Featured Application
This study will highlight the importance of selecting suitable porcine by-products and implementing enzymatic hydrolysis to produce high-quality protein hydrolyzate with promising antioxidant properties. Potential candidates include Alcalase-hydrolyzed porcine kidney and trypsin-hydrolyzed porcine lung.
Abstract
Porcine by-products have garnered attention as an excellent material for producing antioxidant peptides; however, understanding the antioxidant characteristics of protein hydrolyzates derived from specific parts remains limited. In this study, we compared the antioxidant properties of protein hydrolyzates derived from major porcine organs (heart, kidney, spleen, liver, and lung) and performed classification based on their antioxidative potential. Their chemical composition exhibited significant variations, with a high protein content ranging from 15.90 to 20.30 g/100 g. Alcalase achieved higher hydrolysis efficiency than trypsin, which induced limited degradation of some proteins, such as porcine serum albumin. The hydrolyzates exhibited superior radical scavenging activities compared to the raw materials, although their reducing power remained unaffected or, in some instances, decreased. Hierarchical and k-mean cluster analyses revealed distinct antioxidant profiles and Alcalase-hydrolyzed kidney and trypsin-hydrolyzed lung hydrolyzates were deemed the most promising candidates, with strong radical scavenging activities and reducing power. Our findings indicate that, even when processed in bulk rather than being obtained from specific parts, porcine by-products can produce hydrolyzates rich in antioxidant peptides through enzymatic hydrolysis. However, selectively processing porcine kidneys with Alcalase and lungs with trypsin is recommended to produce premium products with enhanced and balanced antioxidant properties.
1. Introduction
The practical and value-added utilization of animal by-products is crucial to sustainable livestock production goals, particularly considering the significant socio-economic losses and environmental pollution associated with their disposal [1]. Generally, animal by-products are categorized as edible, non-edible, and waste materials [2]. Edible by-products include the blood and organs (e.g., liver, kidney, heart, lung, and intestine), which are both nutritious and protein-rich and are traditionally consumed as delicacies or used as valuable feed ingredients [1]. Meanwhile, despite their low cost, non-edible animal by-products are industrially rendered to produce carbonaceous and nitrogenous compounds without a value-adding process [2,3]. Industrial efforts have mainly focused on identifying novel ways to commercialize these underutilized by-products and developing advanced technologies to enhance their value.
Protein hydrolyzate from animal by-products has been identified as a potential animal feed resource due to its nutritional benefits (such by-products are high in protein, with a balanced amino acid profile), high digestibility, growth-promoting effects, immune support, palatability, and competitive cost [3]. Traditionally, protein hydrolysis has primarily been used to improve the solubility and digestibility of low-value by-products such as bone, skin, and feathers [2]. Recently, however, enzymatic hydrolysis has been utilized to generate bioactive peptides, enhancing the physiological benefits of edible by-products. In particular, the application of enzymatic hydrolyzates derived from edible by-products has markedly increased in sectors that place greater emphasis on anti-allergenic and functional properties, such as in pet food production, whereas such processes are not considered when creating traditional animal feed for livestock [3]. Moreover, protein hydrolyzates derived from animal by-products containing antioxidant peptides enhance the antioxidant content in animal feed [3]. Previous studies have reported that incorporating antioxidant protein hydrolyzates could delay lipid oxidation in feed or produce antioxidant effects in vivo in animals [4,5].
Previous studies have indicated that porcine liver is a promising edible by-product which produces protein hydrolyzates rich in antioxidative peptides; this can be attributed to its high protein content and balanced amino acids [6,7,8,9]. Verma et al. [6] reported that trypsin outperformed Alcalase and papain in terms of enhancing the antioxidant and antimicrobial activities of porcine liver hydrolyzates. Meanwhile, Borrajo et al. [7] observed that Alcalase-hydrolyzed porcine liver had a better antioxidant capacity compared to hydrolyzates treated with bromelain or papain. According to López-Pedrouso et al. [8], ferritin and trypsinogen in porcine liver are parent proteins that play major roles in the generation of antioxidative peptides. Moreover, previous research found that whole trypsin-mediated porcine hydrolyzate exhibited higher antioxidant capacity than specific molecular weight fractions [9]. Given that peptide fractionation involves additional costs associated with the separation and purification processes, the applicability of whole protein hydrolyzates could be highly encouraging from an industrial perspective. Moreover, previous reports suggest that Alcalase and trypsin are industrially applicable proteases capable of ensuring effective protein hydrolysis [8,9].
Animal by-products collected from slaughterhouses are often gathered and distributed in bulk without undergoing any form of sorting or categorization. In Korea, it is common practice to separate edible pork by-products into red offal (thoracic organs, such as liver, heart, and lung) and white offal (abdominal organs, such as stomach, pancreas, spleen, kidney, and intestines) to prevent cross-contamination. However, separating and collecting specific organs requires either additional labor or the implementation of automated separation systems, which may increase product costs. A previous study revealed differences in protein content (e.g., 16.6 g/100 g in the lung and 22.05 g/100 g in the liver) and amino acid composition [10]. The variations in such biological characteristics between porcine by-products may affect the hydrolytic characteristics and the antioxidant properties of their hydrolyzates. As such, classifying porcine organs during raw material selection is a key concern in the production of by-product hydrolyzates. While previous studies have examined the antioxidant properties of hydrolyzates derived from specific porcine organs, such as the heart, liver, spleen, and lungs [6,7,8,9], comparative analyses of hydrolyzates from various organs and their classification based on these properties remain limited.
Based on the aforementioned concerns, the objective of this study was to investigate the hydrolytic and antioxidant characteristics of whole hydrolyzates derived from porcine organs (heart, kidney, spleen, liver, and lung) and to classify the organs (and their hydrolyzates) based on their antioxidant properties. Our findings provide valuable insights into the necessity of sorting and selecting specific porcine organs to produce antioxidant protein hydrolyzates with practical applicability.
