1. Introduction
Archery, as a sport, requires the precise coordination of muscular activity to attain accuracy and precision [
1]. Some studies suggest the pivotal involvement of key muscle groups, including the deltoids, trapezius, and rotator cuff muscles, particularly the infraspinatus, in ensuring shoulder stability and control throughout the draw and release phases [
2]. Research emphasizes the critical role of appropriate muscular recruitment in sustaining a consistent performance across successive shots [
3]. Fatigue-induced alterations in muscle activation patterns have been shown to significantly impact shooting accuracy and precision [
4], highlighting the importance of muscular endurance and resistance to fatigue, particularly in the shoulder and forearm muscles responsible for drawing and releasing the bowstring [
5].
During training sessions, archery athletes often encounter repeated shooting conditions, presenting challenges to ensure the consistent and precise repetition of movements [
3]. Repeated conditions of shots in archery can induce muscle fatigue, leading to alterations in electromyographic (EMG) patterns and potentially compromising movement control [
4], thereby possibly impairing performance and ultimately increasing injury risk among athletes.
Previous research conducted on trained archers found that prolonged archery sessions led to decreased EMG amplitude and altered muscle activation patterns, particularly in the shoulder and forearm muscles responsible for drawing and releasing the bowstring [
6]. These fatigue-induced changes in neuromuscular function may disrupt the coordinated firing of muscles essential for precise shooting, consequently impairing movement control and accuracy [
7]. Furthermore, altered EMG patterns may contribute to an increased injury risk in athletes [
3].
In the archery training context, the relationship between training intensity and fatigue management is a topic of significance. Exposing archers to a greater number of shots per session can influence their ability to cope with fatigue while using appropriate distribution of practice, namely using different regimens can play a role to favor adaptations [
5]. Training with higher shot volumes may provide opportunities for skill refinement and the implementation of effective recovery strategies while helping athletes accommodate more extreme conditions better [
8]. While the direct causal link may not be absolute, the training habits of those exposured to a greater number of shots per session in archery training can play a role in sustaining an archer’s capacity to cope with fatigue and ultimately enhance performance in competitive settings.
Based on this hypothesis, understanding how fatigue occurs and influences muscle activity patterns during repeated exposure to shots in archery, while also considering how training habits can contribute to the observed adaptations, is of particular interest in regulating training practices. This understanding can provide valuable insights into establishing optimal thresholds for shot volume during training sessions and identifying potential risks associated with extreme training habits. Given the significance of these factors in enhancing the training process, improving performance, and mitigating the risk of fatigue-induced muscular changes, this study aimed to (i) assess the impact of 50 and 100 archery shots in a single session on the EMG parameters of trained archery athletes and (ii) investigate how the training routines of these athletes influence their ability to cope with fatigue induced by accumulated shots, as reflected in EMG parameters.
2. Materials and Methods
The present study adhered to the reporting guidelines outlined in Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) for cross-sectional studies [
9].
2.1. Study Design
The present study utilized a cross-sectional study design, wherein experienced and trained archers participated in a single session consisting of 100 shots. This design incorporated a repeated measures approach, with each archer undergoing multiple assessments of maximal voluntary isometric contraction in the middle deltoid, posterior deltoid, upper trapezius, middle trapezius, lower trapezius, and infraspinatus. These assessments were conducted at baseline, after 50 shots, and finally, after 100 shots (
Figure 1). The number of 100 shots was determined based on regular coaching advice not to exceed this number of sessions.
2.2. Setting
The evaluation was conducted at the turn of 2023–2024 at the archers’ training facility. The initial assessment took place on the training day, immediately following the players’ warm-up and just before the start of the session. The second assessment occurred five minutes after the first series of 50 shots, and the third assessment took place after the second series, following another 50 shots. The assessment took place after 48 h of rest, with each player being evaluated individually in the afternoon (~4 pm). A convenience sampling strategy was adopted by inviting experienced archers from the regional club. Recruitment was conducted through announcements at the clubs and on social media.
2.3. Ethical Procedures
The study was approved by the Independent Bioethics Committee for Scientific Research at the Medical University of Gdańsk on 14 December 2023 (Resolution No. KB/750/2023–2024). Participants received a detailed explanation of the study, including a simplified description of the protocol. Before participating, they provided written informed consent, confirming their voluntary participation and understanding that they could withdraw from the study at any time without penalty. The study adhered to the ethical principles outlined in the Declaration of Helsinki.
