Berry Seeds—A By-Product of the Fruit Industry as a Source of Oils with Beneficial Nutritional Characteristics
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
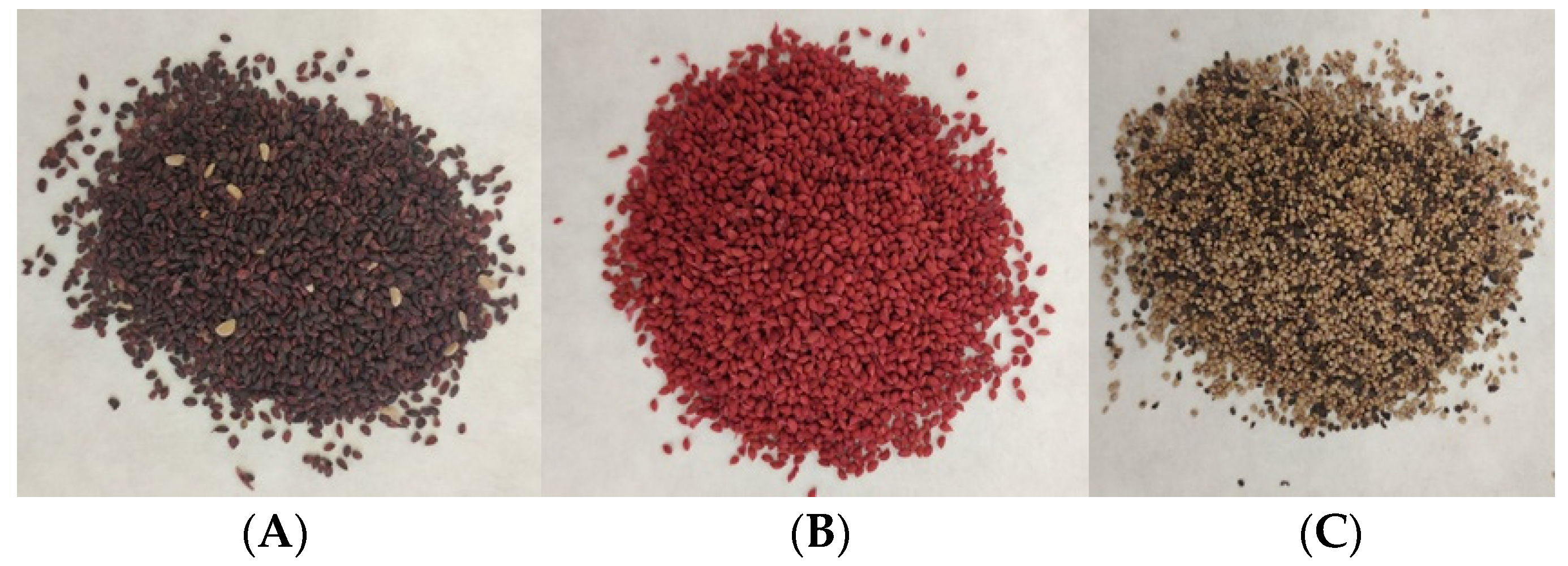
2.1. Materials
- black currant (Ribes nigrum L.);
- strawberries (Fragaria L.);
- cranberries (Vaccinum macrocarpon).
