mm layer of gold using a Leica EM ACE200 (Leica Microsystems GmbH, Wetzlar, Germany). An SEM XL (Phenom) was used for the observations of the cross-section at a magnification of 220×. A vacuum of 60 Pa and a voltage of 10 kV were maintained. For each sample, ten photographs were captured.
2.3.7. Bioactive Compounds and Antioxidant Activity
Vitamin C
The determination of vitamin C content was carried out through ultra-performance liquid chromatography (UPLC) with the use of the UPLC-PDA system (WATERS Acquity H-Class, Milford, MA, USA) [35]. Following the procedure, 10 mL of cooled extraction reagent containing 3% metaphosphoric acid and 8% acetic acid was added to around 0.3 g of dried material. Then, the solution was stirred with a vortex for 5 min, centrifuged for 2 min at 5 °C, filtered through 0.2 μm GHP syringe filters (Acrodisc, Pall Corporation, Port Washington, NY, USA), and diluted twice with eluent (Milli-Q water with 0.1% formic acid). In the next step, separation on a Waters HSS T3 chromatographic column (100 mm × 2.1 mm, 1.8 μm; Waters, Ireland) for 5 min at 35 °C with a mobile phase flow rate of 0.25 mL/min was conducted. The quantitative content of ascorbic acid was accomplished by analysis of the spectrum at 245 nm and the usage of the calibration curve for the L(-) ascorbic acid standard. The analysis was performed twice for each sample.
Total Polyphenol Content (TPC)
The determination of TPC was made via the spectroscopic method, which used a color reaction of the analyte with the Folin–Ciocalteu (F-C) reagent [36]. In order to prepare the material for the analysis, the dried fruits were ground in an analytical mill (IKA A11 basic; IKA-Werke GmbH, Staufen, Germany). The extraction was conducted for 12 h at 20 °C on a shaker (Multi Reax, Heidolph Instruments, Schwabac, Germany) with the use of a mixture of 80% ethyl alcohol and 0.1 M hydrochloric acid (85:15, v/v). Then, the solutions underwent centrifugation in a laboratory centrifuge (MegaStar 600, VWR, Leuven, Belgium) for 2 min at 4350 rpm. For each sample, the extraction procedure was performed in duplicate.
The extracts of 10 µL were diluted twice with distilled water and placed in a 96-well plate. The 5-fold-diluted Folin–Ciocalteu reagent of 40 µL was added to the solutions, followed by supersaturated calcium carbonate of 250 µL after 3 min. Then, the reactions were carried out for 1 h at room temperature, protected from light. Absorbance at a wavelength of 750 nm was measured for each sample against a reagent blank on a multiplate spectrophotometer (Multiskan Sky, Thermo Electron Co., Waltham, MA, USA). The quantitative content of polyphenols was assessed with reference to a calibration curve prepared for chlorogenic acid in the concentration range of 0 to 100 µg/mL. The analysis was performed in two repetitions.
Total Flavonoid Content (TFC)
The aluminum (III) chloride method was employed to assess the total flavonoid content [37]. Initially, 20 µL of the extract was combined with 80 µL of distilled water and mixed with 10 µL of 5% (w/v) NaNO2 solution. After 5 min, 10 µL of 10% (w/v) AlCl3 was added and mixed. Following an additional 6 min, 40 µL of 1 M NaOH solution was introduced and thoroughly mixed. After 20 min, the absorbance was recorded at 510 nm. The flavonoid concentration was determined using a calibration curve for quercetin within the range of 0–500 µg/mL. All measurements were performed in duplicate.
Antioxidant Activity (DPPH Radical)
The evaluation of antioxidant activity was carried out using a spectrophotometric method that quantified the ability of the sample to neutralize the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical [37]. The initial DPPH solution was prepared by dissolving 0.025 g of 2,2-diphenyl-1-picrylhydrazyl in 100 mL of 99% methanol. To allow the formation of radicals, the solution was stored at 4 °C in the dark for at least 24 h. Before the analysis, the working solution was prepared by diluting 9 mL of the stock solution with 80% ethanol to obtain an absorbance of 0.700 ± 0.020 at 515 nm. The experiment was performed in 96-well plates, where 250 μL of the radical solution was mixed with 10 μL of the sample extract, previously diluted fivefold. The reaction mixtures were incubated for 30 min at room temperature in the dark. Subsequently, the absorbance was measured at 515 nm using a plate reader (Multiskan Sky, Thermo Electron Co., St. Louis, MO, USA). Each extract was analyzed twice. The antioxidant activity is expressed as the Trolox equivalent antioxidant capacity (TEAC), which corresponds to the amount of Trolox required to achieve an equivalent antioxidant effect as the analyzed sample (mg Trolox/g d.m.).
2.3.8. Sugar Content
The sugar content was analyzed using liquid chromatography with refractive index detection [38]. The system included a quadruple pump (Waters 515, Milford, MA, USA), an autosampler (Waters 717, Milford, MA, USA), a column thermostat, and a refractive index detector (Waters 2414, Milford, MA, USA). A portion of 0.2–0.3 g of the ground sample was weighed on an analytical balance and mixed with distilled water at 80 °C. The mixture was placed on a circular-vibrating shaker, and the extraction of sugars proceeded for 4 h. The solution was centrifuged (5 min, 6000 rpm) and filtered through a 0.22 μm PTFE syringe filter, and 10 μL was injected onto the column. Separation was conducted on a Waters Sugar Pak I column (300 × 6.5 mm) equipped with a Sugar-Pak pre-column. The mobile phase consisted of Milli-Q redistilled water, with a flow rate of 0.6 mL/min. The detector and column temperatures were maintained at 50 °C and 90 °C, respectively. Quantitative determination was performed using calibration curves for glucose, fructose, and sucrose standards (Sigma-Aldrich, Steinheim, Germany). All measurements were performed in duplicate.
2.3.9. FTIR Analysis
The FTIR spectra of apple samples were acquired by using the Cary 630 Fourier transform infrared spectrometer (Agilent Technologies Inc., Santa Clara, CA, USA) with single-reflection diamond attenuated total reflection (ATR). The wavenumber range was set at 650–4000 cm−1 with a cumulative scan of 32 times and a resolution of 4 cm−1 [39]. Dried apples were inserted into the measuring window and pressed against the crystal. The analysis was performed thrice for each material. Then, the data were analyzed in MicroLab FTIR software (version 1.1.13).
2.4. Statistical Analysis
Statistica 13.3 (TIBCO company software, Palo Alto, CA, USA) was used for the statistical analysis. To assess the significance of the differences in results, one-way ANOVA and Tukey’s tests were performed separately for each ultrasound power level, with ultrasound exposure time as the independent variable and the studied properties of the dried material as the dependent variables. Additionally, to determine the influence of sonication time and ultrasound power, a two-way ANOVA was performed. A response surface model was also applied to the studied parameters, presenting the model of the investigated properties as a function of sonication time and ultrasound power used during hybrid drying. Statistical analyses were performed at a significance level of α = 0.05.
3. Results and Discussion
3.1. Drying Kinetics
Drying kinetic curves indicated a change in the water content in the samples depending on the drying time (Figure 2a). At first, the moisture was evaporated from the surface of the samples, so the drying rate was constant. Then, the water evaporated from deeper layers of the dried material, and the water vapor moved to the surface of the apple, which resulted in a decreasing rate of drying. The samples subjected to 10 min of US pre-treatment and US-assisted drying with 200 W of US power (T_10_D_200W) and 30 min of US pre-treatment and US-assisted drying with 120 W (T_30_D_120W) were characterized with the shortest drying time of 125 min. As a result of the ultrasound application, while processing the samples, the drying time was reduced by 11%. This effect was linked with cavitation and/or the sponge effect [2]. For the sample subjected to 10 min of US pre-treatment and US-assisted drying with 200 W of US power (T_10_D_200W), high cavitation occurred during the short pre-treatment time (10 min). The applied high ultrasound power (200 W) during drying intensified cavitation, where the rapid formation and collapse of microbubbles occurred and led to the disruption of the cell walls and enhanced water diffusion. Though the pre-treatment duration was short (10 min), the strong cavitation effect during drying was compensated, allowing for rapid moisture removal. However, in the case of apples treated with 30 min of US pre-treatment and US-assisted drying with 120 W (T_30_D_120W), a longer US pre-treatment time (30 min) promoted the sponge effect, where cyclic compression and expansion of the tissue structure form microscopic tunnels, through which water was removed from the tissue more easily. Even though the ultrasound power during drying was relatively low (120 W), the pre-formed microchannels significantly improved water transport and effectively reduced drying time.
