Clitoria ternatea Flower and Its Bioactive Compounds: Potential Use as Microencapsulated Ingredient for Functional Foods
Abstract
:Featured Application
Abstract
1. Introduction
2. Botanical and Cultivation Characteristics
3. Phytochemical Composition
3.1. Polyphenols in C. ternatea
3.2. Anthocyanins in C. ternatea
4. Health-Promoting Benefits
4.1. Anti-Cholesterol Activity
4.2. Anti-Inflammatory Activity
4.3. Nootropic Activity
4.4. Antidiabetic Activity
4.5. Antioxidant Potential of C. ternatea Components
5. Safety and Toxicity Issues
6. Bioavailability of C. ternatea Components—Anthocyanins
7. Application in Traditional Food and Food Industry
8. Microencapsulation of C. ternatea’s Phytochemical
8.1. Coating Materials
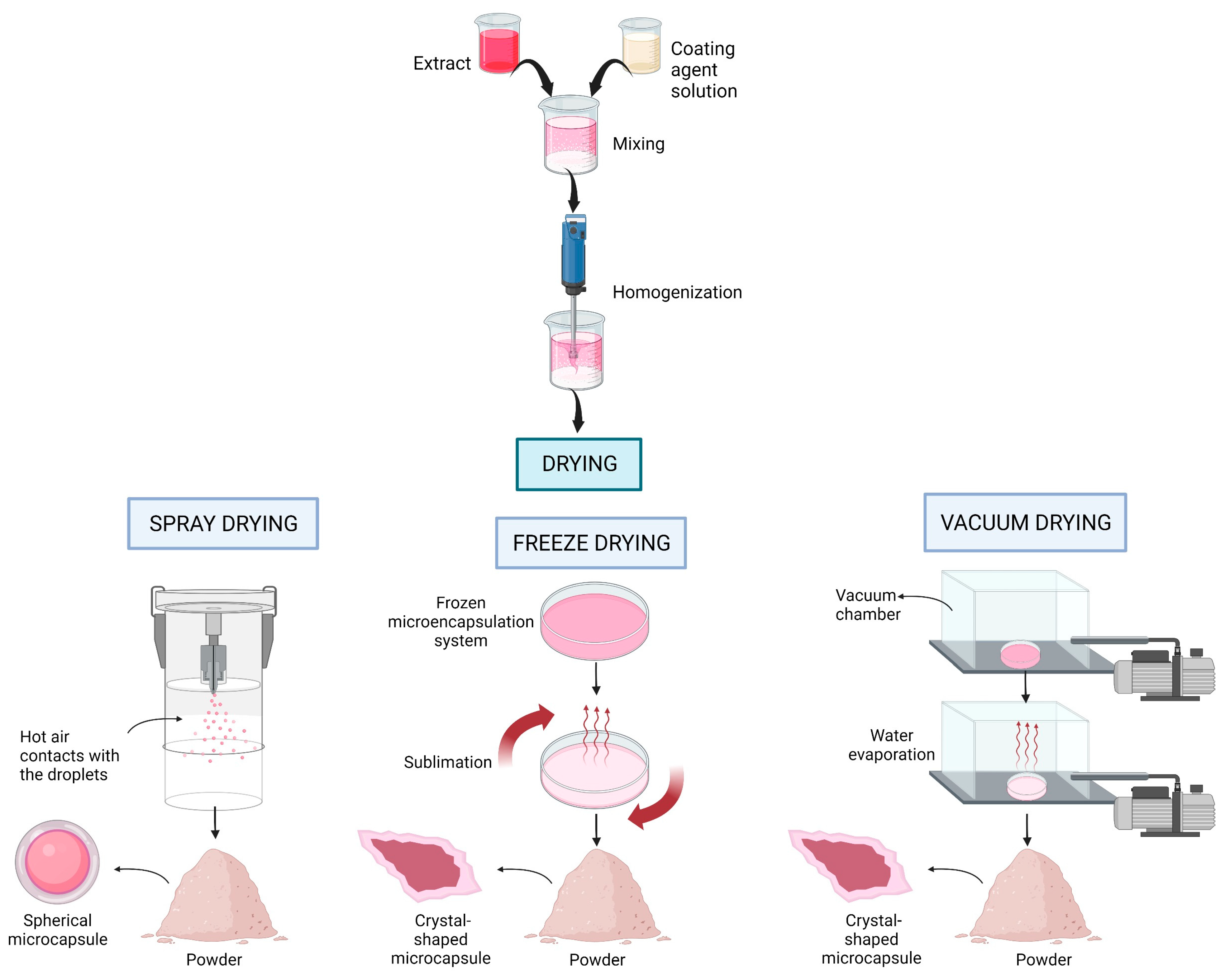
8.2. Drying Methods
9. Effects of Microencapsulation Methods on the Physicochemical and Biological Properties of C. ternatea
9.1. Physicochemical Properties of Microencapsulated C. ternatea
9.2. Antioxidant Activity of Microencapsulated C. ternatea Extract
9.3. Antimicrobial Activity of Microencapsulated C. ternatea Extract
10. Limitations and Future Prospects
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukherjee, P.K.; Kumar, V.; Kumar, N.S.; Heinrich, M. The Ayurvedic Medicine Clitoria ternatea-From Traditional Use to Scientific Assessment. J. Ethnopharmacol. 2008, 120, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Jamil, N.; Mohd Zairi, M.N.; Mohd Nasim, N.A.; Pa’ee, F. Influences of Environmental Conditions to Phytoconstituents in Clitoria ternatea (Butterfly Pea Flower)—A Review. J. Sci. Technol. 2018, 10, 208–228. [Google Scholar] [CrossRef]
- Gupta, G.K.; Kumar Gupta, G. Clitoria ternatea (L.): Old and New Aspects Anticancer Drug Discovery View Project. J. Pharm. Res. 2010, 3, 2610–2614. [Google Scholar]
- Jeyaraj, E.J.; Lim, Y.Y.; Choo, W.S. Extraction Methods of Butterfly Pea (Clitoria ternatea) Flower and Biological Activities of Its Phytochemicals. J. Food Sci. Technol. 2021, 58, 2054–2067. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.K.M.S.; Saha, R.; Talukder, N.; Khaleque, S.M.A.; Ali, H.A. Bioactivity Guided Cytotoxic Activity of Clitoria ternatea Utilizing Brine Shrimp Lethality Bioassay. Bangladesh J. Physiol. Pharmacol. 2006, 22, 18–21. [Google Scholar] [CrossRef]
- Sukri, N.; Multisona, R.R.; Zaida; Saputra, R.A.; Mahani; Nurhadi, B. Effect of Maltodextrin and Arabic Gum Ratio on Physicochemical Characteristic of Spray Dried Propolis Microcapsules. Int. J. Food Eng. 2021, 17, 159–165. [Google Scholar] [CrossRef]
- Gramza-Michalowska, A.; Korczak, J.; Regula, J. Use of Plant Extracts in Summer and Winter Season Butter Oxidative Stability Improvement. Asian Pac. J. Clin. Nutr. 2007, 16, 85–88. [Google Scholar]
- Uwineza, P.A.; Gramza-Michalowska, A.; Bryła, M.; Waśkiewicz, A. Antioxidant Activity and Bioactive Compounds of Lamium Album Flower Extracts Obtained by Supercritical Fluid Extraction. Appl. Sci. 2021, 11, 7419. [Google Scholar] [CrossRef]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as Antimicrobial Agents of Natural Plant Origin. Nat. Prod. Commun. 2011, 6, 1934578X1100600136. [Google Scholar] [CrossRef]
- Setiawati, A.E.; Kusnadi, J. Optimization of Fermentation Time and Grain Concentration for Water Kefir Production from Butterfly Pea Flower (Clitoria ternatea). In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 924. [Google Scholar]
- Thanh, V.T.; Tran, N.Y.T.; Linh, N.T.V.; Vy, T.A.; Truc, T.T. Application of Anthocyanin Natural Colors from Butterfly Pea (Clitoria ternatea L.) Extracts to Cupcake. In IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Bristol, UK, 2020; Volume 736. [Google Scholar]
- Lakshan, S.A.T.; Jayanath, N.Y.; Abeysekera, W.P.K.M.; Abeysekera, W.K.S.M. A Commercial Potential Blue Pea (Clitoria ternatea L.) Flower Extract Incorporated Beverage Having Functional Properties. Evid. Based Complement. Altern. Med. 2019, 2019, 2916914. [Google Scholar] [CrossRef]
- Fuzetti, C.G.; de Castilhos, M.B.M.; Nicoletti, V.R. Microencapsulation of Natural Blue Dye from Butterfly Pea (Clitoria ternatea L.) Flowers: The Application of Different Carriers. J. Food Process. Preserv. 2022, 46, e16420. [Google Scholar] [CrossRef]
- Hamzah, Y.; Jumat, N.A.; Zaliha, W.; Sembok, W. Effect of Drying on the Storage Stability of Encapsulated Anthocyanins Powder Extract from Butterfly Pea Flower (Clitoria ternatea). In Proceedings of the 13th ASEAN Food Conference, Singapore, 9–11 September 2013. [Google Scholar]