2.2. Methods
2.2.1. Oil Extraction from Black Currant, Cranberry and Strawberry Seeds
2.2.2. Determination of the Fatty Acid Composition of Oils by Gas Chromatography
2.2.3. Determination of the Distribution of Fatty Acids between the sn-2 and sn-1,3 Positions of Triacylglycerols by Enzymatic Hydrolysis
2.2.4. Assessment of Oxidative Stability of Oils by Means of Pressure Differential Scanning Calorimetry
2.2.5. Determination of the Melting Characteristics of Oils by Differential Scanning Calorimetry
2.2.6. Health Indices of Black Currant, Cranberry and Strawberry Seed Oils
2.2.7. Statistical Analysis
3. Results
3.1. Fatty Acid Composition and Distribution between sn-2 and sn-1,3 Positions of Triacylglycerols
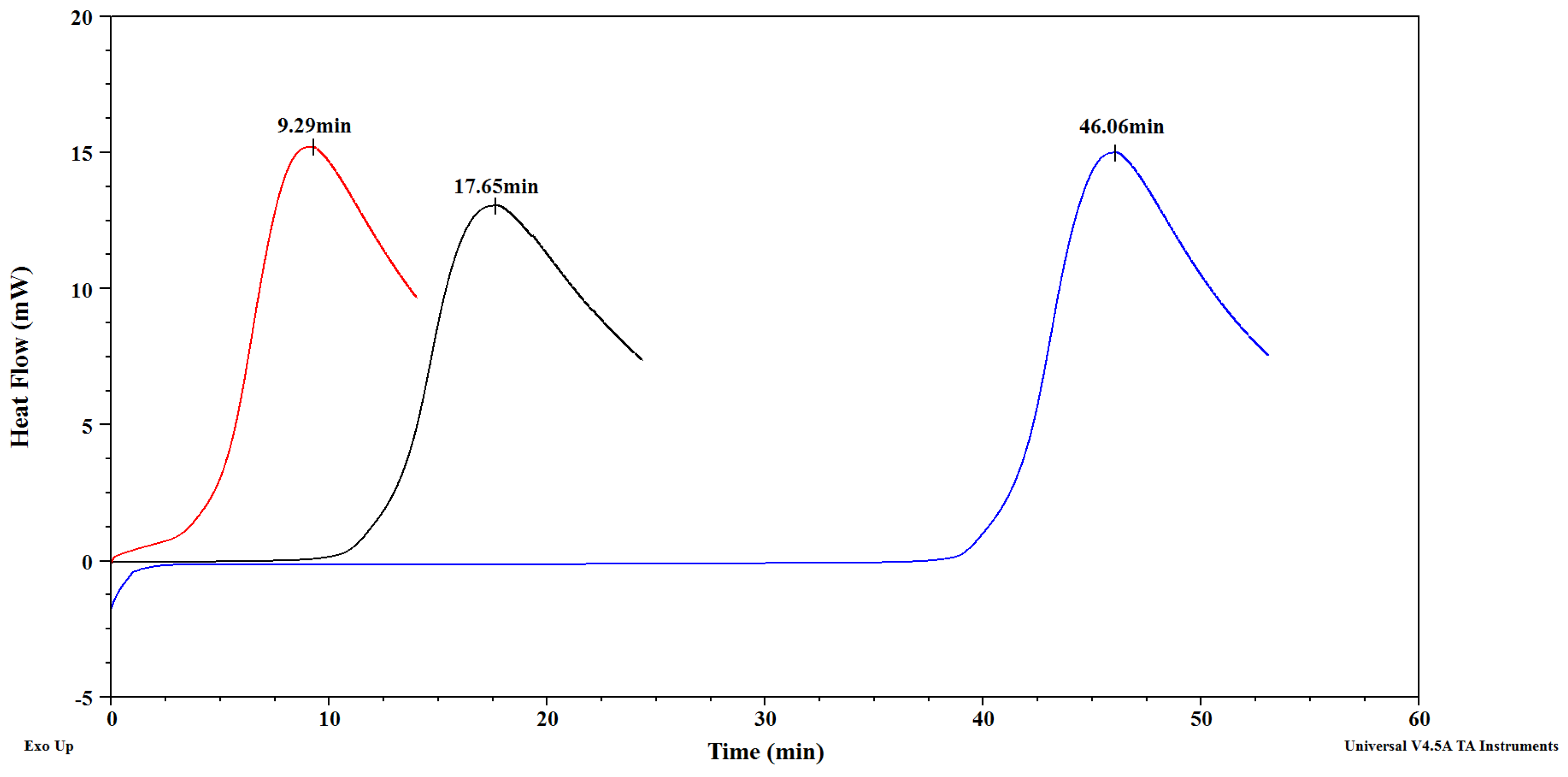
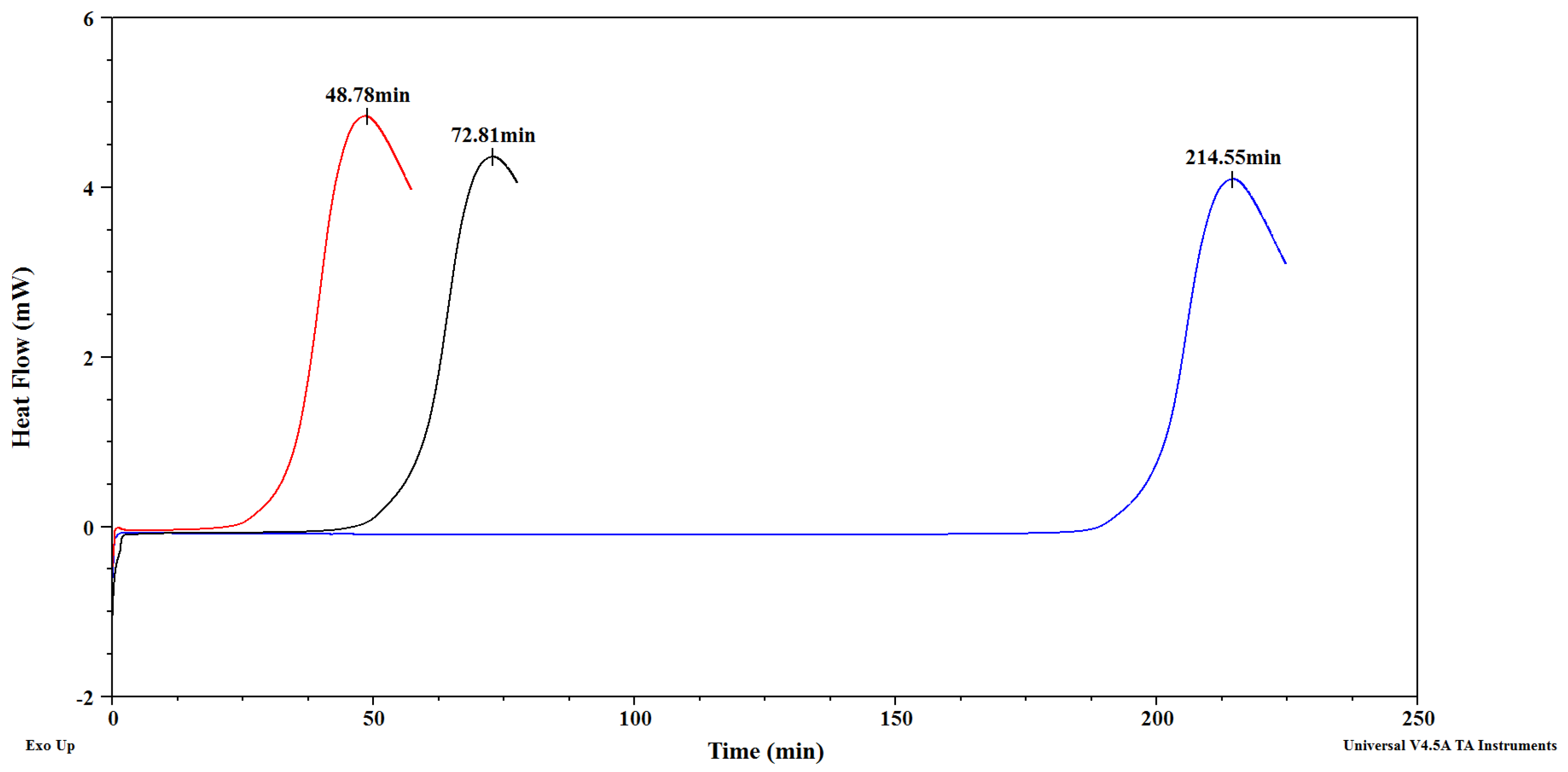
3.2. Oxidative Stability of Cranberry, Black Currant and Strawberry Seed Oils
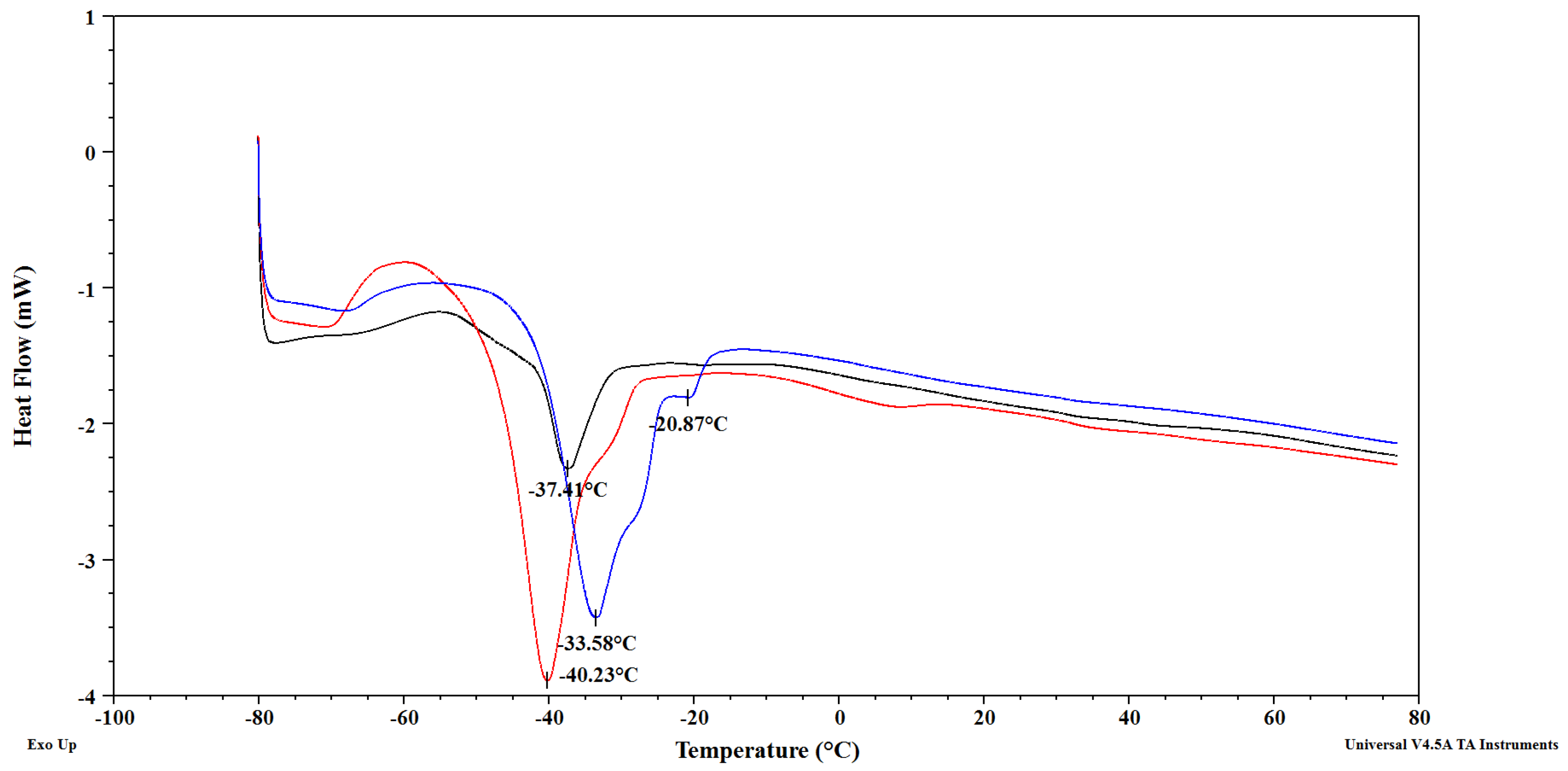
3.3. Melting Characteristics of Cranberry, Black Currant and Strawberry Seed Oils
3.4. Health Indices of Blackcurrant, Cranberry and Strawberry Seed Oils
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Comission. Comission Staff Working Document. Drivers of Food Security; European Comission: Brussels, Belgium, 2023. [Google Scholar]