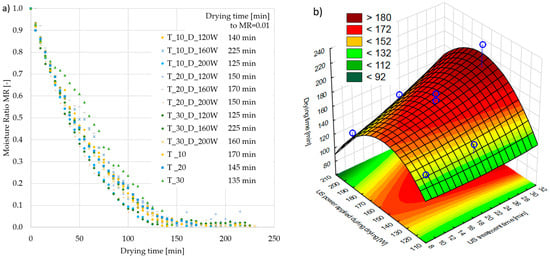
Figure 2.
(a) The kinetics of apple drying using ultrasonic treatment with a hybrid method of drying with drying time in min to obtain MR = 0.01; (b) the effect of US treatment time and the US power applied during drying on the RSM for apple drying time to obtain MR = 0.01. Blue dots on the figure shows research data.
Several studies in the literature report the use of ultrasound pre-treatment and ultrasound-assisted convective drying as methods to shorten the drying time. Tao et al. [40] observed a decrease in processing time and an enhancement in drying when samples of mulberry leaves were pre-treated with ultrasound. Sabarez et al. [26] stated that the application of ultrasounds during convective drying significantly shortened the drying time of apples, depending on the power used. An increase in the drying rate may be influenced by an increase in the permeability of the cell membrane and damage to the structure of the cell walls of the material. In addition, using sonication caused a sponge effect and the formation of microscopic tunnels in the plant tissue, which facilitated water evaporation [41].
Based on Figure 2b, it can be presumed that the highest and lowest tested ultrasonic power applied during drying provided the shortest drying time. This finding was further confirmed by a Pareto plot analysis (Figure S1), according to which a quadratic effect of the impact of the ultrasonic power used during on drying time was found. The shortening of drying time was a result of the presence of a greater or lesser sponge effect in individual samples according to the mechanism of the sonication [42,43]. Based on the RSM graph, it can be stated that the shortest drying time was achieved for the highest and the lowest ultrasound power, regardless of the sonication time before drying. Therefore, the evidence from this study implies that the time of ultrasound treatment was not crucial for shortening the drying time when hybrid drying with a sonication effect (power of US 120, 160, and 200 W) was used as a drying technique for apples.
3.2. Dry Matter Content and Water Activity
The average content of dry matter in the fresh apple was 16.00 ± 0.01%. A similar result was obtained by Fijałkowska et al. [44], who obtained a value of 15.10 ± 0.80% for a raw apple (variety ‘Idared’). Figure 3a shows the impact of US pre-treatment time and US power applied during drying on the dry matter content of hybrid dried apples (Figure S2a). Dry matter content changes in the apple tissue were linked to US pre-treatment, which occurred in the water surrounding it. Drying and pre-treatment with the US resulted in a significantly increasing dry substance content (to above 90%). The response surface plot showed that the highest dry matter content was at the average US pre-treatment time (20 min) and the highest US power applied during drying (200 W). The significant influence of the US power during drying on the dry matter content results was confirmed by a two-factor analysis of variance (p < 0.05). Fijałkowska et al. [44] and Nowacka et al. [45] found that extending the time of US pre-treatment from 10 to 30 min resulted in a higher content of dry matter. Changes occurring in plant tissue after US pre-treatment may be explained by transferring soluble substances from dry matter to the water environment, in which the US processing is conducted [19].
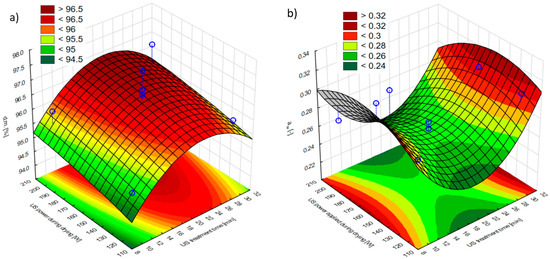
Figure 3.
Effects of the US treatment time and the US power applied during drying (a) on the dry matter content (d.m.) of hybrid dried apples and (b) on the water activity (aw) of hybrid dried apples. Blue dots on the figure shows research data.
Figure 3b shows the impact of US pre-treatment time and US power applied during drying on the water activity of hybrid-dried apples. The water activity of dried apples ranged from 0.24 to 0.31. This means that the obtained materials were microbiologically stable because all samples were below the water activity of 0.6, which prevents the microorganism’s growth [46]. The lowest water activity values were obtained for the average US pre-treatment time. A correlation between water content and water activity was noticed—the lowest water content was also recorded for materials after the average US pre-treatment time. A significant effect of the ultrasonic pre-treatment time on the water activity content was found by the Pareto graph (the relationship was square, Figure S2b). Rani and Tripathy [47], using US pre-treatment with a frequency of 40 kHz for 20 min and 30 min for pineapple prior to convective drying, found a significant decrease in water activity only when the US pre-treatment was given to pineapple slices for 20 min. In turn, Yildiz and Izli [48] studied the effect of US treatment with a frequency of 28 kHz for 10 min, 20 min, and 30 min for quince before freeze-drying. Although they showed an important decrease in water activity as a result of the US application, they noted that the duration of pre-treatment did not significantly affect this parameter.
3.3. Rehydration Properties and Hygroscopic Properties
The drying process damages the structure of the material, reduces the ability to bind water, and makes it impossible to restore the original volume of the raw material. There are two important properties in the case of rehydration: increasing the weight of the dried material and the loss of soluble components of the dry matter. They depend on the kind of tested material, pre-treatment, and drying conditions [49].
The values of the relative weight gain expressed as rehydration rate (RR) and the loss of soluble dry matter components (SSL) were determined in dried apples. The response surface plots of the RR and SSL of hybrid dried apples are presented in Figure 4. The RR on the reproducibility of the dried apples showed that US pre-treatment and US power during drying had a significant (quadratic) effect on the reproducibility of the dried material (Figure S3a). The highest weight gain was characteristic for the samples obtained after the average US treatment time (20 min) and at the lowest US power during drying (120 W), and a slightly lower RR was noted for samples subjected to 20 min of US pre-treatment and US-assisted drying with 200 W (T_20_D_200W). However, the sample treated for 20 min of US pre-treatment and US-assisted drying with 120 W (T_20_D_120W) was significantly different from the others and was characterized by the best reconstitution properties. The formation of microchannels appears to be the key structural change driving improved water-binding during rehydration. Ultrasound applied both before and during drying facilitates the drying process and causes the structural changes of the plant material [2].
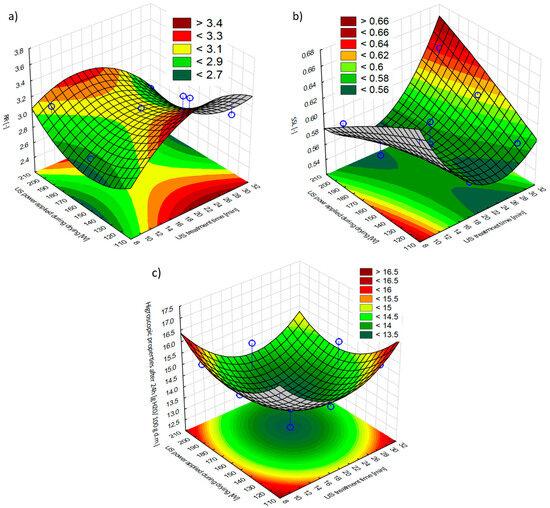
Figure 4.
The effect of the US treatment time and the US power applied during drying on the (a) rehydration rate (RR) of hybrid dried apples; (b) the soluble solid loss (SSL) of hybrid dried apples; and (c) the hygroscopic properties of hybrid dried apples. Blue dots on the figure shows research data.
Better rehydration properties of dried fruit or vegetables after using the US were observed by Mothibe et al. [19] for apples, Ricce et al. [50] and Chen et al. [51] for carrots, and Szadzińska et al. [52] for beetroot. Ricce et al. [50] explain the higher weight gain during the rehydration of US-pre-treated dried materials by the formation of US microchannels, which causes higher porosity and facilitates the penetration of water into the cell. Increasing the time of US pre-treatment from 20 min to 30 min resulted in a decrease in weight gain. Mothibe et al. [19] also found that increasing the duration of pre-treatment from 5 to 15 min resulted in a decrease in the ability to rehydrate the dried apples. The same conclusions were reached by Ricce et al. [50], who indicated that a shorter US treatment of 30 min allowed a higher water-binding capacity of dried carrots to be obtained, compared to the initial process carried out for 60 min. In our study, the result of the best rehydration rate of dried materials obtained after US treatment (with an average time of treatment of 20 min) and US-assisted drying (with the lowest US power applied during drying) can be explained by structural modifications caused by the combination of sonication before and during drying.