2. Materials and Methods
2.1. Raw Material
The porcine organs used in this study were obtained from female or castrated commercial crossbred pigs (Landrace × Yorkshire × Duroc; LYD) with an average live weight exceeding 110 kg. The animals were slaughtered at a domestic slaughterhouse in Gwangju, Korea. On the same day, approximately 20 kg each of heart, kidney, spleen, liver, and lung was randomly collected, sealed in plastic bags, stored in a cooler with ice, and transported to the laboratory. Upon arrival, the porcine organs were washed several times to remove contaminants and manually cut into small cubes (approximately 5 × 5 × 5 cm). The cubes were frozen at −70 °C and lyophilized using a freeze dryer (80 × 10–3 Torr pressure, PVTFD10R, Ilshin Lab, Daejeon, Republic of Korea). The freeze-dried samples were then powdered using a commercial food blender, individually vacuum-packaged in PA/PE bags, and stored at −20 °C until further use.
2.2. Enzymatic Hydrolysis Procedure
The sample powder was suspended in deionized distilled water (DDW) at 5% concentration (w/v), and the pH was adjusted to 8.0 using 0.1 N sodium hydroxide, considering the pH ranges for optimal activation (pH 8.0 for trypsin and pH 7.0–8.5 for Alcalase) [8]. For each porcine organ, the sample mixture was divided into three 500 mL volumes and assigned to non-hydrolyzed control, Alcalase-hydrolyzed treatment, and trypsin-hydrolyzed treatment groups. Two types of proteases, Alcalase 2.4 L (with a declared activity of 2.4 AU/kg, density of 1.18 g/mL, Novyzymes, Bagsværd, Denmark) and trypsin from porcine pancreas (EC 3.4.21.4, 1500 U/mg, Sigma-Aldrich, Saint Louis, MO, USA) were added in a 1:100 enzyme–substrate (protein concentration) ratio (E:S) [8]. For Alcalase-hydrolyzed treatments, the sample mixture was enzymatically hydrolyzed in a 50 °C incubator for 2 h, whereas the trypsin-mediated hydrolysis was performed at 37 °C for 2 h. Next, the samples were heated in an 85 °C water bath for 20 min to deactivate the proteases. The hydrolyzates were cooled at room temperature for 30 min and filtered through a mesh sieve (size 500 μm/35, line thickness 315 μm, 885705, Chung-Gye Sanggong Sa, Seoul, Republic of Korea) to remove the insoluble residues. The filtrates were freeze-dried and the sample powder was vacuum-packaged and stored at −20 °C to await further experiments. Three batches underwent this process on different days.
2.3. Chemical Composition and Extractable Protein of Porcine Organs
2.3.1. Chemical Composition
The moisture, protein, fat, ash, and collagen contents of lyophilized porcine organs were measured in triplicate using a Food ScanTM2 (Foss Tecator, Höganäs, Sweden) as described by Hoa et al. [11].
2.3.2. Extractable Protein
The amount of extractable protein in porcine by-products was determined in triplicate according to a modified version of the protein solubility method described by Kim et al. [12]. Firstly, 3 g of lyophilized sample powder was homogenized with 30 mL of DDW at 10,000 rpm for 1 min. The homogenate was centrifuged at 3500× g for 20 min (4 °C), and the supernatant was filtered through filter papers (Whatman No. 4). The protein concentration was determined by the biuret method, and extractable protein was expressed as mg per g of dry matter (mg/g dry matter).
2.4. Analysis of Hydrolytic Characteristics
2.4.1. Degree of Hydrolysis
The degree of hydrolysis was determined in triplicate according to the o-phthalaldehyde (OPA) spectrophotometric assay described by Morais et al. [13]. Ten microliters of the sample were reacted with 3.4 mL of OPA reagent, and the absorbance of the reactant was read at 340 nm. The degree of hydrolysis was calculated using the following equation:
where As is the absorbance of each sample, d is the dilution factor, and c is the protein content of the sample (g/L).
Degree of hydrolysis (%) = (As × 1.934 × d)/c
2.4.2. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Protein SDS-PAGE was performed with the Laemmli method described by Song et al. [14]. All samples were dissolved in DDW, mixed with sample buffer, and heated for 5 min, in which the final protein concentration was equally fixed to 2 μg/μL. Fifteen microliters of each sample was injected and loaded onto the 5% polyacrylamide stacking gel (pH 6.8) at 80 V for approximately 10 min, followed by a 12% separating gel (pH 8.8) at 120 V. After loading, the gel was stained with a staining solution containing 0.25% (w/v) Coomassie blue R-250, 50% (v/v) methanol, 40% (v/v) DDW, and 10% (v/v) acetic acid and destained with a destaining solution containing 50% (v/v) methanol, 40% (v/v) DDW, and 10% (v/v) acetic acid. The same volume of standard protein marker (Dokdo-mark EBM-1032, Elpis, Daejeon, Republic of Korea) was also loaded to compare the molecular weight of protein bands.
2.5. Antioxidant Assay of Porcine By-Products Hydrolyzates
2.5.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
The DPPH radical scavenging activity of the hydrolyzates was determined in triplicate [15]. Four different concentrations (0.25, 0.5, 0.75, and 1 mg/mL) of samples were prepared, and 1.5 mL of the diluted sample, 1.5 mL of 99.5% (v/v) ethanol, and 375 μL of 0.02% (w/v) ethanolic 0.5 mM DPPH solution were mixed. As a control, 1.5 mL of DDW was added instead of the diluted sample. The mixture was incubated in a dark room (22 °C) for 50 min and centrifuged at 2500× g for 10 min to spin down insoluble fractions. The absorbance of the supernatant was read at 517 nm against a blank using a spectrophotometer (Libia S22 UV/Vis Spectrophotometer, Biochrom Ltd., Cambridge, UK), and DPPH radical scavenging activity was calculated using the following equation:
where Ab is the absorbance of control and As is the absorbance of each sample.