- Gaonkar, A.; Vasisht, N.; Kharre, A.; Sobel, R. Microencapsulation in the Food Industry: A Practical Implementation Guide; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Mukherjee, P.K.; Rai, S.; Kumar, V.; Mukherjee, K.; Hylands, P.J.; Hider, R.C. Plants of Indian Origin in Drug Discovery. Expert Opin. Drug Discov. 2007, 2, 633–657. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Bishop, H.G.; Clem, R.L.; Conway, M.J.; Cook, B.G.; Moore, K.; Pengelly, B.C. Measurements of Nutritive Value of a Range of Tropical Legumes and Their Use in Legume Evaluation. Trop. Grassl. 2000, 34, 78–90. [Google Scholar]
- Nguyen, G.K.T.; Zhang, S.; Nguyen, N.T.K.; Nguyen, P.Q.T.; Chiu, M.S.; Hardjojo, A.; Tam, J.P. Discovery and Characterization of Novel Cyclotides Originated from Chimeric Precursors Consisting of Albumin-1 Chain a and Cyclotide Domains in the Fabaceae Family. J. Biol. Chem. 2011, 286, 24275–24287. [Google Scholar] [CrossRef]
- Neda, G.D.; Rabeta; Ong, M.T. Chemical Composition and Anti-Proliferative Properties of Flowers of Clitoria ternatea. Int. Food Res. J. 2013, 20, 1229–1234. [Google Scholar]
- Manjula, P.; Mohan, C.H.; Sreekanth, D.; Keerthi, B.; Devi, B.P. Phytochemicalanalysis of Clitoria ternatea Linn., a Valuable Medicinal Plant. J. Indian Bot. 2013, 92, 173–178. [Google Scholar]
- Kazuma, K.; Noda, N.; Suzuki, M. Malonylated Flavonol Glycosides from the Petals of Clitoria ternatea. Phytochemistry 2003, 62, 229–237. [Google Scholar] [CrossRef]
- Kazuma, K.; Noda, N.; Suzuki, M. Flavonoid Composition Related to Petal Color in Different Lines of Clitoria ternatea. Phytochemistry 2003, 64, 1133–1139. [Google Scholar] [CrossRef]
- Shen, Y.; Du, L.; Zeng, H.; Zhang, X.; Prinyawiwatkul, W.; Alonso-Marenco, J.R.; Xu, Z. Butterfly Pea (Clitoria ternatea) Seed and Petal Extracts Decreased HEp-2 Carcinoma Cell Viability. Int. J. Food Sci. Technol. 2016, 51, 1860–1868. [Google Scholar] [CrossRef]
- López Prado, A.S.; Shen, Y.; Ardoin, R.; Osorio, L.F.; Cardona, J.; Xu, Z.; Prinyawiwatkul, W. Effects of Different Solvents on Total Phenolic and Total Anthocyanin Contents of Clitoria ternatea L. Petal and Their Anti-Cholesterol Oxidation Capabilities. Int. J. Food Sci. Technol. 2019, 54, 424–431. [Google Scholar] [CrossRef]
- Jayanti, M.; Bharatkumar, Z.D.; Narendra, A.G.; Ashok Kumar, B.; Saravanan, R. Assessment of Chemical Diversity in Clitoria ternatea Accessions by an Improved and Validated HPTLC Method Assessment of Chemical Diversity in Clitoria ternatea Accessions by an Improved and Validated HPTLC Method. Indian J. Agric. Sci. 2016, 86, 1133–1139. [Google Scholar]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Pharmacological Importance of Clitoria ternatea—A Review. IOSR J. Pharm. 2016, 6, 68–83. [Google Scholar]
- Tuan Putra, T.N.M.; Zainol, M.K.; Mohdisa, N.S.; Mohdmaidin, N. Chemical Characterization of Ethanolic Extract of Butterfly Pea Flower (Clitoria ternatea). Food Res. 2021, 5, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Havananda, T.; Luengwilai, K. Variation in Floral Antioxidant Activities and Phytochemical Properties among Butterfly Pea (Clitoria ternatea L.) Germplasm. Genet. Resour. Crop Evol. 2019, 66, 645–658. [Google Scholar] [CrossRef]
- Warner, L. Handbook of Anthocyanins: Food Sources, Chemical Applications and Health Benefits; Nova Science Publishers: New York, NY, USA, 2015. [Google Scholar]
- Pasukamonset, P.; Kwon, O.; Adisakwattana, S. Alginate-Based Encapsulation of Polyphenols from Clitoria ternatea Petal Flower Extract Enhances Stability and Biological Activity under Simulated Gastrointestinal Conditions. Food Hydrocoll. 2016, 61, 772–779. [Google Scholar] [CrossRef]
- Zakaria, N.N.A.; Okello, E.J.; Howes, M.J.; Birch-Machin, M.A.; Bowman, A. In Vitro Protective Effects of an Aqueous Extract of Clitoria ternatea L. Flower against Hydrogen Peroxide-Induced Cytotoxicity and UV-Induced MtDNA Damage in Human Keratinocytes. Phyther. Res. 2018, 32, 1064–1072. [Google Scholar] [CrossRef]
- Nair, V.; Bang, W.Y.; Schreckinger, E.; Andarwulan, N.; Cisneros-Zevallos, L. Protective Role of Ternatin Anthocyanins and Quercetin Glycosides from Butterfly Pea (Clitoria ternatea Leguminosae) Blue Flower Petals against Lipopolysaccharide (LPS)-Induced Inflammation in Macrophage Cells. J. Agric. Food Chem. 2015, 63, 6355–6365. [Google Scholar] [CrossRef]
- Terahara, N.; Toki, K.; Saito, N.; Honda, T.; Matsui, T.; Osajima, Y. Eight New Anthocyanins, Ternatins C1–C5 and D3 and Preternatins A3 and C4 from Young Clitoria ternatea Flowers. J. Nat. Prod. 1998, 61, 1361–1367. [Google Scholar] [CrossRef]
