Enhancing the Protein, Mineral Content, and Bioactivity of Wheat Bread through the Utilisation of Microalgal Biomass: A Comparative Study of Chlorella vulgaris, Phaeodactylum tricornutum, and Tetraselmis chuii
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Volatile Organic Compounds Microalgae Profile
2.3. Bread Preparation
2.4. Dough Rheology
2.4.1. Mixolab—Mixing and Pasting Curves
2.4.2. Viscoelastic Behaviour
2.4.3. Extensional Evaluation
2.5. Bread Quality
2.5.1. Texture, Volume, and Colour Measurements
2.5.2. Nutritional Composition
2.5.3. Determination of Total Phenolic Compounds and Antioxidant Capacity
2.6. Statistical Analysis
3. Results
3.1. Volatile Organic Compounds Profile
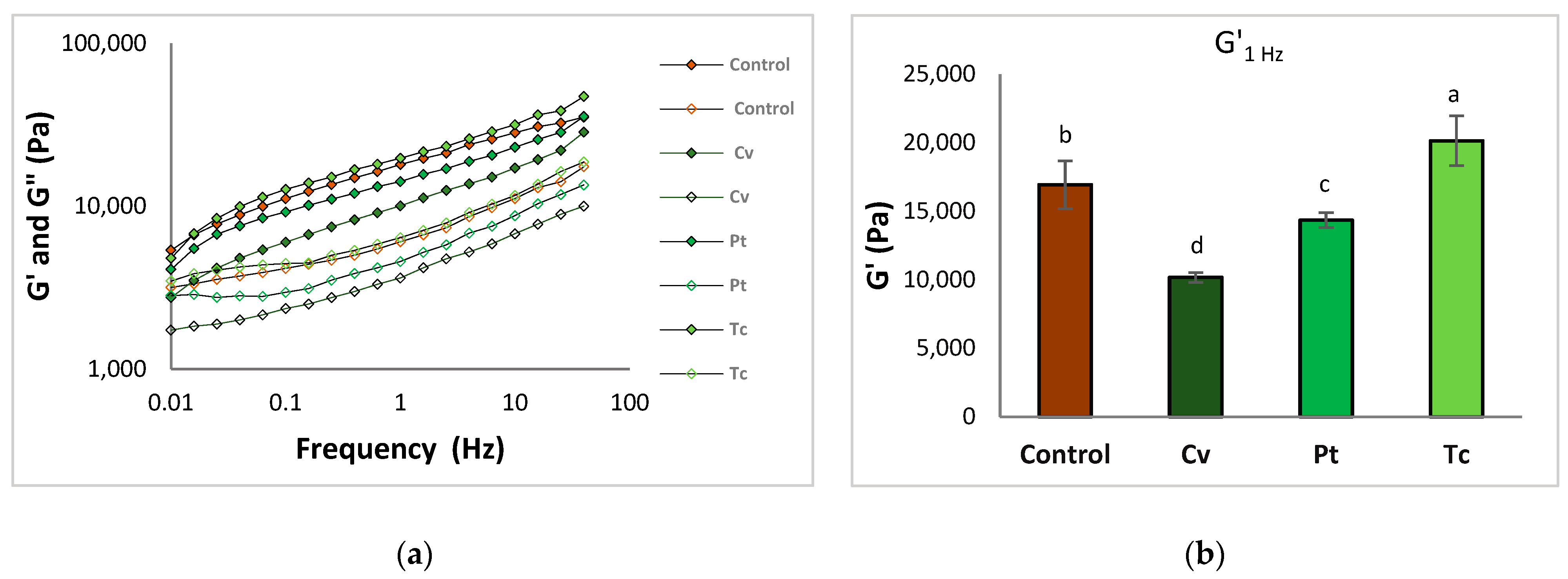
3.2. Dough Rheology
3.2.1. Mixing and Pasting Properties
3.2.2. Dough Viscoelastic Behaviour
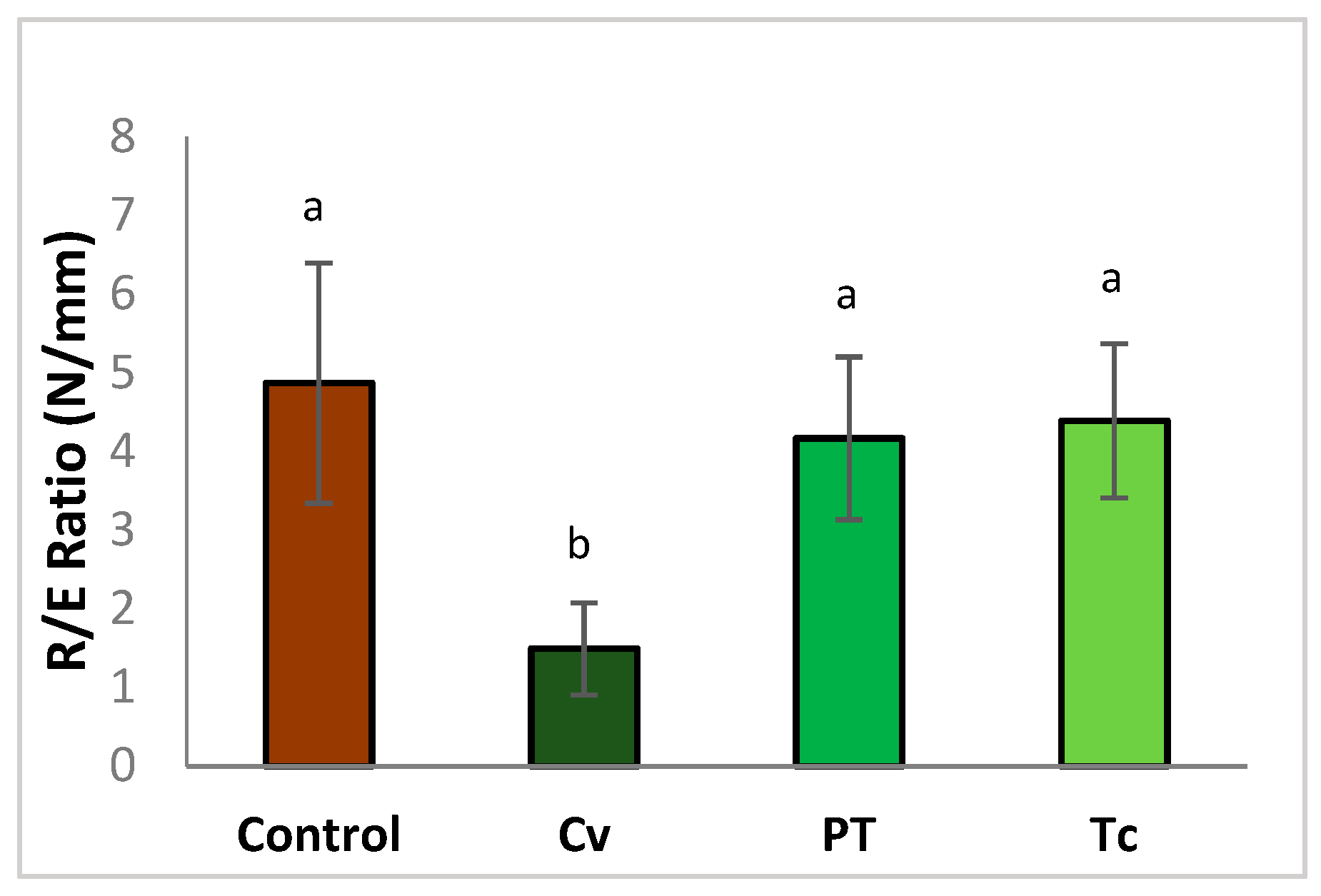
3.2.3. Dough Extensibility Properties
3.3. Technological and Chemical Properties of Bread
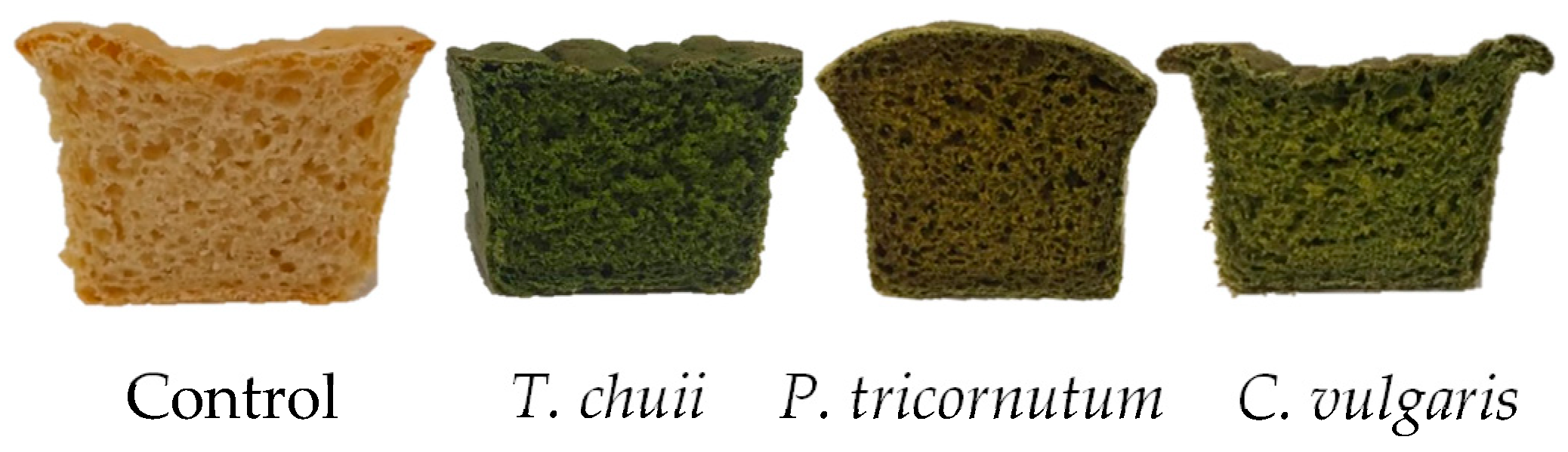
3.3.1. Bread Colour and Volume
3.3.2. Bread Texture
3.3.3. Bread Moisture and Water Activity
3.4. Nutritional Composition and Bioactivity
3.4.1. Nutritive Value
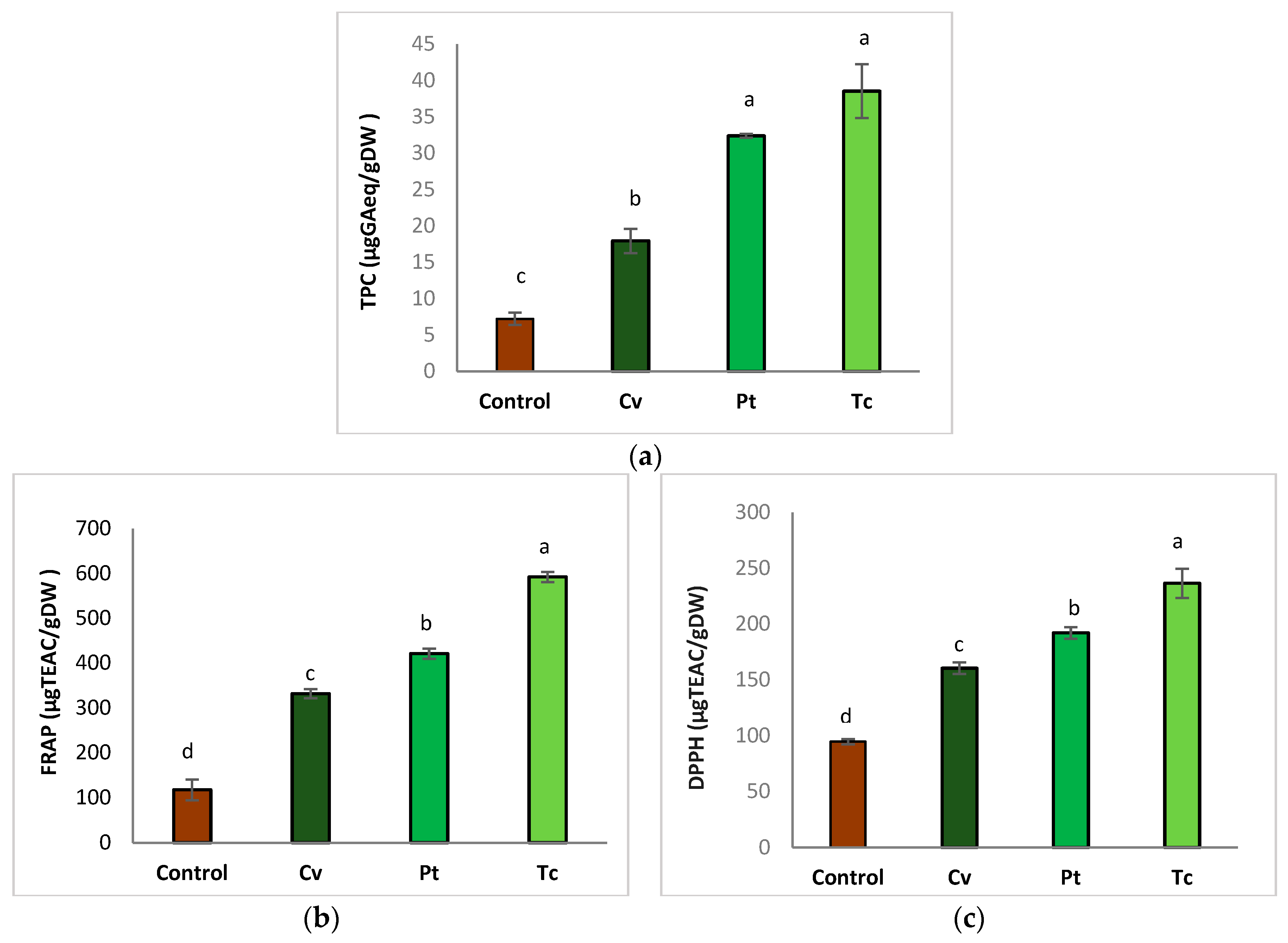
3.4.2. Total Phenolic Compounds and Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garzon, R.; Skendi, A.; Lazo-Velez, M.A.; Papageorgiou, M.; Rosell, C.M. Interaction of dough acidity and microalga level on bread quality and antioxidant properties. Food Chem. 2021, 344, 128710. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.; Graça, C.; Vlaisavljević, S.; Tenreiro, A.; Sousa, I.; Raymundo, A. Microalgal cell disruption: Effect on the bioactivity and rheology of wheat bread. Algal Res. 2020, 45, 101749. [Google Scholar] [CrossRef]
- Demarco, M.; Oliveira de Moraes, J.; Matos, Â.P.; Derner, R.B.; de Farias Neves, F.; Tribuzi, G. Digestibility, bioaccessibility and bioactivity of compounds from algae. Trends Food Sci. Technol. 2022, 121, 114–128. [Google Scholar] [CrossRef]
