Abstract
Essential oils (EOs), the odorous and volatile products of a plant’s secondary metabolism, have wide applications in folk medicine, in food flavoring and preservation, and in fragrance industries. The aim of this study was to analyze the chemical composition of the EO from the aerial parts (including the inflorescences) of wild Teucrium luteum subsp. flavovirens from Tunisia. The EO obtained by the hydrodistillation of air-dried plant material in a Clevenger-type apparatus was analyzed using gas chromatography coupled with mass spectrometry (GC-MS). Fifty-three components representing 83.9% of the total constituents were identified. The EO of T. luteum subsp. flavovirens is characterized by the presence of β-elemol (7.2%), (+)-α-pinene (6%), β-eudesmol (5.5%), guaiol (4.2%), α-bisabolol (4.2%), and β-caryophyllene (4.1%) as principal chemical components. In vitro (DPPH and β-carotene bleaching assays), it showed significantly higher radical scavenging and antioxidant properties than the reference compound, BHT. To the best of our knowledge, this is the first report describing the composition and antioxidant properties of the EO from Tunisian T. luteum subsp. flavovirens. Our preliminary data will help to valorize this potentially useful plant species from Tunisia and represent a starting point for further studies on its volatile fraction.
1. Introduction
In recent years, there has been increasing interest in the use of natural substances for their potential therapeutic effects. Medicinal plants are universally considered to be important sources of phytochemicals and are widely used to cure human diseases. The essential oils (EOs) of various species of edible and medicinal plants represent an important source of biologically active secondary metabolites that are useful for the treatment of various health issues [1,2]. In this context, the flora of Tunisia is still exhibiting a wide diversity of non-studied medicinal and odorous plant species that produce EOs and bioactive principles. Of particular interest, plants from the Lamiaceae Martinov (Labiatae Juss.) family have been extensively explored in folkloric medicine, cosmetics, and culinary applications and for the commercial production of EOs [3]. The Teucrium genus, which belongs to the Lamiaceae family, comprises about 300 species that are widespread all around the world [4,5]; these mostly include perennial plants and rarely include annual or biennial plants.
Many species of the genus Teucrium possess several biological activities. Some of the properties are antinociceptive, antihypertensive, anticancer, anti-inflammatory, hypoglycemic, antioxidant, and antibacterial [6,7,8,9]. Moreover, plant species from this genus are used in traditional medicine for their diaphoretic, tonic, antipyretic, antispasmodic, and cholagogue purposes [10]. From the chemical point of view, previous investigations have shown that Teucrium species are rich in EOs, sesquiterpenes, monoterpenes, flavonoids, polyphenolic compounds, iridoids, fatty acids, sterols, saponins, and alkaloids [10,11]. The genus Teucrium is one of the richest sources of neoclerodane diterpenes: more than 220 diterpenes have been described, many of them being of interest due to their insect-repellent and medicinal properties [12,13].
In Tunisia, the genus Teucrium is represented by twenty-four taxa. Among them, Teucrium polium (poley) includes three subspecies: polium, gabesianum, and flavovirens [14]. The plant is a perennial herb known by the name of “germander” and is known in Arabic as “Gattabet Al-Ajrah” [15]. A survey conducted by herbalists identified that in Tunisian folk medicine, a decoction of the leaves of T. polium is used as a cardiac analeptic [16]. T. polium has also been used in folk remedies to treat various diseases and illnesses such as smallpox, malarial fever, biliary retention, upset stomach, diarrhea, colic, and gastric and intestinal pains [15]. Among the other subspecies, T. polium subsp. flavovirens (syn. T. luteum subsp. flavovirens (Batt.) Greuter & Burdet) is of particular interest since it is one of the most important perennial plants growing in Tunisia. From a botanical point of view, the unusual features of this species compared to other members of Lamiaceae are that it is a yellowish green plant with prostrate stems, yellow flowers (7–8 mm), crenate leaves, and ramified indumentum [14].
Several papers have reported the extraction of EOs from subspecies of T. polium, such as T. polium subsp. valentinum from Spain [11], T. polium subsp. capitatum (L.) from Corsica [17], T. polium subsp. aurasiacum from Algeria [18], T. polium subsp. capitatum (L.) from Iran [19], and T. polium subsp. gabesianum from Tunisia [20]. Regarding T. luteum subsp. flavovirens, several authors have already investigated the chemical composition of its EO, focusing on the plants growing in Morocco [21,22,23,24] and Algeria [25]. To the best of our knowledge, no reports regarding T. luteum subsp. flavovirens from Tunisia have been published until now.