- Terahara, N.; Oda, M.; Matsui, T.; Osajima, Y.; Saito, N.; Toki, K.; Honda, T. Five New Anthocyanins, Ternatins A3, B4, B3, B2, and D2, from Clitoria ternatea Flowers. J. Nat. Prod. 1996, 59, 139–144. [Google Scholar] [CrossRef]
- Gould, K.; Davies, K.M.; Winefield, C. Anthocyanins: Biosynthesis, Functions, and Applications; Winefield, C., Davies, K., Gould, K., Eds.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Saptarini, N.M.; Suryasaputra, D.; Nurmalia, H. Application of Butterfly Pea (Clitoria ternatea Linn) Extract as an Indicator of Acid-Base Titration. J. Chem. Pharm. Res. 2015, 7, 275–280. [Google Scholar]
- Oguis, G.K.; Gilding, E.K.; Jackson, M.A.; Craik, D.J. Butterfly Pea (Clitoria ternatea), a Cyclotide-Bearing Plant with Applications in Agriculture and Medicine. Front. Plant Sci. 2019, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Escher, G.B.; Marques, M.B.; do Carmo, M.A.V.; Azevedo, L.; Furtado, M.M.; Sant’Ana, A.S.; da Silva, M.C.; Genovese, M.I.; Wen, M.; Zhang, L.; et al. Clitoria ternatea L. Petal Bioactive Compounds Display Antioxidant, Antihemolytic and Antihypertensive Effects, Inhibit α-Amylase and α-Glucosidase Activities and Reduce Human LDL Cholesterol and DNA Induced Oxidation. Food Res. Int. 2020, 128, 108763. [Google Scholar] [CrossRef] [PubMed]
- Shyamkumar, I.B.; Ishwar, B. Anti-Inflammatory, Analgesic, and Phytochemical Studies of Clitoria ternatea Linn Flower Extract. Int. Res. J. Pharm. 2012, 3, 208–210. [Google Scholar]
- Devi, B.P.; Boominathan, R.; Mandal, S.C. Anti-Inflammatory, Analgesic and Antipyretic Properties of Clitoria ternatea Root. Fitoterapia 2003, 74, 345–349. [Google Scholar] [CrossRef]
- Raghu, K.S.; Shamprasad, B.R.; Kabekkodu, S.P.; Paladhi, P.; Joshi, M.B.; Valiathan, M.S.; Guruprasad, K.P.; Satyamoorthy, K. Age Dependent Neuroprotective Effects of Medhya Rasayana Prepared from Clitoria ternatea Linn. in Stress Induced Rat Brain. J. Ethnopharmacol. 2017, 197, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Chusak, C.; Thilavech, T.; Henry, C.J.; Adisakwattana, S. Acute Effect of Clitoria ternatea Flower Beverage on Glycemic Response and Antioxidant Capacity in Healthy Subjects: A Randomized Crossover Trial. BMC Complement. Altern. Med. 2018, 18, 6. [Google Scholar] [CrossRef]
- Kavitha, R. Biochemical Studies on The Effect of Ethanolic Extracts of Trichosanthes Dioica and Clitoria ternatea in Streptozotocin Induced Diabetic Male Wistar Rats. Int. J. Pharm. Sci. Res. 2018, 9, 4682. [Google Scholar]
- Singh, N.K.; Garabadu, D.; Sharma, P.; Shrivastava, S.K.; Mishra, P. Anti-Allergy and Anti-Tussive Activity of Clitoria ternatea L. in Experimental Animals. J. Ethnopharmacol. 2018, 224, 15–26. [Google Scholar] [CrossRef]
- Bhatia, M.; Chahal, J.; Gupta, S. Analgesic and Anti-Inflammatory Activities of Clitoria ternatea Linn. Leaves Extract on Rat Model. Int. J. Pharm. Sci. Res. 2014, 5, 600. [Google Scholar]
- Jain, N.N.; Ohal, C.C.; Shroff, S.K.; Bhutada, R.H.; Somani, R.S.; Kasture, V.S.; Kasture, S.B. Clitoria ternatea and the CNS. Pharmacol. Biochem. Behav. 2003, 75, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Taranalli, A.D.; Cheeramkuzhy, T.C. Influence of Clitoria ternatea Extracts on Memory. Pharm. Biol. 2000, 38, 51–56. [Google Scholar] [CrossRef]
- Rai, K. Clitoria ternatea (Linn) Root Extract Treatment during Growth Spurt Period Enhances Learning and Memory in Rats Prenatal Stress View Project Clitoria ternatea View Project. Indian J. Physiol. Pharmacol. 2001, 45, 305–313. [Google Scholar] [PubMed]
- Phrueksanan, W.; Yibchok-Anun, S.; Adisakwattana, S. Protection of Clitoria ternatea Flower Petal Extract against Free Radicalinduced Hemolysis and Oxidative Damage in Canine Erythrocytes. Res. Vet. Sci. 2014, 97, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Pasukamonset, P.; Kwon, O.; Adisakwattana, S. Oxidative Stability of Cooked Pork Patties Incorporated with Clitoria ternatea Extract (Blue Pea Flower Petal) During Refrigerated Storage. J. Food Process. Preserv. 2017, 41, e12751. [Google Scholar] [CrossRef]
- Pasukamonset, P.; Pumalee, T.; Sanguansuk, N.; Chumyen, C.; Wongvasu, P.; Adisakwattana, S.; Ngamukote, S. Physicochemical, Antioxidant and Sensory Characteristics of Sponge Cakes Fortified with Clitoria ternatea Extract. J. Food Sci. Technol. 2018, 55, 2881–2889. [Google Scholar] [CrossRef]
- EFSA. Notification of Dried Flowers of Clitoria ternatea L. as a Traditional Food from a Third Country Pursuant to Article; European Food Safety Authority: Parma, Italy, 2022; No. December 2021.