DPPH radical scavenging activity (%) = ((Ab − As)/Ab) × 100
The DPPH radical IC50 value, which was the sample concentration required to achieve 50% inhibition, was calculated based on the results.
2.5.2. 2-Azino-bis-3-ethylbenzothiazoline-6-sulfonic Acid (ABTS) Radical Scavenging Activity
The ABTS radical-scavenging activity of the hydrolyzates was performed in triplicate [16]. Forty microliters of serially diluted samples (1, 5 and 10 mg/mL) or DDW (as control) were mixed with 5 mL of ABTS+ reagents (1:1 mixture of 7.4 mM ABTS reagent and 2.6 mM potassium persulfate) and reacted at room temperature (dark condition) for 6 min. The absorbance of the reactant was read at 734 nm using the spectrophotometer. The ABTS radical scavenging activity was calculated using the following equation:
where Ab is the absorbance of the control and As represents the absorbance of each sample.
ABTS radical scavenging activity (%) = ((Ab − As)/Ab) × 100
Based on the results, the ABTS radical IC50 value, defined as the sample concentration at 50% inhibition, was calculated.
2.5.3. Hydroxyl Radical Scavenging Activity
The hydroxyl (OH) radical scavenging activity of the hydrolyzates was determined in triplicate according to the method described by Cui et al. [16]. One milliliter of serially diluted sample (1, 2.5, and 5 mg/mL) or DDW (as control) was mixed with 1 mL of 6 mM hydrogen peroxide (H2O2), and the mixture was incubated at 37 °C for 10 min. After incubation, 1 mL of 6 mM salicylic acid was added and reacted at 37 °C for 30 min. The absorbance of the reactant was read at 510 nm, and hydroxyl radical scavenging activity was calculated using the following equation.
where Ab is the absorbance of the control and As is the absorbance of each sample.
Hydroxyl radical scavenging activity (%) = ((Ab − As)/Ab) × 100
The OH radical IC50 value, the sample concentration at 50% inhibition, was calculated based on the results.
2.5.4. Reducing Power
The reducing power of the hydrolyzates was determined in triplicate [17]. Two milliliters of diluted sample (1 mg/mL) were mixed with 2 mL of 0.2 M sodium phosphate buffer (pH 6.6) and 2 mL of 1% potassium ferricyanide. The mixture was incubated at 50 °C for 20 min, and then 2 mL of 10% trichloroacetic acid (TCA) was mixed. Two milliliters of aliquots from the reactant were mixed with 2 mL of DDW and 0.4 mL of 0.1% (w/v) ferric chloride and then incubated at 50 °C for 10 min. The absorbance of the final reactant was read at 700 nm using the spectrophotometer.
2.6. Statistical Analysis
The experimental design of this study was a completely randomized block design with three independent batches. Data were expressed as mean ± standard deviation. Analysis of variance (ANOVA) was performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA), where raw material effect (five different porcine organs), hydrolysis effect (non-hydrolyzed control, Alcalase-hydrolyzed treatment, and trypsin-hydrolyzed treatment), and/or their interaction were set as fixed main effects. When a significant effect was observed, Duncan’s multiple range test was applied to determine significant differences between treatment means at p < 0.05. Cluster analysis was conducted to classify the antioxidant characteristics of raw materials and their hydrolyzates. Hierarchical cluster analysis of the Z-scores of the measured variables related to antioxidant properties was performed using the SPSS program to visualize the similarities in a dendrogram. Subsequently, k-means cluster analysis was performed to obtain the final cluster centers and assign treatments to each cluster based on the cluster membership number.
3. Results and Discussion
3.1. Chemical Composition and Extractable Protein of Porcine Organs
3.1.1. Chemical Composition
The chemical compositions of porcine heart, kidney, spleen, liver, and lung are shown in Table 1. Significant variations in moisture, protein, fat, and ash content were observed among the porcine organs, while no significant difference was found in terms of collagen content. Notably, the protein content was ranked as follows: spleen ≥ liver > lung > heart ≥ kidney. The lowest fat content was found in the spleen (p < 0.05), whereas the liver had the highest ash content (p < 0.05); this is likely attributable to its mineral-rich profile, which includes phosphorus, potassium, iron, manganese, zinc, and copper [10]. Previous studies reported similar protein and fat content in porcine heart, liver, lung, and spleen [10,18]. These findings confirm that porcine organs could be valuable raw materials for producing protein hydrolyzates, particularly considering their high protein content.

Table 1.
Chemical composition of porcine organ.
3.1.2. Extractable Protein
The porcine organs contained significantly different quantities of extractable protein (Figure 1a). The highest amount was identified in the heart (262.7 mg/g dry matter), whereas the lung contained the lowest amount of extractable protein (155.1 mg/g dry matter) (p < 0.05). Generally, the solubility and extractability of food proteins are related to intrinsic (protein content, amino acid composition/sequence, and modifications) and extrinsic factors (pH, ionic strength, temperature, and solvents), which influence the protein–protein and protein–water interactions [19]. Collagen, which is commonly found in the connective tissues of organs, is a highly stable macromolecule under neutral-pH and low-temperature conditions [20]. Thus, although there were no statistically significant differences in collagen content (Table 1), the relationship between the collagen content and the extractable protein indicates that collagen content could be one of the critical factors influencing the amount of protein that can be extracted from porcine organs.
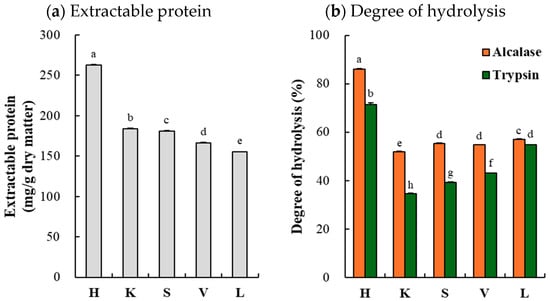
Figure 1.
