Bioconversion of Apple Pomace to Meyerozyma guilliermondii and Scheffersomyces stipitis Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Dry Matter Analysis in the Raw Material
2.3. Cellulose, Hemicellulose, and Lignin Assay in the AP
2.4. Pretreatment and Enzymatic Hydrolysis of the AP
2.5. Yeast Strains, Media, and SCP Cultivations
2.6. Yeast Cell Biomass Concentration
2.7. HPLC Analysis of Hydrolysates and Post-Cultivation Effluents
2.8. Number of Samples and Statistical Data Treatment
2.9. Sample Designations
- W—sample after aqueous pretreatment;
- A—sample after pretreatment with 2% sulphuric acid;
- WE50—sample after aqueous pretreatment and enzymatic hydrolysis at 50 °C;
- AE50—sample after pretreatment with 2% sulphuric acid and enzymatic hydrolysis at 50 °C;
- WE30—sample after aqueous pretreatment and enzymatic hydrolysis at 30 °C;
- AE30—sample after pretreatment with 2% sulphuric acid and enzymatic hydrolysis at 30 °C;
- MgWE50—effluent after M. guilliermondii cultivation in the hydrolysate after aqueous pretreatment and enzymatic hydrolysis at 50 °C;
- MgAE50—effluent after M. guilliermondii cultivation in the hydrolysate after pretreatment with 2% sulphuric acid and enzymatic hydrolysis at 50 °C;
- MgWE30—effluent after M. guilliermondii cultivation in the hydrolysate after aqueous pretreatment and enzymatic hydrolysis at 30 °C;
- MgAE30—effluent after M. guilliermondii cultivation in the hydrolysate after pretreatment with 2% sulphuric acid and enzymatic hydrolysis at 30 °C;
- SsWE50—effluent after S. stipitis yeast cultivation in the hydrolysate after aqueous pretreatment and enzymatic hydrolysis at 50 °C;
- SsAE50—effluent after S. stipitis yeast cultivation in the hydrolysate after pretreatment with 2% sulphuric acid and enzymatic hydrolysis at 50 °C;
- SsWE30—effluent after S. stipitis yeast cultivation in the hydrolysate after aqueous pretreatment and enzymatic hydrolysis at 30 °C;
- SsAE30—effluent after S. stipitis yeast cultivation in the hydrolysate after pretreatment with 2% sulphuric acid and enzymatic hydrolysis at 30 °C.
3. Results and Discussion
3.1. The Basic Parameters of the Apple Pomace
3.2. Sugars, Organic Acids, and Alcohols in the AP Hydrolysates
3.3. Sugars, Organic Acids, and Alcohols in the Post-Cultivation Effluents
3.4. The Results of the Yeast SCP Cultivation in the AP Hydrolysates
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 18 May 2024).
- Zhang, F.; Wang, T.; Wang, X.; Lü, X. Apple Pomace as a Potential Valuable Resource for Full-Components Utilization: A Review. J. Clean. Prod. 2021, 329, 129676. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Salama, M.; El Kersh, D.; Salem, M. Apple Pomace as a Source of Nutraceuticals. In Food and Agricultural Byproducts as Important Source of Valuable Nutraceuticals; Egbuna, C., Sawicka, B., Khan, J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 75–86. ISBN 978-3-030-98759-6. [Google Scholar]
- Kołodziejczyk, K.; Markowski, J.; Kosmala, M.; Król, B.; Płocharski, W. Apple Pomace as a Potential Source of Nutraceutical Products. Pol. J. Food Nutr. Sci. 2007, 57, 291–295. [Google Scholar]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.-M.; Tou, J.C. A Comprehensive Analysis of the Composition, Health Benefits, and Safety of Apple Pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Waldbauer, K.; McKinnon, R.; Kopp, B. Apple Pomace as Potential Source of Natural Active Compounds. Planta Med. 2017, 83, 994–1010. [Google Scholar] [CrossRef] [PubMed]
- Kauser, S.; Murtaza, M.A.; Hussain, A.; Imran, M.; Kabir, K.; Najam, A.; An, Q.U.; Akram, S.; Fatima, H.; Batool, S.A.; et al. Apple Pomace, a Bioresource of Functional and Nutritional Components with Potential of Utilization in Different Food Formulations: A Review. Food Chem. Adv. 2024, 4, 100598. [Google Scholar] [CrossRef]
- Gołębiewska, E.; Kalinowska, M.; Yildiz, G. Sustainable Use of Apple Pomace (AP) in Different Industrial Sectors. Materials 2022, 15, 1788. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.M.; Forster-Carneiro, T. Valorization of Apple Pomace By-Products from the Juice Processing Industry Using Pressurized Liquid Technology. J. Environ. Chem. Eng. 2023, 11, 110907. [Google Scholar] [CrossRef]
- Costa, J.M.; Ampese, L.C.; Ziero, H.D.D.; Sganzerla, W.G.; Forster-Carneiro, T. Apple Pomace Biorefinery: Integrated Approaches for the Production of Bioenergy, Biochemicals, and Value-Added Products—An Updated Review. J. Environ. Chem. Eng. 2022, 10, 108358. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Che Yunus, M.A.; Veza, I.; Harny, I.; Tirta, A. Waste to Wealth of Apple Pomace Valorization by Past and Current Extraction Processes: A Review. Sustainability 2023, 15, 830. [Google Scholar] [CrossRef]
- Borujeni, N.E.; Karimi, K.; Denayer, J.F.M.; Kumar, R. Apple Pomace Biorefinery for Ethanol, Mycoprotein, and Value-Added Biochemicals Production by Mucor Indicus. Energy 2022, 240, 122469. [Google Scholar] [CrossRef]
- Martău, G.A.; Teleky, B.-E.; Ranga, F.; Pop, I.D.; Vodnar, D.C. Apple Pomace as a Sustainable Substrate in Sourdough Fermentation. Front. Microbiol. 2021, 12, 742020. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.; Landberg, M. Production of Bio-Plastic Materials from Apple Pomace- a New Application for the Waste Material. Univ. Boras. 2018. Available online: https://www.diva-portal.org/smash/get/diva2:1327184/FULLTEXT01.pdf (accessed on 15 May 2024).