- Simonetto, M.; Sgarbossa, F.; Battini, D.; Govindam, K. Closed loop supply chains 4.0: From risks to benefits through advanced technologies. A literature review and research agenda. Int. J. Prod. Econ. 2022, 253, 108582. [Google Scholar] [CrossRef]
- Zhang, Q.; Dhir, A.; Kaur, P. Circular economy and the food sector: A systematic literature review. Sustain. Prod. Consum. 2022, 32, 655–668. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Asioli, D.; Banovic, M.; Perito, M.A.; Peschel, A.O.; Stancu, W. Defining upcycled food: The dual role of upcycling in reducing food loss and waste. Trends Food Sci. Technol. 2023, 132, 132–137. [Google Scholar] [CrossRef]
- Olivares-Marín, M.; Fernández, J.A.; Lázaro, M.J.; Fernández-González, C.; Macías-García, A.; Gómez-Serrano, V.; Stoeckli, F.; Centeno, T.A. Cherry stones as precursor of activated carbons for supercapacitors. Mater. Chem. Phys. 2009, 114, 223–227. [Google Scholar] [CrossRef]
- Oomah, B.D.; Ladet, S.; Godfrey, D.V.; Liang, J.; Girard, B. Characteristics of raspberry (Rubus idaeus L.) seed oil. Food Chem. 2000, 69, 187–193. [Google Scholar]