- Srichaikul, B. Ultrasonication Extraction, Bioactivity, Antioxidant Activity, Total Flavonoid, Total Phenolic and Antioxidant of Clitoria ternatea Linn Flower Extract for Anti-Aging Drinks. Pharmacogn. Mag. 2018, 14, 322–327. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Ichiyanagi, T.; Rahman, M.M.; Kashiwada, Y.; Ikeshiro, Y.; Shida, Y.; Hatano, Y.; Matsumoto, H.; Hirayama, M.; Tsuda, T.; Konishi, T. Absorption and Metabolism of Delphinidin 3-O-β-d-Glucopyranoside in Rats. Free Radic. Biol. Med. 2004, 36, 930–937. [Google Scholar] [CrossRef]
- Ichiyanagi, T.; Shida, Y.; Rahman, M.M.; Sekiya, M.; Hatano, Y.; Matsumoto, H.; Hirayama, M.; Konishi, T.; Ikeshiro, Y. Effect on Both Aglycone and Sugar Moiety towards Phase II Metabolism of Anthocyanins. Food Chem. 2008, 110, 493–500. [Google Scholar] [CrossRef]
- Ichiyanagi, T.; Shida, Y.; Rahman, M.M.; Hatano, Y.; Matsumoto, H.; Hirayama, M.; Konishi, T. Metabolic Pathway of Cyanidin 3-O-β-D-Glucopyranoside in Rats. J. Agric. Food Chem. 2005, 53, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Ichiyanagi, T.; Terahara, N.; Rahman, M.M.; Konishi, T. Gastrointestinal Uptake of Nasunin, Acylated Anthocyanin in Eggplant. J. Agric. Food Chem. 2006, 54, 5306–5312. [Google Scholar] [CrossRef] [PubMed]
- Ichiyanagi, T.; Kashiwada, Y.; Shida, Y.; Sekiya, M.; Hatano, Y.; Takaishi, Y.; Ikeshiro, Y. Structural Elucidation and Biological Fate of Two Glucuronyl Metabolites of Pelargonidin 3-O-β-D-Glucopyranoside in Rats. J. Agric. Food Chem. 2013, 61, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Terahara, N.; Matsui, T. Structures and Functionalities of Acylated Anthocyanins. ACS Symp. Ser. 2008, 993, 90–101. [Google Scholar]
- Jing, P.; Qian, B.; Zhao, S.; Qi, X.; Ye, L.; Giusti, M.M.; Wang, X. Effect of Glycosylation Patterns of Chinese Eggplant Anthocyanins and Other Derivatives on Antioxidant Effectiveness in Human Colon Cell Lines. Food Chem. 2015, 172, 183–189. [Google Scholar] [CrossRef]
- Suda, I.; Oki, T.; Masuda, M.; Nishiba, Y.; Furuta, S.; Matsugano, K.; Sugita, K.; Terahara, N. Direct Absorption of Acylated Anthocyanin in Purple-Fleshed Sweet Potato into Rats. J. Agric. Food Chem. 2002, 50, 1672–1676. [Google Scholar] [CrossRef]
- Charron, C.S.; Clevidence, B.A.; Britz, S.J.; Novotny, J.A. Effect of Dose Size on Bioavailability of Acylated and Nonacylated Anthocyanins from Red Cabbage (Brassica oleracea L. Var. Capitata). J. Agric. Food Chem. 2007, 55, 5354–5362. [Google Scholar] [CrossRef]
- Ichiyanagi, T.; Shida, Y.; Rahman, M.M.; Hatano, Y.; Konishi, T. Bioavailability and Tissue Distribution of Anthocyanins in Bilberry (Vaccinium myrtillus L.) Extract in Rats. J. Agric. Food Chem. 2006, 54, 6578–6587. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Kano, M.; Takayanagi, T.; Yamakawa, O.; Ishikawa, F. Absorption of Acylated Anthocyanins in Rats and Humans after Ingesting an Extract of Ipomoea Batatas Purple Sweet Potato Tuber. Biosci. Biotechnol. Biochem. 2004, 68, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Ichiyanagi, T.; Nashimoto, M.; Terahara, N. Gastrointestinal Absorption of Ternatins, Polyacylated Anthocyanins Derived from Butterfly Pea (Clitoria ternatea L.) Petals in Rats. BPB Rep. 2021, 4, 136–141. [Google Scholar] [CrossRef]
- Ravindran, P.N. The Encyclopedia of Herbs and Spices; CAB International: Wallingford, UK, 2017; Volume 1. [Google Scholar]
- Martirosyan, D.M.; Singh, J. A New Definition of Functional Food by FFC: What Makes a New Definition Unique? Funct. Foods Health Dis. 2015, 5, 209–223. [Google Scholar] [CrossRef]
- Shiau, S.; Yu, Y.; Pan, W.; Li, G. Colorful and Health Improving Chinese Steamed Bread Fortified by Anthocyanin-rich Extract of Butterfly Pea Flower. J. Food Process. Preserv. 2022, 46, e16925. [Google Scholar] [CrossRef]
- Ab Rashid, S.; Tong, W.Y.; Leong, C.R.; Abdul Ghazali, N.M.; Taher, M.A.; Ahmad, N.; Tan, W.N.; Teo, S.H. Anthocyanin Microcapsule from Clitoria ternatea: Potential Bio-Preservative and Blue Colorant for Baked Food Products. Arab. J. Sci. Eng. 2021, 46, 65–72. [Google Scholar] [CrossRef]
- Lonez, H.E. Butterfly Pea (Clitoria ternatea): A Natural Colorant for Soft Candy (Gummy Candy). Indian J. Sci. Technol. 2021, 14, 239–244. [Google Scholar] [CrossRef]
- Sutakwa, A.; Nadia, L.S.; Suharman, S. Addition of Blue Pea Flower (Clitoria ternatea L.) Extract Increase Antioxidant Activity in Yogurt from Various Types of Milk. J. Agercolere 2021, 3, 31–37. [Google Scholar] [CrossRef]
- Ramli, M.E.; Salleh, R.M.; Tajarudin, H.A.; Zulkurnain, M. Influence of Amylose Content on Phenolics Fortification of Different Rice Varieties with Butterfly Pea (Clitoria ternatea) Flower Extract through Parboiling. LWT 2021, 147, 111493. [Google Scholar] [CrossRef]