- Świątkiewicz, S. (Ed.) Chów i Hodowla Zwierząt Gospodarskich na Przestrzeni 70 Lat: Problemy i Wyzwania; Zakład Żywienia Zwierząt i Paszoznawstwa Instytutu Zootechniki PIB: Monografia; Instytut Zootechniki, Państwowy Instytut Badawczy: Kraków, Poland, 2020; ISBN 978-83-7607-333-0. [Google Scholar]
- Manrich, A. Apple Industry: Wastes and Possibilities. Int. J. Agri. Res. Env. Sci. 2024, 5, 1–10. [Google Scholar]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple Pomace as a Functional and Healthy Ingredient in Food Products: A Review. Processes 2020, 8, 319. [Google Scholar] [CrossRef]
- Shalini, R.; Gupta, D.K. Utilization of Pomace from Apple Processing Industries: A Review. J. Food Sci. Technol. 2010, 47, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Beigh, Y.A.; Ganai, A.M.; Ahmad, H.A. Utilisation of Apple Pomace as Livetock Feed: A Review. Indi. J. Small Rumin. 2015, 21, 165. [Google Scholar] [CrossRef]
- Luo, J.; Xu, Y.; Fan, Y. Upgrading Pectin Production from Apple Pomace by Acetic Acid Extraction. Appl. Biochem. Biotechnol. 2019, 187, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Piwowarek, K.; Lipińska, E.; Hać-Szymańczuk, E.; Pobiega, K. Propionic Acid Production from Apple Pomace in Bioreactor Using Propionibacterium Freudenreichii: An Economic Analysis of the Process. 3 Biotech 2021, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Rubin, E.M. Genomics of Cellulosic Biofuels. Nature 2008, 454, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, J. Hydrolysis of Lignocellulosic Materials for Ethanol Production: A Review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Sun, L.; Wu, B.; Zhang, Z.; Yan, J.; Liu, P.; Song, C.; Shabbir, S.; Zhu, Q.; Yang, S.; Peng, N.; et al. Cellulosic Ethanol Production by Consortia of Scheffersomyces Stipitis and Engineered Zymomonas Mobilis. Biotechnol. Biofuels 2021, 14, 221. [Google Scholar] [CrossRef]
- Ojo, A. An Overview of Lignocellulose and Its Biotechnological Importance in High-Value Product Production. Fermentation 2023, 9, 990. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and Sustainable Pretreatment Methods for Cellulose Extraction from Lignocellulosic Biomass and Its Applications: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Lonnie, M.; Hooker, E.; Brunstrom, J.; Corfe, B.; Green, M.; Watson, A.; Williams, E.; Stevenson, E.; Penson, S.; Johnstone, A. Protein for Life: Review of Optimal Protein Intake, Sustainable Dietary Sources and the Effect on Appetite in Ageing Adults. Nutrients 2018, 10, 360. [Google Scholar] [CrossRef]
- Pomeroy, J.; Jose, D.; Tyler, A.; Bloxham, P.; Culling, J. The Future of Food. Can We Meet the Needs of 9bn People? Available online: https://www.research.hsbc.com/C/1/1/320/WgCK7Wv (accessed on 20 May 2024).
- Galanakis, C.M. The Future of Food. Foods 2024, 13, 506. [Google Scholar] [CrossRef] [PubMed]
- Robins, S.; Iley, R.; Michell, L.; Smith, S.; Gilbert, G.; McCarthy, M.; Stein, U.; Wells, P.; Lawton, J.; Landridge, A.; et al. The Future of Food: Are Food Businesses on Track to Deliver a Sustainable Protein System by 2040? Available online: https://www.forumforthefuture.org/Handlers/Download.ashx?IDMF=f2a9339c-8a62-4462-a886-f7de0e3fd729 (accessed on 13 June 2024).