During the rehydration process, a loss of soluble components of the dry substance, such as vitamins, sugars, or acids, is also observed. The outflow of these substances into the surrounding solution is determined by the chemical composition and internal structure of the material [44]. The loss of soluble components ranged from 0.54 ± 0.02 to 0.64 ± 0.03 (Figure 4b). The response surface of the loss of soluble components (SSL) of the dry substance (Figure S3b) shows that US treatment time had a significant, quadratic effect on the obtained values. The lowest SSL was obtained for samples after 20 min of US pre-treatment. Nowacka et al. [45] found that the extension of sonication time from 10 to 30 min resulted in a higher loss of soluble dry substance components in the apples obtained by convection drying at 70 °C. This was explained by the higher structural damage of the raw material that occurred during the longer pre-treatment.
The water content after 24 h was in the range of 13.25 g/100 g d.m. to 16.71 g/100 g d.m. The response surface of the water content in the dried apples after 24 h is presented in Figure 4c. According to a storage point of view, the best variant of the material was obtained in the case of using the average time of US treatment (20 min) and the average US power level during drying (160 W), which absorbed the lowest water vapor from the surroundings. Fijałkowska et al. [44], while convection drying an apple (at 70 °C), observed that the use of US pre-treatment (10, 20, 30 min) reduced the water vapor adsorption capacity of dried apples, while the lowest hygroscopicity was characteristic for apples subjected to 30 min of US pre-treatment. However, in our study, there was no significant effect of the US treatment time and the power applied by the US during drying (Figure S3c).
3.4. Color Changes and Textural Properties
Consumers tend to make their first assessment of a food product based on its appearance. Therefore, color is one of the characteristics that has a great impact on the acceptability of foods. The natural pigment compounds present in food that are mainly responsible for the color of food matrices can be degraded by both drying and various pre-treatment methods [53].
Figure 5a shows the impact of US pre-treatment time and US power applied during drying on the total color difference of hybrid-dried apples. Regardless of the time of US pre-treatment and the US power applied during drying, substantial color changes were recorded in relation to the fresh sample. As a result of the ultrasound pre-treatment and hybrid drying, the total color difference between fresh and processed apple changed from 15.71 ± 0.74 (sample treated for 20 min with US pre-treatment and US-assisted drying with 160 W—T_20_D_160W) to 18.55 ± 0.23 (sample treated for 20 min with US pre-treatment and US-assisted drying with 200 W—T_20_D_200W). This means that a standard observer will see apples of two different colors when seeing a fresh and a processed sample [54]. There were numerous possible factors that affected the color changes as a result of US treatment and hybrid drying, e.g., Maillard reaction, pigment degradation, enzymatic and non-enzymatic browning, and ascorbic acid oxidation [49,55].
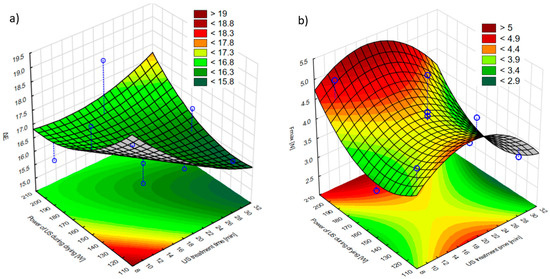
Figure 5.
Effects of the US treatment time and the US power applied during drying on the (a) total color change (ΔE) of hybrid dried apples and the (b) texture properties (Fmax recorded during a 50% deformation test) of hybrid dried apples. Blue dots on the figure shows research data.
Ultrasound can induce diverse effects on the fruits and vegetables. Typically, US pre-treatment may contribute to high-quality dried products as a mild technique to facilitate the removal of water from plant tissue. For instance, Kahraman et al. [49] compared the non-thermal ultrasound contact drying (US-CD) of apple slices with hot-air drying and concluded that the better color retention in the US-CD samples could be the result of the shorter drying time combined with the reduced temperature of drying. Furthermore, Zhang et al. [56] applied low-intensity ultrasound combined with heat (LIUH) pre-treatment prior to microwave vacuum drying and stated that it reduced the color change of dried materials. Additionally, the researchers observed that by using LIUH with a higher power intensity (0.4 W/cm2), a smaller color change could be reached than with the lower one (0.2 W/cm2). However, the physical destruction and membrane deformation of cells caused by the US application may also lead to the leakage of pigments from plant tissue. This may be the explanation why, in the study by Yildiz and Izli [48], a 10 min or 20 min US pre-treatment of quince followed by freeze drying led to a statistically smaller color change than a 30 min treatment. In our study, based on the Pareto chart (Figure S4a), it can be found that neither ultrasonic pre-treatment time nor the power of sonication applied during drying had any significant linear or quadratic effect on the color change of apple tissue.
The textural properties of all the samples were evaluated in terms of the maximum force needed to deform the material by 50%. The fresh sample was characterized by a maximum force equal to 2.39 ± 0.53 N. Generally, both ultrasonic pre-treatment and sonication applied during drying did not significantly alter the textural properties in comparison to fresh apple, except the sample sonicated for 10 min before drying and with an ultrasound of 200 W during drying (T_10_D_200W). Fijałkowska et al. [44] reported similar observations for sonicated apples prior to convective drying regarding maximum cutting force. The authors noted no statistically significant difference between fresh apples and different sonication times (10 min and 20 min). However, the apple after 30 min of ultrasound pre-treatment was less dense in comparison to the fresh sample, which is in contrast to the results presented in this research. Sabarez et al. [26], who also dried apples with the application of ultrasound during the process, similarly noted no statistically significant differences in the texture of the studied samples. Nevertheless, the authors observed that apples dried with ultrasound were less dense than apples dried conventionally, as lower force was needed to deform the sample.
Generally, the response surface plot showed that the highest power of applied ultrasound during convective drying combined with the middle sonication time affected the highest maximum force needed to deform the material (Figure 5b). However, both ultrasonic pre-treatment and sonication applied during drying did not have a significant effect as shown by the Pareto chart (Figure S4b). Finally, it is likely that the reason for no differences between the textural properties of samples may lie in quite large standard deviations, as the raw material varied significantly internally after pre-treatment and ultrasound application.
3.5. Microstructure
The internal microstructure is an important quality parameter of dried materials, as it can affect drying kinetics, physical properties (texture, rehydration), and the content of bioactive compounds (the washout from the damaged structure is easier) [57]. The microstructure of dehydrated samples was observed by two methods—XRCT and SEM. Pictures of horizontal and vertical cross-sections of apple slices scanned by XRCT, together with corresponding SEM images, are presented in Figure 6.
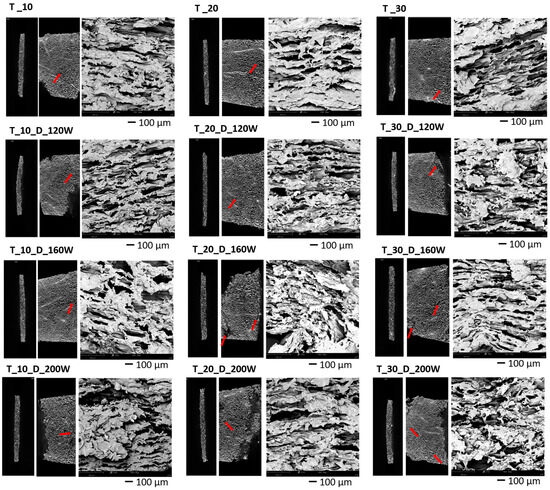
Figure 6.
Microstructures of dried apple samples based on XRCT visualization (horizontal and vertical cross-sections on a black background), and SEM pictures (third picture for each sample, magnification of 220×). The microchannels are marked on the images with the red arrows.
Significant changes in the structure of apple tissue were caused by both drying-induced shrinkage and US-treatment effects. Moreover, there were significant differences between samples treated only before drying and those that were also treated during the dehydration process. According to previously published data, structural modifications due to US are caused by different mechanisms [58]. One of them is the “sponge effect” related to rapid compressions and expansions in the tissue in a similar way to a sponge, which is squeezed and released repeatedly. Another mechanism is cavitation, based on the formation of thousands of cavitation bubbles in liquids, causing in turn very high, local alterations of pressure and temperature that lead to damage to the cells [59]. As a result of those effects, microchannels were formed in the structure, which can be observed in the pictures of samples subjected only to sonication for 10 min, 20 min, and 30 min (T_10, T_20, and T_30, respectively), which were treated only before drying. Moreover, subsequent drying combined with US led to further changes in microstructure, as more prominent cell disruption can be observed.
