Differences in the Impact of Plantar Fasciopathy on the Spatio-Temporal Gait Parameters between Participants with Bilateral Plantar Fasciopathy and Healthy Subjects: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Training Phase
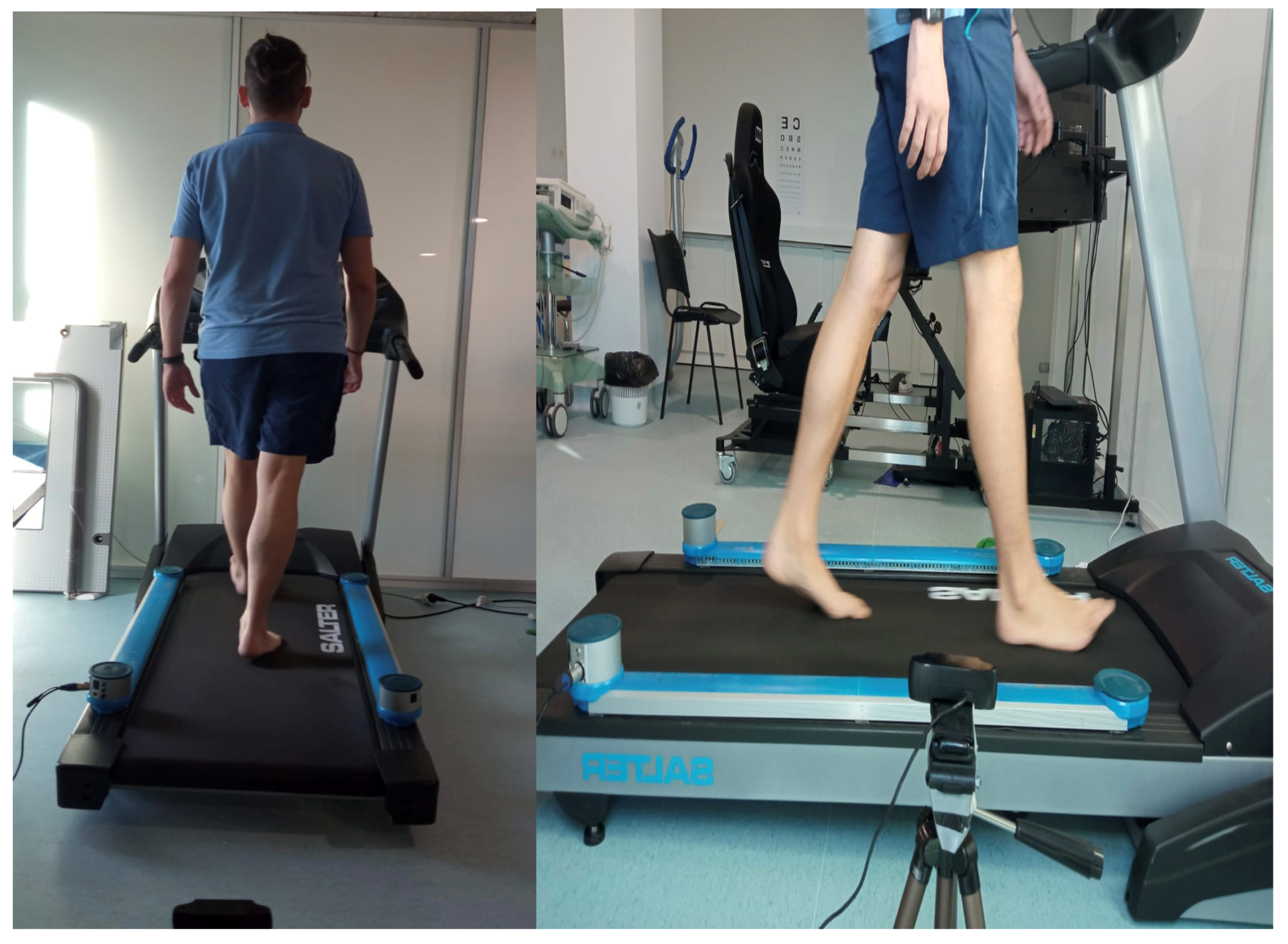
2.4. Instrumentation
2.5. Procedures
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sean, C.; Gary, S.; Gregory, F.; Karl, T. Proximal Plantar Intrinsic Tendinopathy: Anatomical and Biomechanical Considerations in Plantar Heel Pain. J. Am. Podiatr. Med. Assoc. 2019, 109, 412–415. [Google Scholar] [CrossRef]
- Ryan, C.; Pedro, A.R.; Richard, E.A.V.E.; Joseph, H. Multi-Segment Foot Kinematics and Ground Reaction Forces during Gait of Individuals with Plantar Fasciitis. J. Biomech. 2014, 47, 2571–2577. [Google Scholar] [CrossRef]
- Seung Don, Y.; Hee Sang, K.; Jong Ha, L.; Dong Hwan, Y.; Dong Hwan, K.; Jinmann, C.; Seung Ah, L.; Yoo Jin, H.; Yun Soo, S.; Yong, K.; et al. Biomechanical Parameters in Plantar Fasciitis Measured by Gait Analysis System with Pressure Sensor. Ann. Rehabil. Med. 2017, 41, 979–989. [Google Scholar] [CrossRef]
- Wei-Yi, Y.; Yan-Hong, H.; Xue-Wei, C.; Jian-Ke, P.; Ling-Feng, Z.; Jiong-Tong, L.; Jun, L. Platelet-Rich Plasma as a Treatment for Plantar Fasciitis: A Meta-Analysis of Randomized Controlled Trials. Medicine 2017, 96, e8475. [Google Scholar] [CrossRef]
- Morgan, H.; Ivan, U.; Vwaire, O.; Mariam Salisu, O.; Joseph, B.; Stephen, G.; Lukas, F.; Laxmaiah, M.; Alan, D.K.; Rachel, J.K.; et al. Current Concepts of Minimally Invasive Treatment Options for Plantar Fasciitis: A Comprehensive Review. Curr. Pain Headache Rep. 2020, 24, 55. [Google Scholar] [CrossRef]
- Jacquelin, P. Gait Analysis: Normal and Pathological Function; SLACK: Thorofare, NJ, USA, 2010; ISBN 978-1-55642-766-4. [Google Scholar]
- Hicks, J.H. The Mechanics of the Foot. II. The Plantar Aponeurosis and the Arch. J. Anat. 1954, 88, 25–30. [Google Scholar] [PubMed]
- Christian, G.; Dorianne, S.; Bert, O.; van Laurens, K.; Erik, V.; Klaas, P.; Rienk, D.; Juha, M.H. Biomechanical Effects of Rocker Shoes on Plantar Aponeurosis Strain in Patients with Plantar Fasciitis and Healthy Controls. PLoS ONE 2019, 14, e0222388. [Google Scholar] [CrossRef]