- Chusak, C.; Henry, C.J.; Chantarasinlapin, P.; Techasukthavorn, V.; Adisakwattana, S. Influence of Clitoria ternatea Flower Extract on the in Vitro Enzymatic Digestibility of Starch and Its Application in Bread. Foods 2018, 7, 102. [Google Scholar] [CrossRef]
- Dhia, F.; Karim, A. Microencapsulation of Clitoria ternatea, Curcuma Longa, Brassica Oleracea and Hibiscus Sabdariffa Using Thermal Effect Ionic Gelation Technique. Int. J. Eng. Adv. Res. 2022, 4, 57–65. [Google Scholar]
- Choudhury, N.; Meghwal, M.; Das, K. Microencapsulation: An Overview on Concepts, Methods, Properties and Applications in Foods. Food Front. 2021, 2, 426–442. [Google Scholar] [CrossRef]
- Musdalifa; Chairany, M.; Haliza, N.; Bastian, F. Microencapsulation of Three Natural Dyes from Butterfly Pea, Sappan Wood, and Turmeric Extracts and Their Mixture Base on Cyan, Magenta, Yellow (CMY) Color Concept. Canrea J. Food Technol. Nutr. Culin. J. 2021, 4, 91–101. [Google Scholar]
- Zainol, M.K.; Lew, H.W.; Mohd Zin, Z.; Abd Razak, S.B.; Mohd Maidin, N.; Mamat, H. Ramification of Gum Arabic Microencapsulation on the Physicochemical and Microbiological Properties of Butterfly Pea (Clitoria ternatea) Flowers Using Ultrasonic Spray Dryer. Food Res. 2020, 4, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.Y.; Mohd Zin, Z.; Mohd Maidin, N.M.; Mamat, H.; Zainol, M.K. Effect of the Different Encapsulation Methods on the Physicochemical and Biological Properties of Clitoria ternatea Flowers Microencapsulated in Gelatine. Food Res. 2020, 4, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Marsin, A.M.; Mohd Jusoh, Y.M.; Abang Zaidel, D.N.; Hashim, Z.; Mohd Yusof, A.H.; Muhamad, I.I. Microwave-Assisted Encapsulation of Blue Pea Flower (Clitoria ternatea) Colourant: Maltodextrin Concentration, Power, and Time. Chem. Eng. Trans. 2020, 78, 199–204. [Google Scholar]
- Veerathummanoon, N. Anthocyanin Retention Improvement of Microencapsulated Butterfly Pea Flower Crude Extract by Using Freeze Drying and β-Cyclodextrin. Bachelor’s Thesis, Assumption University, Bangkok, Thailand, 2015. [Google Scholar]
- Gibbs, B.F.; Kermasha, S.; Alli, I.; Mulligan, C.N. Encapsulation in the Food Industry: A Review. Int. J. Food Sci. Nutr. 1999, 50, 213–224. [Google Scholar] [PubMed]
- Pang, S.F.; Yusoff, M.M.; Gimbun, J. Assessment of Phenolic Compounds Stability and Retention during Spray Drying of Orthosiphon Stamineus Extracts. Food Hydrocoll. 2014, 37, 159–165. [Google Scholar] [CrossRef]
- Krishnan, S.; Kshirsagar, A.C.; Singhal, R.S. The Use of Gum Arabic and Modified Starch in the Microencapsulation of a Food Flavoring Agent. Carbohydr. Polym. 2005, 62, 309–315. [Google Scholar] [CrossRef]
- Da Silva, F.C.; Da Fonseca, C.R.; De Alencar, S.M.; Thomazini, M.; Balieiro, J.C.D.C.; Pittia, P.; Favaro-Trindade, C.S. Assessment of Production Efficiency, Physicochemical Properties and Storage Stability of Spray-Dried Propolis, a Natural Food Additive, Using Gum Arabic and OSA Starch-Based Carrier Systems. Food Bioprod. Process. 2013, 91, 28–36. [Google Scholar] [CrossRef]
- Bicudo, M.O.P.; Jó, J.; de Oliveira, G.A.; Chaimsohn, F.P.; Sierakowski, M.R.; de Freitas, R.A.; Ribani, R.H. Microencapsulation of Juçara (Euterpe edulis M.) Pulp by Spray Drying Using Different Carriers and Drying Temperatures. Dry. Technol. 2015, 33, 153–161. [Google Scholar] [CrossRef]
- Righi da Rosa, J.; Nunes, G.L.; Motta, M.H.; Fortes, J.P.; Cezimbra Weis, G.C.; Rychecki Hecktheuer, L.H.; Muller, E.I.; Ragagnin de Menezes, C.; Severo da Rosa, C. Microencapsulation of Anthocyanin Compounds Extracted from Blueberry (Vaccinium spp.) by Spray Drying: Characterization, Stability and Simulated Gastrointestinal Conditions. Food Hydrocoll. 2019, 89, 742–748. [Google Scholar] [CrossRef]
- Do, H.T.T.; Nguyen, H.V.H. Effects of Spray-Drying Temperatures and Ratios of Gum Arabic to Microcrystalline Cellulose on Antioxidant and Physical Properties of Mulberry Juice Powder. Beverages 2018, 4, 101. [Google Scholar] [CrossRef]
- Arpagaus, C.; Collenberg, A.; Rütti, D.; Assadpour, E.; Jafari, S.M. Nano Spray Drying for Encapsulation of Pharmaceuticals. Int. J. Pharm. 2018, 546, 194–214. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of Polyphenols—A Review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Ceballos, A.M.; Giraldo, G.I.; Orrego, C.E. Effect of Freezing Rate on Quality Parameters of Freeze Dried Soursop Fruit Pulp. J. Food Eng. 2012, 111, 360–365. [Google Scholar] [CrossRef]
- Berk, Z. Freeze Drying (Lyophilization) and Freeze Concentration. Food Process Eng. Technol. 2018, 2, 567–581. [Google Scholar]
