Mechanism of Iron Transport in the Triticum aestivum L.–Soil System: Perception from a Pot Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Solution Preparation
2.3. Sample Analysis
2.4. Evaluation Indicators
2.4.1. Enrichment Coefficient
2.4.2. Translocation Factor
2.5. Data Processing
3. Results
3.1. Characteristics of Element Contents in Soil and Different Parts of Triticum aestivum L.
3.2. Correlation Analysis between Total Element Contents in Soil and Specific Contents in Various Parts of Triticum aestivum L.
3.2.1. The Jointing Stage
3.2.2. The Maturity Stage
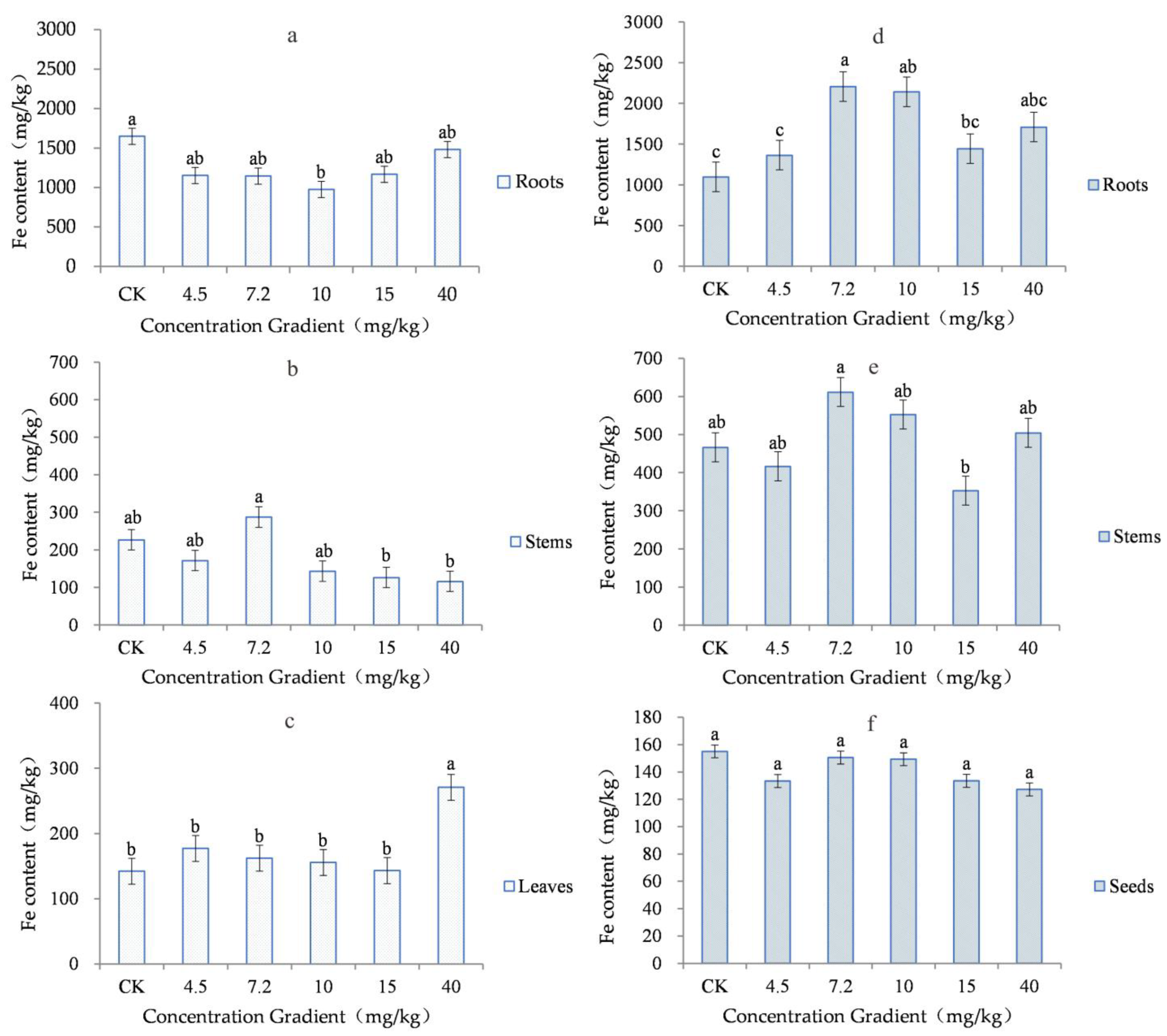
3.3. Variation in Element Contents in Roots, Stems, Leaves and Grains of Triticum aestivum L.
3.4. Enrichment Coefficients of Iron in Different Parts of Enrichment Coefficients of Iron in Different Parts of Triticum aestivum L.