The present research is part of an ongoing study on the valorization of medicinal and aromatic/odorous plants of native Tunisian flora [26,27,28], with the purpose of identifying more bioactive natural products. In this paper, we investigate the chemical composition of Tunisian T. luteum subsp. flavovirens for the first time through a study of its volatile compounds, which were extracted by hydrodistillation and analyzed using gas chromatography coupled with mass spectrometry (GC-MS). Furthermore, a preliminary assessment of its biological properties was performed by assaying its antioxidant and radical scavenging activities through DPPH and β-carotene bleaching assays.
2. Materials and Methods
2.1. Plant Material and Extraction of Essential Oil
A 350 g sample of aerial parts of T. luteum subsp. flavovirens (syn. T. polium subsp. flavovirens) was collected in central-east Tunisia (Monastir Governorate) during the flowering–fruiting period (late spring, 2020. Figure 1). Coordinates of the site are 35°43′39″ N, 10°49′14″ E; altitude: about 1 m above sea level. The selection, collection, and identification of the plant material were carried out by Dr. Ridha El Mokni, a botanist in the Laboratory of Botany, Cryptogamy and Plant Biology in the Faculty of Pharmacy of Monastir (Tunisia), where some voucher specimens were deposited (LAM/Teu.lu.-flav.; 23-0105/2020, 24-0105/2020 and 25-0105/2020). The collected material was dried in the shade at ambient temperature until a constant weight was achieved. An amount of 100 g of dried plant material was cut into small parts and then subjected to hydrodistillation in a Clevenger-type apparatus for 3 h after the first drop of distillate and continued until the complete exhaustion of the plant material was achieved [18,27]. The oil was stored at 4 °C in sealed brown glass vials until analysis.
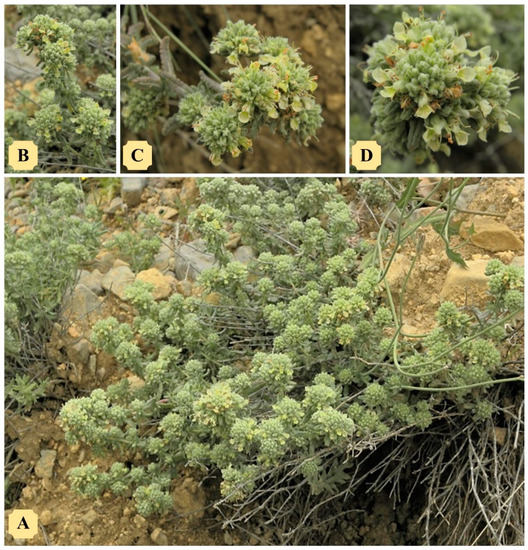
Figure 1.
Tunisian Teucrium luteum subsp. flavovirens: (A) plant in its natural habitat; (B) leafy stem topped by the inflorescences of the year; (C) flowers gathered in pseudo-capitula at the top of the stems; (D) hemispherical inflorescence with yellow corolla flowers and exert stamens. Photos by Ridha El Mokni.
2.2. GC-MS Analysis
The GC analysis of the EO was carried out on a Varian 3900 gas chromatograph coupled with a Varian Saturn 2100T ion trap MS equipped with an Agilent DB-1 fused-silica capillary column (30 mm × 0.25 mm i.d., 0.25 µm film thickness). The analysis was carried out under the following conditions: injector and detector temperatures of 210 °C and 290 °C, respectively; helium was the carrier gas and was used at a flow rate of 0.8 mL/min; split ratio, 1:10; injection volume, 1 µL. A temperature gradient was programmed as follows: 60 °C with a 3 min initial hold, then 280 °C at a rate of 3 °C/min, and finally held isothermally for 5 min. Regarding the MS conditions, the ionization mode had an electronic impact of 70 eV. The mass range was set from 45 to 650 Da. The components were identified by comparing their mass spectra to those of the NIST Library (2014) [29] of the instrument, by evaluating their linear retention indices (LRI) relative to C6-C24 n-alkanes, and, when available, by comparing them to authentic standards from Sigma-Aldrich (Milano, Italy). The percentage composition of the oil was calculated from the GC peak areas using the normalization method without correction factors. The data are reported as the mean value of three oil injections.