2.4. Participants
Prior to the study, inclusion criteria were established as follows: (i) participants must have trained at least twice a week for the past 6 months, confirming their active status as archers; (ii) participants must be in good health with no history of injury or illness in the past 6 months; (iii) participants must be over 18 years of age; and (iv) participants must not skip any of the recurring phases established for the study. Exclusion criteria included (i) reporting an injury or illness during the study; (ii) having had an upper limb injury within the last 6 months (including shoulder, elbow, or wrist), experiencing pain in the shoulder, elbow, or wrist joint, hypermobility of a lower limb joint, or any neurological or connective tissue disease.
Of the 50 archers initially considered, 30 were excluded due to upper limb injuries or refusal to participate. Consequently, twenty archers were included in the study (men, n = 14; women, n = 6), with an average age of 30.1 ± 6.8 years old, a body mass of 78.0 ± 17.2 kg, and a height of 175.7 ± 9.1 cm. All participants were active sport archers (training experience: 5.5 ± 4.8 years old), part of a group of traditional archers who participate in traditional tournaments, and engage in consistent training practices (weekly training frequency: 1.6 ± 0.8 days/week; shots per training: 111.0 ± 46.4) focused on accuracy and conditioning tailored to these events.
2.5. Independent Variable
The participants were categorized into two groups: those reporting ≤100 shots per training session (n = 13) and those performing >100 shots per session (n = 7). This information was collected individually through surveys conducted before the study began, with participants asked to report the average number of shots per session over the past month. The group reporting ≤100 shots per training session averaged 86.2 ± 20.6 shots per session, while the group performing >100 shots per session averaged 157.1 ± 46.4 shots per session.
2.6. Procedures
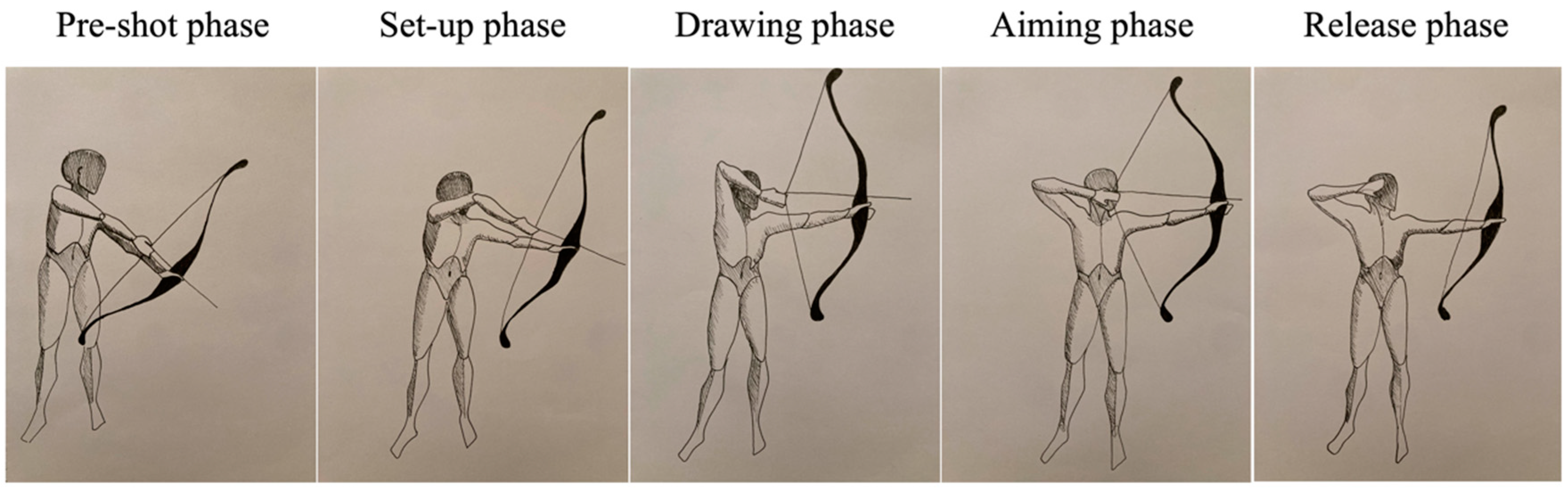
The muscle fatigue assessment focused on the string limb (right-handed: 19, left-handed: 1) during shooting. Each shot consists of five phases: pre-shot, set-up, drawing, aiming, and release. The pre-shot phase involves preparing the bow and loading the arrow. In the subsequent phases, the archer performs the set-up and then draws the bowstring by bending the horizontal string arm. During the aiming phase, the string is maximally stretched, with the isometric work of the muscles maintaining this position predominating. Finally, in the release phase, the string is released by extending the fingers, and the arms are lowered, thus relaxing the muscles.