- Kowalczyk, R.; Piwnicki, Ł. Pestki owoców jako cenny surowiec wtórny przemysłu spożywczego. (Fruit seeds as a valuable by-product of the food industry). Postępy Tech. Przetwórstwa Spożywczego 2007, 2, 62–66. [Google Scholar]
- Helbig, D.; Bohm, V.; Wagner, A.; Schubert, R.; Jahreis, G. Berry seed press residues and their valuable ingredients with special regard to black currant seed press residues. Food Chem. 2008, 111, 1043–1049. [Google Scholar] [CrossRef]
- Pachołek, B.; Małecka, M. Pestki czarnej porzeczki jako źródło naturalnych przeciwutleniaczy. (Blackcurrant seeds as a source of natural antioxidants). Rośliny Oleiste 2000, 21, 675–682. [Google Scholar]
- Goffman, G.D.; Galletti, S. Gamma-linolenic acid and tocopherol contents in the seed oil of 47 accessions from several rubes species. J. Agric. Food Chem. 2001, 49, 349–354. [Google Scholar] [CrossRef]
- Tahvonen, R.L.; Schwab, U.S.; Linderborg, K.M.; Mykkänen, H.M.; Kallio, H.P. Black currant seed oil and fish oil supplements differ in their effects on fatty acid of plasma lipids, and concentrations of serum total and lipoprotein lipids, plasma glucose and insulin. J. Nutr. Biochem. 2005, 16, 353–359. [Google Scholar] [CrossRef]
- Martysiak-Żurowska, D.; Drapała, Ł. Struktura triacylogliceroli olejów z nasion truskawki, maliny i czarnej porzeczki. (Structure of triacylglycerols of strawberry, raspberry and blackcurrant seed oils). Tłuszcze Jadalne 2010, 45, 11–20. [Google Scholar]