- Smith, K.; Watson, A.W.; Lonnie, M.; Peeters, W.M.; Oonincx, D.; Tsoutsoura, N.; Simon-Miquel, G.; Szepe, K.; Cochetel, N.; Pearson, A.G.; et al. Meeting the Global Protein Supply Requirements of a Growing and Ageing Population. Eur. J. Nutr. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bedsaul-Fryer, J.R.; Monroy-Gomez, J.; Van Zutphen-Küffer, K.G.; Kraemer, K. Editorial: An Introduction to Traditional and Novel Alternative Proteins for Low- and Middle-Income Countries. Curr. Dev. Nutr. 2023, 8, 102014. [Google Scholar] [CrossRef] [PubMed]
- Sijpestijn, G.F.; Wezel, A.; Chriki, S. Can Agroecology Help in Meeting Our 2050 Protein Requirements? Livest. Sci. 2022, 256, 104822. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef]
- Bratosin, B.C.; Darjan, S.; Vodnar, D.C. Single Cell Protein: A Potential Substitute in Human and Animal Nutrition. Sustainability 2021, 13, 9284. [Google Scholar] [CrossRef]
- Šovljanski, O.; Saveljić, A.; Tomić, A.; Šeregelj, V.; Lončar, B.; Cvetković, D.; Ranitović, A.; Pezo, L.; Ćetković, G.; Markov, S.; et al. Carotenoid-Producing Yeasts: Selection of the Best-Performing Strain and the Total Carotenoid Extraction Procedure. Processes 2022, 10, 1699. [Google Scholar] [CrossRef]
- Chreptowicz, K.; Mierzejewska, J.; Tkáčová, J.; Młynek, M.; Čertik, M. Carotenoid-Producing Yeasts: Identification and Characteristics of Environmental Isolates with a Valuable Extracellular Enzymatic Activity. Microorganisms 2019, 7, 653. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. From Yeast to Biotechnology. Bioengineering 2022, 9, 751. [Google Scholar] [CrossRef]
- Fernandes, N.D.A.T.; Simões, L.A.; Dias, D.R. Biosurfactants Produced by Yeasts: Fermentation, Screening, Recovery, Purification, Characterization, and Applications. Fermentation 2023, 9, 207. [Google Scholar] [CrossRef]
- Jia, L.L.; Brough, L.; Weber, J.L. Saccharomyces Cerevisiae Yeast-Based Supplementation as a Galactagogue in Breastfeeding Women? A Review of Evidence from Animal and Human Studies. Nutrients 2021, 13, 727. [Google Scholar] [CrossRef] [PubMed]
- Tullio, V. Yeast Genomics and Its Applications in Biotechnological Processes: What Is Our Present and Near Future? J. Fungi 2022, 8, 752. [Google Scholar] [CrossRef] [PubMed]
- Thiviya, P.; Gamage, A.; Kapilan, R.; Merah, O.; Madhujith, T. Single Cell Protein Production Using Different Fruit Waste: A Review. Separations 2022, 9, 178. [Google Scholar] [CrossRef]
- Walker, G.; Stewart, G. Saccharomyces Cerevisiae in the Production of Fermented Beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Stewart, G. Saccharomyces Species in the Production of Beer. Beverages 2016, 2, 34. [Google Scholar] [CrossRef]
- Pauley, M.; Maskell, D. Mini-Review: The Role of Saccharomyces Cerevisiae in the Production of Gin and Vodka. Beverages 2017, 3, 13. [Google Scholar] [CrossRef]