Extractable protein (a) of porcine organs and degree of hydrolysis (b) of their hydrolyzates. H, heart; K, kidney; S, spleen; V, liver; L, lung. Each error bar indicates the standard deviation (n = 3). a–h means with the same letter are not significantly different (p ≥ 0.05).
3.2. Hydrolytic Characteristics
3.2.1. Degree of Hydrolysis
Significant interaction effects on the degree of hydrolysis of porcine organ hydrolyzates were found (Figure 1b). The porcine heart exhibited a remarkably higher degree of hydrolysis than the other organs (p < 0.05), which could be associated with the increased extractable protein in this organ. Numerous studies have noted that enzymatic hydrolysis could improve the solubility of food proteins as a result of exposure to free amino acids and carboxyl groups from peptide bonds [21]. On the contrary, our results indicate that the high extractable protein content could also increase the degree of hydrolysis. Mora and Toldrá [22] noted that a combination of advanced technologies, such as ultrasonication and high hydrostatic pressure, which facilitate conformational changes in food protein, could enhance the efficiency of enzymatic hydrolysis and lead to significant increases in the degree of hydrolysis. Another noticeable trend highlighting the more efficient enzymatic hydrolysis of porcine organs was observed for Alcalase compared to trypsin (p < 0.05). Similarly, Wang et al. [23] suggested that Alcalase is more suitable for hydrolyzing porcine liver than trypsin, based on the efficiency of its hydrolysis. Furthermore, our results indicate that Alcalase could have greater hydrolysis efficiency than trypsin in porcine liver, as well as in other porcine organs.
3.2.2. SDS-PAGE
Protein SDS-PAGE (Figure 2) confirmed the degradation of major proteins contained in porcine organs; a visual representation of the main effects, showing good agreement with the findings on the degree of hydrolysis, is presented in Figure 1b. In the non-hydrolyzed control, distinct protein bands were observed, most of which disappeared after enzymatic hydrolysis. This was likely due to the hydrolysis of the observed proteins into low-molecular-weight peptides and free amino acids. These types of alterations in protein patterns were more prominent in the porcine heart treatments, which consistently aligned with the increased degree of hydrolysis. Notably, a protein band with a molecular weight of ≈65–70 kDa (indicated by the arrow in Figure 2) remained even after enzymatic hydrolysis, particularly in trypsin-mediated treatment, and a similar phenomenon was observed in all porcine organs used in this study. Based on its molecular weight, abundance, and prevalence, the protein band can be presumed to represent porcine serum albumin (≈66 kDa). Kim et al. [24] found that trypsin treatment alone cannot completely degrade porcine serum albumin; extrinsic factors are required to facilitate structural changes. Extending the duration of hydrolysis may lead to further degradation of trypsin-resistant albumin and other similar proteins. As mentioned earlier, utilizing pretreatment technologies to induce conformational changes in proteins within porcine organs could serve as a practical approach to enhance the hydrolysis efficiency of proteins resistant to trypsin-mediated hydrolysis. Furthermore, the current results are limited; thus, it is necessary to explore the impact of trypsin-mediated selective hydrolysis on the degradation of major proteins present in porcine organs by observing the low-molecular-weight region to determine the peptide distribution.
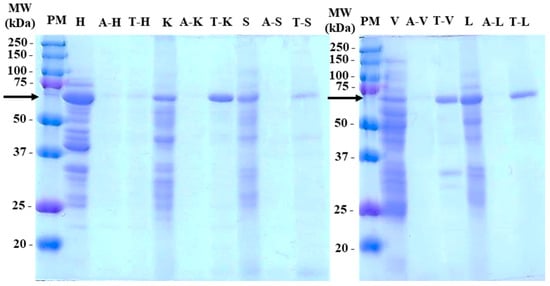
Figure 2.
A representative photo of protein SDS-PAGE (5% stacking gel and 12% separating gel) of Alcalase- and trypsin-medicated protein hydrolyzates from pork organs. PM, standard protein market; H, heart; K, kidney; S, spleen; V, liver; L, lung; A-, Alcalase-hydrolyzed; T-, trypsin-hydrolyzed. The arrows indicate the intact protein bands after hydrolysis.
3.3. Antioxidant Capacities of Porcine Organ Hydrolyzates
3.3.1. DPPH Radical Scavenging Activity
DPPH radical scavenging activity revealed a dose-dependent increase in inhibitory effects (Figure 3a–e). In most instances, the porcine hydrolyzates achieved greater radical scavenging than the non-hydrolyzed control. In Figure 3f, with the exception of the porcine lung, the Alcalase-mediated hydrolyzates exhibited lower IC50 values for DPPH radical scavenging activity; only Alcalase-mediated porcine heart (4.93 mg/mL) and liver (4.69 mg/mL) hydrolyzates had significantly lower values compared to their non-hydrolyzed counterparts. Previously, López-Pedrouso et al. [8] reported that the DPPH IC50 of the non-hydrolyzed porcine liver was 4.73 mg/mL, but the IC50 value decreased to 5.42 mg/mL after autolysis at pH 4.8. Verma et al. [9] observed that the DPPH radical scavenging activity of porcine liver hydrolyzates noticeably increased after 2 h of enzymatic hydrolysis, indicating that 2 h of enzymatic hydrolysis might be sufficient to generate antioxidant peptides in this study. However, according to the authors of [8], trypsin was more effective than Alcalase when it came to enhancing the DPPH radical scavenging activity of porcine liver hydrolyzates, which contradicted the findings of the current study.