4. Discussion
4.1. Mechanism of Iron Transport in Soil and Triticum aestivum L. Plants
4.2. Mechanism of Iron Accumulation in Various Parts of Triticum aestivum L. under Iron Deficiency Stress Conditions
4.3. Transport and Enrichment of Iron in Triticum aestivum L.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.J.; Hoffland, E.; Kuyper, T.; Yu, Y.; Zhang, C.C.; Li, H.G.; Zhang, F.S.; Werf, W. Syndromes of production in intercropping impact yield gains. Nat. Plants 2020, 6, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.Q.; Wang, T.Q.; Chen, Y.; Wang, M.; Lu, Q.F.; Wang, K.G.; Dou, Z.C.; Chi, Z.G.; Qiu, W.; Dai, J.; et al. Microbiome convergence enables siderophore-secreting-rhizobacteria to improve iron nutrition and yield of peanut intercropped with maize. Nat. Commun. 2024, 15, 839. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, L.J.; Li, M.X. Study on soil water and salt movement and its effect on wheat production under saline water irrigation in North China Plain. N. China Geol. 2023, 46, 76–81. (In Chinese) [Google Scholar] [CrossRef]
- Ray, D.; Mueller, N.; West, P.; Foley, J. Yield Trends Are Insufficient to Double Global Crop Production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- Donaire, G.; Vanzetti, L.; Conde, M.; Bainotti, C.; Mir, L.; Borrás, L.; Chicaiza, O.; Helguera, M. Dissecting genetic loci of yield, yield components, and protein content in bread wheat nested association mapping population. Euphytica 2023, 219, 65. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhang, S.R.; Liu, J.H.; Xing, Y.; Yang, J.Q. Evaluation of the characteristic land resources with Zn, Se and their ecological effects in Raoyang county of Hebei province. Geol. Surv. Res. 2019, 42, 49–56. (In Chinese) [Google Scholar]
- Fei, X.F.; Lou, Z.H.; Xiao, R.; Ren, Z.Q.; Lv, X.N. Estimating the spatial distribution of soil available trace elements by combining auxiliary soil property data through the Bayesian maximum entropy technique. Stoch. Environ. Res. Risk Assess. 2021, 36, 2015–2026. [Google Scholar] [CrossRef]
- Sindireva, A. Local Biogeochemical Cycles of Trace Elements in Agroecosystems of Western Siberia. Geochem. Int. 2023, 61, 1048–1060. [Google Scholar] [CrossRef]
- Ofori, K.; Antoniello, S.; English, M.; Aryee, A. Improving nutrition through biofortification—A systematic review. Front. Nutr. 2022, 9, 1043655. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Khokhar, A.; Maqsood, M.; Shahbaz, M.; Naz, N.; Sara, M.; Maqsood, S.; Sahar, S.; Hussain, S.; Ahmad, M. Genetic biofortification: Advancing crop nutrition to tackle hidden hunger. Funct. Integr. Genom. 2024, 24, 34. [Google Scholar] [CrossRef]
- Cohen, C.; Fox, T.; Garvin, D.; Kochia, L. The Role of Iron-Deficiency Stress Responses in Stimulating Heavy-Metal Transport in Plants. Plant Physiol. 1998, 116, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, L.H.; Wang, M.Y. Iron and zinc biofortification in polished rice and accumulation in rice plant (Oryza sativa L.) as affected by nitrogen fertilization. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2008, 58, 267–272. [Google Scholar] [CrossRef]
- Ma, F.L.; Song, L.M.; Wang, J.M. Overview of Research on Trace Elements in Soil. Qinghai Sci. Technol. 2009, 16, 32–36. (In Chinese) [Google Scholar]
- Zhang, S.R.; Wang, D.M.; Yang, J.Q.; Zhang, J.; Wang, J.H.; Zhang, D.H.; Tong, Y.X.; Jin, Z.B.; Chen, D.L. Progress and Prospects for Remote Sensing Quantitative Inversion Research on Soil Elements. Geol. China. 2023. accepted (In Chinese). Available online: https://link.cnki.net/urlid/11.1167.P.20231205.1103.002 (accessed on 30 May 2024).