- Raffak, A.; Chafai, Y.; Hamouda, A.; Ouazzani Touhami, A.; Mounir, M. Assessment of the Fermentative Performance of Traditional Fresh Moroccan Sourdoughs and Their Freeze-Dried Forms Using Online Monitoring Device: Panigraph. Appl. Sci. 2023, 13, 12453. [Google Scholar] [CrossRef]
- Reed, G.; Nagodawithana, T.W. Yeast Technology; Springer: Dordrecht, The Netherlands, 1990; ISBN 978-94-011-9773-1. [Google Scholar]
- Dygas, D.; Kręgiel, D.; Berłowska, J. Sugar Beet Pulp as a Biorefinery Substrate for Designing Feed. Molecules 2023, 28, 2064. [Google Scholar] [CrossRef] [PubMed]
- Dygas, D.; Liszkowska, W.; Steglińska, A.; Sulyok, M.; Kręgiel, D.; Berłowska, J. Rapeseed Meal Waste Biomass as a Single-Cell Protein Substrate for Nutritionally-Enhanced Feed Components. Processes 2023, 11, 1556. [Google Scholar] [CrossRef]
- Patelski, P.; Berłowska, J.; Balcerek, M.; Dziekońska-Kubczak, U.; Pielech-Przybylska, K.; Dygas, D.; Jędrasik, J. Conversion of Potato Industry Waste into Fodder Yeast Biomass. Processes 2020, 8, 453. [Google Scholar] [CrossRef]
- Henriques, T.; Pereira, S.; Serafim, L.; Xavier, A. Two-Stage Aeration Fermentation Strategy to Improve Bioethanol Production by Scheffersomyces Stipitis. Fermentation 2018, 4, 97. [Google Scholar] [CrossRef]
- Salazar-Cerezo, S.; De Vries, R.P.; Garrigues, S. Strategies for the Development of Industrial Fungal Producing Strains. J. Fungi 2023, 9, 834. [Google Scholar] [CrossRef] [PubMed]
- Bajić, B.; Vučurović, D.; Vasić, Đ.; Jevtić-Mučibabić, R.; Dodić, S. Biotechnological Production of Sustainable Microbial Proteins from Agro-Industrial Residues and By-Products. Foods 2022, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.A.; El-Baky, N.A. Fungi as a Source of Edible Proteins and Animal Feed. J. Fungi 2023, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Valentino, M. Mycota of Distillery Yeast Sludge as Source of Single Cell Protein. Mycosphere 2015, 6, 241–247. [Google Scholar] [CrossRef]
- Kut, A.; Demiray, E.; Ertuğrul Karatay, S.; Dönmez, G. Second Generation Bioethanol Production from Hemicellulolytic Hydrolyzate of Apple Pomace by Pichia stipitis. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 5574–5585. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2022/1104 of 1 July 2022 Amending Regulation (EU) No 68/2013 on the Catalogue of Feed Materials. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX%3A32022R1104 (accessed on 6 July 2024).
- Patelski, P.; Berlowska, J.; Dziugan, P.; Pielech-Przybylska, K.; Balcerek, M.; Dziekonska, U.; Kalinowska, H. Utilisation of Sugar Beet Bagasse for the Biosynthesis of Yeast SCP. J. Food Eng. 2015, 167, 32–37. [Google Scholar] [CrossRef]
- Patelski, P.; Stanisz, M.; Antczak, A.; Balcerek, M.; Pielech-Przybylska, K.; Sapinska, E.; Dziekonska, U. Conversion of Sugar Beet Leaf Polysaccharides into Single Cell Protein. RSC Adv. 2015, 5, 20961–20965. [Google Scholar] [CrossRef]
- Papini, M.; Nookaew, I.; Uhlén, M.; Nielsen, J. Scheffersomyces Stipitis: A Comparative Systems Biology Study with the Crabtree Positive Yeast Saccharomyces Cerevisiae. Microb. Cell Fact. 2012, 11, 136. [Google Scholar] [CrossRef]