- Pieszka, M.; Migdał, W.; Gąsior, R.; Rudzińska, M.; Bederska-Łojewska, D.; Pieszka, M.; Szczurek, P. Native oils from apple, blackcurrant, raspberry, and strawberry seeds as a source of polyenoic fatty acids, tocochromanols, and phytosterols: A health implication. J. Chem. 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Ahmad, N.; Anwar, F.; Abbas, A. Fruit Oils: Chemistry and Functionality, 1st ed.; Springer: Cham, Switzerland, 2019; pp. 663–674. [Google Scholar]
- Yu, L.L.; Zhou, K.K.; Parry, J. Antioxidant properties of cold-pressed black caraway, carrot, cranberry, and hemp seed oils. Food Chem. 2005, 91, 723–729. [Google Scholar] [CrossRef]
- Heeg, T.; Lager, H.; Bernard, G. Cranberry Seed Oil, Cranberry Seed Flour and a Method for Making. U.S. Patent US 2012/0148687 A1, 14 June 2012. [Google Scholar]
- PN-EN ISO 659:2010; Oilseeds-Determination of Oil Content (Reference Method). Polish Committee for Standardization: Warsaw, Poland, 2010.
- PN-EN ISO 5509:2001; Vegetable and Animal Oils and Fats. Preparation of Fatty Acid Methyl Esters. Polish Committee for Standardization: Warsaw, Poland, 2001.