The deformation of the structure of the analyzed materials and the appearance of microchannels facilitated the process of water loss and shortened the drying time. However, it was assumed that the sponge effect was the main factor contributing to the improved drying process, rather than the cavitation effect. When analyzing the drying kinetics of the tested materials, it can be seen that there was no relationship between the extension of the sonication time and the shortening of the drying time of apple slices. This could result from the presence of a greater or lesser sponge effect in individual samples.
3.6. Bioactive Compounds and Antioxidant Activity in Hybrid Dried Apples
Bioactive components are crucial to human health, so it is important to consume fruits and vegetables that are their main sources. The preservation of antioxidant substances in dried foods contributes to their stability during long-term storage [60]. Changes in bioactive compound content in the apple tissue were assessed based on the content of vitamin C, polyphenols, and flavonoids, whereas the antioxidant activity was evaluated with the DPPH assay (Figure 7). Untreated apples contained 143.2 ± 5.0 mg of vitamin C, 1683 ± 2 mg of polyphenols, and 715 ± 8 mg of flavonoids per 100 g of dry matter, while the antioxidant activity was 4.33 ± 0.06 mg TE/g d.m.
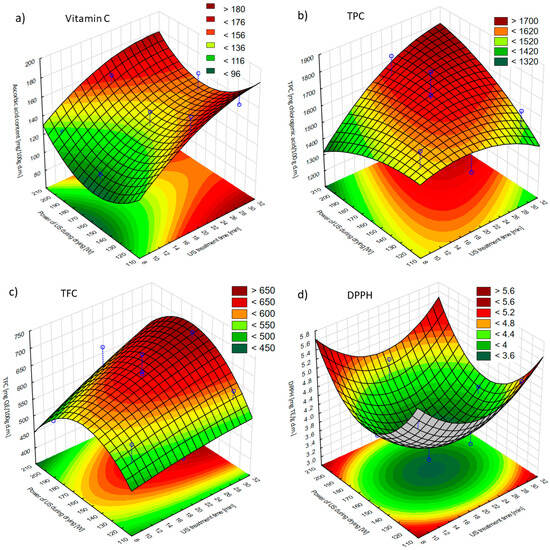
Figure 7.
Effects of the US treatment time and the US power applied during drying on (a) the vitamin C content of hybrid dried apples; (b) the total polyphenol content (TPC) of hybrid dried apples; (c) the total flavonoid content (TFC) of hybrid dried apples; and (d) the antioxidant capacity with DPPH radicals of hybrid dried apples. Blue dots on the figure shows research data.
Vitamin C was used as a criterion for nutritional quality estimation due to its high sensitivity to temperature, oxygen, and light [61]. As a result of US pre-treatment and hybrid drying, the vitamin C content changed and ranged from 107.5 ± 0.2 mg/100 g d.m. to 171.5 ± 8.2 mg/100 g d.m. For all of the US power values applied during drying, when US pre-treatment lasted 10 min, the vitamin C content was significantly lower as compared to those with US pre-treatment lasting 20 min or 30 min (Figure 7a). This finding was in agreement with the Pareto chart (Figure S5a), according to which the US treatment time had a significant linear effect on the vitamin C content of dried apples. A different finding was reported by Wang et al. [61]. The researchers used US pre-treatment for 10 min, 20 min, or 30 min before the hot-air drying of kiwifruit slices and observed the highest ascorbic acid content for the 20 min US pre-treatment, while its level did not differ significantly when the US treatment duration was 10 min or 30 min. In contrast, Rodríguez et al. [62] reported no significant differences in ascorbic acid content in convection-dried pineapples subjected to US pre-treatment in distilled water for 10 min, 20 min, or 30 min. Additionally, in the present study, for all variants with a US pre-treatment of 30 min and for the two variants with a US treatment of 20 min, when the US power during drying was 120 or 200 W, the vitamin C content was higher than for the fresh sample. This can be explained by the fact that the cavitation produced during US pre-treatment could eliminate the oxygen that might affect the degradation of vitamin C [63].
Polyphenols play an integral role in the antioxidant activity of foods by capturing active oxygen species [64]; however, they are thermolabile compounds [65]. As a result of processing, the total polyphenol content decreased in most cases. The exceptions were samples subjected to 20 min of US pre-treatment and US-assisted drying with 160 W (T_20_D_160W), 30 min of US pre-treatment and US-assisted drying with 200 W (T_30_D_200W), and 20 min of US pre-treatment and US-assisted drying with 200 W (T_20_D_200W), for which it slightly increased by 14.5 mg/100 g d.m., 40 mg/100 g d.m., and 98.3 mg/100 g d.m., respectively. This increase could be attributed to the release of more bound phenolic compounds due to the breakdown of cellular components. Although the breakdown of cell walls releases oxidative and hydrolytic enzymes that can degrade antioxidants, thermal processing may deactivate these enzymes, helping preserve phenolic acids [66]. Also, there was no noticeable correlation between the TPC and antioxidant activity (DPPH), which means that other compounds had a more substantial effect on antioxidant activity, e.g., vitamin C content. According to the response surface plot (Figure 7b), TPC reached the highest values for the longest US treatment time and the highest power of US during drying. This finding was in contrast with the results obtained by Rodríguez et al. [15]. They stated that apples subjected to drying with the application of ultrasound resulted in the loss of the TPC, around 20–39% of the polyphenols in the raw material. Furthermore, the application of a higher power of ultrasound resulted in a higher loss of TPC, especially when the drying temperature was 50 or 70 °C.
Flavonoids are one of the major groups of polyphenols in fruits and vegetables [60]. Similarly to TPC, TFC was lowered by the processing methods employed to values varying from 471 ± 21 mg/100 g d.m. to 701 ± 29 mg/100 g d.m. The highest TFC was achieved by samples subjected to 30 min of US pre-treatment and US-assisted drying with 160 W (T_30_D_160W), and the TFC usually decreased when the US treatment time decreased or the power of US during drying increased or decreased (Figure 7c). Nevertheless, for both polyphenol and flavonoid contents, neither the US treatment time nor the power of US during drying had a statistically significant linear or quadratic effect on the contents of the mentioned bioactive components (Figure S5b,c). Likewise, when the sonication in distilled water was applied for 10 min, 20 min, or 30 min prior to the hot-air drying of pineapple, the TPC and TFC were not significantly different to the duration of US application [62]. As regards the power of US during drying, Rodríguez et al. [15] conducted a study that allowed them to note statistically significant changes—when apples were hot-air-dried at 70 °C, the highest values of TPC and TFC were achieved without the use of ultrasound, while the higher the power of ultrasound, the greater the loss of the discussed bioactive components. The authors suggested that the highest US power could lead to the most deteriorative treatment and cellular damage.
The antioxidant activity of dried apples was lowest for the middle values of both US treatment time and US power during drying (Figure 7d). Although antioxidant activity increased for both increases and decreases in US treatment time and US power during drying, it can be stated from the Pareto chart (Figure S5d) that only the power of US during drying had a significant quadratic effect on antioxidant activity as measured by DPPH radical scavenging. In the study of Ren et al. [66], hot-air-dried and freeze-dried onion slices were subjected to 1 min, 3 min, or 5 min of US pre-treatment. Although there were no significant differences in antioxidant activity between freeze-dried samples subjected to sonication at different times, in the case of hot-air-dried samples, the antioxidant activity was lower for hot-air-dried samples treated with US for a longer time. Rodríguez et al. [15] stated that better antioxidant activity was observed at higher drying temperatures without the use of ultrasound due to shorter drying times, whereas at a higher acoustic density of 30.8 kW/m3, lower drying temperatures were more effective in minimizing the degradation of these quality parameters. Furthermore, when the sonication was applied during convective drying, Tao et al. [67] noted that a medium intensity (902.7 W/m2) of US enables the obtaining of dried garlic slices of higher antioxidant capacity than lower (216.8 W/m2) or higher (1513.5 W/m2) intensity. This finding was shown using ABTS and FRAP methods. Tao et al. investigated the effect of US-assisted air drying at 70 °C on broccoli florets. It was observed that the lower intensity of US (125.2 W/dm2) resulted in a significantly higher antioxidant capacity of broccoli than the higher intensity of ultrasound (180.1 W/dm2). However, it is worth mentioning that none of these variants had a significantly different antioxidant capacity than broccoli dried without sonication. The authors stated that all dried samples were characterized by similar antioxidant activity. In general, the antioxidant activity decreased as a result of drying. However, the decrease was similar for all applied drying methods. On one hand, a high level of ultrasound may have a destructive effect on phytochemicals due to the generation of free radicals [68]. On the other hand, it may reduce drying time, limit oxidation reactions, and maintain antioxidant activity [69].