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. An emerging trend in functional foods for the prevention of cardiovascular disease and diabetes: Marine algal polyphenols. Crit. Rev. Food Sci. Nutr. 2018, 58, 1342–1358. [Google Scholar] [CrossRef] [PubMed]
- Verni, M.; Demarinis, C.; Rizzello, C.G.; Pontonio, E. Bioprocessing to preserve and improve microalgae nutritional and functional potential: Novel insight and perspectives. Foods 2023, 12, 983. [Google Scholar] [CrossRef] [PubMed]
- Loke Show, P. Global market and economic analysis of microalgae technology: Status and perspectives. Bioresour. Technol. 2022, 357, 127329. [Google Scholar] [CrossRef]
- Lafarga, T.; Mayre, E.; Echeverria, G.; Vinas, I.; Villaro, S.; Acien-Fernandez, F.G.; Castellari, M.; Aguilo-Aguayo, I. Potential of the microalgae Nannochloropsis and Tetraselmis for being used as innovative ingredients in baked goods. LWT-Food Sci. Technol. 2019, 115, 108439. [Google Scholar] [CrossRef]
- Ampofo, J.; Abbey, L. Microalgae: Bioactive Composition, Health benefits, safety and prospects as potential high-value ingredients for the functional food industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in microalgae incorporation into innovative food products with potential health benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef]
- Qazi, W.M.; Ballance, S.; Kousoulaki, K.; Uhlen, A.K.; Kleinegris, D.M.M.; Skjanes, K.; Rieder, A. Protein enrichment of wheat bread with microalgae: Microchloropsis gaditana, Tetraselmis chuii and Chlorella vulgaris. Foods 2021, 10, 3078. [Google Scholar] [CrossRef]
- Pereira, T.; Costa, S.; Barroso, S.; Teixeira, P.; Mendes, S.; Gil, M.M. Development and optimization of high-protein and low-saturated fat bread formulations enriched with lupin and microalgae. LWT-Food Sci. Technol. 2024, 191, 115612. [Google Scholar] [CrossRef]
- Graça, C.; Fradinho, P.; Sousa, I.; Raymundo, A. Impact of Chlorella vulgaris on the rheology of wheat flour dough and bread texture. LWT-Food Sci. Technol. 2017, 89, 466–474. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2012, 35, 265–278. [Google Scholar] [CrossRef]