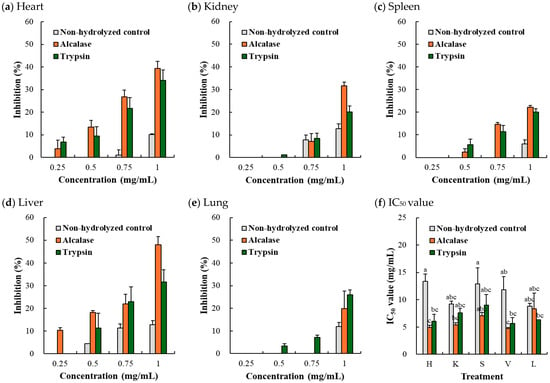
Figure 3.
DPPH radical scavenging activity (a–e) and DPPH radical IC50 value (f) of Alcalase- and trypsin-medicated protein hydrolyzates from porcine organs. H, heart; K, kidney; S, spleen; V, liver; L, lung. Each error bar indicates the standard deviation (n = 3). a–c means with the same letter within the result for IC50 value are not significantly different (p ≥ 0.05).
3.3.2. ABTS Radical Scavenging Activity
Regarding ABTS radical scavenging activity (Figure 4a–e), a concentration-dependent increase in inhibitory activity was observed, similar to the results for DPPH radical scavenging activity. At the highest concentration of 10 mg/mL, no dramatic differences in ABTS radical scavenging activity were observed for any porcine organ, regardless of the type of protease used. In all tissues, the hydrolyzates exhibited significantly lower IC50 values than the non-hydrolyzed control (Figure 4f), indicating that enzymatic hydrolysis improved ABTS radical scavenging activity. In particular, the Alcalase-mediated porcine kidney hydrolyzate had the lowest ABTS IC50 (2.06 mg/mL) (p < 0.05). Previously, Damgaard et al. [25] observed no difference in ABTS radical scavenging activity (41.0–44.6%) in Alcalase- and Protamex-hydrolyzed bovine lung, bovine kidney, and porcine heart. Furthermore, they suggested that the distribution of low-molecular-weight peptides had no connection to the ABTS radical scavenging activity [9]. Likewise, in our study, no clear correlation between the degree of hydrolysis and ABTS radical scavenging activity could be identified, which supported the previous theory.
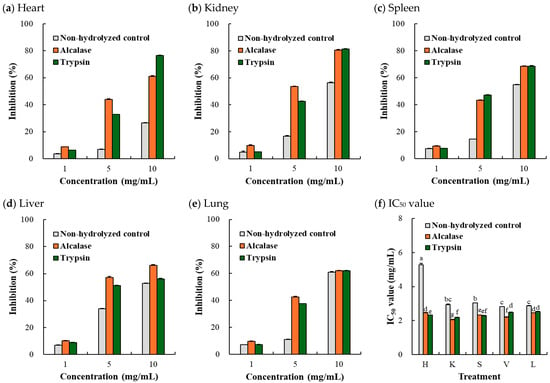
Figure 4.
ABTS radical scavenging activity (a–e) and ABTS radical IC50 value (f) of Alcalase- and trypsin-medicated protein hydrolyzates from porcine organs. H, heart; K, kidney; S, spleen; V, liver; L, lung. Each error bar indicates the standard deviation (n = 3). a–g means with the same letter within the result for IC50 value are not significantly different (p ≥ 0.05).
3.3.3. Hydroxyl Radical Scavenging Activity
Hydroxyl radical scavenging activity is widely used to determine antioxidant capacity because hydroxyl radicals are among the reactive free radicals present in living organisms [26]. Regardless of the type of porcine organ, the hydroxyl radical scavenging activity of the hydrolyzates was higher than that of the non-hydrolyzed control, even at concentrations as low as 1 mg/mL (Figure 5a–e). As a result, with the exception of the porcine liver, the trypsin-mediated hydrolyzates exhibited over 50% lower IC50 values than their control counterparts. Notably, in the porcine kidney and spleen, trypsin outperformed Alcalase in terms of improving hydroxyl radical scavenging activity. The lowest OH radical IC50 value (1.06 mg/mL) was recorded for trypsin-mediated porcine spleen hydrolyzate (p < 0.05). The porcine spleen has recently been identified as a novel source for producing antioxidant peptides [27,28]. The trypsin-treated spleen hydrolyzate, composed of low-molecular-weight peptides (<3000 Da), exhibited strong hydroxyl radical scavenging activity (396.40 U/mL), and its antioxidant mechanism may involve the Keap1-Nrf2 pathway [28].
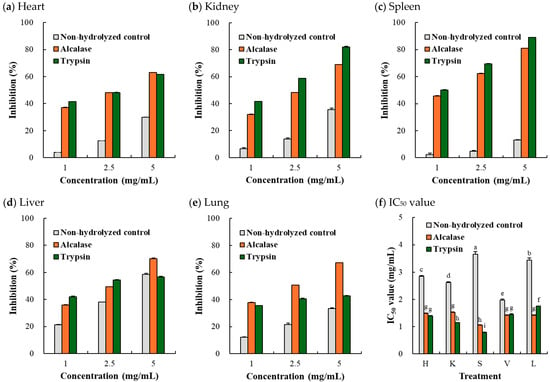
Figure 5.
Hydroxyl radical scavenging activity (a–e) and OH radical IC50 value (f) of Alcalase- and trypsin-medicated protein hydrolyzates from porcine organs. H, heart; K, kidney; S, spleen; V, liver; L, lung. Each error bar indicates the standard deviation (n = 3). a–i means with the same letter within the result for IC50 values are not significantly different (p ≥ 0.05).