3.7. Sugar Content and Fourier-Transform Infrared Spectroscopy
The total sugar content (TSC) in the fresh material was equal to 36.71 g/100 g of dry matter (4.94 g of sucrose/100 g d.m., 24.80 g of fructose/100 g d.m., and 6.11 g of glucose/100 g d.m.) (Figure 8), whereas the TSC in dried fruits ranged from 23.81 g/100 g d.m. to 31.64 g/100 g d.m. The US pre-treatment and US power applied during the drying process resulted in a reduction in the sugar content. The water-soluble saccharides may have been washed out by the water during the US pre-treatment. The obtained effect could also be influenced by the phenomenon of cavitation erosion, which increased the access of water to sugars and increased their outflow outside the tissue [70]. The results showed that the use of shorter ultrasound pre-treatment times and medium US powers during drying resulted in a higher sugar content in the material. Similarly, the use of shorter US pre-treatment times and the lower and medium US powers resulted in the highest fructose content in the material. The largest percentage of sugars in the dried apple tissue was fructose, respectively, in the range of 15.86 g/100 g d.m. to 21.85 g/100 g d.m. The more ripe the analyzed fruit was, the more simple sugars and less sucrose it contained [71,72], whereas the glucose content in the dried materials ranged from 2.63 g/100 g d.m. to 5.24 g/100 g d.m. and the use of average US pre-exposure times and average US powers during drying resulted in higher glucose content.
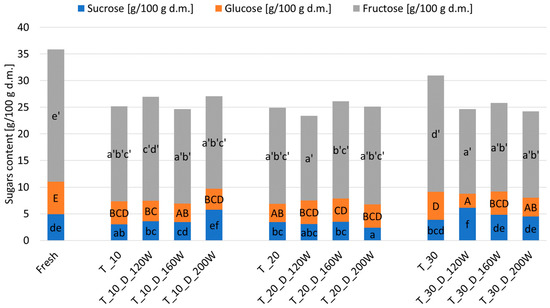
Figure 8.
The effect of the US treatment time and the US power applied during drying on the sugar content of hybrid dried apples. Different letters on columns (a–f for sucrose; A–E for glucose; a′–e′ for fructose) show the significant groups (α = 0.05).
As mentioned earlier, a decrease in sugars in dried apples compared with raw ones was found. Hot-air drying had an effect on the content of glucose and fructose in the dried material. At high temperatures, reducing sugars reacted with amino acids, peptides, or proteins containing a free amino group. Formed products of the Maillard reaction decreased the content of monosaccharides in the dried apple tissue. Reducing the sugar content may also have been related to the caramelization process [73]. Furthermore, the smallest amounts of sucrose occurred with the middle US pre-treatment time and the lower US power used during drying. This may have been related to the fact that sucrose had broken down into simple sugars (glucose, fructose), which were observed in large numbers in this area. Higher glucose and fructose contents in dried fruits can be explained by sucrose hydrolysis. In addition, the amount of sucrose was affected by the degree of apple ripeness and storage time [71,72].
On the basis of FTIR analysis, the chemical changes of the dried material were determined (Figure 9). The dried materials exhibited almost identical FTIR spectral patterns, with slight differences in the heights of the transmittance peaks across different wavenumber ranges. Almost identical infrared spectral patterns indicated the presence of the same functional groups in the analyzed fruits.
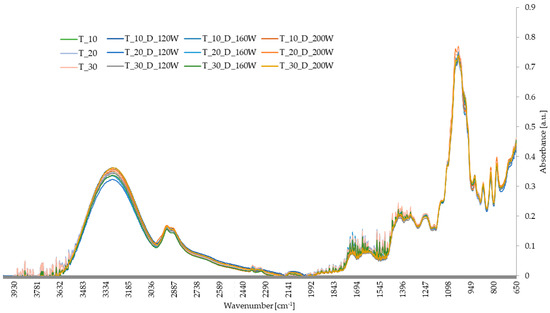
Figure 9.
The effect of the US treatment time and the US power applied during drying on the FTIR spectra of hybrid dried apples.
The first peak, in the wavenumber range of 3000–3500 cm−1, indicated O-H bond stretching, suggesting the presence of phenols, carboxylic acids, and alcohols in the analyzed fruits [74]. A broad peak with relatively high absorbance confirmed a significant content of polyphenols and flavonoids in the dried material. Another peak, within the wavenumber range of 2800–3000 cm−1, corresponded to the C–H stretching vibrations of aliphatic methyl and methylene groups (–CH3 and –CH2) present in the dried fruit material [75,76]. Additionally, a peak in the range of 1300–1500 cm−1 indicated the presence of these compounds in the apple tissue, specifically related to C-H bond bending. The absorbance in this range indicated the presence of proteins, organic acids, and polysaccharides (e.g., pectin) in the apple tissue [77]. The peak in the wavenumber range of 1700–1750 cm−1 indicated C=O bond stretching and the presence of aldehydes, ketones, and carboxylic acids in the dried material. Another peak, around 1720 cm−1, indicated the presence of organic acids in the fruits, whereas the peak in the range of 1600–1700 cm−1 was related to the presence of alkenes in the apple tissue, indicating C=C bond stretching [78]. Peaks in the wavenumber range of 1200–900 cm−1 were related to C-O bond bending and C-C bond stretching, suggesting the presence of compounds such as alcohols, ethers, and carboxylic acids, as well as sugars in the apple tissue [79].
The peak corresponding to a wavenumber of approximately 922 cm−1 belonged to the α-anomeric bond between glucose and fructose in sucrose, while the peak at 990 cm−1 related to the glycosidic bond in sucrose. The peak at 1030 cm−1 indicated C-O and C-C bond stretching in glucose. The band at 1046 cm−1 indicated exocyclic deformation of the C-O ring in fructose molecules. The peak at 1048 cm−1 was related to the C-O bond in sucrose, and the peak at 1102 cm−1 was due to C-O and C-C bond stretching, and C-O-H bond bending in glucose [78,80,81].
Previous studies indicated varying effects of sonication on biological materials. The application of ultrasound might have led to tissue deconstruction or left its original structure unchanged [82]. Zheng et al. [83], by analyzing infrared spectra, demonstrated the effect of single-frequency sonication on material deconstruction without altering functional groups, whereas using dual-frequency ultrasound resulted in additional changes in the functional groups of sweet potato tissue. Additionally, the stability of non-covalent bonds in materials increases with the frequency of ultrasonic waves [84].
4. Conclusions
This study uniquely integrated US pre-treatment and US-assisted drying and evaluated how ultrasound pre-treatment time (10 min, 20 min, 30 min), as well as ultrasound power during drying (120 W, 160 W, 200 W), influenced the quality of apples dried using a hybrid ultrasound-assisted convective drying method at 70 °C.
The conducted research showed that, depending on the parameters of the ultrasonic waves, the obtained dried product had different structures and physicochemical properties. The use of ultrasound could affect both the shortening and the prolongation of the mass exchange process. The combination of shorter pre-treatment (10 min) with higher ultrasound power during drying (200 W) or longer pre-treatment (30 min) with lower power (120 W) significantly reduced the drying time. A 20 min ultrasound pre-treatment combined with US-assisted hot-air drying with a US power of 120–160 W provided the optimal balance between drying efficiency and the preservation of key quality attributes, such as texture, color, soluble solids, and water vapor absorption. However, the retention of bioactive compounds was generally higher with longer ultrasound pre-treatments, although antioxidant activity showed a more complex, non-linear relationship with treatment parameters.
There was an attempt to examine the relationship between the tested properties in order to identify the most optimal parameters, i.e., ultrasound pre-treatment time and ultrasound power during drying. Due to low significance, neither the principal component analysis nor the correlation matrix was included. However, it has been demonstrated that it would be inappropriate to explicitly indicate the optimal US parameters in the treatments used. Based on the presented results, some patterns could be seen that might be valuable for future experiments and processes. A hybrid method based on US pre-treatment with US-assisted drying offers a promising approach to improving fruit processing; however, further research is essential to realize its full industrial potential and to establish standardized processing protocols.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app15105309/s1. Figure S1: The Pareto chart effects on US treatment time and the US power applied during drying for apple drying time to obtain MR = 0.01; Figure S2: The Pareto chart effects on US treatment time and the US power applied during drying for (a) the dry matter content (d.m.) of hybrid dried apples and (b) the water activity (aw) of hybrid dried apples; Figure S3: The Pareto chart effects on US treatment time and the US power applied during drying for (a) the rehydration rate (RR) of hybrid dried apples, (b) the soluble solid loss (SSL) of hybrid dried apples, and (c) the hygroscopic properties of hybrid dried apples; Figure S4: The Pareto chart effects on US treatment time and the US power applied during drying for (a) the total color change (ΔE) of hybrid dried apples and (b) the texture properties (Fmax recorded during a 50% deformation test) of hybrid dried apples; Figure S5: The Pareto chart effects on US treatment time and the US power applied during drying for (a) the vitamin C content of hybrid dried apples; (b) the total polyphenol content (TPC) of hybrid dried apples; (c) the total flavonoid content (TFC) of hybrid dried apples; and (d) the antioxidant capacity with DPPH radicals of hybrid dried apples.