- Justo, G.Z.; Silva, M.R.; Queiroz, M.L.S. Effects of the green algae Chlorella vulgaris on the response of the host hematopoietic system to intraperitoneal Ehrlich ascites tumor transplantation in mice. Immunopharmacol. Immunotoxicol. 2001, 23, 119–132. [Google Scholar] [CrossRef]
- de Souza Queiroz, J.; Barbosa, C.M.V.; da Rocha, M.C.; Bincoletto, C.; Paredes-Gamero, E.J.; de Souza Queiroz, M.L.; Palermo Neto, J. Chlorella vulgaris treatment ameliorates the suppressive effects of single and repeated stressors on hematopoiesis. Brain Behav. Immun. 2013, 29, 39–50. [Google Scholar] [CrossRef]
- Pina-Pérez, M.C.; Rivas, A.; Martínez, A.; Rodrigo, D. Antimicrobial potential of macro and microalgae against pathogenic and spoilage microorganisms in food. Food Chem. 2017, 235, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Toro, V.; Siquier-Coll, J.; Bartolomé, I.; Robles-Gil, M.C.; Rodrigo, J.; Maynar-Mariño, M. Effects of Tetraselmis chuii microalgae supplementation on ergospirometric, haematological and biochemical parameters in amateur soccer players. Int. J. Environ. Res. Public Health 2020, 17, 6885. [Google Scholar] [CrossRef]
- Sharp, M.; Wilson, J.; Stefan, M.; Gheith, R.; Lowery, R.; Ottinger, C.; Reber, D.; Orhan, C.; Sahin, N.; Tuzcu, M.; et al. Marine phytoplankton improves recovery and sustains immune function in humans and lowers proinflammatory immunoregulatory cytokines in a rat model. Phys. Act. Nutr. 2021, 25, 42–55. [Google Scholar] [CrossRef] [PubMed]
- García, Á.; Toro-Román, V.; Siquier-Coll, J.; Bartolomé, I.; Muñoz, D.; Maynar-Mariño, M. Effects of Tetraselmis chuii microalgae supplementation on anthropometric, hormonal and hematological parameters in healthy young men: A double-blind study. Int. J. Environ. Res. Public Health 2022, 19, 6060. [Google Scholar] [CrossRef] [PubMed]