2.3. Determination of Antioxidant and Antiradical Activities
The free radical scavenging activity of the EO from T. luteum subsp. flavovirens was determined spectrophotometrically by the 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assay, following a protocol that was previously reported elsewhere [30,31]. In brief, 100 μL of EO dissolved in methanol was added to 1900 μL of a methanol DPPH solution. After mixing by vortexing, the solution was kept at room temperature for 30 min in the dark. The absorbance was measured at 517 nm using a solution of DPPH in methanol as the control. The scavenging activity of the EO on DPPH was calculated as reported in [31] and was expressed as IC50 (50% inhibitory concentration, μg/mL). Results were compared to those previously obtained using ascorbic acid and 3,5-di-tert-butyl-4-hydroxytoluene (BHT) as reference compounds.
The β-carotene bleaching assay was also performed to assess the antioxidant activity of the EO, following a protocol that was previously reported elsewhere [32] with minor modifications. This assay is based on the ability of the EO to decrease oxidative losses of β-carotene in a β-carotene/linoleic acid emulsion [32]. In brief, 4 mg of β-carotene was dissolved in 10 mL of chloroform by keeping the mixture in an ultrasound bath. Then, 120 mg of linoleic acid and 1200 mg of Tween 80 were then added to 10 mL of this solution, which, after mixing by vortexing, was dried under vacuum at 40 °C. The residue of 300 mL of oxygenated ultra-pure water (MilliQ, 18.2 MΩ) was added, and then the emulsion was vigorously shaken. Then, 5 mL aliquots of the emulsion were added to 15 mL Falcon tubes containing 0.2 mL of the EO at different concentrations (i.e., 200, 400, 600, 800, and 1200 μg/mL). The emulsions were incubated in a water bath at 50 °C for 120 min. The absorbance of the emulsions was measured at 470 nm at 0, 30, 60, 90, and 120 min. The antioxidant activity of the EO was evaluated in terms of the bleaching of the β-carotene using a formula that was previously reported elsewhere [32]. A blank solution where the EO was substituted with ethanol was used as a negative control, while emulsions containing either BHT or ascorbic acid were used as positive controls.
2.4. Statistical Analysis
Antioxidant and antiradical assays were performed in triplicate, and the results were expressed as the mean ± standard deviation (SD). A two-sample t-test assuming equal variances was performed using SPSS Statistics 25 software (IBM) to determine the significant differences between the means, and p-values < 0.05 were considered statistically significant.
3. Results and Discussion
3.1. Chemical Composition of Essential Oil
The hydrodistillation of the aerial parts of T. luteum subsp. flavovirens, including the inflorescences, produced an EO with a yield of 2 × 10−2 % (w/w), equivalent to 0.02 g. The chemical composition of the EO was analyzed via GC-MS, and the components were identified based on their LRI values compared to those reported in the literature. Thus, 53 components representing 83.9% of the whole chromatographic area were identified. The composition and percentages of those components are summarized in Table 1. The major compounds constituting the EO from T. luteum subsp. flavovirens were β-elemol (7.2%), (+)-α-pinene (6%), β-eudesmol (5.5%), guaiol (4.2%), α-bisabolol (4.2%), and β-caryophyllene (4.1%) (Figure 2). The identified volatiles were mainly terpenic compounds (82.4%), which were represented by high percentages of oxygenated sesquiterpenes (43.8%) and sesquiterpene hydrocarbons (16.7%) and lower proportions of monoterpene hydrocarbons (10.5%) and oxygenated monoterpenes (8.9%). The non-terpenic components accounted for 1.5%. Taking previous works into account and comparing the present results to those reported in the literature for T. luteum subsp. flavovirens from Morocco [21,22,23,24], we noticed that the two species share several volatile markers. Specifically, β-elemol, β-eudesmol, α-pinene, and β-caryophyllene are present in similar amounts in the EOs obtained from the plants from both regions. Conversely, guaiol and α-bisabolol can be considered more specific markers of the Tunisian species. Comparing our results with the data available for the EO of Algerian T. luteum subsp. flavovirens, more differences were observed since τ-cadinol (16.33%), caryophyllene oxide (13.23%), eudesma-4(15), 7-dien-1.β.-ol (7.64%), and β-pinene (6.77%) have been reported as the most abundant constituents [25].