Author Contributions
Conceptualization, D.W.-R. and M.N.; methodology, M.N. and K.R.; software, K.R. and M.N.; validation, K.R.; formal analysis, A.J., K.S., A.B.-D., A.S. and M.T.; investigation, K.R. and M.N.; resources, D.W-R. and M.N.; data curation, A.J., K.R., K.S., A.B-D., A.S. and M.T.; writing—original draft preparation, A.J., K.R., K.S., A.B-D., A.S., M.T. and M.N.; writing—review and editing, M.N. and D.W-R.; visualization, A.J., K.S., A.S., A.B-D., M.T. and M.N.; supervision, D.W-R. and M.N.; project administration, M.N.; funding acquisition, M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by transnational funding bodies, partners of the H2020 ERA-NETs SUSFOOD2 and CORE Organic Cofunds, under the Joint SUSFOOD2/CORE Organic Call 2019 (MILDSUSFRUIT) as well as the National Centre for Research and Development (POLAND, decision DWM/SF-CO/31/2021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed at the corresponding author.
Acknowledgments
The authors would like to thank Marta Sierpatowska for her help. The research for this publication was carried out with the use of research equipment purchased as part of the “Food and Nutrition Centre—modernization of the WULS campus to create a Food and Nutrition Research and Development Centre (CŻiŻ)” co-financed by the European Union from the European Regional Development Fund under the Regional Operational Programme of the Mazowieckie Voivodeship for 2014–2020 (Project No. RPMA.01.01.00-14-8276/17).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abbaspour-Gilandeh, Y.; Kaveh, M.; Fatemi, H.; Khalife, E.; Witrowa-Rajchert, D.; Nowacka, M. Effect of Pretreatments on Convective and Infrared Drying Kinetics, Energy Consumption and Quality of Terebinth. Appl. Sci. 2021, 11, 7672. [Google Scholar] [CrossRef]
- Miano, A.C.; Ibarz, A.; Augusto, P.E.D. Mechanisms for improving mass transfer in food with ultrasound technology: Describing the phenomena in two model cases. Ultrason. Sonochem. 2016, 29, 413–419. [Google Scholar] [CrossRef]
- Abbaspour-Gilandeh, Y.; Kaveh, M.; Jahanbakhshi, A. The effect of microwave and convective dryer with ultrasound pre-treatment on drying and quality properties of walnut kernel. J. Food Process. Preserv. 2019, 43, e14178. [Google Scholar] [CrossRef]
- Sledz, M.; Wiktor, A.; Nowacka, M.; Witrowa-Rajchert, D. Drying Kinetics, Microstructure and Antioxidant Properties of Basil Treated by Ultrasound. J. Food Process Eng. 2017, 40, e12271. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, P.; Wang, Y.; Kadam, S.U.; Han, Y.; Wang, J.; Zhou, J. Power ultrasound as a pretreatment to convective drying of mulberry (Morus alba L.) leaves: Impact on drying kinetics and selected quality properties. Ultrason. Sonochem. 2016, 31, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Mane, S.; Bremner, D.H.; Tziboula-Clarke, A.; Lemos, M.A. Effect of ultrasound on the extraction of total anthocyanins from Purple Majesty potato. Ultrason. Sonochem. 2015, 27, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Perera, C.O.; Alzahrani, M.A.J. Ultrasound as a pre-treatment for extraction of bioactive compounds and food safety: A review. LWT 2021, 142, 111114. [Google Scholar] [CrossRef]
- Liao, X.; Cullen, P.J.; Muhammad, A.I.; Jiang, Z.; Ye, X.; Liu, D.; Ding, T. Cold Plasma–Based Hurdle Interventions: New Strategies for Improving Food Safety. Food Eng. Rev. 2020, 12, 321–332. [Google Scholar] [CrossRef]
- Bi, X.; Wang, X.; Chen, Y.; Chen, L.; Xing, Y.; Che, Z. Effects of combination treatments of lysozyme and high power ultrasound on the Salmonella typhimurium inactivation and quality of liquid whole egg. Ultrason. Sonochem. 2020, 60, 104763. [Google Scholar] [CrossRef]
- Kowalski, S.J.; Pawłowski, A. Intensification of apple drying due to ultrasound enhancement. J. Food Eng. 2015, 156, 1–9. [Google Scholar] [CrossRef]
- Szadzińska, J.; Łechtańska, J.; Kowalski, S.J.; Stasiak, M. The effect of high power airborne ultrasound and microwaves on convective drying effectiveness and quality of green pepper. Ultrason. Sonochem. 2017, 34, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Vega-Gálvez, A.; Ah-Hen, K.; Chacana, M.; Vergara, J.; Martínez-Monzó, J.; García-Segovia, P.; Lemus-Mondaca, R.; Di Scala, K. Effect of temperature and air velocity on drying kinetics, antioxidant capacity, total phenolic content, colour, texture and microstructure of apple (var. Granny Smith) slices. Food Chem. 2012, 132, 51–59. [Google Scholar] [CrossRef]
- Available online: https://agridata.ec.europa.eu/extensions/DashboardFruitAndVeg/FruitandVegetableProduction.html (accessed on 3 March 2025).
- Moreno, C.; Brines, C.; Mulet, A.; Rosselló, C.; Cárcel, J.A. Antioxidant potential of atmospheric freeze-dried apples as affected by ultrasound application and sample surface. Dry. Technol. 2017, 35, 957–968. [Google Scholar] [CrossRef]
- Rodríguez, Ó.; Santacatalina, J.V.; Simal, S.; Garcia-Perez, J.V.; Femenia, A.; Rosselló, C. Influence of power ultrasound application on drying kinetics of apple and its antioxidant and microstructural properties. J. Food Eng. 2014, 129, 21–29. [Google Scholar] [CrossRef]
- Rodríguez, Ó.; Llabrés, P.J.; Simal, S.; Femenia, A.; Rosselló, C. Intensification of Predrying Treatments by Means of Ultrasonic Assistance: Effects on Water Mobility, PPO Activity, Microstructure, and Drying Kinetics of Apple. Food Bioprocess Technol. 2015, 8, 503–515. [Google Scholar] [CrossRef]
- Oliveira, F.I.P.; Gallão, M.I.; Rodrigues, S.; Fernandes, F.A.N. Dehydration of Malay Apple (Syzygium malaccense L.) Using Ultrasound as Pre-treatment. Food Bioprocess Technol. 2011, 4, 610–615. [Google Scholar] [CrossRef]
- Mierzwa, D.; Kowalski, S.J. Ultrasound-assisted osmotic dehydration and convective drying of apples: Process kinetics and quality issues. Chem. Process Eng. 2016, 37, 383–391. [Google Scholar] [CrossRef]
- Mothibe, K.J.; Zhang, M.; Mujumdar, A.S.; Wang, Y.C.; Cheng, X. Effects of Ultrasound and Microwave Pretreatments of Apple Before Spouted Bed Drying on Rate of Dehydration and Physical Properties. Dry. Technol. 2014, 32, 1848–1856. [Google Scholar] [CrossRef]
- Szadzińska, J.; Łechtańska, J.; Pashminehazar, R.; Kharaghani, A.; Tsotsas, E. Microwave- and ultrasound-assisted convective drying of raspberries: Drying kinetics and microstructural changes. Dry. Technol. 2019, 37, 1–12. [Google Scholar] [CrossRef]
- Magalhães, M.L.; Cartaxo, S.J.M.; Gallão, M.I.; García-Pérez, J.V.; Cárcel, J.A.; Rodrigues, S.; Fernandes, F.A.N. Drying intensification combining ultrasound pre-treatment and ultrasound-assisted air drying. J. Food Eng. 2017, 215, 72–77. [Google Scholar] [CrossRef]
- Opalić, M.; Domitran, Z.; Komes, D.; Belščak, A.; Horžić, D.; Karlović, D. The Effect of Ultrasound Pre-Treatment and Air-Drying on the Quality of Dried Apples. Czech J. Food Sci. 2009, 27, S297–S300. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Rodrigues, S.; Cárcel, J.A.; García-Pérez, J.V. Ultrasound-Assisted Air-Drying of Apple (Malus domestica L.) and Its Effects on the Vitamin of the Dried Product. Food Bioprocess Technol. 2015, 8, 1503–1511. [Google Scholar] [CrossRef]
- Ozuna, C.; Álvarez-Arenas, T.G.; Riera, E.; Cárcel, J.A.; Garcia-Perez, J.V. Influence of material structure on air-borne ultrasonic application in drying. Ultrason. Sonochem. 2014, 21, 1235–1243. [Google Scholar] [CrossRef]
- Santacatalina, J.V.; Contreras, M.; Simal, S.; Cárcel, J.A.; Garcia-Perez, J.V. Impact of applied ultrasonic power on the low temperature drying of apple. Ultrason. Sonochem. 2016, 28, 100–109. [Google Scholar] [CrossRef]
- Sabarez, H.T.; Gallego-Juarez, J.A.; Riera, E. Ultrasonic-Assisted Convective Drying of Apple Slices. Dry. Technol. 2012, 30, 989–997. [Google Scholar] [CrossRef]
- Santacatalina, J.V.; Rodríguez, O.; Simal, S.; Cárcel, J.A.; Mulet, A.; García-Pérez, J.V. Ultrasonically enhanced low-temperature drying of apple: Influence on drying kinetics and antioxidant potential. J. Food Eng. 2014, 138, 35–44. [Google Scholar] [CrossRef]
- Rybak, K.; Wiktor, A.; Kaveh, M.; Dadan, M.; Witrowa-Rajchert, D.; Nowacka, M. Effect of Thermal and Non-Thermal Technologies on Kinetics and the Main Quality Parameters of Red Bell Pepper Dried with Convective and Microwave–Convective Methods. Molecules 2022, 27, 2164. [Google Scholar] [CrossRef] [PubMed]
- AOAC 920.151; AOAC Official Method 920.151 Solids (Total) in Fruits and Fruit Products. AOAC Publications: New York, NY, USA, 2023. Available online: https://doi.org/10.1093/9780197610145.003.3365 (accessed on 20 January 2025).