- Velemis, D.; Almaliotis, D.; Bladenopoulou, S.; Karapetsas, N. Leaf nutrient levels of apple orchards (cv. Starkrimson) in relation to crop yield. Adv. Hort. Sc. 1999, 13, 147–150. [Google Scholar]
- Dai, J.; Qiu, W.; Wang, N.Q.; Wang, T.Q.; Nakanishi, H.; Zuo, Y.M. From Leguminosae/Gramineae Intercropping Systems to See Benefits of Intercropping on Iron Nutrition. Front. Plant Sci. 2019, 10, 605. [Google Scholar] [CrossRef]
- Jiang, W.J.; Meng, L.S.; Liu, F.T.; Sheng, Y.Z.; Chen, S.M.; Yang, J.L.; Mao, H.R.; Zhang, J.; Zhang, Z.; Ning, H. Distribution, source investigation, and risk assessment of topsoil heavy metals in areas with intensive anthropogenic activities using the positive matrix factorization (PMF) model coupled with self-organizing map (SOM). Environ. Geochem. Health 2023, 45, 6353–6370. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, C.P.; Sheng, Y.Z.; Dong, S.S.; Chen, N.; Hao, C. Effect of Fe(II) on reactivity of heterotrophic denitrifiers in the remediation of nitrate- and Fe(II)-contaminated groundwater. Ecotoxicol. Environ. Saf. 2018, 166, 437–445. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, Y.Z.; Feng, C.P.; Chen, N.; Liu, T. Distinct functional microbial communities mediating the heterotrophic denitrification in response to the excessive Fe(II) stress in groundwater under wheat-rice stone and rock phosphate amendments. Environ. Res. 2020, 185, 109391. [Google Scholar] [CrossRef]
- Liu, Y.J.; Cao, L.M.; Li, Z.L.; Wang, H.N.; Chu, T.Q.; Zhang, J.R. Element Geochemistry, 1st ed.; Science Press: Beijing, China, 1984; pp. 86–88. [Google Scholar]
- Marschner, H.; Romheld, V.; Kissel, M. Different strategies in higher plants in mobilization and uptake of iron. J. Plant Nutr. 1986, 9, 695–713. [Google Scholar] [CrossRef]
- Li, L.M.; Wu, L.H.; Ma, G.R. The Progress on Iron-absorbing Mechanism and Related Gene in Plant. Chin. J. Soil Sci. 2010, 41, 994–999. (In Chinese) [Google Scholar] [CrossRef]
- Shen, H.Y.; Xiong, H.C.; Guo, X.T.; Zuo, Y.M. Progress of molecular and physiological mechanism of iron uptake and translocation in plants. Plant Nutr. Fertil. Sci. 2011, 17, 1522–1530. (In Chinese) [Google Scholar]
- Briat, J.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef]
- Chang, J.B.; Ma, Z.Y.; Ding, Z.J.; Zheng, S.J. Research progresses on molecular mechanisms of storage, transportation and reutilization of plant seed iron. J. Zhejiang Univ. (Agric. Life Sci.) 2021, 47, 473–480. (In Chinese) [Google Scholar] [CrossRef]
- Römheld, V.; Marschner, H. Mechanism of Iron Uptake by Peanut Plants. Plant Physiol. 1983, 71, 949–954. [Google Scholar] [CrossRef]
- Brown, J.; Chaney, R. Effect of Iron on the Transport of Citrate into the Xylem of Soybeans and Tomatoes. Plant Physiol. 1971, 47, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Tiffin, L.O. Iron Translocation II. Citrate/Iron Ratios in Plant Stem Exudates. Plant Physiol. 1966, 41, 515–518. [Google Scholar] [CrossRef]
- Hell, R.; Stephan, U.W. Iron uptake, trafficking and homeostasis in plants. Planta 2003, 216, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Kobayashi, T.; Takahashi, M.; Nagasaka, S.; Usuda, K.; Kakei, Y.; Ishimaru, Y.; Nakanishi, H.; Mori, S.; Nishizawa, N. OsYSL18 is a rice iron(III)-deoxymugineic acid transporter specifically expressed in reproductive organs and phloem of lamina joints. Plant Mol. Biol. 2009, 70, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.; Baxter, I.; Lee, J.; Li, L.; Lahner, B.; Grotz, N.; Kaplan, J.; Guerinot, S. The Ferroportin Metal Efflux Proteins Function in Iron and Cobalt Homeostasis in Arabidopsis. Plant Cell 2009, 21, 3326–3338. [Google Scholar] [CrossRef]
- Kim, S.; Guerinot, L.M. Mining iron: Iron uptake and transport in plants. Febs Lett. 2007, 581, 2273–2280. [Google Scholar] [CrossRef]
- Krüger, C.; Berkowitz, O.; Stephan, U.W.; Hell, R. A metal-binding member of the late embryogenesis abundant protein family transports iron in the phloem of Ricinus communis L. J. Biol. Chem. 2002, 277, 25062–25069. [Google Scholar] [CrossRef] [PubMed]
- DZ/T 0295-2016; Specification for Geochemical Evaluation of Land Quality. Ministry of Land and Resources of the People’s Republic of China. Geological Press: Beijing, China, 2016.