Table 1.
Volatile compounds identified in the essential oil from the aerial parts of Teucrium luteum subsp. flavovirens growing wild in Tunisia. The most abundant constituents are reported in bold, and their structures are shown in Figure 2.
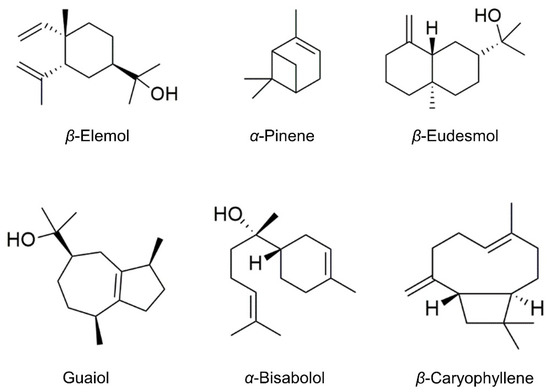
Figure 2.
Chemical structures of the most abundant constituents of the essential oil from the aerial parts of Tunisian Teucrium luteum subsp. flavovirens.
Our results were also compared to those obtained for EOs from other Tunisian Teucrium species. Specifically, the EO of T. polium harvested in Kef (north-western Tunisia), for which the authors did not specify the subspecies [33], is richer in monoterpene hydrocarbons (48.7%) compared to that from the population collected from Monastir in central-eastern Tunisia (10.5%). Oxygenated sesquiterpenes are the main constituents of T. luteum subsp. flavovirens, but they are less abundant in the essential oil of T. polium from Kef (5.54%). Furthermore, β-pinene (12.68%), limonene (6.65%), β-myrcene (6.07%), and germacrene-D (5.89%), which were identified as the main organic volatiles in the Kef population, are completely absent in the EO from T. luteum subsp. flavovirens collected in the central-east part of the country. The volatile compound α-pinene, which was quantified at a relative amount of 17.04% in the EO of T. pollium collected from Kef, is less abundant in T. luteum subsp. flavovirens (6%). Moreover, α-bisabolol, (+)-γ-gurjunene, zierone, and τ–muurolol are not present in the essential oil of T. pollium from Kef at all [33]. On the other hand, the EO of T. polium subsp. gabesianum from Gafsa (southwest Tunisia) [20] is characterized by a high abundance of β-pinene (35.97%), which was not even detected in the EO from T. luteum subsp. flavovirens. Furthermore, large discrepancies were observed for other abundant compounds since the main constituents of the EO from T. luteum subsp. flavovirens have not been detected in the EO from the aerial parts of T. polium subsp. gabesianum.
Overall, these data show high chemical variation in the species within the Teucrium genus originating from Tunisia and other North African Mediterranean regions. Variations in the chemical composition of EOs might be due to several factors: environmental factors (temperature, humidity and altitude), geographical origin, plant organ, time of picking, storage of plant material, and individual genetic variability [27,28,33]. Finally, different extraction protocols can also contribute to the observed variations, as already reported by other authors [34,35].
3.2. Evaluation of Antioxidant Activity
The results of the antioxidant and antiradical assays for the EO from Tunisian T. luteum subsp. flavovirens are reported in Table 2.

Table 2.
Antioxidant activity of essential oil from the aerial parts of Teucrium luteum subsp. flavovirens growing wild in Tunisia. Results are reported as means ± SD.
Overall, the EO exerted significant antioxidant activity in both the DPPH and β-carotene bleaching assays and was more effective than the lipophilic BHT. However, if compared to the polar reference compound, i.e., ascorbic acid, the antioxidant activity of the EO held modest results. These results are in partial agreement with what has been previously observed by other authors for the EO of the same plant species from Morocco [21]. In this work, the authors reported the efficacy of the EO in reducing the free radical DPPH, which was significantly higher than BHT and comparable to ascorbic acid. Conversely, the EO was not active in the β-carotene bleaching assay. In the same work, the authors associated the antioxidant effects of the EO with its content in some monoterpenoids, such as elemol, α- and β-pinene, trans-caryophyllene, α-humulene, and γ-eudesmol, and/or synergistic effects between all of the compounds [21]. Considering that the EO from Tunisian T. luteum subsp. flavovirens presents a high abundance of monoterpenoids and shares several of these constituents, we can propose a similar correlation between the chemical content of the EO and its antioxidant properties.