3.3.4. Reducing Power
The reducing power of porcine organ hydrolyzates is shown in Figure 6; a high absorbance level indicates excellent reducing power. Unlike the radical scavenging results, the hydrolyzates in each tissue showed little or no significant difference in reducing power compared to the non-hydrolyzed control. Similarly, López-Pedrouso et al. [8] reported the decreased ferric-reducing ability of porcine liver after autolysis and explained that electron-transferring compounds such as vitamins and endogenous enzymes could be related to the higher reducing power of the control porcine liver. In addition, the reducing power of protein hydrolyzates is strongly associated with molecular weight distribution, in which the redox reaction occurs with multifaceted molecular weight compounds [29]. Previously, Borrajo et al. [7] found the highest ferric-reducing power at a higher-molecular-weight fraction (30 kDa) than 5 and 10 kDa fractions. Thus, the decreased reducing power in the porcine organ hydrolyzates might be attributable to an excessive level of hydrolysis. Thus, our results imply that extensive and untargeted enzymatic hydrolysis can weaken the reducing power of porcine organ hydrolyzates.
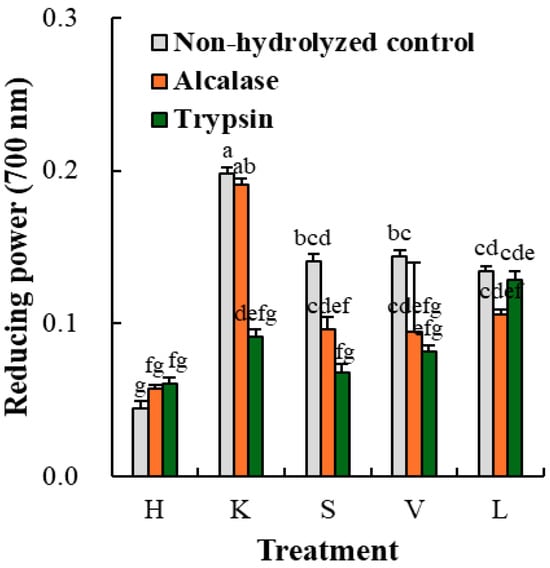
Figure 6.
Reducing power of Alcalase- and trypsin-medicated protein hydrolyzates (1 mg/mL) from porcine organs. H, heart; K, kidney; S, spleen; V, liver; L, lung. Each error bar indicates the standard deviation (n = 3). a–g means with the same letter are not significantly different (p ≥ 0.05).
3.4. Cluster Analysis and Raw Material Classification
Hierarchical clustering analysis was conducted to explore the structural relationships of the raw materials and hydrolyzates of porcine organs based on the Z-scores of measured variables with regard to their antioxidant properties (DPPH, ABTS, and OH radical IC50 values and reducing power). The Euclidean distance was used to calculate the dissimilarity, which was visualized as a dendrogram (Figure 7) using Ward’s linkage method. The hierarchical clustering results clearly distinguished non-hydrolyzed controls (K, L, S, V, and H) and their hydrolyzates. There were demonstrable differences in antioxidant properties between the control group and the hydrolyzates. In non-hydrolyzed control groups, porcine heart characteristics can be easily distinguished from those of other offal. Moreover, Alcalase-mediated kidney (A-K) and trypsin-mediated lung (T-L) hydrolyzates possessed highly similar antioxidant properties, differentiating them from the other hydrolyzates.
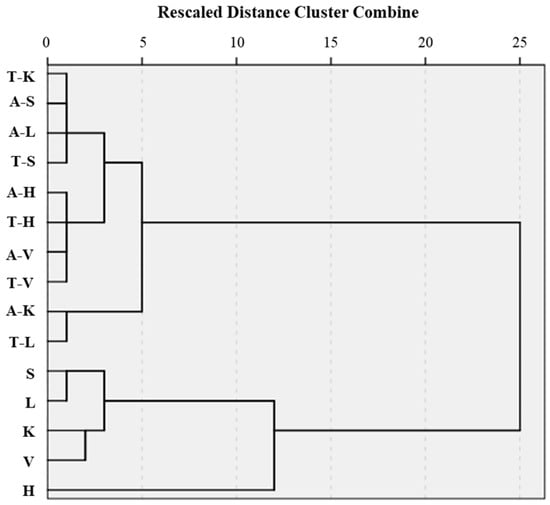
Figure 7.
Dendrogram on antioxidant property-related variables (DPPH, ABTS, and OH radical IC50 values and reducing power) of Alcalase- and trypsin-medicated protein hydrolyzates from pork by-products. H, heart; K, kidney; S, spleen; V, liver; L, lung; A-, Alcalase-mediated hydrolyzate; T-, trypsin-mediated hydrolyzate.
Based on the dendrogram, the data were divided into four clusters with distinct antioxidant profiles, and the treatment for each cluster was confirmed via the cluster membership number (Table 2). The key features of each cluster can be explained as follows:

Table 2.
Z-scores of antioxidant factors in four different clusters and their assigned treatment.
Cluster 1: low radical scavenging activities and reducing power (porcine heart);
Cluster 2: moderate radical scavenging activities but high reducing power (porcine kidney, spleen, liver, and lung);
Cluster 3: balanced radical scavenging activities but low reducing power (Alcalase- and trypsin-hydrolyzed porcine heart, trypsin-hydrolyzed kidney, Alcalase- and trypsin-hydrolyzed spleen, Alcalase- and trypsin-hydrolyzed liver, and Alcalase-hydrolyzed lung);
Cluster 4: strong DPPH and ABTS radical scavenging activities and high reducing power (Alcalase-hydrolyzed kidney and trypsin-hydrolyzed lung).
As demonstrated, the distinct antioxidant properties of porcine organs and their hydrolyzates are important considerations when selecting initial raw materials for future industrial applications. Specifically, since all hydrolyzates exhibited improved radical scavenging activities compared to the intact raw materials, it may be possible to produce hydrolyzates with antioxidant properties without separating specific porcine organs from bulk raw material supplies. However, when producing premium hydrolyzates with superior antioxidant properties, it may be preferable to separately process porcine kidneys and lungs with Alcalase and trypsin, respectively, as supported by Cluster 4 in Table 2. Thus, this study suggests that the selection of porcine organs as raw materials and the application of specific enzymes should be tailored to ensure that the desired antioxidant properties are achieved.