After following a standardized warm-up protocol led by a researcher—consisting of 10 min of dynamic stretching focused on the upper limbs and 5 min of isometric exercises for the upper limbs—the participants were assessed at the baseline. Assessments were consistently conducted in the same shooting range, within a dedicated room housing the research equipment (sEMG), with a maintained temperature of 23 °C and a relative humidity of 55%. To minimize disruption to athletes, assessments were carried out individually. Two investigators, one specializing in sEMG instrumentation and the other in physical performance assessment, conducted the assessments.
During the initial assessment (prior to training), participants were introduced to the tests and familiarized with the devices. They were given the opportunity to repeat each test without recording to become acquainted with them. Participants were tested while performing the sequence of shooting phases depicted in
Figure 2 (pre-shot phase, set-up phase, drawing phase, aiming phase, and release phase), during which sEMG activity of selected muscles was assessed: middle deltoid, posterior deltoid, upper trapezius, middle trapezius, lower trapezius, and infraspinatus. Each tested muscle was assessed three times during the aiming phase.
2.7. Assessment of Surface EMG—Outcomes
Surface EMG data were recorded for the middle deltoid, posterior deltoid, upper trapezius, middle trapezius, lower trapezius, and infraspinatus during the maximum voluntary contraction (3 s was recorded). The normalization method, which used maximum voluntary contractions as the reference level, was the approach for comparing muscle activity levels and activation patterns across different muscles, tasks, and individuals [
10]. This method ensured that comparisons were accurate and meaningful, as long as maximum neural activation was achieved in all muscles and individuals being tested. By setting maximum voluntary contraction as the baseline, this normalization technique allowed for consistent and reliable assessments of muscle function and performance. The SEMG data were collected and differentially amplified with a gain of 500 using TeleMyo DTS (Noraxon, Scottsdale, AZ, USA) and Ag/AgCl 1-cm2 surface electrodes (Sorimex, Toruń, Poland). The SEMG signals were band-pass filtered (15–500 Hz) and sampled at 1500 Hz (16-bit resolution) using an analogue-to-digital converter. Finally, SEMG data were archived and further processed using MyoResearch 2.8 software (Noraxon). Electrode placement and skin preparation including shaving, abrasing and cleaning with the alcohol took place according to the SENIAM recommendations. Processing of the signal included full rectification and smoothing by the root mean square (EMGRMS) method with a 300 ms moving time window. The following SEMG outcomes were analyzed: mean and maximal amplitude of EMGRMS (µV) and the median frequency of raw SEMG signal power spectrum (EMGMED, Hz).
2.8. Statistical Procedures
After examining potential outliers, descriptive statistics were presented, including means and standard deviations. Prior to conducting inferential statistics, the normality of the sample was assessed, and confirmation was obtained through the Kolmogorov–Smirnov test (
p > 0.05). Similarly, the assumption of homogeneity was verified using Levene’s test (
p > 0.05). Due to the study’s design (two assessments for three groups), a mixed ANOVA was employed to analyze interactions between time and groups. This analysis also entailed calculating partial eta squared (
). The magnitudes of
were interpreted as follows [
11]: trivial (<0.01), small (0.01–0.06), moderate (0.06–0.14), and large (>0.14). Additionally, post hoc comparisons were performed using the Bonferroni test. All statistical analyses were conducted using JASP software (version 0.18.3, the University of Amsterdam, the Netherlands), with a predetermined significance level of
p < 0.05.