- Celi, C.; Fino, D.; Savorani, F. Phaeodactylum tricornutum as a source of value-added products: A review on recent developments in cultivation and extraction technologies. Bioresour. Technol. Rep. 2022, 19, 101122. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Qazi, M.W.; de Sousa, I.G.; Nunes, M.C.; Raymundo, A. Improving the nutritional, structural, and sensory properties of gluten-free bread with different species of microalgae. Foods 2022, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, P.; Gururani, P.; Parveen, A.; Gautam, P.; Kumar, V. Algae: A promising and sustainable protein-rich food ingredient for bakery and dairy products. Food Chem. 2023, 441, 138322. [Google Scholar] [CrossRef] [PubMed]
- Sukhikh, S.; Ivanova, S.; Dolganyuk, V.; Pilevinova, I.; Prosekov, A.; Ulrikh, E.; Noskova, S.; Michaud, P.; Babich, O. Evaluation of the prospects for the use of microalgae in functional bread production. Appl. Sci. 2022, 12, 12563. [Google Scholar] [CrossRef]
- Švec, I.; Hrušková, M. The Mixolab parameters of composite wheat/hemp flour and their relation to quality features. LWT-Food Sci. Technol. 2015, 60, 623–629. [Google Scholar] [CrossRef]
- Grácio, M.; Ferreira, J.; Pagarete, A.; Nunes, C.; Raymundo, A. Potential health benefits of different microalgae strains. In Proceedings of the Young Algaeneers Symposium (YAS), Faro, Portugal, 9–11 May 2023. [Google Scholar]
- AACC International Method. Determination of Rheological Behavior as a Function of Mixing and Temperature Increase in Wheat Flour and Whole Wheat Meal by Mixolab; Method 54–60; AACC Int: St. Paul, MN, USA, 2010; pp. 1–6. [Google Scholar]
- Macedo, C.; Nunes, M.C.; Sousa, I.; Raymundo, A. Rheology methods as a tool to study the impact of whey powder on the dough and breadmaking performance of wheat flour. Fluids 2020, 5, 50. [Google Scholar] [CrossRef]
- Khemiri, S.; Khelifi, N.; Nunes, M.C.; Ferreira, A.; Gouveia, L.; Smaali, I.; Raymundo, A. Microalgae biomass as an additional ingredient of gluten-free bread: Dough rheology, texture quality and nutritional properties. Algal Res. 2020, 50, 101998. [Google Scholar] [CrossRef]
- AACC. Approved Methods of Analysis 2023. 11th ed. Available online: https://www.cerealsgrains.org/resources/Methods/Pages/default.aspx (accessed on 17 September 2023).