4. Conclusions
This study confirms that major porcine organs (heart, kidney, spleen, liver, and lung) with high protein content (15.90–20.30 g/100 g) are valuable raw materials for producing antioxidant hydrolyzates. In particular, the high extractable protein content of the heart could be tentatively associated with its increased degree of hydrolysis. Trypsin achieved a lower degree of hydrolysis compared to Alcalase, likely due to the limited degradation of porcine serum albumin. The hydrolyzates exhibited significantly higher DPPH, ABTS, and hydroxyl radical scavenging activities than their non-hydrolyzed counterparts, whereas the enzymatic hydrolysis did not positively influence reducing power. The complexity of antioxidant properties, linked to raw materials, applied proteases, and antioxidant assay, observed in this study was clarified through cluster analysis. Hierarchical and k-means cluster analysis revealed distinct differences in antioxidant properties between non-hydrolyzed controls and hydrolyzates, where A-K and T-L treatments were classified as showing strong DPPH and ABTS radical scavenging activities and reducing power. Therefore, from an industrial perspective, our findings suggest that porcine organs obtained in bulk could be used to produce antioxidant hydrolyzates distinct from raw materials without necessitating additional separation of raw materials. However, in the interests of developing a high-quality, premium product with more pronounced antioxidant effects, it is recommended that kidneys be selectively processed with Alcalase (A-K) and lungs with trypsin (T-L). Further studies are warranted to elucidate the fundamental mechanisms underlying the antioxidant properties of the A-K and T-L treatments by improving the scientific understanding of the free amino acid profile and specific bioactive peptides. In addition, for the purpose of commercialization, safety evaluations and parallel in vivo studies should be conducted to confirm the suitability of hydrolyzates for use in food or feed applications.
Author Contributions
Conceptualization, E.J.J., K.-S.K., E.-C.S., K.-W.L. and H.-W.K.; data curation, J.H., W.-Y.S. and D.-H.S.; formal analysis, J.H., W.-Y.S. and D.-H.S.; funding acquisition, E.J.J., K.-S.K., E.-C.S., K.-W.L. and H.-W.K.; investigation, E.J.J., K.-S.K., E.-C.S., K.-W.L. and H.-W.K.; methodology, J.H., W.-Y.S. and H.-W.K.; project administration, K.-W.L. and H.-W.K.; supervision, K.-W.L. and H.-W.K.; validation, E.J.J., K.-S.K. and E.-C.S.; visualization, J.H. and H.-W.K.; writing—original draft, J.H.; writing—review and editing, E.J.J., K.-S.K., E.-C.S., D.-H.S., K.-W.L. and H.-W.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through the Project for Development of Development of companion animal feed additives to improve palatability using byproducts, funded by the Ministry of Agriculture, Food and Rural Affairs (322089031HD020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the Ministry of Agriculture, Food and Rural Affairs (322089031HD020) for the financial support through national funds. Furthermore, this work was supported by the Gyeongsang National University Fund for Professors on Sabbatical Leave, 2024.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Irshad, A.; Sharma, B.D. Abattoir By-Product Utilization for Sustainable Meat Industry: A Review. J. Anim. Pro. Adv. 2015, 5, 681–696. [Google Scholar]
- Alao, B.O.; Falowo, A.B.; Chulayo, A.; Muchenje, V. The Potential of Animal By-Products in Food Systems: Production, Prospects, and Challenges. Sustainability 2017, 9, 1089. [Google Scholar] [CrossRef]
- Martínez-Alvarez, O.; Chamorro, S.; Brenes, A. Protein Hydrolysates from Animal Processing By-Products as a Source of Bioactive Molecules with Interest in Animal Feeding: A Review. Food Res. Int. 2015, 73, 204–212. [Google Scholar] [CrossRef]
- Zheng, K.; Liang, M.; Yao, H.; Wang, J.; Chang, Q. Effect of Size-Fractionated Fish Protein Hydrolysate on Growth and Feed Utilization of Turbot (Scophthalmus maximus L.). Aquac. Res. 2012, 44, 895–902. [Google Scholar] [CrossRef]
- Hu, R.; Dunmire, K.M.; Truelock, C.N.; Paulk, C.B.; Aldrich, G.; Li, Y. Antioxidant Performances of Corn Gluten Meal and DDGS Protein Hydrolysates in Food, Pet Food, and Feed Systems. J. Agric. Food Res. 2020, 2, 100030. [Google Scholar] [CrossRef]
- Verma, A.K.; Chatli, M.K.; Kumar, P.; Mehta, N. Antioxidant and Antimicrobial Activity of Protein Hydrolysate Extracted from Porcine Liver. Indian J. Anim. Sci. 2017, 87, 711–717. [Google Scholar] [CrossRef]
- Borrajo, P.; Pateiro, M.; Gagaoua, M.; Franco, D.; Zhang, W.; Lorenzo, J.M. Evaluation of the Antioxidant and Antimicrobial Activities of Porcine Liver Protein Hydrolysates Obtained Using Alcalase, Bromelain, and Papain. Appl. Sci. 2020, 10, 2290. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Borrajo, P.; Pateiro, M.; Lorenzo, J.M.; Franco, D. Antioxidant Activity and Peptidomic Analysis of Porcine Liver Hydrolysates Using Alcalase, Bromelain, Flavourzyme and Papain Enzymes. Food Res. Int. 2020, 137, 109389. [Google Scholar] [CrossRef]