- Sun, X.; Guo, P.C.; Tao, L.N.; Wang, F.W.; Zhang, Y.D.; Li, X.B. Plant Nutrition and Fertilizers, 1st ed.; Agricultural Press: Beijing, China, 1988; pp. 154–155. [Google Scholar]
- GB5009.268-2016; National Food Safety Standards—Determination of Multiple Elements in Food. The National Health and Family Planning Commission of the People’s Republic of China and State Food and Drug Administration: Beijing, China, 2016.
- Zhang, J.J. Study on Monitoring the Change of Heavy Metal Content in Soil Crop System of Farmland by Hyperspectral Remote Sensing. Master’s Thesis, Nanjing University, Nanjing, China, May 2019. (In Chinese). [Google Scholar]
- Pan, Y.H.; Wang, H.B.; Gu, Z.P.; Xiong, G.H.; Yi, F. Accumulation and translocation of heavy metals by macrophytes. Acta Ecol. Sin. 2010, 30, 6430–6441. [Google Scholar]
- Yan, L.; Li, L.S.; Ni, X.L.; Li, C.X.; Li, J. Accumulation of Soil Heavy Metals in Five Species of Wetland Plants. Acta Bot. Boreal.-Occident. Sin. 2016, 36, 2078–2085. (In Chinese) [Google Scholar] [CrossRef]
- Fan, Y.J.; Wei, L.M.; Liang, Z.; Gu, J.W.; Qiu, S.B.; Wang, T.J. A green synthetic route to fischer-tropsch catalysts by complexing and dissolving iron powder with citric acid. Renew. Energy Resour. (In Chinese). 2024, 42, 448–454. [Google Scholar] [CrossRef]
- Bai, Y.J.; Mu, S.H. Uptake and Transport of Iron in Plants and Relations between Iron and Chlorophyll. J. Hebei Agric. Univ. 1994, 17, 121–125. (In Chinese) [Google Scholar]
- Curie, C.; Cassin, G.; Couch, D.; Divol, F.; Higuchi, K.; Jean, M.; Misson, J.; Schikora, A.; Czernic, P.; Mari, S. Metal movement within the plant: Contribution of nicotianamine and yellow stripe 1-like transporters. Ann. Bot. 2009, 103, 1–11. [Google Scholar] [CrossRef]
- Staiger, D. Chemical Strategies for Iron Acquisition in Plants. Angew. Chem. Int. Ed. 2002, 41, 2259–2264. [Google Scholar] [CrossRef]
- Lopez-millan, A.; Morales, F.; Gogorcena, Y.; Abadía, A.; Abadía, J. Iron resupply-mediated deactivation of Fe-deficiency stress responses in roots of sugar beet. Aust. J. Plant Physiol. 2001, 28, 171–180. [Google Scholar] [CrossRef]