- Ratti, C. Hot Air and Freeze-Drying of High-Value Foods: A Review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An Overview of Encapsulation Technologies for Food Applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- El-Messery, T.M.; El-Said, M.M.; Demircan, E.; Ozçelik, B. Microencapsulation of Natural Polyphenolic Compounds Extracted from Apple Peel and Its Application in Yoghurt. Acta Sci. Pol. Technol. Aliment. 2019, 18, 25–34. [Google Scholar] [PubMed]
- Ibrahim, U.K.; Kamarrudin, N.; Suzihaque, M.U.H.; Abd Hashib, S. Local Fruit Wastes as a Potential Source of Natural Antioxidant: An Overview. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 12040. [Google Scholar] [CrossRef]
- Moghrovyan, A.; Sahakyan, N.; Babayan, A.; Chichoyan, N.; Petrosyan, M.; Trchounian, A. Essential Oil and Ethanol Extract of Oregano (Origanum vulgare L.) from Armenian Flora as a Natural Source of Terpenes, Flavonoids and Other Phytochemicals with Antiradical, Antioxidant, Metal Chelating, Tyrosinase Inhibitory and Antibacterial Activity. Curr. Pharm. Des. 2019, 25, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Gramza-Michalowska, A.; Suliburska, J.; Sidor, A. Puerarin—An Isoflavone with Beneficial Effects on Bone Health. Front. Biosci. 2021, 26, 1653–1667. [Google Scholar] [CrossRef]
- Yu, J. Effect of Drying on the Bioactive Compounds and Antioxidant Activity of Rubus Lambertianus. Int. J. Food Eng. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. Cold Spring Harbor Perspect. Biol 2010, 2, a000414. [Google Scholar]
- Kamilla, L.; Mnsor, S.M.; Ramanathan, S.; Sasidharan, S. Antimicrobial Activity of Clitoria ternatea (L.) Extracts. Pharmacologyonline 2009, 1, 731–738. [Google Scholar]
- Kaushik, P.; Verma, R.; Mittal, V.; Bhatia, S.; Pratap-Singh, A.; Kaushik, D. Flavor Microencapsulation for Taste Masking in Medicated Chewing Gums—Recent Trends, Challenges, and Future Perspectives. Coatings 2022, 12, 1656. [Google Scholar] [CrossRef]
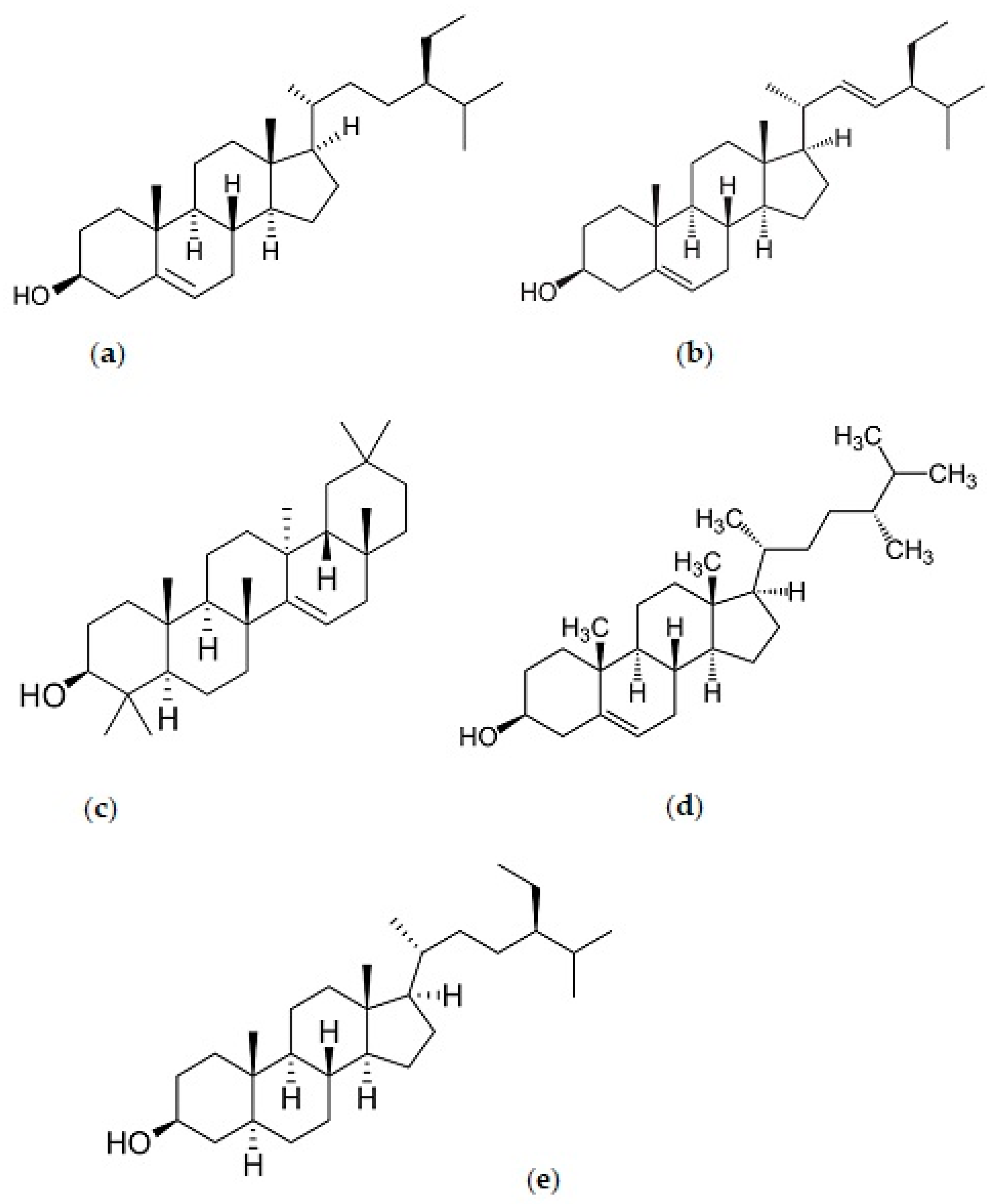

| Group | Compound | Concentration (mg/g) | ||
|---|---|---|---|---|
| [23] | [24] | [22] | ||
| Anthocyanin | Cyanidin-3-sophoroside | 0.31 | N.D. | N.D. |
| Ternatin A1 | 0.51 | 0.39 | 0.61 | |
| Ternatin A2 | N.D. | N.D. | 0.75 | |
| Ternatin A3 | N.D. | N.D. | 0.46 | |
| Ternatin B1 | N.D. | N.D. | 1.42 | |
| Ternatin B2 | 0.32 | 0.73 | 1.52 | |
| Ternatin B3 | 0.50 | N.D. | 0.48 | |
| Ternatin B4 | N.D. | N.D. | 0.40 | |
| Ternatin C2 | 1.81 | N.D. | 0.11 | |
| Ternatin D2 | 1.45 | 0.67 | 0.63 | |
| Ternatin D3 | 0.54 | N.D. | 0.24 | |
| Anthocyanidin | Delphinidin derivative | 2.13 | N.D. | N.D. |
| Flavanol | Rutin | 0.89 | N.D. | N.D. |
| Kaempferol 3-neohesperidoside | 1.76 | 1.29 | 4.89 | |
| Kaempferol 3-rutinoside | N.D. | N.D. | 0.04 | |
| Kaempferol 3-(2G-rhamnosylrutinoside) | N.D. | N.D. | 2.7 | |
| Quercetin 3-(2G-rhamnosylrutinoside) | 0.37 | 0.89 | 0.39 | |
| Quercetin 3-glucoside | N.D. | N.D. | 0.15 | |
| Quercetin 3-rutinoside | N.D. | N.D. | 0.27 | |
| Quercetin 3-neohesperidoside | N.D. | N.D. | 0.39 | |
| Ellagic acid | 0.21 | N.D. | N.D. | |
| Caffeoylmalic acid | N.D. | 1.37 | N.D. | |
| Group | Compound | Concentration | |
|---|---|---|---|
| [23] | [25] | ||
| Fatty Acid (mg/g) | Palmitic acid (C16:0) | 2.13 | N.E. |
| Stearic acid (C18:0) | 1.99 | N.E. | |
| Petroselinic acid (C18:2n6c) | 1.01 | N.E. | |
| Linolenic acid (C18:2n6c) | 4.72 | N.E. | |
| Arachidic acid (C22:0) | 0.36 | N.E. | |
| Behenic acid (C22:0) | 0.30 | N.E. | |
| Phytanic acid | 0.81 | N.E. | |
| Phytosterol (mg/100 g) | Campesterol | 1.24 | N.E. |
| Stigmasterol | 6.70 | N.E. | |
| β-Sitosterol | 6.77 | 18.3–33.4 | |
| Sitostanol | 1.20 | N.E. | |
| Taraxerol | N.D. | 35.8–104.0 | |
| Tocols (mg/100 g) | α-tocopherol | 0.20 | N.E. |
| γ-tocopherol | 0.24 | N.E. | |
| Study Model | Findings | Mode of Action | References |
|---|---|---|---|
| Examination of human copper-reduced low-density-lipoprotein (LDL) cholesterol | 50µL of 2.5µL of C.ternatea flower crude lyophilized extracts (CLE) and partially purified extract (PPE) were used respectively. PPE showed higher inhibition compared to CLE. Both demonstrated the phenolic compounds’ protection against human LDL cholesterol oxidation | Anti-cholesterol activity | [39] |
| Emulsion model observation. | C. ternatea flower extract was used to inhibit cholesterol oxidation and determined after 24 and 48 h. The extract was made by 0.2 g of C. ternatea petal and 4 mL of distilled water, methanol, and both in combination (1:1) after different soaking times. The combined solvents yielded 63.9 µg/mL of anthocyanin in the extract after 6 h of soaking time and inhibited 89.8% of 7-ketocholesterol production in emulsion. | Anti-cholesterol activity | [24] |