3. Results
Table 1 shows the descriptive statistics of the EMG measures for the middle, posterior deltoid, and infraspinatus across different conditions (baseline, after 50 shots, and after 100 shots). No significant interaction between time and group was found in the middle deltoid when considering the mean amplitude EMGRMS (F = 2.579;
p = 0.090;
.125), maximal amplitude EMGRMS (F = 1.323;
p = 0.279;
= 0.068), and EMGMED (F = 1.408;
p = 0.258;
.073). Significant differences over time were observed in the middle deltoid for the mean amplitude EMGRMS (F = 8.225;
p = 0.001;
.314) and maximal amplitude EMGRMS (F = 4.432;
p = 0.033;
.198), although no significant differences were found in EMGMED (F = 2.884;
p = 0.085;
.138). Specifically, after 100 shots, the mean amplitude EMGRMS significantly increased compared to the baseline (182.3 vs. 224.6 µV;
p = 0.006).
No significant interaction between time and group was found in the posterior deltoid when considering the mean amplitude EMGRMS (F = 0.299; p = 0.743;.016), maximal amplitude EMGRMS (F = 1.567; p = 0.223;.080), and EMGMED (F = 1.264; p = 0.295;.066). No significant differences over time were observed in the posterior deltoid for the mean amplitude EMGRMS (F = 2.647; p = 0.085;.128), although significant differences were found in maximal amplitude EMGRMS (F = 4.313; p = 0.021; .193) and in EMGMED (F = 9.925; p < 0.001; .355). Specifically, after 100 shots, the maximal amplitude EMGRMS significantly increased compared to the baseline (742.9 vs. 619.4 µV; p = 0.020). Moreover, at the baseline, the EMGMED was significantly smaller than after 50 shots (61.9 vs. 66.5 Hz; p < 0.001) and 100 shots (61.9 vs. 65.6 Hz; p = 0.028).
No significant interaction between time and group was found in the infraspinatus when considering the mean amplitude EMGRMS (F = 0.895; p = 0.418; .047), maximal amplitude EMGRMS (F = 1.180; p = 0.319; .062), and EMGMED (F = 3.879; p = 0.060; .177). Significant differences over time were observed in the infraspinatus for the mean amplitude EMGRMS (F = 5.620; p = 0.008;.238), maximal amplitude EMGRMS (F = 6.227; p = 0.005;.257), and EMGMED (F = 6.762; p = 0.016;.273). Post hoc comparisons revealed a significantly greater mean amplitude EMGRMS after 50 shots compared to 100 shots (162.0 vs. 113.2 µV; p = 0.033). Similar results were observed for maximal amplitude EMGRMS (357.7 vs. 218.5 µV; p = 0.018). For EMGMED, greater values were observed after 50 shots compared to the baseline (58.1 vs. 60.7 Hz; p = 0.045) and after 100 shots (60.7 vs. 49.3 Hz; p = 0.021).
Table 2 shows the descriptive statistics of the EMG measures for the upper, middle and low trapezius across different conditions (baseline, after 50 shots, and after 100 shots). No significant interaction between time and group was found in the upper trapezius when considering the mean amplitude EMG
RMS (
F = 0.851;
p = 0.435;
.045), maximal amplitude EMG
RMS (
F = 0.021;
p = 0.980;
.001), and EMG
MED (
F = 0.197;
p = 0.822;
.011). Significant differences over time were observed in the upper trapezius for the mean amplitude EMG
RMS (
F = 12.836;
p < 0.001;
.416) and maximal amplitude EMG
RMS (
F = 7.492;
p = 0.008;
.294), although no significant differences were found in EMG
MED (
F = 2.948;
p = 0.822;
.011). Post hoc comparisons revealed a significantly smaller mean amplitude EMG
RMS at the baseline compared to after 50 shots (130.2 vs. 164.9 µV;
p = 0.008) and 100 shots (130.2 vs. 175.5 µV;
p < 0.001). Similar results were observed for maximal amplitude EMG
RMS, with the baseline presenting smaller values than after 50 shots (325.0 vs. 404.5 µV;
p = 0.013) and 100 shots (325.0 vs. 498.4 µV;
p = 0.005).