- Enomoto, Y.; Hodoshima, H.; Shimada, H.; Shoji, K.; Yoshihara, T.; Goto, F. Long-distance signals positively regulate the expression of iron uptake genes in tobacco roots. Planta 2007, 227, 81–89. [Google Scholar] [CrossRef]
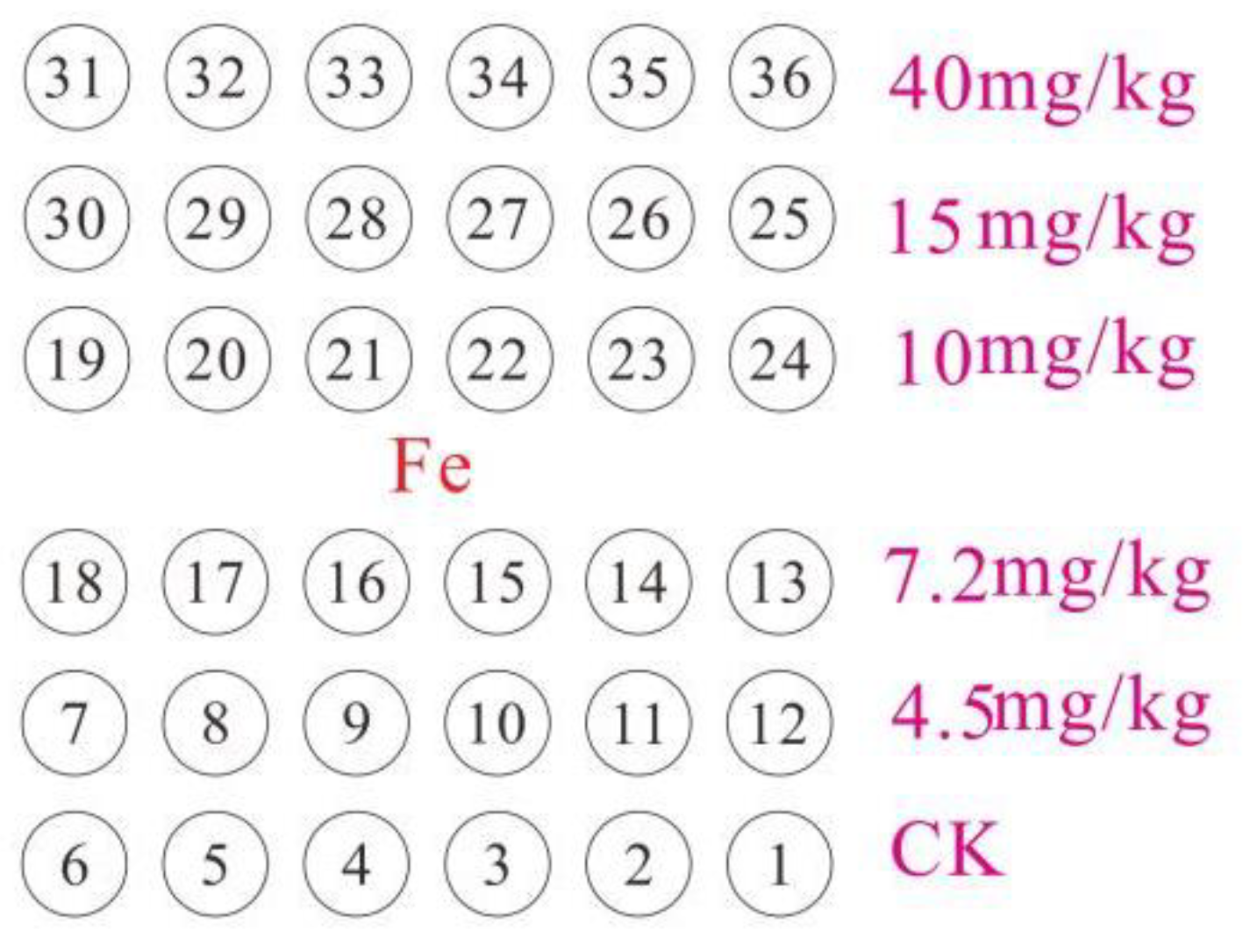

| Indices | Class A (Adequate) | Class B (Moderately Adequate) | Class C (Medium) | Class D (Moderately Deficient) | Class E (Deficient) |
|---|---|---|---|---|---|
| Available iron (mg/kg) | >20 | >10~20 | >4.5~10 | >2.5~4.5 | ≤2.5 |
| Indices | Class A (Adequate) | Class B (Moderately Adequate) | Class C (Medium) | Class D (Moderately Deficient) | Class E (Deficient) |
|---|---|---|---|---|---|
| Available iron (mg/kg) | >40 | >15~40 | >10~15 | >7.2~10 | ≤4.5 |
| Indicator | Measured Available Content in Soil | Presumed Available Content in Soil | Solution Preparation | |||||
|---|---|---|---|---|---|---|---|---|
| Available iron (mg/kg) | 11.9 | 2.38 | Target concentration | 40 | 15 | 10 | 7.2 | 4.5 |
| Compensation concentration | 37.62 | 12.62 | 7.62 | 4.82 | 2.12 | |||
| Application concentration | 376.2 | 126.2 | 76.2 | 48.2 | 21.2 | |||
| Element | Raw Materials | Class A | Class B | Class C | Class D | Class E | ||
|---|---|---|---|---|---|---|---|---|
| Compound | Mol. Formula | At.w./ Cont. % | Conc./Weight | Conc./Weight | Conc./Weight | Conc./Weight | Conc./Weight | |
| Available iron | Fe(II) sulfate | FeSO4·7H2O | 55.85 | 376.2 | 126.2 | 76.2 | 48.2 | 21.2 |
| M.W. | 278.05 | 20.09 | 1872.57 | 628.17 | 379.29 | 239.92 | 105.53 | |
| Growth Stage | Analyte | Minimum | Maximum | Mean | Standard Deviation |
|---|---|---|---|---|---|
| The jointing stage (n = 36) | Total iron in soil | 3.82 | 3.93 | 3.87 | 0.03 |
| Available iron in soil | 9.70 | 30.40 | 15.13 | 5.21 | |
| Root | 592 | 3437 | 1262 | 551 | |
| Stem | 86 | 872 | 179 | 131 | |
| Leaf | 112 | 401 | 175 | 61 | |
| The maturity stage (n = 36) | Total iron in soil | 3.64 | 3.75 | 3.70 | 0.03 |
| Available iron in soil | 10.20 | 18.60 | 13.13 | 2.26 | |
| Root | 801 | 3848 | 1661 | 693 | |
| Stem | 187 | 1017 | 484 | 215 | |
| Seed | 93 | 216 | 141 | 29 |
| Iron Content | Total Iron | Available Iron | Root | Stem |
|---|---|---|---|---|
| Total iron | 1 | 0.691 ** | −0.110 | −0.271 |