The antioxidant properties of the EOs of several members of the genus Teucrium have been extensively reported in the literature and are exhaustively reviewed in [36]. The studies are mostly concerned with the use of the DPPH radical scavenging test. Nevertheless, what emerges from the revision of this mole of data is the lack of specific molecular markers associated with the antioxidant activity of Teucrium EO. Although several authors have attempted to correlate specific constituents (e.g., β-caryophyllene, dolichodial, phenols, allylic alcohols, and unsaturated hydrocarbons) with bioactivity, the synergism among all of the constituents is usually purported to be responsible for the antioxidant effects [36], although further studies are needed for confirmation.
4. Conclusions
In conclusion, the present study provides important data about the chemical composition of the EO of the Teucrium luteum subsp. flavovirens growing wild in Tunisia. Qualitative and quantitative variations in the chemical composition have been noted for the EOs from different species from Tunisia as well as from the same species growing in other Mediterranean regions. This could mainly be due to genetic variability in favor of deep taxonomic segregation carried out within the Teucrium polium aggregate and due to the influence of local environmental, geographical, and climatic factors in the second stage.
Author Contributions
Conceptualization, S.M., R.E.M. and S.H.; methodology, S.H., G.P. and S.D.; software, G.P.; validation, S.H., S.D. and G.P.; formal analysis, G.P.; investigation, S.M., R.E.M. and S.H.; resources, S.H. and S.D.; data curation, G.P. and S.H.; writing—original draft preparation, S.M., S.H. and G.P.; writing—review and editing, G.P.; visualization, G.P. and R.E.M.; supervision, S.H.; project administration, S.H.; funding acquisition, S.H. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially financed by the Ministry of Higher Education and Scientific Research of Tunisia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Ministry of Higher Education and Scientific Research of Tunisia for the financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, J.N.; Gomez, M.C.V.; Rolón, M.; Coronel, C.; Almeida-Bezerra, J.W.; Fidelis, K.R.; de Menezes, S.A.; da Cruz, R.P.; Duarte, A.E.; Ribeiro, P.R.V.; et al. Chemical composition, Evaluation of Antiparasitary and Cytotoxic Activity of the essential oil of Psidium brownianum MART EX. DC. Biocatal. Agric. Biotechnol. 2022, 39, 102247. [Google Scholar] [CrossRef]
- Chhetri, B.K.; Ali, N.A.A.; Setzer, W.N. A Survey of Chemical Compositions and Biological Activities of Yemeni Aromatic Medicinal Plants. Medicines 2015, 2, 67–92. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, R.; Genovese, C.; Amodeo, A.; Tomasello, B.; Malfa, G.; Sorrenti, V.; Tempera, G.; Addamo, A.P.; Ragusa, S.; Rosa, T.; et al. Biological activities of Teucrium flavum L., Teucrium fruticans L., and Teucrium siculum rafin crude extracts. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2018, 152, 720–727. [Google Scholar] [CrossRef]
- Bezerra, J.W.A.; Costa, A.R.; da Silva, M.A.P.; Rocha, M.I.; Boligon, A.A.; da Rocha, J.B.T.; Barros, L.M.; Kamdem, J.P. Chemical composition and toxicological evaluation of Hyptis suaveolens (L.) Poiteau (LAMIACEAE) in Drosophila melanogaster and Artemia salina. S. Afr. J. Bot. 2017, 113, 437–442. [Google Scholar] [CrossRef]