No significant interaction between time and group was found in the middle trapezius when considering the mean amplitude EMGRMS (F = 0.677; p = 0.452; .036), maximal amplitude EMGRMS (F = 0.586; p = 0.471; .032), and EMGMED (F = 0.596; p = 0.456; .032). No significant differences over time were observed in the middle trapezius for the mean amplitude EMGRMS (F = 0.352; p = 0.706; .019), maximal amplitude EMGRMS (F = 1.729; p = 0.205; .088), and EMGMED (F = 0.437; p = 0.524; .024).
No significant interaction between time and group was found in the low trapezius when considering the mean amplitude EMGRMS (F = 0.496; p = 0.515; .027), maximal amplitude EMGRMS (F = 0.445; p = 0.585; .024), and EMGMED (F = 0.640; p = 0.530; .034). Significant differences over time were observed in the low trapezius for the mean amplitude EMGRMS (F = 4.388; p = 0.044; .196) and maximal amplitude EMGRMS (F = 7.358; p = 0.006; .290), although no significant differences were found in EMGMED (F = 0.483; p = 0.621; .026). Post hoc comparisons revealed a significantly smaller mean amplitude EMGRMS after 100 shots compared to baseline (258.3 vs. 375.0 µV; p = 0.014) and after 50 shots (258.3 vs. 408.5 µV; p = 0.029).
Figure 3 shows the mean amplitude variations in the electromyography (EMG) root mean square (RMS) values which differed across all the regions analyzed across the three repeated measures.
4. Discussion
Our research into muscle activity during repeated archery shots revealed interesting and different patterns among various muscle groups. While the middle and posterior deltoid, along with the upper trapezius, showed a significant rise in both mean and maximal amplitude EMGRMS after 50 shots, this trend continued after 100 shots compared to the baseline. In contrast, a different trajectory was observed in the infraspinatus and lower trapezius. For these muscles, there was a significant increase in EMG parameters after 50 shots, followed by a subsequent decrease after 100 shots, particularly in comparison to the baseline. Interestingly, the EMG activity of the middle trapezius had no significant variations across time. Another noteworthy finding from our study is that the comparison between individuals practicing more shots (>100 shots per session) during regular training sessions and those practicing fewer shots does not show significant differences in the observed trends in EMG measurements. However, the within-group analysis revealed that in the group with regular practice sessions of less than 100 shots, there were more significant differences between the baseline, after 50 shots, and after 100 shots, whereas the other group exhibited greater stability and less variability in EMG measurements across the conditions.
Our findings highlight significant differences in the infraspinatus and lower trapezius, particularly in the mean and maximal amplitude of EMG
RMS over time. Specifically, there was a visible trend wherein the values were significantly higher after 50 shots compared to the subsequent conditions, followed by a significant decrease after 100 shots. Fatigue during repeated shots in archery can induce complex changes in EMG parameters [
7]. This may include an increase in the mean and maximal amplitude of EMG
RMS, indicating higher muscle activation levels as the primary muscles compensate for decreased efficiency followed by a significant decrease reflecting a shift towards lower-frequency components in the EMG signal, leading to the obvious identification of fatigue effect [
12]. This decline in EMG
RMS may result from both central and peripheral fatigue mechanisms, including impaired neuromuscular transmission and alterations in muscle contractility [
13]. Additionally, fatigue may lead to a decrease in the median frequency of the raw surface EMG signal power spectrum, reflecting a shift towards lower-frequency components in the EMG signal due to changes in muscle fiber recruitment patterns and conduction velocity [
14]. As fatigue sets in, there is a possible shift towards recruiting slower-twitch muscle fibers and a decrease in muscle conduction velocity, leading to alterations in the EMG signal frequency spectrum [
15].
However, this trend may not always be consistent. For instance, a simulation study [
16] demonstrated a slightly higher amplitude under fatigue to achieve the same force. In an actual experiment [
17], higher amplitude was observed under fatigue compared to non-fatigue conditions, accompanied by a noticeable decrease in zero-crossing corresponding to median frequency. Therefore, the variability in EMG signals should not be interpreted as a universal mechanism, and further research is needed to enhance our understanding of the phenomenon of muscle fatigue and its interaction with changes in EMG signals.