| Paw edema method in healthy rates | Healthy albino rats of either gender were dosed with 200 and 400 mg/kg of C. ternatea flower extract. The doses significantly inhibited paw edema compared to control untreated group. The study demonstrated the possibility that the extract may have protective benefits against the release of prostaglandins, kinnins, and other chemicals. | Anti-inflammatory activity | [40] |
| Examination of the inhibition of carrageenin-induced rat paw oedema and acetic acid-induced vascular permeability in rats. | After oral administration of 200 and 400 mg/kg methanolic root extracts C. ternatea, carrageenin-induced rat paw oedema and acetic acid-induced vascular permeability in rats were considerable reduced. | Anti-inflammatory activity | [41] |
| Autophagy measurement | Rats fed with “medhya rasayana” for 60 days, a 1:1 mixture of crushed the whole plant of C. ternatea and jaggery, had significantly lower autophagy in the brain, which indicates that C. ternatea protects the brain by affecting the autophagy-directed pathway. | Nootropic activity | [42] |
| Examination of human plasma glucose and insulin levels | 15 healthy males found that when 1 or 2 g of C. ternatea flower extract were combined with 50 g of sugar, plasma glucose and insulin levels were reduced. | Antidiabetic activity | [43] |
| Examination of blood glucose, insulin, glycosylated hemoglobin, urea, and creatinine levels in rats | Wistar rats given 400 mg/kg ethanolic leaf extracts of C. ternatea weight per day for 28 days indicated considerably lower blood glucose, insulin, glycosylated hemoglobin, urea, and creatinine levels than diabetic control. | Antidiabetic activity | [44] |
| Food Products | Main Findings | ||
|---|---|---|---|
| C. ternatea Extract Concentration | Notes for Recommendation | References | |
| Chinese steam bread | 20–30% of flower water extract added to the bread dough | Total anthocyanins and free polyphenols, as well as antioxidant activities, were increased as the extract concentration increased. However, 30% extract highly reduced the springiness, cohesiveness, and elasticity of the bread. Overall, all concentrations are acceptable sensory attributes. | [70] |
| Muffin | 5 g of spray dried flower acetic water extract to the muffin dough | Providing inhibitory activity on foodborne bacteria, both gram-positive bacteria such as Bacillus cereus, Staphylococcus aureus, Streptococcus sp., and Bacillus coagulans, and gram-negative bacteria such as Yesirnia sp., Proteus mirabilis, Pseudomonas aeruginosa, and Escherichia coli, as well as longer shelf life of product. Physical attributes are acceptable. | [71] |
| Gummy candy | 100 mL of concentration 30 g/1000 mL water extract added into gummy candy ingredients | The highest level of acceptability in color and appearance. | [72] |
| Yogurt | Addition of dried flower extracted with water 3:1 (g/L) ratio to skim milk to produce yogurt. | Showing the highest antioxidant activity in yoghurt made from skim milk or added skim milk compared to other types of milk without the addition of skimmed milk. | [73] |
| Parboiled milled rice | 1% (w/v) of flower water extract used to water to soak 20 g of rice at ratio (1:2). | For maximum phenolic compounds fortification from the C. ternatea flower extract, it is suggested to use low amylose milled rice. | [74] |
| Water kefir | 2 g of flower/250 mL of water before kefir strain is added. | Improved antioxidant activity and TPC. | [10] |
| Cupcake | 50 g of diluted flower water and ethanolic mixture extract (ratio 1:80 with concentrated flower extract) | Preferred by consumers over the traditional mixture due to the color changes, aroma, flavor, and overall organoleptic assessment. | [11] |
| Functional beverage | Ratio of flower water extract, stevia extract, and lime is respectively 983.25 mL/L:1.75 mL/L:15 g/L. | Significantly most acceptable for sensory attributes, possesses an antioxidant activity and is shelf stable for a period of 28 days without preservatives. | [12] |
| Flours (potato, rice, glutinous rice, wheat, and corn) | Addition of 1% and 2% (w/v) flower water extract into each flour. | Inhibition of the pancreatic α-amylase activity in all flours, reduction in glucose release, hydrolysis index, and predicted glycemic index. | [75] |
| Wheat bread | 5%, 10%, and 20% (w/w) flower water extract of wheat flour basis. | Significant reduction in starch digestibility of the bread. | [75] |
| Sponge cakes | 5% of spray dried flower extract (commercially bought from Thai market) of wheat flower basis. | Organoleptically more satisfactory than control, 10%, 15% and 20% concentration. Overall, as the concentration increased, it provided higher total phenolic and anthocyanins content as well as antioxidant activity. | [52] |