- Verma, A.K.; Chatli, M.K.; Kumar, P.; Mehta, N. In-Vitro Assessment of Antioxidant and Antimicrobial Activity of Whole Porcine-Liver Hydrolysates and Its Fractions. Anim. Prod. Sci. 2018, 59, 641–646. [Google Scholar] [CrossRef]
- Seong, P.N.; Park, K.M.; Cho, S.H.; Kang, S.M.; Kang, G.H.; Park, B.Y.; Moon, S.S.; Ba, H.V. Characterization of Edible Pork By-products by Means of Yield and Nutritional Composition. Korean J. Food Sci. Anim. Resour. 2014, 34, 297–306. [Google Scholar] [CrossRef]
- Hoa, V.B.; Kim, D.G.; Song, D.H.; Ko, J.H.; Kim, H.W.; Bae, I.S.; Kim, Y.S.; Cho, S.H. Quality Properties and Flavor-Related Components of Beef Longissimus Lumborum Muscle from Four Korean Native Cattle Breeds. Food Sci. Anim. Resour. 2024, 44, 832–848. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Kim, T.-K.; Yun, H.-J.; Kim, J.; Cha, J.Y.; Lee, J.H.; Choi, Y.-S. Effects of Grafted Myofibrillar Protein as a Phosphate Replacer in Brined Pork Loin. Meat Sci. 2023, 199, 109142. [Google Scholar] [CrossRef] [PubMed]
- Morais, H.A.; Silvestre, M.P.C.; Silva, V.D.M.; Silva, M.R.; Simoes e Silva, A.C.; Silveira, J.N. Correlation between the Degree of Hydrolysis and the Peptide Profile of Whey Protein Concentrate Hydrolysates: Effect of the Enzyme Type and Reaction Time. Am. J. Food Technol. 2013, 8, 1–16. [Google Scholar] [CrossRef]
- Song, D.-H.; Ham, Y.-K.; Noh, S.-W.; Chin, K.B.; Kim, H.-W. Evaluation of NaCl and KCl Salting Effects on Technological Properties of Pre- and Post-Rigor Chicken Breasts at Various Ionic Strengths. Foods 2020, 9, 721. [Google Scholar] [CrossRef] [PubMed]
- de Castro, R.J.S.; Sato, H.H. A Response Surface Approach on Optimization of Hydrolysis Parameters for the Production of Egg White Protein Hydrolysates with Antioxidant Activities. Biocatal. Agric. Biotechnol. 2015, 4, 55–62. [Google Scholar] [CrossRef]
- Cui, Q.; Sun, Y.; Cheng, J.; Guo, M. Effect of Two-Step Enzymatic Hydrolysis on the Antioxidant Properties and Proteomics of Hydrolysates of Milk Protein Concentrate. Food Chem. 2022, 366, 130711. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jin, Y.; Lin, S.; Jones, G.S.; Chen, F. Purification and Identification of Novel Antioxidant Peptides from Egg White Protein and Their Antioxidant Activities. Food Chem. 2015, 175, 258–266. [Google Scholar] [CrossRef]
- Rivera, J.A.; Sebranek, J.G.; Rust, R.E.; Tabatabai, L.B. Composition and Protein Fractions of Different Meat By-Products Used for Pet Food Compared with Mechanically Separated Chicken (MSC). Meat Sci. 2000, 55, 53–59. [Google Scholar] [CrossRef]
- Grossmann, L.; McClements, D.J. Current Insights into Protein Solubility: A Review of Its Importance for Alternative Proteins. Food Hydrocoll. 2023, 137, 108416. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, B.; Xiong, Y.L.; Xia, X. Antioxidant Activity and Functional Properties of Porcine Plasma Protein Hydrolysate as Influenced by the Degree of Hydrolysis. Food Chem. 2010, 118, 403–410. [Google Scholar] [CrossRef]
- Mora, L.; Toldrá, F. Advanced Enzymatic Hydrolysis of Food Proteins for the Production of Bioactive Peptides. Curr. Opin. Food Sci. 2023, 49, 100973. [Google Scholar] [CrossRef]
- Wang, Z.; Xing, L.; Cai, J.; Toldrá, F.; Hao, Y.; Zhang, W. Identification of Hepatoprotective Peptides from Porcine Liver and Its Interaction with Ethanol Metabolizing Enzymes In Vitro. Food Biosci. 2023, 55, 103036. [Google Scholar] [CrossRef]
- Kim, K.-B.-W.; Song, E.-J.; Lee, S.-Y.; Park, J.-G.; Lee, J.-W.; Byun, M.-W.; Ahn, D.-H. Changes in Antigenicity of Porcine Serum Albumin in Gamma-Irradiated Sausage Extract by Treatment with Pepsin and Trypsin. Radiat. Phys. Chem. 2011, 80, 1258–1262. [Google Scholar] [CrossRef]
- Damgaard, T.; Lametsch, R.; Otte, J. Antioxidant Capacity of Hydrolyzed Animal By-Products and Relation to Amino Acid Composition and Peptide Size Distribution. J. Food Sci. Technol. 2015, 52, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.C.; Jen, J.F.; Tsai, T.H. Hydroxyl Radical in Living Systems and Its Separation Methods. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 781, 481–496. [Google Scholar] [CrossRef]
- Liu, C.; Ding, W.-J.; Huo, Y.; Liu, A.-J. Comprehensive Assessment of Peptide Derived from Pig Spleen: Preparation, Bioactivity and Structure-Activity Relationships. Food Biosci. 2023, 56, 103361. [Google Scholar] [CrossRef]
- Liu, C.; An, H.-X.; Liu, A.-J. Functional Development of Porcine Spleen as a By-Product of Pig Slaughterhouse: Preparation, Identification and Bioactive Activities of a Novel Peptide. Food Biosci. 2023, 56, 103448. [Google Scholar] [CrossRef]
- Czelej, M.; Garbacz, K.; Czernecki, T.; Wawrzykowski, J.; Waśko, A. Protein Hydrolysates Derived from Animals and Plants—A Review of Production Methods and Antioxidant Activity. Foods 2022, 11, 1953. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).