- ISO 16634-2:2016; Food Products. Determination of the Total Nitrogen Content by Combustion according to the Dumas Principle and Calculation of the Crude Protein Content. Part 2: Cereals, Pulses And Milled Cereal Products. ISO: Geneva, Switzerland, 2016.
- Martins, R.B.; Gouvinhas, I.; Nunes, M.C.; Peres, J.A.; Raymundo, A.; Barros, A.I.R.N.A. Acorn flour as a source of bioactive compounds in gluten-free bread. Molecules 2020, 25, 3568. [Google Scholar] [CrossRef]
- Khemiri, S.; Khelifi, N.; Messaoud, C.; Smaali, I. Bioprospecting of microalgae for a potential use as enzyme inhibitors, anti-ageing and prebiotic agents. Biocatal. Agric. Biotechnol. 2023, 51, 102759. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2002, 48, 3597–3604. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nunes, M.C.; Ferreira, J.; Raymundo, A. Volatile fingerprint impact on the sensory properties of microalgae and development of mitigation strategies. Curr. Opin. Food Sci. 2023, 51, 101040. [Google Scholar] [CrossRef]
- Cichoński, J.; Chrzanowski, G. Microalgae as a source of valuable phenolic compounds and carotenoids. Molecules 2022, 27, 8852. [Google Scholar] [CrossRef] [PubMed]
- Lafarge, C.; Cayot, N. Insight on a comprehensive profile of volatile compounds of Chlorella vulgaris extracted by two “green” methods. Food Sci. Nutr. 2019, 7, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Sudha, M.L.; Srivastava, A.K.; Vetrimani, R.; Leelavathi, K. Fat replacement in soft dough biscuits: Its implications on dough rheology and biscuit quality. J. Food Eng. 2007, 80, 922–930. [Google Scholar] [CrossRef]
- Batista, A.P.; Nunes, M.C.; Raymundo, A.; Gouveia, L.; Sousa, I.; Cordobés, F.; Guerrero, A.; Franco, J.M. Microalgae biomass interaction in biopolymer gelled systems. Food Hydrocoll. 2011, 25, 817–825. [Google Scholar] [CrossRef]
- Qazi, W.M.; Ballance, S.; Uhlen, A.K.; Kousoulaki, K.; Haugen, J.E.; Rieder, A. Protein enrichment of wheat bread with the marine green microalgae Tetraselmis chuii—Impact on dough rheology and bread quality. LWT-Food Sci. Technol. 2021, 143, 111115. [Google Scholar] [CrossRef]
- Lazo-Velez, M.A.; Mata-Ramírez, D.; Serna-Saldivar, S.O.; Chuck-Hernandez, C. Functional effects of soybean concentrates obtained from sprouted seeds enriched in selenium in wheat breadmaking. Cereal Chem. 2017, 94, 740–745. [Google Scholar] [CrossRef]
- Amjid, M.R.; Shehzad, A.; Hussain, S.; Shabbir, M.A.; Khan, M.R.; Shoaib, M. A comprehensive review on wheat flour dough rheology. Pak. J. Food Sci. 2013, 23, 105–123. [Google Scholar]
- Rosell, C.M.; Collar, C.; Haros, M. Assessment of hydrocolloid effects on the thermo-mechanical properties of wheat using the Mixolab. Food Hydrocoll. 2007, 21, 452–462. [Google Scholar] [CrossRef]
- Dhaka, V.; Khatkar, B.S. Mixolab thermomechanical characteristics of dough and bread making quality of Indian wheat varieties. Qual. Assur. Saf. Crops Foods 2013, 5, 311–323. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Fabra, M.J.; Gómez-Mascaraque, L.G.; López-Rubio, A. Structural effects of microalgae additives on the starch gelatinisation process. Food Hydrocoll. 2018, 77, 257–269. [Google Scholar] [CrossRef]
- Oprea, O.B.; Tolstorebrov, I.; Claussen, I.C.; Sannan, S.; Apostol, L.; Moșoiu, C.; Gaceu, L. Potential for Saccharina latissima flour as a functional ingredient in the baking sector. Foods 2023, 12, 4498. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Kumar, V. Effect of high-pressure treatment on oscillatory rheology, particle size distribution and microstructure of microalgae Chlorella vulgaris and Arthrospira platensis. Algal Res. 2022, 62, 102617. [Google Scholar] [CrossRef]