- Yan, W.; Gao, H.; Qian, X.; Jiang, Y.; Zhou, J.; Dong, W.; Xin, F.; Zhang, W.; Jiang, M. Biotechnological Applications of the Non-Conventional Yeast Meyerozyma Guilliermondii. Biotechnol. Adv. 2021, 46, 107674. [Google Scholar] [CrossRef] [PubMed]
- Molwitz, M.; Silva, S.S.; Ribeiro, J.D.; Roberto, I.C.; Felipe, M.G.A.; Prata, A.M.R.; Mancilha, I.M. Aspects of the Cell Growth of Candida Guilliermondii in Sugar Cane Bagasse Hydrolysate. Z. Für Naturforschung C 1996, 51, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Roberto, I.C.; Sato, S.; Mancilha, I.M. Effect of Inoculum Level on Xylitol Production from Rice Straw Hemicellulose Hydrolysate byCandida Guilliermondii. J. Ind. Microbiol. 1996, 16, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.D.V.D.; Mancilha, I.M.D.; Silva, S.S.D.; Felipe, M.D.G.D.A. Improvement of Biotechnological Xylitol Production by Glucose during Cultive of Candida Guilliermondii in Sugarcane Bagasse Hydrolysate. Braz. Arch. Biol. Technol. 2007, 50, 207–215. [Google Scholar] [CrossRef]
- Martini, C.; Tauk-Tornisielo, S.M.; Codato, C.B.; Bastos, R.G.; Ceccato-Antonini, S.R. A Strain of Meyerozyma Guilliermondii Isolated from Sugarcane Juice Is Able to Grow and Ferment Pentoses in Synthetic and Bagasse Hydrolysate Media. World J. Microbiol. Biotechnol. 2016, 32, 80. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.O.; Scarlata, C.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Ma, Y.; Luo, J.; Xu, Y. Co-Preparation of Pectin and Cellulose from Apple Pomace by a Sequential Process. J. Food Sci. Technol. 2019, 56, 4091–4100. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Paniagua-García, A.I.; Díez-Antolínez, R. Biobutanol Production from Apple Pomace: The Importance of Pretreatment Methods on the Fermentability of Lignocellulosic Agro-Food Wastes. Appl. Microbiol. Biotechnol. 2017, 101, 8041–8052. [Google Scholar] [CrossRef]
- Gama, R.; Van Dyk, J.S.; Pletschke, B.I. Optimisation of Enzymatic Hydrolysis of Apple Pomace for Production of Biofuel and Biorefinery Chemicals Using Commercial Enzymes. 3 Biotech 2015, 5, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of Apple Pomace for Bioactive Molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Nawirska, A.; Kwaśniewska, M. Dietary Fibre Fractions from Fruit and Vegetable Processing Waste. Food Chem. 2005, 91, 221–225. [Google Scholar] [CrossRef]
- Marchwicka, M. Influence of pH and Cellic® CTec2 Enzymes Dose on the Glucose Yield after Enzymatic Hydrolysis of Cellulose at 50 °C. For. Wood Technol. 2021, 114, 53–58. [Google Scholar] [CrossRef]
- Magyar, M.; Da Costa Sousa, L.; Jin, M.; Sarks, C.; Balan, V. Conversion of Apple Pomace Waste to Ethanol at Industrial Relevant Conditions. Appl. Microbiol. Biotechnol. 2016, 100, 7349–7358. [Google Scholar] [CrossRef] [PubMed]