- Bryś, J.; Vaz Flores, I.F.; Górska, A.; Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Bryś, A. Use of GC and PDSC methods to characterize human milk fat substitutes obtained from lard and milk thistle oil mixtures. J. Therm. Anal. Calorim. 2017, 130, 319–327. [Google Scholar] [CrossRef]
- Dolatowska-Żebrowska, K.; Ostrowska-Ligęza, E.; Wirkowska-Wojdyła, M.; Bryś, J.; Górska, A. Characterization of thermal properties of goat milk fat and goat milk chocolate by using DSC, PDSC and TGA methods. J. Therm. Anal. Calorim. 2019, 138, 2769–2779. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs: II. Fatty acid composition of meat. Livest. Prod. Sci. 2022, 77, 187–194. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J. Nutr. Metab. 2012, 2012, 1–16. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Dubois, V.; Breton, S.; Linder, M.; Fanni, J.; Parmentier, M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur. J. Lipid Sci. Technol. 2007, 109, 710–732. [Google Scholar] [CrossRef]
- Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Górska, A.; Bryś, J. Application of Chromatographic and Thermal Methods to Study Fatty Acids Composition and Positional Distribution, Oxidation Kinetic Parameters and Melting Profile as Important Factors Characterizing Amaranth and Quinoa Oils. Appl. Sci. 2022, 12, 2166. [Google Scholar] [CrossRef]
- Brzezińska, R.; Górska, A.; Gotowicka, K.; Bryś, J.; Ostrowska-Ligęza, E.; Wirkowska-Wojdyła, M. Quality Assessment of Avocado Pulp Oils during Storage. Proceedings 2021, 70, 14. [Google Scholar]
- Kamal-Eldin, A. Effect of fatty acids and tocopherols on the oxidative stability of vegetable oils. Eur. J. Lipid Sci. Technol. 2006, 58, 1051–1061. [Google Scholar] [CrossRef]
- Piasecka, I.; Górska, A.; Ostrowska-Ligęza, E.; Kalisz, S. The study of thermal properties of blackberry, chokeberry and raspberry seeds and oils. Appl. Sci. 2021, 11, 7704. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Kourimská, L. Assessment of the Nutritional Quality of Plant Lipids Using Atherogenicity and Thrombogenicity Indices. Nutrients 2022, 14, 3795. [Google Scholar] [CrossRef] [PubMed]
- Acay, A.; Ulu, M.S.; Ahsen, A.; Ozkececi, G.; Demir, K.; Ozuguz, U.; Yuksel, S.; Acarturk, G. Atherogenic index as a predictor of atherosclerosis in subjects with familial Mediterranean fever. Medicina 2014, 50, 329–333. [Google Scholar] [CrossRef]
- Kourimska, L.; Pokhrel, K.; Bozik, M.; Khalili Tilami, S.; Horcicka, P. Fat content and fatty acid profiles of recently registered varieties of naked and hulled oats with and without husks. J. Cereal Sci. 2020, 99, 103216. [Google Scholar] [CrossRef]
| Fatty Acid | Cranberry Seed Oil | Black Currant Seed Oil | Strawberry Seed Oil |
|---|---|---|---|
| 12:0 | 0.01 | 0.06 | - |
| 14:0 | 0.04 | 0.19 | 0.13 |
| 15:0 | 0.01 | 0.05 | 0.03 |
| 16:0 | 5.89 | 7.95 | 4.71 |
| 16:1 | 0.07 | 0.10 | 0.23 |
| 17:0 | 0.05 | 0.09 | 0.05 |
| 17:1 | 0.04 | 0.05 | 0.06 |
| 18:0 | 1.34 | 1.93 | 1.87 |
| 18:1 n-9c | 23.99 | 16.09 | 17.26 |
| 18:2 n-6c | 36.59 | 48.07 | 44.01 |