- dos Brasil, B.S.A.F.; de Siqueira, F.G.; Salum, T.F.C.; Zanette, C.M.; Spier, M.R. Microalgae and cyanobacteria as enzyme biofactories. Algal Res. 2017, 25, 76–89. [Google Scholar] [CrossRef]
- Dura, A.; Rosell, C.M. Enzymes in baking. In Microbrobial Enzyme Technology in Food Applications; CRC Press: Boca Raton, FL, USA, 2017; pp. 295–314. [Google Scholar] [CrossRef]
- Thapa, S.; Li, H.; OHair, J.; Bhatti, S.; Chen, F.C.; Nasr, K.A.; Johnson, T.; Zhou, S. Biochemical characteristics of microbial enzymes and their significance from industrial perspectives. Mol. Biotechnol. 2019, 61, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Dapcevic, T.; Pojic, M.; Hadnaev, M.; Torbic, A. The Role of Empirical Rheology in Flour Quality Control. In Wide Spectra of Quality Control; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Caffe-Treml, M.; Glover, K.D.; Krishnan, P.G.; Hareland, G.A.; Bondalapati, K.D.; Stein, J. Effect of wheat genotype and environment on relationships between dough extensibility and breadmaking quality. Cereal Chem. 2011, 88, 201–208. [Google Scholar] [CrossRef]
- Jaekel, L.Z.; da Silva, C.B.; Steel, C.J.; Chang, Y.K. Influência da adição de xilanase nas características de pão de forma preparado com farinha de trigo comum ou farinha de trigo de grão inteiro. Cienc. Tecnol. Aliment. 2012, 32, 844–849. [Google Scholar] [CrossRef]
- Ayub, M.; Wahab, S.; Durrani, Y. Effect of water activity (Aw), moisture content and total microbial count on the overall quality of bread. Int. J. Agric. Biol. 2003, 5, 274–278. [Google Scholar]
- Ibrahim, U.K.; Rahman, N.A.A.; Suzihaque, M.U.H.; Hashib, S.A.; Aziz, R.A.A. Effect of baking conditions on the physical properties of bread incorporated with green coffee beans (GCB). IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 062019. [Google Scholar] [CrossRef]
- Allen, L.V. Quality Control: Water Activity Considerations for Beyond-use Dates. Int. J. Pharm. Compd. 2018, 22, 288–293. [Google Scholar]
- European Parliament & Council. Regulation (EC) 1924/2006 on nutrition and health claims made on foods. Off. J. Eur. Communities 2006, L404, 1–15. [Google Scholar]
- Cobalchin, F.; Volpato, M.; Modena, A.; Finotti, L.; Manni, F.; Panozzo, A.; Vamerali, T. Biofortification of common wheat grains with combined Ca, Mg, and K through foliar fertilisation. Agronomy 2021, 11, 1718. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Silva, M.; Kamberovic, F.; Uota, S.T.; Kovan, I.M.; Viegas, C.S.B.; Simes, D.C.; Gangadhar, K.N.; Varela, J.; Barreira, L. Microalgae as potential sources of bioactive compounds for functional foods and pharmaceuticals. Appl. Sci. 2022, 12, 5877. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenço-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.; Simal-Gandara, J. Main bioactive phenolic compounds in marine algae and their mechanisms of action supporting potential health benefits. Food Chem. 2021, 341 Pt 2, 128262. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Jerez-Martel, I.; García-Poza, S.; Rodríguez-Martel, G.; Rico, M.; Afonso-Olivares, C.; Gómez-Pinchetti, J.L. Phenolic profile and antioxidant activity of crude extracts from microalgae and cyanobacteria strains. J. Food Qual. 2017, 2017, 2924508. [Google Scholar] [CrossRef]