- Vaez, S.; Karimi, K.; Denayer, J.F.M.; Kumar, R. Evaluation of Apple Pomace Biochemical Transformation to Biofuels and Pectin through a Sustainable Biorefinery. Biomass Bioenergy 2023, 172, 106757. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gołebiewska, E.; Zawadzka, M.; Choińska, R.; Koronkiewicz, K.; Piasecka-Jóźwiak, K.; Bujak, M. Sustainable Extraction of Bioactive Compound from Apple Pomace through Lactic Acid Bacteria (LAB) Fermentation. Sci. Rep. 2023, 13, 19310. [Google Scholar] [CrossRef] [PubMed]
- González-García, S.; Argiz, L.; Míguez, P.; Gullón, B. Exploring the Production of Bio-Succinic Acid from Apple Pomace Using an Environmental Approach. Chem. Eng. J. 2018, 350, 982–991. [Google Scholar] [CrossRef]
- Maslov-Bandić, L.; Žulj, M.M.; Fruk, G.; Babojelić, M.S.; Jemrić, T.; Jeromel, A. The Profile of Organic Acids and Polyphenols in Apple Wines Fermented with Different Yeast Strains. J. Food Sci. Technol. 2019, 56, 599–606. [Google Scholar] [CrossRef]
- Mahgoub, S.A.; Kedra, E.G.A.; Abdelfattah, H.I.; Abdelbasit, H.M.; Alamoudi, S.A.; Al-Quwaie, D.A.; Selim, S.; Alsharari, S.S.; Saber, W.I.A.; El-Mekkawy, R.M. Bioconversion of Some Agro-Residues into Organic Acids by Cellulolytic Rock-Phosphate-Solubilizing Aspergillus Japonicus. Fermentation 2022, 8, 437. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of Lignocellulose: Formation of Inhibitory by-Products and Strategies for Minimizing Their Effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of Lignocellulose: Inhibitors and Detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of Lignocellulosic Hydrolysates. II: Inhibitors and Mechanisms of Inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Vanmarcke, G.; Demeke, M.M.; Foulquié-Moreno, M.R.; Thevelein, J.M. Identification of the Major Fermentation Inhibitors of Recombinant 2G Yeasts in Diverse Lignocellulose Hydrolysates. Biotechnol. Biofuels 2021, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Trzcinski, A.P.; Stuckey, D.C. Contribution of Acetic Acid to the Hydrolysis of Lignocellulosic Biomass under Abiotic Conditions. Bioresour. Technol. 2015, 185, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid Hydrolysis of Lignocellulosic Biomass: Sugars and Furfurals Formation. Catalysts 2020, 10, 437. [Google Scholar] [CrossRef]
- Dziekońska-Kubczak, U.; Berłowska, J.; Dziugan, P.; Patelski, P.; Balcerek, M.; Pielech-Przybylska, K.; Robak, K. Two-Stage Pretreatment to Improve Saccharification of Oat Straw and Jerusalem Artichoke Biomass. Energies 2019, 12, 1715. [Google Scholar] [CrossRef]
- Dziekońska-Kubczak, U.; Berłowska, J.; Dziugan, P.; Patelski, P.; Pielech-Przybylska, K.; Balcerek, M. Nitric Acid Pretreatment of Jerusalem Artichoke Stalks for Enzymatic Saccharification and Bioethanol Production. Energies 2018, 11, 2153. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W. The Yeasts, A Taxonomic Study, 4th ed.; Elsevier: Amsterdam, The Netherlands, 1998; ISBN 0-444-81312-8. [Google Scholar]
- Matos, I.T.S.R.; Cassa-Barbosa, L.A.; Medeiros Galvao, R.S.; Nunes-Silva, C.G.; Astolfi Filho, S. “Isolation, Taxonomic Identification and Investigation of the Biotechnological Potential of Wild-Type Meyerozyma Guilliermondii Associated with Amazonian Termites Able to Ferment D-Xylose. Biosci.J. 2014, 30, 260–266. [Google Scholar]