| Available iron | 0.691 ** | 1 | −0.058 | −0.252 |
| Root | −0.110 | −0.058 | 1 | 0.039 |
| Stem | −0.271 | −0.252 | 0.039 | 1 |
| Leaf | 0.504 ** | 0.626 ** | 0.007 | −0.086 |
| Iron Content | Total Iron | Available Iron | Root | Stem | Seed |
|---|---|---|---|---|---|
| Total iron | 1 | −0.079 | −0.143 | −0.033 | −0.102 |
| Available iron | −0.079 | 1 | 0.141 | 0.096 | −0.351 * |
| Root | −0.143 | 0.141 | 1 | 0.638 ** | −0.153 |
| Stem | −0.033 | 0.096 | 0.638 ** | 1 | −0.303 |
| Seed | −0.102 | −0.351 * | −0.153 | −0.303 | 1 |
| Concentration Gradient (mg/kg) | The Jointing Stage | The Maturity Stage | |||||
|---|---|---|---|---|---|---|---|
| Roots | Stems | Leaves | Roots | Stems | Seeds | ||
| CK (n = 6) | Minimum | 918 | 120 | 118 | 816 | 193 | 113 |
| Maximum | 3437 | 306 | 164 | 1398 | 735 | 180 | |
| Mean | 1649 | 227 | 142 | 1098 | 467 | 155 | |
| Standard Deviation | 945 | 72 | 17 | 261 | 176 | 23 | |
| Sig. | a | ab | b | c | ab | a | |
| 4.5 (n = 6) | Minimum | 763 | 112 | 139 | 863 | 187 | 114 |
| Maximum | 1932 | 212 | 240 | 1778 | 724 | 155 | |
| Mean | 1152 | 172 | 177 | 1364 | 417 | 133 | |
| Standard Deviation | 426 | 38 | 35 | 383 | 176 | 16 | |
| Sig. | ab | ab | b | c | ab | a | |
| 7.2 (n = 6) | Minimum | 676 | 135 | 113 | 926 | 501 | 112 |
| Maximum | 1623 | 872 | 222 | 2926 | 830 | 188 | |
| Mean | 1145 | 288 | 162 | 2206 | 612 | 151 | |
| Standard Deviation | 388 | 288 | 40 | 767 | 166 | 28 | |
| Sig. | ab | a | b | a | a | a | |
| 10 (n = 6) | Minimum | 592 | 88 | 112 | 871 | 225 | 97 |
| Maximum | 1177 | 189 | 201 | 3848 | 1017 | 216 | |
| Mean | 975 | 143 | 156 | 2142 | 553 | 149 | |
| Standard Deviation | 206 | 45 | 35 | 1067 | 342 | 45 | |
| Sig. | b | ab | b | ab | ab | a | |
| 15 (n = 6) | Minimum | 802 | 97 | 114 | 801 | 267 | 105 |
| Maximum | 1780 | 196 | 190 | 1764 | 620 | 181 | |
| Mean | 1167 | 127 | 143 | 1444 | 353 | 134 | |
| Standard Deviation | 330 | 36 | 30 | 370 | 134 | 28 | |
| Sig. | ab | b | b | bc | b | a | |
| 40 (n = 6) | Minimum | 751 | 86 | 192 | 1257 | 281 | 93 |
| Maximum | 2429 | 180 | 401 | 2209 | 848 | 150 | |
| Mean | 1481 | 116 | 271 | 1709 | 505 | 127 | |