| 18:3 n-6c | 0.12 | 8.92 | - |
| 18:3 n-3c | 31.39 | 15.02 | 29.76 |
| 20:0 | 0.11 | - | 1.09 |
| 20:1 | 0.23 | 1.19 | 0.34 |
| 22:1 n-9c | 0.12 | - | 0.07 |
| other | - | 0.29 | 0.39 |
| ΣSFA | 7.45 ± 0.46 a | 10.27 ± 1.21 b | 7.88 ± 0.74 a |
| ΣMUFA | 24.45 ± 1.89 b | 17.43 ± 0.97 a | 17.96 ± 1.02 a |
| ΣPUFA | 68.1 ± 2.28 a | 72.01 ± 2.75 b | 73.77 ± 2.03 b |
| n-3:n-6 ratio | 1:1.2 | 1:4 | 1:1.5 |
| Health indices | |||
| IA | 0.065 | 0.098 | 0.057 |
| IT | 0.058 | 0.122 | 0.055 |
| HH | 15.50 | 10.74 | 18.81 |
| Fatty Acid | Fatty Acid Composition in sn-2 Position of TAG [%] | Fatty Acid Composition in sn-1,3 Positions of TAG [%] | Fatty Acid Share in sn-2 Position of TAG [%] |
|---|---|---|---|
| 16:0 | 2.19 | 7.74 | 12.39 |
| 18:1 n-9c | 29.53 | 21.22 | 41.03 |
| 18:2 n-6c | 40.85 | 34.46 | 37.21 |
| 18:3 n-3c | 26.78 | 33.70 | 28.44 |
| Fatty Acid | Fatty Acid Composition in sn-2 Position of TAG [%] | Fatty Acid Composition in sn-1,3 Positions of TAG [%] | Fatty Acid Share in sn-2 Position of TAG [%] |
|---|---|---|---|
| 16:0 | 4.43 | 9.71 | 18.57 |
| 18:1 n-9c | 21.63 | 13.32 | 44.82 |
| 18:2 n-6c | 49.04 | 47.59 | 34.01 |
| 18:3 n-3c | 13.47 | 15.80 | 29.90 |
| 18:3 n-6c | 9.17 | 8.80 | 34.27 |
| Fatty Acid | Fatty Acid Composition in sn-2 Position of TAG [%] | Fatty Acid Composition in sn-1,3 Positions of TAG [%] | Fatty Acid Share in sn-2 Position of TAG [%] |
|---|---|---|---|
| 16:0 | 3.57 | 5.29 | 25.25 |
| 18:1 n-9c | 20.82 | 15.48 | 40.21 |
| 18:2 n-6c | 49.85 | 41.09 | 37.76 |
| 18:3 n-3c | 24.38 | 32.45 | 27.31 |
| Oil | OIT at 100 °C [min] | OIT at 120 °C [min] |
|---|---|---|
| Cranberry seed oil | 212.04 ± 3.55 e | 46.09 ± 0.63 c |
| Blackcurrant seed oil | 73.00 ± 2.29 d | 17.33 ± 0.41 b |
| Strawberry seed oil | 50.04 ± 2.33 c | 9.39 ± 0.46 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górska, A.; Piasecka, I.; Wirkowska-Wojdyła, M.; Bryś, J.; Kienc, K.; Brzezińska, R.; Ostrowska-Ligęza, E. Berry Seeds—A By-Product of the Fruit Industry as a Source of Oils with Beneficial Nutritional Characteristics. Appl. Sci. 2023, 13, 5114. https://doi.org/10.3390/app13085114
Górska A, Piasecka I, Wirkowska-Wojdyła M, Bryś J, Kienc K, Brzezińska R, Ostrowska-Ligęza E. Berry Seeds—A By-Product of the Fruit Industry as a Source of Oils with Beneficial Nutritional Characteristics. Applied Sciences. 2023; 13(8):5114. https://doi.org/10.3390/app13085114
Chicago/Turabian StyleGórska, Agata, Iga Piasecka, Magdalena Wirkowska-Wojdyła, Joanna Bryś, Kinga Kienc, Rita Brzezińska, and Ewa Ostrowska-Ligęza. 2023. "Berry Seeds—A By-Product of the Fruit Industry as a Source of Oils with Beneficial Nutritional Characteristics" Applied Sciences 13, no. 8: 5114. https://doi.org/10.3390/app13085114
APA StyleGórska, A., Piasecka, I., Wirkowska-Wojdyła, M., Bryś, J., Kienc, K., Brzezińska, R., & Ostrowska-Ligęza, E. (2023). Berry Seeds—A By-Product of the Fruit Industry as a Source of Oils with Beneficial Nutritional Characteristics. Applied Sciences, 13(8), 5114. https://doi.org/10.3390/app13085114