- Duarte, L.C.; Lopes, F.C.S.; Neves, I.; Girio, F.M. Yeast Biomass Production in Brewery’s Spent Grains Hemicellulosic Hydrolyzate. Appl. Biochem. Biotechnol. 2008, 148, 119–129. [Google Scholar] [CrossRef]
- Joshi, V.; Bhushan, S. Apple Pomace Utilization for the Production of Baker’s Yeast: Effect of Substrate Concentrations and Growth Stimulators. Indian. J. Biotechnol. 2003, 2, 220–226. [Google Scholar]
| Value | |
|---|---|
| Dry matter [%] | 90.68 ± 2.7 |
| Cellulose [% DM] | 15.42 ± 0.3 |
| Hemicellulose [% DM] | 12.45 ± 0.09 |
| Lignin [% DM] | 15.23 ± 0.12 |
| Sample | CEL | GLU | XYL | ARA | GA |
|---|---|---|---|---|---|
| [g/L] | |||||
| W | 0.081 ± 0.003 | 0.06 ± 0.0032 | 0.21 ± 0.0052 | 0.131 ± 0.0038 | 0.315 ± 0.0029 |
| A | 0.91 ± 0.052 | 7.258 ± 0.1798 | 7.19 ± 0.3013 | 5.659 ± 0.1159 | 0.947 ± 0.0272 |
| WE50 | 0.787 ± 0.0291 | 18.55 ± 0.6058 | 5.76 ± 0.1958 | 5.348 ± 0.0665 | 7.013 ± 0.3713 |
| AE50 | 1.44 ± 0.0246 | 18.325 ± 0.8415 | 7.61 ± 0.2352 | 5.592 ± 0.17 | 6.23 ± 0.3561 |
| WE30 | 1.228 ± 0.0365 | 19.311 ± 0.8692 | 4.96 ± 0.1071 | 5.15 ± 0.2943 | 6.785 ± 0.277 |
| AE30 | 1.485 ± 0.0583 | 14.866 ± 0.6147 | 7.92 ± 0.2332 | 5.669 ± 0.3002 | 6.438 ± 0.2378 |
| Sample | CA | SA | LA | FA | AA |
|---|---|---|---|---|---|
| [g/L] | |||||
| W | 0.185 ± 0.007 | 0.127 ± 0.0016 | 1.27 ± 0.0415 | 0.067 ± 0.0019 | 0.262 ± 0.0043 |
| A | 0.043 ± 0.0023 | 0.091 ± 0.0038 | 1.2 ± 0.0098 | 0.083 ± 0.001 | 1.115 ± 0.0374 |
| WE50 | 0.184 ± 0.0082 | 0.054 ± 0.0011 | 1.18 ± 0.0096 | 0.042 ± 0.001 | 0.905 ± 0.0275 |
| AE50 | 0.085 ± 0.0026 | 0.062 ± 0.0018 | 1.11 ± 0.024 | 0.056 ± 0.002 | 1.141 ± 0.0244 |
| WE30 | 0.215 ± 0.0063 | 0.007 ± 0.0003 | 1.19 ± 0.0541 | 0.045 ± 0.0018 | 0.858 ± 0.0337 |
| AE30 | 0.101 ± 0.003 | 0.09 ± 0.0015 | 1.24 ± 0.061 | 0.065 ± 0.0023 | 1.178 ± 0.0386 |
| Sample | GOH | EOH |
|---|---|---|
| [g/L] | ||
| W | 0.679 ± 0.0249 | 0.036 ± 0.0013 |
| A | 0.678 ± 0.0206 | 0.011 ± 0.0004 |
| WE50 | 0.742 ± 0.0061 | 0.01 ± 0.0003 |
| AE50 | 0.74 ± 0.0255 | 0.012 ± 0.0005 |
| WE30 | 0.687 ± 0.0216 | 0.022 ± 0.0009 |
| AE30 | 0.775 ± 0.0249 | 0.056 ± 0.0015 |
| Sample | CEL | GLU | XYL | ARA | GA |
|---|---|---|---|---|---|
| [g/L] | |||||
| MgWE30 | 0.411 ± 0.0218 | 0.01 ± 0.0002 | 0.04 ± 0.0018 | 0.433 ± 0.0073 | 3.307 ± 0.0722 |
| MgAE30 | 0.685 ± 0.0117 | n.d. | 0.04 ± 0.0016 | 0.376 ± 0.0142 | 6.506 ± 0.1486 |
| MgWE50 | 0.358 ± 0.0119 | 0.01 ± 0.0002 | 0.04 ± 0.001 | 0.634 ± 0.0157 | 5.733 ± 0.1525 |
| MgAE50 | 0.061 ± 0.0027 | 0.131 ± 0.0032 | 0.11 ± 0.0036 | 0.04 ± 0.0013 | 1.707 ± 0.0725 |
| SsAE30 | 0.821 ± 0.0213 | n.d. | 0.01 ± 0.0002 | 0.01 ± 0.0004 | 6.166 ± 0.0897 |
| SsWE30 | 0.118 ± 0.0043 | 0.111 ± 0.0029 | 0.06 ± 0.0027 | 0.02 ± 0.0007 | 3.141 ± 0.0916 |
| SsWE50 | 0.02 ± 0.0001 | 0.151 ± 0.0025 | 0.05 ± 0.002 | 0.02 ± 0.0005 | 1.972 ± 0.06 |