- Kręcisz, M.; Kolniak-Ostek, J.; Łyczko, J.; Stępień, B. Evaluation of bioactive compounds, volatile compounds, drying process kinetics and selected physical properties of vacuum impregnation celery dried by different methods. Food Chem. 2023, 413, 135490. [Google Scholar] [CrossRef]
- Matys, A.; Dadan, M.; Witrowa-Rajchert, D.; Parniakov, O.; Wiktor, A. Response Surface Methodology as a Tool for Optimization of Pulsed Electric Field Pretreatment and Microwave-Convective Drying of Apple. Appl. Sci. 2022, 12, 3392. [Google Scholar] [CrossRef]
- Feng, S.; Bi, J.; Yi, J.; Li, X.; Li, J.; Ma, Y. Cell wall polysaccharides and mono-/disaccharides as chemical determinants for the texture and hygroscopicity of freeze-dried fruit and vegetable cubes. Food Chem. 2022, 395, 133574. [Google Scholar] [CrossRef]
- Fauster, T.; Giancaterino, M.; Pittia, P.; Jaeger, H. Effect of pulsed electric field pretreatment on shrinkage, rehydration capacity and texture of freeze-dried plant materials. LWT 2020, 121, 108937. [Google Scholar] [CrossRef]
- Rybak, K.; Parniakov, O.; Samborska, K.; Wiktor, A.; Witrowa-Rajchert, D.; Nowacka, M. Energy and Quality Aspects of Freeze-Drying Preceded by Traditional and Novel Pre-Treatment Methods as Exemplified by Red Bell Pepper. Sustainability 2021, 13, 2035. [Google Scholar] [CrossRef]
- Spínola, V.; Mendes, B.; Câmara, J.S.; Castilho, P.C. An improved and fast UHPLC-PDA methodology for determination of L-ascorbic and dehydroascorbic acids in fruits and vegetables. Evaluation of degradation rate during storage. Anal. Bioanal. Chem. 2012, 403, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Bochnak-Niedźwiecka, J.; Szymanowska, U.; Świeca, M. The Protein-Rich Powdered Beverages Stabilized with Flax Seeds Gum—Antioxidant and Antiproliferative Properties of the Potentially Bioaccessible Fraction. Appl. Sci. 2022, 12, 7159. [Google Scholar] [CrossRef]
- Tian, W.; Chen, G.; Tilley, M.; Li, Y. Changes in phenolic profiles and antioxidant activities during the whole wheat bread-making process. Food Chem. 2021, 345, 128851. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, H.; Woźniak, Ł.; Masiarz, E.; Stelmach, A.; Salamon, A.; Kowalska, J.; Piotrowski, D.; Marzec, A. The impact of using polyols as osmotic agents on mass exchange during osmotic dehydration and their content in osmodehydrated and dried apples. Dry. Technol. 2020, 38, 1620–1631. [Google Scholar] [CrossRef]
- Maceda, A.; Soto-Hernández, M.; Terrazas, T. Cellulose in Secondary Xylem of Cactaceae: Crystalline Composition and Anatomical Distribution. Polymers 2022, 14, 4840. [Google Scholar] [CrossRef]
- Tao, Y.; Han, M.; Gao, X.; Han, Y.; Show, P.-L.; Liu, C.; Ye, X.; Xie, G. Applications of water blanching, surface contacting ultrasound-assisted air drying, and their combination for dehydration of white cabbage: Drying mechanism, bioactive profile, color and rehydration property. Ultrason. Sonochem. 2019, 53, 192–201. [Google Scholar] [CrossRef]
- Amanor-Atiemoh, R.; Zhou, C.; Abdullaleef Taiye, M.; Sarpong, F.; Wahia, H.; Amoa-Owusu, A.; Ma, H.; Chen, L. Effect of ultrasound-ethanol pretreatment on drying kinetics, quality parameters, functional group, and amino acid profile of apple slices using pulsed vacuum drying. J. Food Process Eng. 2020, 43, e13347. [Google Scholar] [CrossRef]
- Rajewska, K.; Mierzwa, D. Influence of ultrasound on the microstructure of plant tissue. Innov. Food Sci. Emerg. Technol. 2017, 43, 117–129. [Google Scholar] [CrossRef]
- Pieczywek, P.M.; Kozioł, A.; Konopacka, D.; Cybulska, J.; Zdunek, A. Changes in cell wall stiffness and microstructure in ultrasonically treated apple. J. Food Eng. 2017, 197, 1–8. [Google Scholar] [CrossRef]
- Fijałkowska, A.; Nowacka, M.; Witrowa-Rajchert, D. The physical, optical and reconstitution properties of apples subjected to ultrasound before drying. Ital. J. Food Sci. 2017, 29, 343–356. [Google Scholar] [CrossRef]
- Nowacka, M.; Wiktor, A.; Śledź, M.; Jurek, N.; Witrowa-Rajchert, D. Drying of ultrasound pretreated apple and its selected physical properties. J. Food Eng. 2012, 113, 427–433. [Google Scholar] [CrossRef]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of Water Activity (a w) on Microbial Stability as a Hurdle in Food Preservation. In Water Activity in Foods; Barbosa-Cánovas, G.V., Fontana, A.J., Jr., Schmidt, S.J., Labuza, T.P., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 323–355. ISBN 9781118765982. [Google Scholar]
- Rani, P.; Tripathy, P.P. Effect of ultrasound and chemical pretreatment on drying characteristics and quality attributes of hot air dried pineapple slices. J. Food Sci. Technol. 2019, 56, 4911–4924. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, G.; Izli, G. The effect of ultrasound pretreatment on quality attributes of freeze-dried quince slices: Physical properties and bioactive compounds. J. Food Process Eng. 2019, 42, e13223. [Google Scholar] [CrossRef]
- Kahraman, O.; Malvandi, A.; Vargas, L.; Feng, H. Drying characteristics and quality attributes of apple slices dried by a non-thermal ultrasonic contact drying method. Ultrason. Sonochem. 2021, 73, 105510. [Google Scholar] [CrossRef]
- Ricce, C.; Rojas, M.L.; Miano, A.C.; Siche, R.; Augusto, P.E.D. Ultrasound pre-treatment enhances the carrot drying and rehydration. Food Res. Int. 2016, 89, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, M.; Xu, B.; Sun, J.; Mujumdar, A.S. Artificial intelligence assisted technologies for controlling the drying of fruits and vegetables using physical fields: A review. Trends Food Sci. Technol. 2020, 105, 251–260. [Google Scholar] [CrossRef]
- Szadzińska, J.; Mierzwa, D.; Pawłowski, A.; Musielak, G.; Pashminehazar, R.; Kharaghani, A. Ultrasound- and microwave-assisted intermittent drying of red beetroot. Dry. Technol. 2020, 38, 93–107. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, M.; Shi, W. Evaluation of ultrasound pretreatment and drying methods on selected quality attributes of bitter melon (Momordica charantia L.). Dry. Technol. 2019, 37, 387–396. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Colour difference ∆E-A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Nahimana, H.; Zhang, M. Shrinkage and Color Change during Microwave Vacuum Drying of Carrot. Dry. Technol. 2011, 29, 836–847. [Google Scholar] [CrossRef]