- Rahmouni, F.; Hamdaoui, L.; Rebai, T. In vivo anti-inflammatory activity of aqueous extract of Teucrium polium against carrageenan-induced inflammation in experimental models. Arch. Physiol. Biochem. 2017, 123, 313–321. [Google Scholar] [CrossRef]
- Zlatić, N.; Stanković, M. Anticholinesterase, Antidiabetic and Anti-inflammatory Activity of Secondary Metabolites of Teucrium Species. In Teucrium Species: Biology and Applications; Stanković, M., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 391–411. ISBN 978-3-030-52159-2. [Google Scholar]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Lyoussi, B.; Oumokhtar, B.; Abdellaoui, A. Phytochemistry, antioxidant and antibacterial activities of two Moroccan Teucrium polium L. subspecies: Preventive approach against nosocomial infections. Arab. J. Chem. 2020, 13, 3866–3874. [Google Scholar] [CrossRef]
- Abdollahi, M.; Karimpour, H.; Monsef-Esfehani, H.R. Antinociceptive effects of Teucrium polium L. total extract and essential oil in mouse writhing test. Pharmacol. Res. 2003, 48, 31–35. [Google Scholar] [CrossRef]
- Kamel, A.; Sandra, P. Gas chromatography—Mass spectrometry analysis of the volatile oils of two Teucrium polium varieties. Biochem. Syst. Ecol. 1994, 22, 529–532. [Google Scholar] [CrossRef]
- Pérez-Alonso, M.J.; Velasco-Negueruela, A.; López-Sáez, J.A. The Essential Oils of Two Iberian Teucrium Species. J. Essent. Oil Res. 1993, 5, 397–402. [Google Scholar] [CrossRef]
- Coll, J.; Tandrón, Y. Isolation and structure elucidation of three neo-clerodane diterpenes from Teucrium fruticans L. (LABIATAE). Phytochemistry 2005, 66, 2298–2303. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Rosselli, S.; Maggio, A.; Piozzi, F.; Scaglioni, L.; Arnold, N.A.; Simmonds, M.S.J. Neoclerodanes from Teucrium orientale. Chem. Pharm. Bull. 2004, 52, 1497–1500. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pottier-Alapetite, G. Flore de la Tunisie: Angiospermes-Dicotyledones, Gamopétales; Ministère de l’Enseignement Supérieur et de la Recherche Scientifique et le Ministère de l’Agriculture: Tunis, Tunisia, 1981.
- Le Floc’h, E. Contribution à une Étude Ethnobotanique de la Flore Tunisienne; Imprimerie Officielle de la République Tunisienne: Radès, Tunisia, 1983.
- Boukef, M. Les Plantes dans la Médecine Traditionnelle Tunisienne; Agence de Coopération Culturelle et Technique: Paris, France, 1986. [Google Scholar]
- Cozzani, S.; Muselli, A.; Desjobert, J.-M.; Bernardini, A.-F.; Tomi, F.; Casanova, J. Chemical composition of essential oil of Teucrium polium subsp. capitatum (L.) from Corsica. Flavour Fragr. J. 2005, 20, 436–441. [Google Scholar] [CrossRef]
- Kabouche, A.; Kabouche, Z.; Ghannadi, A.; Sajjadi, S.E. Analysis of the Essential Oil of Teucrium polium ssp. aurasiacum from Algeria. J. Essent. Oil Res. 2007, 19, 44–46. [Google Scholar] [CrossRef]
- Khani, A.; Heydarian, M. Fumigant and repellent properties of sesquiterpene-rich essential oil from Teucrium polium subsp. capitatum (L.). Asian Pac. J. Trop. Med. 2014, 7, 956–961. [Google Scholar] [CrossRef]
- Ben Othman, M.; Bel Hadj Salah-Fatnassi, K.; Ncibi, S.; Elaissi, A.; Zourgui, L. Antimicrobial activity of essential oil and aqueous and ethanol extracts of Teucrium polium L. subsp. gabesianum (L.H.) from Tunisia. Physiol. Mol. Biol. Plants 2017, 23, 723–729. [Google Scholar] [CrossRef]
- Ouknin, M.; Majidi, L.; Jean-Marie, D.; Costa, J.; Mustapha, C. Chemical profiling study and antioxidant activity of wild Teucrium luteum subsp. flavovirens essential oil from Morocco. J. Appl. Pharm. Sci. 2019, 9, 10. [Google Scholar] [CrossRef]