- Hernández-López, I.; Alamprese, C.; Cappa, C.; Prieto-Santiago, V.; Abadias, M.; Aguiló-Aguayo, I. Effect of Spirulina in bread formulated with wheat flours of different alveograph strength. Foods 2023, 12, 3724. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez De Marco, E.; Steffolani, M.E.; Martínez, C.S.; León, A.E. Effects of spirulina biomass on the technological and nutritional quality of bread wheat pasta. LWT-Food Sci. Technol. 2014, 58, 102–108. [Google Scholar] [CrossRef]
- Wang, H.M.; Pan, J.L.; Chen, C.Y.; Chiu, C.C.; Yang, M.H.; Chang, H.W.; Chang, J.S. Identification of anti-lung cancer extract from Chlorella vulgaris C-C by antioxidant property using supercritical carbon dioxide extraction. Process Biochem. 2010, 45, 1865–1872. [Google Scholar] [CrossRef]
- Coulombier, N.; Blanchier, P.; Le Dean, L.; Barthelemy, V.; Lebouvier, N.; Jauffrais, T. The effects of CO2-induced acidification on Tetraselmis biomass production, photophysiology and antioxidant activity: A comparison using batch and continuous culture. J. Biotechnol. 2021, 325, 312–324. [Google Scholar] [CrossRef] [PubMed]
| Sample | Moisture % | Carbohydrate % | Protein % | Fat % | Ash % |
|---|---|---|---|---|---|
| C. vulgaris | 4.9 ± 0.14 a | 48.0 ± 0.01 a | 43.6 ± 0.00 a | 0.2 ± 0.09 b | 16.5 ± 1.93 a |
| P. tricornutum | 4.7 ± 0.00 a | 38.7 ± 0.00 b | 43.5 ± 0.05 a | 0.7 ± 0.05 b | 8.9 ± 0.47 b |
| T. chuii | 4.6 ± 0.00 a | 47.9 ± 0.55 a | 31.8 ± 0.20 b | 3.1 ± 0.08 a | 15.4 ± 2.23 a |
| Compound | Cv | Pt | Tc |
|---|---|---|---|
| Aldehydes | 17.2 ± 1.28 | 8.6 ±2.03 | 10.4 ± 1.03 |
| Alcohols | 11.5 ± 1.66 | 31.4 ± 3.50 | 6.7 ± 0.80 |
| Ketones | 2.2 ± 0.56 | 4.1 ± 0.48 | 22.9 ± 9.48 |
| Alkanes | 16.8 ± 4.00 | 5.6 ± 1.34 | 1.2 ± 0.22 |
| Alkenes | 22.1 ± 3.46 | 0.7 ± 0.00 | 0.2 ± 0.03 |
| Alkynes | 12.0 ± 0.90 | - | 0.1 ± 0.00 |
| S-based compounds | 4.2 ± 0.31 | - | 3.0 ± 0.01 |
| N-based compounds | 9.4 ± 1.60 | 32.4 ± 4.75 | 47.5 ± 6.58 |
| Terpenoids | 2.1 ± 0.36 | 12.9 ± 2.12 | 6.9 ± 0.89 |
| Other | 0.4 ± 0.13 | 0.6 ± 0.40 | 0.6 ± 0.44 |
| Total identified compounds | 97.8 ± 7.44 | 96.2 ± 12.46 | 99.4 ± 14.93 |
| Non-identified compounds | 2.2 | 3.8 | 0.5 |
| Total | 100.0 | 100.0 | 100.0 |
| Sample | WA % | DDT (s) | DS (s) | C2 (N.m) | C3 | C4 (N.m) | C5 (N.m) | ||
|---|---|---|---|---|---|---|---|---|---|
| Time (s) | Torque (N.m) | T (°C) | |||||||
| Control | 59.0 | 86 ± 19 c | 574 ± 4 a | 0.44 ± 0.01 a | 1376 ± 6 b | 2.58 ± 0.01 b | 71.50 ± 0.76 c | 2.00 ± 0.00 c | 4.09 ± 0.02 ab |
| Cv | 60.5 | 221 ± 23 b | 496 ± 0 b | 0.23 ± 0.00 d | 1465 ± 10 a | 2.12 ± 0.03 c | 72.80 ± 2.42 bc | 2.11 ± 0.02 bc | 3.06 ± 0.04 c |
| Pt | 58.5 | 299 ± 5 a | 592 ± 15 a | 0.34 ±0.03 b | 1452 ± 40 a | 2.58 ± 0.07 b | 76.20 ± 0.66 a | 2.24 ± 0.91 b | 4.27 ± 0.12 a |
| Tc | 57.0 | 286 ± 23 a | 568 ± 32 a | 0.28 ± 0.00 c | 1478 ± 21 a | 2.74 ± 0.02 a | 76.80 ± 0.14 a | 2.41 ± 0.45 a | 3.98 ± 0.04 b |
| Sample | Crust | Crumb | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |
| Control | 63.26 ± 2.31 a | 7.90 ± 1.88 a | 32.23 ± 2.52 a | 63.76 ± 3.28 a | −1.05 ± 0.26 b | 17.70 ± 0.84 c |
| Cv | 32.79 ± 1.78 c | 0.24 ± 0.19 b | 18.21 ± 1.45 c | 31.01 ± 2.32 b | −4.32 ± 0.23 c | 25.77 ± 1.04 b |
| Pt | 39.63 ± 1.30 b | −1.51 ± 0.72 c | 22.95 ± 1.97 b | 29.63 ± 1.65 b | 0.67 ± 0.15 a | 27.50 ± 1.43 a |
| Tc | 38.70 ± 2.40 b | 0.40 ± 0.70 b | 22.98 ± 2.00 b | 31.87 ± 1.88 b | −6.46 ± 0.92 d | 28.83 ± 1.88 a |
| Sample | Firmness (N) | Cohesiveness | Elasticity | Volume (cm³) | Moisture (%) | aw |