- Chen, Y.N.; Chang, C.W.; Li, C.T.; Chang, C.H.; Lin, C.F. Finite Element Analysis of Plantar Fascia during Walking: A Quasi-Static Simulation. Foot Ankle Int. 2015, 36, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Harutaichun, P.; Boonyong, S.; Pensri, P. Differences in Lower-Extremity Kinematics between the Male Military Personnel with and without Plantar Fasciitis. Phys. Ther. Sport 2021, 50, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Caravaggi, P.; Pataky, T.; Günther, M.; Savage, R.; Crompton, R. Dynamics of Longitudinal Arch Support in Relation to Walking Speed: Contribution of the Plantar Aponeurosis. J. Anat. 2010, 217, 254–261. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hertel, J.; Lee, S.C. Rearfoot Eversion Has Indirect Effects on Plantar Fascia Tension by Changing the Amount of Arch Collapse. Foot 2010, 20, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Taunton, J.E.; Ryan, M.B.; Clement, D.B.; McKenzie, D.C.; Lloyd-Smith, D.R.; Zumbo, B.D. A Retrospective Case-Control Analysis of 2002 Running Injuries. Br. J. Sports Med. 2002, 36, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Petraglia, F.; Ramazzina, I.; Costantino, C. Plantar Fasciitis in Athletes: Diagnostic and Treatment Strategies. A Systematic Review. Muscles Ligaments Tendons J. 2017, 7, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Patrick, O.R.; Gabriele, P.; Ugo, D.C.; Kate, W.P.; Kerrigan, D.C. A Kinematic and Kinetic Comparison of Overground and Treadmill Walking in Healthy Subjects. Gait Posture 2007, 26, 17–24. [Google Scholar] [CrossRef]
- Patrick, O.R.; Jay, D.; Jason, F.; Ugo, D.C.; Robert, P.W.; Kerrigan, D.C. A Kinematics and Kinetic Comparison of Overground and Treadmill Running. Med. Sci. Sports Exerc. 2008, 40, 1093–1100. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Trojian, T.; Tucker, A.K. Plantar Fasciitis—American Family Physician. Drexel Univ. Coll. Med. 2019, 99, 744–750. [Google Scholar]
- Neufeld, S.K.; Cerrato, R. Plantar Fasciitis: Evaluation and Treatment. J. Am. Acad. Orthop. Surg. 2008, 16, 338–346. [Google Scholar] [CrossRef]
- Rosa, N. Manual de Uso. Signa Rev. Asoc. Española Semiótica 2022, 7, 335–346. [Google Scholar] [CrossRef]
- McNally, E.G.; Shetty, S. Plantar Fascia: Imaging Diagnosis and Guided Treatment. Semin. Musculoskelet. Radiol. 2010, 14, 334–343. [Google Scholar] [CrossRef]
- Neumann, D.A.; Kelly, E.R.; Kiefer, C.L.; Martens, K.; Grosz, C.M. Kinesiology of the Musculoskeletal System: Foundations for Rehabilitation; Mosby: Maryland Heights, MO, USA, 2016. [Google Scholar]
- Evaluación de La Marcha. Clave Musculoesquelético. Available online: https://musculoskeletalkey.com/assessment-of-gait/ (accessed on 26 November 2022).