| Standard Deviation | 609 | 33 | 83 | 320 | 217 | 24 | |
| Sig. | ab | b | a | abc | ab | a | |
| Concentration Gradient | Enrichment Coefficient of Root | Enrichment Coefficient of Stem | Enrichment Coefficient of Leaf | Underground to Aboveground Translocation Factor |
|---|---|---|---|---|
| CK | 0.043 | 0.006 | 0.004 | 0.286 |
| 4.5 mg/kg | 0.030 | 0.004 | 0.005 | 0.321 |
| 7.2 mg/kg | 0.030 | 0.007 | 0.004 | 0.393 |
| 10 mg/kg | 0.025 | 0.004 | 0.004 | 0.330 |
| 15 mg/kg | 0.030 | 0.003 | 0.004 | 0.248 |
| 40 mg/kg | 0.038 | 0.003 | 0.007 | 0.317 |
| Mean | 0.033 | 0.005 | 0.005 | 0.316 |
| Concentration Gradient | Enrichment Coefficient of Root | Enrichment Coefficient of Stem/Leaf | Enrichment Coefficient of Seed | Underground to Aboveground Translocation Factor |
|---|---|---|---|---|
| CK | 0.030 | 0.013 | 0.004 | 0.570 |
| 4.5 mg/kg | 0.037 | 0.011 | 0.004 | 0.415 |
| 7.2 mg/kg | 0.060 | 0.017 | 0.004 | 0.389 |
| 10 mg/kg | 0.058 | 0.015 | 0.004 | 0.352 |
| 15 mg/kg | 0.039 | 0.010 | 0.004 | 0.356 |
| 40 mg/kg | 0.046 | 0.014 | 0.003 | 0.381 |
| Mean | 0.045 | 0.013 | 0.004 | 0.411 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Yang, J.; Wang, D.; Liu, J.; Wang, J.; Duan, X.; Yang, L. Mechanism of Iron Transport in the Triticum aestivum L.–Soil System: Perception from a Pot Experiment. Appl. Sci. 2024, 14, 6059. https://doi.org/10.3390/app14146059
Zhang S, Yang J, Wang D, Liu J, Wang J, Duan X, Yang L. Mechanism of Iron Transport in the Triticum aestivum L.–Soil System: Perception from a Pot Experiment. Applied Sciences. 2024; 14(14):6059. https://doi.org/10.3390/app14146059
Chicago/Turabian StyleZhang, Surong, Junquan Yang, Daming Wang, Jihong Liu, Jianhua Wang, Xiaolong Duan, and Lingzhi Yang. 2024. "Mechanism of Iron Transport in the Triticum aestivum L.–Soil System: Perception from a Pot Experiment" Applied Sciences 14, no. 14: 6059. https://doi.org/10.3390/app14146059
APA StyleZhang, S., Yang, J., Wang, D., Liu, J., Wang, J., Duan, X., & Yang, L. (2024). Mechanism of Iron Transport in the Triticum aestivum L.–Soil System: Perception from a Pot Experiment. Applied Sciences, 14(14), 6059. https://doi.org/10.3390/app14146059