|---|---|---|---|---|---|---|
| Control | 1.80 ± 0.36 b | 0.76 ± 0.03 a | 0.99 ± 0.02 a | 437.5 ± 46.30 a | 43 ± 0.13 a | 0.97 ±0.00 a |
| Cv | 2.42 ± 0.72 a | 0.74 ± 0.02 a | 1.04 ± 0.10 a | 338.0 ± 22.80 b | 42.8 ± 0.21 a | 0.97 ± 0.00 a |
| Pt | 2.28 ± 0.23 a | 0.76 ± 0.02 a | 1.02 ± 0.05 a | 372.5 ± 45.00 ab | 42.9 ± 0.50 a | 0.96 ± 0.00 b |
| Tc | 1.69 ± 0.14 b | 0.77 ± 0.03 a | 0.99 ± 0.02 a | 390.0 ± 42.81 ab | 41.5 ± 0.11 b | 0.95 ± 0.00 c |
| Sample | Moisture g/100 g | Carbohydrates g/100 g | Protein g/100 g | Lipids g/100 g | Ash g/100 g | Energy kJ/100 g |
|---|---|---|---|---|---|---|
| Control | 42.97 ± 0.12 a | 44.75 ± 0.06 a | 10.62 ± 0.05 d | 0.03 ± 0.00 d | 1.63 ± 0.02 c | 221.62 |
| Cv | 42.75 ± 0.15 a | 42.30 ± 0.21 c | 12.18 ± 0.02 a | 0.16 ± 0.00 a | 2.61 ± 0.06 a | 219.19 |
| Pt | 42.93 ± 0.50 a | 43.87 ± 0.47 b | 11.31 ± 0.02 b | 0.04 ± 0.00 c | 1.84 ± 0.03 b | 221.23 |
| Tc | 41.47 ± 0.12 b | 44.75 ± 0.17 a | 11.08 ± 0.03 c | 0.14 ± 0.00 b | 2.56 ± 0.04 a | 224.46 |
| Major Minerals (mg/100 g) | ||||||
|---|---|---|---|---|---|---|
| Na | K | Ca | Mg | P | S | |
| Control | 166.40 ± 0.45 d | 111.97 ± 0.61 d | 9.56 ± 0.07 d | 12.27 ± 0.24 d | 60.63 ± 0.87 d | 58.27 ± 0.27 d |
| Cv | 208.36 ± 1.31 c | 132.73 ± 1.91 c | 11.07 ± 0.30 c | 14.05 ± 0.06 c | 74.28 ± 1.24 a | 72.50 ± 1.08 c |
| Pt | 281.18 ± 2.19 a | 186.62 ± 1.05 a | 18.33 ± 0.65 b | 25.10 ± 0.44 a | 66.82 ± 0.67 b | 112.53 ± 1.41 a |
| Tc | 260.05 ± 1.28 b | 141.01 ± 1.62 b | 40.61 ± 0.32 a | 24.89 ± 0.11 b | 61.78 ± 0.20 c | 84.82 ± 0.38 b |
| 15% RDV (mg/100 g) | Nd | 300.0 | 120.0 | 56.3 | 105.0 | Nd |
| Trace Minerals (mg/100 g) | ||||||
| Fe | Cu | Zn | Mn | |||
| Control | 0.93 ± 0.04 b | 0.06 ± 0.00 b | 0.69 ± 0.00 b | 0.37 ± 0.00 c | ||
| Cv | 1.14 ± 0.09 a | 0.09 ± 0.01 a | 0.71 ± 0.00 a | 0.43 ± 0.00 b | ||
| Pt | 0.93 ± 0.00 b | 0.07 ± 0.00 ab | 0.71 ± 0.02 a | 0.50 ± 0.00 a | ||
| Tc | 0.96 ± 0.00 b | 0.08 ± 0.00 ab | 0.68 ± 0.01 b | 0.41 ± 0.00 b | ||
| 15% RDV (mg/100 g) | 2.1 | 0.2 | 1.5 | 0.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, N.; Ferreira, J.; Raymundo, A.; Nunes, M.C. Enhancing the Protein, Mineral Content, and Bioactivity of Wheat Bread through the Utilisation of Microalgal Biomass: A Comparative Study of Chlorella vulgaris, Phaeodactylum tricornutum, and Tetraselmis chuii. Appl. Sci. 2024, 14, 2483. https://doi.org/10.3390/app14062483
Mahmoud N, Ferreira J, Raymundo A, Nunes MC. Enhancing the Protein, Mineral Content, and Bioactivity of Wheat Bread through the Utilisation of Microalgal Biomass: A Comparative Study of Chlorella vulgaris, Phaeodactylum tricornutum, and Tetraselmis chuii. Applied Sciences. 2024; 14(6):2483. https://doi.org/10.3390/app14062483
Chicago/Turabian StyleMahmoud, Nancy, Joana Ferreira, Anabela Raymundo, and Maria Cristiana Nunes. 2024. "Enhancing the Protein, Mineral Content, and Bioactivity of Wheat Bread through the Utilisation of Microalgal Biomass: A Comparative Study of Chlorella vulgaris, Phaeodactylum tricornutum, and Tetraselmis chuii" Applied Sciences 14, no. 6: 2483. https://doi.org/10.3390/app14062483
APA StyleMahmoud, N., Ferreira, J., Raymundo, A., & Nunes, M. C. (2024). Enhancing the Protein, Mineral Content, and Bioactivity of Wheat Bread through the Utilisation of Microalgal Biomass: A Comparative Study of Chlorella vulgaris, Phaeodactylum tricornutum, and Tetraselmis chuii. Applied Sciences, 14(6), 2483. https://doi.org/10.3390/app14062483