- Zhang, Z.; Niu, L.; Li, D.; Liu, C.; Ma, R.; Song, J.; Zhao, J. Low intensity ultrasound as a pretreatment to drying of daylilies: Impact on enzyme inactivation, color changes and nutrition quality parameters. Ultrason. Sonochem. 2017, 36, 50–58. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S. Microstructure and its relationship with quality and storage stability of dried foods. In Food Microstructure and Its Relationship with Quality and Stability; Elsevier: Amsterdam, The Netherlands, 2018; pp. 139–159. ISBN 9780081007648. [Google Scholar]
- Rojas, M.L.; Kubo, M.T.K.; Caetano-Silva, M.E.; Augusto, P.E.D. Ultrasound processing of fruits and vegetables, structural modification and impact on nutrient and bioactive compounds: A review. Int. J. Food Sci. Technol. 2021, 56, 4376–4395. [Google Scholar] [CrossRef]
- Fijałkowska, A.; Nowacka, M.; Witrowa-Rajchert, D. Effect of ultrasound waves on drying process and selected properties of beetroot tissue. Food Sci. Technol. Qual. 2015, 21, 138–149. [Google Scholar] [CrossRef]
- Xu, B.; Sylvain Tiliwa, E.; Yan, W.; Roknul Azam, S.M.; Wei, B.; Zhou, C.; Ma, H.; Bhandari, B. Recent development in high quality drying of fruits and vegetables assisted by ultrasound: A review. Food Res. Int. 2022, 152, 110744. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, H.-W.; Ye, J.-H.; Wang, J.; Raghavan, V. Ultrasound Pretreatment to Enhance Drying Kinetics of Kiwifruit (Actinidia deliciosa) Slices: Pros and Cons. Food Bioprocess Technol. 2019, 12, 865–876. [Google Scholar] [CrossRef]
- Rodríguez, Ó.; Gomes, W.; Rodrigues, S.; Fernandes, F.A.N. Effect of acoustically assisted treatments on vitamins, antioxidant activity, organic acids and drying kinetics of pineapple. Ultrason. Sonochem. 2017, 35, 92–102. [Google Scholar] [CrossRef]
- Oladejo, A.O.; Ma, H.; Qu, W.; Zhou, C.; Wu, B. Effects of Ultrasound on Mass Transfer Kinetics, Structure, Carotenoid and Vitamin C Content of Osmodehydrated Sweet Potato (Ipomea batatas). Food Bioprocess Technol. 2017, 10, 1162–1172. [Google Scholar] [CrossRef]
- Huang, M.-T.; Ferraro, T. Phenolic compounds in food and cancer prevention. In Phenolic Compounds in Food and Their Effects on Health II; ASC: Washington, WA, USA, 1992; pp. 8–34. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Ren, F.; Perussello, C.A.; Zhang, Z.; Kerry, J.P.; Tiwari, B.K. Impact of ultrasound and blanching on functional properties of hot-air dried and freeze dried onions. LWT 2018, 87, 102–111. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, J.; Jiang, S.; Xu, Y.; Show, P.-L.; Han, Y.; Ye, X.; Ye, M. Contacting ultrasound enhanced hot-air convective drying of garlic slices: Mass transfer modeling and quality evaluation. J. Food Eng. 2018, 235, 79–88. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, D.-W. Enhancement of Food Processes by Ultrasound: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 570–594. [Google Scholar] [CrossRef]
- Rodríguez, Ó.; Eim, V.; Rosselló, C.; Femenia, A.; Cárcel, J.A.; Simal, S. Application of power ultrasound on the convective drying of fruits and vegetables: Effects on quality. J. Sci. Food Agric. 2018, 98, 1660–1673. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, E.C.; Grillo, G.; Tabasso, S.; Stevanato, L.; Cravotto, G.; Marjamaa, K.; Pihlajaniemi, V.; Koivula, A.; Aro, N.; Uusitalo, J.; et al. Optimization of ultrasound pretreatment and enzymatic hydrolysis of wheat straw: From lab to semi-industrial scale. J. Clean. Prod. 2022, 380, 134897. [Google Scholar] [CrossRef]
- Rincon, A.; Kerr, W.L. Influence of osmotic dehydration, ripeness and frozen storage on physicochemical properties of mango. J. Food Process. Preserv. 2010, 34, 887–903. [Google Scholar] [CrossRef]
- Gonzalez, J.; Fuchs, C.; Betts, J.; Van Loon, L. Glucose Plus Fructose Ingestion for Post-Exercise Recovery—Greater than the Sum of Its Parts? Nutrients 2017, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.; Zieliński, H. Maillard reaction products in food. Food Sci. Technol. Qual. 2007, 2, 5–16. (In Polish) [Google Scholar]
- Masithoh, R.E.; Amanah, H.Z.; Cho, B.K. Application of Fourier Transform Near-Infrared (FT-NIR) and Fourier Transform Infrared (FT-IR) Spectroscopy Coupled with Wavelength Selection for Fast Discrimination of Similar Color of Tuber Flours. Indones. J. Chem. 2020, 20, 680. [Google Scholar] [CrossRef]
- Duan, W.; Zhang, Z.; Zhu, J.; Zhang, D.; Qian, D.; Teng, F.; Zhao, Y.; Chen, F.; Li, R.; Yang, J. Comparative Analysis of the Phenolic Profile of Lycium barbarum L. Fruits from Different Regions in China. Molecules 2022, 27, 5842. [Google Scholar] [CrossRef]
- Kowalska, H.; Trusinska, M.; Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Nowacka, M. Shaping the Properties of Osmo-Dehydrated Strawberries in Fruit Juice Concentrates. Appl. Sci. 2023, 13, 2728. [Google Scholar] [CrossRef]
- Synytsya, A.; Bleha, R.; Skrynnikova, A.; Babayeva, T.; Čopíková, J.; Kvasnička, F.; Jablonsky, I.; Klouček, P. Mid-Infrared Spectroscopic Study of Cultivating Medicinal Fungi Ganoderma: Composition, Development, and Strain Variability of Basidiocarps. J. Fungi 2023, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.S.; Liew, R.K.; Lim, X.Y.; Ani, F.N.; Jusoh, A. Fruit waste as feedstock for recovery by pyrolysis technique. Int. Biodeterior. Biodegrad. 2016, 113, 325–333. [Google Scholar] [CrossRef]
- Ma, F.; Chen, J.; Wu, X.; Zhou, Q.; Sun, S. Rapid discrimination of Panax notogeinseng of different grades by FT-IR and 2DCOS-IR. J. Mol. Struct. 2016, 1124, 131–137. [Google Scholar] [CrossRef]
- Al-Amrani, M.; Al-Alawi, A.; Al-Marhobi, I. Assessment of Enzymatic Browning and Evaluation of Antibrowning Methods on Dates. Int. J. Food Sci. 2020, 2020, 8380461. [Google Scholar] [CrossRef] [PubMed]
- Nizamlioglu, N.M.; Yasar, S.; Bulut, Y. Chemical versus infrared spectroscopic measurements of quality attributes of sun or oven dried fruit leathers from apple, plum and apple-plum mixture. LWT 2022, 153, 112420. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, F. Ultrasound modified polysaccharides: A review of structure, physicochemical properties, biological activities and food applications. Trends Food Sci. Technol. 2021, 107, 491–508. [Google Scholar] [CrossRef]
- Zheng, J.; Li, Q.; Hu, A.; Yang, L.; Lu, J.; Zhang, X.; Lin, Q. Dual-frequency ultrasound effect on structure and properties of sweet potato starch. Starch-Stärke 2013, 65, 621–627. [Google Scholar] [CrossRef]
- Feng, L.; Cao, Y.; Xu, D.; Wang, S.; Zhang, J. Molecular weight distribution, rheological property and structural changes of sodium alginate induced by ultrasound. Ultrason. Sonochem. 2017, 34, 609–615. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).