| SsAE50 | 0.427 ± 0.0177 | 0.149 ± 0.0037 | n.d. | 0.04 ± 0.0009 | 1.366 ± 0.0627 |
| Sample | CA | SA | LA | FA | AA |
|---|---|---|---|---|---|
| [g/L] | |||||
| MgWE30 | 0.022 ± 0.0008 | 0.012 ± 0.0004 | 0.006 ± 0.0002 | 0.074 ± 0.0027 | 0.002 ± 0.0001 |
| MgAE30 | 0.051 ± 0.0021 | 0.094 ± 0.0035 | 0.011 ± 0.0004 | 0.045 ± 0.002 | 0.004 ± 0.0001 |
| MgWE50 | n.d. | 0.01 ± 0.0003 | 0.029 ± 0.0014 | 0.127 ± 0.0057 | 0.004 ± 0.0002 |
| MgAE50 | 0.007 ± 0.0002 | 0.053 ± 0.0017 | 0.03 ± 0.0009 | 0.09 ± 0.0037 | 0.006 ± 0.0001 |
| SsAE30 | 0.066 ± 0.0016 | 0.057 ± 0.0023 | 0.021 ± 0.001 | 0.102 ± 0.0042 | 0.002 ± 0.0001 |
| SsWE30 | 0.058 ± 0.0026 | 0.049 ± 0.0004 | 0.024 ± 0.0006 | 0.097 ± 0.0039 | 0.009 ± 0.0004 |
| SsWE50 | 0.054 ± 0.0205 | 0.045 ± 0.0015 | 0.009 ± 0.0002 | 0.057 ± 0.0012 | 0.023 ± 0.0006 |
| SsAE50 | 0.013 ± 0.0002 | 0.031 ± 0.0004 | 0.032 ± 0.0011 | 0.134 ± 0.0039 | 0.012 ± 0.0005 |
| Sample | GOH | EOH |
|---|---|---|
| [g/L] | ||
| MgWE30 | n.d. | n.d. |
| MgAE30 | n.d. | 0.021 ± 0.0006 |
| MgWE50 | n.d. | 0.014 ± 0.0005 |
| MgAE50 | n.d. | 0.061 ± 0.0023 |
| SsAE30 | n.d. | 0.02 ± 0.0008 |
| SsWE30 | 0.008 ± 0.0005 | 0.019 ± 0.0009 |
| SsWE50 | 0.024 ± 0.001 | 0.062 ± 0.0011 |
| SsAE50 | n.d. | 0.113 ± 0.0019 |
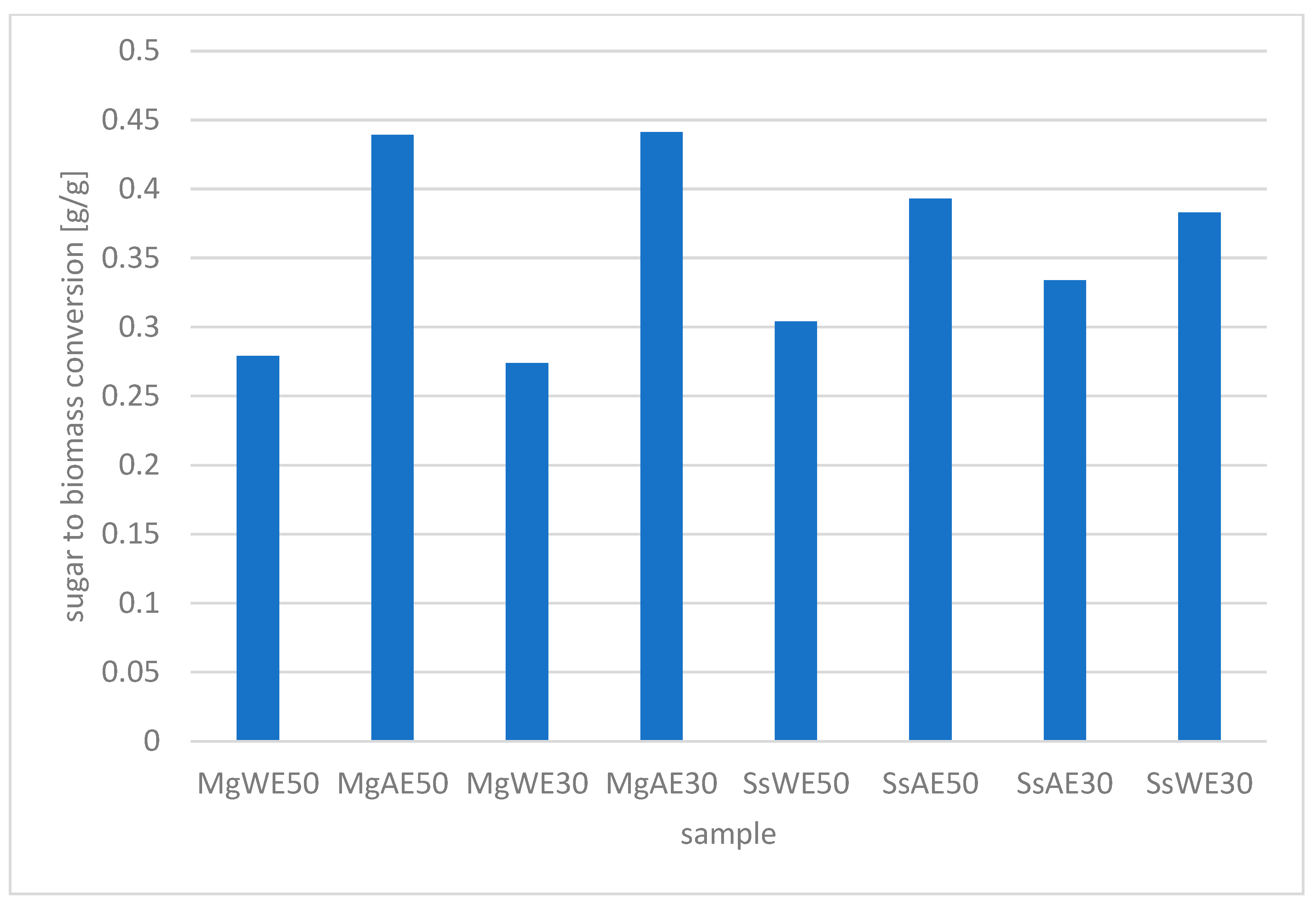
| Sample | Pretreatment Method | Temperature of Enzymatic Hydrolysis [°C] | Yeast Strain | Biomass Concentration [g DM/L] |
|---|---|---|---|---|
| MgWE50 | water | 50 | Meyerozyma guilliermondii | 8.55 ± 0.28 |
| MgAE50 | 2% acid | 50 | 16.29 ± 0.65 | |
| MgWE30 | water | 30 | 9.12 ± 0.48 | |
| MgAE30 | 2% acid | 30 | 12.69 ± 0.53 | |
| SsWE50 | water | 50 | Scheffersomyces stipitis | 10.71 ± 0.23 |
| SsAE50 | 2% acid | 50 | 14.63 ± 0.36 | |
| SsAE30 | 2% acid | 30 | 9.82 ± 0.14 | |
| SsWE30 | water | 30 | 13.03 ± 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patelski, A.M.; Ciach, M.; Dziekońska-Kubczak, U.; Nowak, A.; Balcerek, M.; Pielech-Przybylska, K. Bioconversion of Apple Pomace to Meyerozyma guilliermondii and Scheffersomyces stipitis Biomass. Appl. Sci. 2024, 14, 6108. https://doi.org/10.3390/app14146108
Patelski AM, Ciach M, Dziekońska-Kubczak U, Nowak A, Balcerek M, Pielech-Przybylska K. Bioconversion of Apple Pomace to Meyerozyma guilliermondii and Scheffersomyces stipitis Biomass. Applied Sciences. 2024; 14(14):6108. https://doi.org/10.3390/app14146108
Chicago/Turabian StylePatelski, Andrea Maria, Małgorzata Ciach, Urszula Dziekońska-Kubczak, Agnieszka Nowak, Maria Balcerek, and Katarzyna Pielech-Przybylska. 2024. "Bioconversion of Apple Pomace to Meyerozyma guilliermondii and Scheffersomyces stipitis Biomass" Applied Sciences 14, no. 14: 6108. https://doi.org/10.3390/app14146108
APA StylePatelski, A. M., Ciach, M., Dziekońska-Kubczak, U., Nowak, A., Balcerek, M., & Pielech-Przybylska, K. (2024). Bioconversion of Apple Pomace to Meyerozyma guilliermondii and Scheffersomyces stipitis Biomass. Applied Sciences, 14(14), 6108. https://doi.org/10.3390/app14146108