| Pork patties | 0.02–0.16% (w/w) of spray dried extract (commercially bought from Thai market) to 100 g pork meat | Increased radical scavenging activity | [51] |
| Extract | Microencapsulation Method | Notes for Recommendation | References | |
|---|---|---|---|---|
| Coating Agent | Drying Method | |||
| Water extract | 20% maltodextrin, 19% maltodextrin and 1% cassava starch, 15% maltodextrin, and 5% gelatin (w/w of extract) | Spray drying Inlet temperature 140 °C, Outlet temperature ±92 °C, feed rate 5 mL/min | The retention of anthocyanins for all treatments are >90% and the gelatin–maltodextrin formulation had the best physicochemical and morphological characteristics, as well as better color preservation. | [13] |
| Acidic extract (Acidic acid) | Maltodextrin 100%, Arabic gum 100%, and combination of maltodextrin 60%, and Arabic gum 40% | Vacuum oven drying Under 0.085 pa, 45 °C, 24 h Freeze drying −80 °C, 24 h | Microcapsules produced by vacuum oven drying with combination of maltodextrin and Arabic gum indicates as the most effective in preserving anthocyanins as powder colorant during storage at room temperature. For freeze-dried microcapsules, using maltodextrin also showed to be effective in maintaining anthocyanins. | [14] |
| Water extract | Sodium alginate (1–2% (w/v)) and calcium chloride (1.5–5% (w/v)) | Air drying 25 °C, 24 h | The beads with 10% C. ternatea extract, 1.5% alginate, and 3% CaCl2 showed the highest encapsulation efficiency, maximal antioxidant capacity, physicochemical properties, and improved the biological activity. | [31] |
| Ethanolic extract | 85% maltodextrin and carrageenan, 90% maltodextrin, and 10% carrageenan (w/w of coating materials) | Freeze drying 48 h | The formulation with a ratio of maltodextrin (90%) and carrageenan (10%) indicated the best results compared to maltodextrin (85%) and carrageenan (15%) in maintaining the antioxidant activity and color intensity of microcapsules. | [78] |
| Water extract | Arabic gum 0, 2, 4, 6, 8, and 10% (w/v of extract) | Ultrasonic spray drying Outlet temperature 90 °C, feed rate 8 mL/min | Among the various concentrations, the sample with 6% Arabic gum concentration relative to solid content was the most effective in maintaining the antioxidant activities and microbial activity, and was acceptable physically. | [79] |
| Water extract | 5% gelatin (w/v of 100 mL distilled water) | Ultrasonic spray drying Outlet temperature 100 °C Feed rate 3 mL/min, Convection oven 80 °C, low air pressure, 2 h. Freeze drying −80 °C, 24 h | The highest encapsulation efficiency was shown by the freeze-dried product, according to the anthocyanin contents, antioxidant activity, microbial properties, and color lightness. | [80] |
| Water extract | Maltodextrin 20%, 30%, 40%, and 50% (w/w of distilled water) | Microwave drying 550 W, 6 min 770 W, 7 min 770 W, 8 min | The best encapsulation condition resulted from the concentration of maltodextrin 40%, microwave power 770 W, and 7 min drying, which has high encapsulation efficiency (73.24%), high anthocyanin contents, and low water activity value. | [81] |
| Water extract | Maltodextrin and β-cyclodextrin (75:25, 50:50, and 75:25) | Freeze drying 24 h | The ratio of extract to coating materials 1:1 with composition 75% maltodextrin and 25% β-cyclodextrin showed the highest anthocyanin retention, as high as 88.4%, with good color profile. | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Multisona, R.R.; Shirodkar, S.; Arnold, M.; Gramza-Michalowska, A. Clitoria ternatea Flower and Its Bioactive Compounds: Potential Use as Microencapsulated Ingredient for Functional Foods. Appl. Sci. 2023, 13, 2134. https://doi.org/10.3390/app13042134
Multisona RR, Shirodkar S, Arnold M, Gramza-Michalowska A. Clitoria ternatea Flower and Its Bioactive Compounds: Potential Use as Microencapsulated Ingredient for Functional Foods. Applied Sciences. 2023; 13(4):2134. https://doi.org/10.3390/app13042134
Chicago/Turabian StyleMultisona, Ribi Ramadanti, Shwetali Shirodkar, Marcellus Arnold, and Anna Gramza-Michalowska. 2023. "Clitoria ternatea Flower and Its Bioactive Compounds: Potential Use as Microencapsulated Ingredient for Functional Foods" Applied Sciences 13, no. 4: 2134. https://doi.org/10.3390/app13042134
APA StyleMultisona, R. R., Shirodkar, S., Arnold, M., & Gramza-Michalowska, A. (2023). Clitoria ternatea Flower and Its Bioactive Compounds: Potential Use as Microencapsulated Ingredient for Functional Foods. Applied Sciences, 13(4), 2134. https://doi.org/10.3390/app13042134