- Znini, M.; Ou-Ani, O.; Chebabe, D.; Costa, J.; Majidi, L. Gas Chromatography-Mass Spectrometry Analysis and Comparison of Volatile Components Obtained by Hydrodistillation and Headspace Solid Phase Microextraction (HS-SPME) from Teucrium luteum subsp. flavovirens. Arab. J. Med. Aromat. Plants 2022, 8, 1. [Google Scholar] [CrossRef]
- Znini, M. Chemical Composition, Liquid and Vapor-phase Antifungal Activities of Essential Oil of Teucrium luteum subsp. flavovirens against Three Postharvest Phytopathogenic Fungi. Int. J. Fruit Sci. 2021, 21, 400–412. [Google Scholar] [CrossRef]
- Znini, M.; Costa, J.; Majidi, L. Chemical constituents of the essential oil of endemic Teucrium luteum subsp. flavovirens (batt.) Greuter & burdet collected from two localities in Morocco. J. Essent. Oil Res. 2021, 33, 197–203. [Google Scholar] [CrossRef]
- Gherib, M.; Gordo, B.; Ziane, M.; Braik, O.B.; Bouafia, M.; Chami, K.; Fillali, S.; Amrouche, A.I. Chemical composition and antimicrobial activity of Teucrium luteum subsp. flavovirens essential oil from Northwestern Algeria. Vegetos 2022, 1–8. [Google Scholar] [CrossRef]
- Hammami, S.; Li, Z.; Huang, M.; El Mokni, R.; Dhaouadi, H.; Yin, S. New bioactive labdane diterpenoids from Marrubium aschersonii. Nat. Prod. Res. 2016, 30, 2142–2148. [Google Scholar] [CrossRef] [PubMed]
- El Mokni, R.; Faidi, K.; Joshi, R.K.; Mighri, Z.; El Aouni, M.H.; Hammami, S. Essential oil composition and antioxidant activity of Stachys officinalis subsp. algeriensis (Lamiaceae) from a wild population in Tunisia. Eur. Food Res. Technol. 2018, 244, 1691–1697. [Google Scholar] [CrossRef]
- El Mokni, R.; Hammami, S.; Dall’Acqua, S.; Peron, G.; Faidi, K.; Braude, J.P.; Sebei, H.; El Aouni, M.H. Chemical Composition, Antioxidant and Cytotoxic Activities of Essential Oil of the Inflorescence of Anacamptis coriophora subsp. fragrans (Orchidaceae) from Tunisia. Nat. Prod. Commun. 2016, 11, 857–860. [Google Scholar] [CrossRef]
- NIST/EPA/NIH Mass Spectral Library; National Institute of Standards and Technology: Gaithersburg, MA, USA, 2014.
- Smaili, T.; Bendif, H.; Öztürk, M.; Flamini, G.; Peron, G. Chemical Composition and Antioxidant Activity of Essential Oil from Daucus reboudii Coss., an Endemic Plant of Algeria. Appl. Sci. 2021, 11, 1843. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Moure, A.; Franco, D.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Lema, J.M. Evaluation of Extracts from Gevuina avellana Hulls as Antioxidants. J. Agric. Food Chem. 2000, 48, 3890–3897. [Google Scholar] [CrossRef]
- Bakari, S.; Ncir, M.; Felhi, S.; Hajlaoui, H.; Saoudi, M.; Gharsallah, N.; Kadri, A. Chemical composition and in vitro evaluation of total phenolic, flavonoid, and antioxidant properties of essential oil and solvent extract from the aerial parts of Teucrium polium grown in Tunisia. Food Sci. Biotechnol. 2015, 24, 1943–1949. [Google Scholar] [CrossRef]
- Lemberkovics, E.; Kéry, A.; Kakasy, A.; Szöke, E.; Simái, B. Effect of extraction methods on the composition of essential oils. Acta Hortic. 2003, 597, 49–56. [Google Scholar] [CrossRef]
- Siddique, A.B.; Mizanur Rahman, S.M.; Hossain, M.A. Chemical composition of essential oil by different extraction methods and fatty acid analysis of the leaves of Stevia Rebaudiana Bertoni. Arab. J. Chem. 2016, 9, S1185–S1189. [Google Scholar] [CrossRef]
- Candela, R.G.; Rosselli, S.; Bruno, M.; Fontana, G. A Review of the Phytochemistry, Traditional Uses and Biological Activities of the Essential Oils of Genus Teucrium. Planta Med. 2020, 87, 432–479. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).