The middle and posterior deltoid, along with the upper trapezius, showed a significant rise in both mean and maximal amplitude EMG
RMS after 50 shots; this trend continued after 100 shots compared to the baseline. The deltoid muscles, particularly the middle and posterior portions, play a crucial role in shoulder abduction and extension [
18], respectively, while the upper trapezius assists in shoulder elevation and stabilization [
19]. The significant rise in EMG
RMS may indicate an increase in motor unit recruitment and firing rates within these muscle groups [
20], likely due to the demand for sustained muscle activation to support the repetitive task. Additionally, the observed trend continuing beyond 50 shots to 100 shots emphasizes the persistence of neuromuscular adaptation over prolonged repetitive activities, highlighting the potential for muscular fatigue and overuse injuries if proper rest and recovery protocols are not implemented [
21].
The observed differences in EMG
RMS patterns between different muscular groups during shooting in fatigue conditions can be attributed to the varying functional roles and fatigue susceptibilities of these muscles. In the case of the infraspinatus and lower trapezius, the initial rise may reflect compensatory efforts by these muscles to maintain shooting performance as fatigue sets in, leading to increased muscle activation levels [
22]. However, the subsequent decrease in EMG
RMS could indicate a transition towards fatigue-induced changes, including impaired neuromuscular transmission and alterations in muscle contractility [
23]. Conversely, the middle and posterior deltoid, along with the upper trapezius, exhibit a consistent increase in EMG
RMS over time, suggesting ongoing motor unit recruitment and firing rate enhancement within these muscle groups [
24], reflecting the demand for prolonged muscle activation to support the shooting motion. The persistence of this trend beyond 50 shots to 100 shots suggests the adaptability of these muscles to prolonged repetitive activities.
Our study found no significant differences in the observed trends in EMG measurements between individuals practicing more than 100 shots per session and those practicing fewer shots. It is possible that the training routines did not significantly influence the final ability to cope with changes in fatigue conditions. This suggests that future training routines could benefit from a stronger focus on enhancing the ability to endure and cope with the intermittent muscular endurance required to sustain performance across repeated shots. However, the within-group analysis revealed more significant differences in the group with less than 100 shots per session, suggesting that training routines with fewer shots may expose athletes to greater variability in muscle fatigue patterns. One possible explanation could lie in the training specificity and adaptation. Athletes accustomed to higher shot volumes may have undergone physiological adaptations that enable them to better regulate muscle fatigue and maintain consistent performance over extended training sessions [
25]. In contrast, individuals with lower shot volumes may experience greater variability in muscle fatigue patterns due to their inadequate adaptation to the demands of archery. Another factor to consider is the role of motor learning and skill acquisition. Athletes engaging in more frequent and intense training sessions may have refined their shooting technique to minimize energy expenditure and optimize muscle recruitment patterns [
2], thereby mitigating the onset of fatigue. Conversely, those with fewer shots per session may exhibit less refined motor skills, leading to inefficiencies in movement execution and increased susceptibility to fatigue.
The limitations of our study should be acknowledged when interpreting these findings. Firstly, our sample size was relatively small and may not have fully represented the diverse population of archers. Additionally, while we focused on EMG measurements, other physiological parameters such as muscle fatigue markers or kinematic data could provide a more comprehensive understanding of muscle activity during archery shots. Future research should aim to incorporate these additional measures to enhance the understanding of muscular fatigue in archery. Moreover, our study only examined muscle activity during the shooting phase, neglecting potential differences in muscle recruitment during the aiming phases. Investigating muscle activity throughout the entire archery process could provide valuable insights into the specific demands placed on different muscle groups. Further studies could also explore the impact of individual characteristics such as skill level and physical fitness on muscle fatigue patterns in archers, which may help design training programs to individual needs.
Practical implications for training practices can be derived from our findings. Coaches and athletes should be aware of the differing patterns of muscle fatigue among various muscle groups during repeated archery shots. Incorporating endurance muscle exercises targeting the infraspinatus and lower trapezius, which exhibited a decrease in EMGRMS after 100 shots, into training routines may help prevent overuse injuries and improve shooting performance. Furthermore, implementing proper rest and recovery protocols between training sessions is crucial to mitigate the risk of muscular fatigue and overuse injuries, particularly for the deltoid muscles and upper trapezius, which show sustained increases in EMGRMS over prolonged shooting sessions.