- Brachman, A.; Sobota, G.; Marszałek, W.; Pawłowski, M.; Juras, G.; Bacik, B. Plantar Pressure Distribution and Spatiotemporal Gait Parameters after the Radial Shock Wave Therapy in Patients with Chronic Plantar Fasciitis. J. Biomech. 2020, 105, 109773. [Google Scholar] [CrossRef] [PubMed]
- Wearing, S.C.; Smeathers, J.E.; Sullivan, P.M.; Yates, B.; Urry, S.R.; Dubois, P. Plantar Fasciitis: Are Pain and Fascial Thickness Associated with Arch Shape and Loading? Phys. Ther. 2007, 87, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; van Emmerik, R.; Hamill, J. Chronic Plantar Fasciitis Reduces Rearfoot to Medial-Forefoot Anti-Phase Coordination. Clin. Biomech. 2021, 88, 105439. [Google Scholar] [CrossRef]
- Wearing, S.C.; Smeathers, J.E.; Urry, S.R. The Effect of Plantar Fasciitis on Vertical Foot-Ground Reaction Force. Clin. Orthop. Relat. Res. 2003, 409, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Dudoniene, V.; Balnytė, M.; Kuisma, R. Comparison of Static Balance and Gait between Subjects with Plantar Fasciitis and Age-Matched Controls. J. Back Musculoskelet. Rehabil. 2022, 1–8, ahead of print. [Google Scholar] [CrossRef]
- Kelly, D.K.; Wiegand, K.; Freedman Silvernail, J. Dynamic Stability in Runners with and without Plantar Fasciitis. Gait Posture 2022, 96, 301–305. [Google Scholar] [CrossRef]
- Gefen, A. The in Vivo Elastic Properties of the Plantar Fascia during the Contact Phase of Walking. Foot Ankle Int. 2003, 24, 238–244. [Google Scholar] [CrossRef]
- Justin, S.; Joshua, B.; Roger, A.; Evangelos, P.; Jack, C. Plantar Heel Pain and Foot Loading during Normal Walking. Gait Posture 2015, 41, 688–693. [Google Scholar] [CrossRef]
- Inmaculada, R.-R.; Aurora, C.-M.; Ana María, J.-C.; María Luisa, G.-E.; Inmaculada, C.P.-T.; Manuel, P.-C. Assessment of Selected Spatio-Temporal Gait Parameters on Subjects with Pronated Foot Posture on the Basis of Measurements Using OptoGait. A Case-Control Study. Sensors 2021, 21, 2805. [Google Scholar] [CrossRef]
| Total (SD) N = 74 | Injured (SD) N = 31 | Healthy Control (SD) N = 43 | Kolmogorov Smirnov (p-Value) | |
|---|---|---|---|---|
| Age (years) | 48.52 (7.01) | 47.58 (6.23) | 50.03 (7.69) | 0.006 |
| Height (cm) | 170.55 (9.2) | 170 (8) | 171 (9) | 0.305 |
| Body mass (kg) | 73.32 (17.13) | 69.5 (16.45) | 78 (16.24) | 0.426 |
| BMI (kg/m2) | 24.99 (4.27) | 23.76 (4.16) | 26.31(3.98) | 0.352 |
| Foot size | 40.29 (3.3) | 40.04 (2.84) | 40 (3.62) | 0.015 |
| Total (SD) N = 74 | Injured (SD) N = 31 | Healthy Control (SD) N = 43 | p | Mean Differences (95% CI) | p | |
|---|---|---|---|---|---|---|
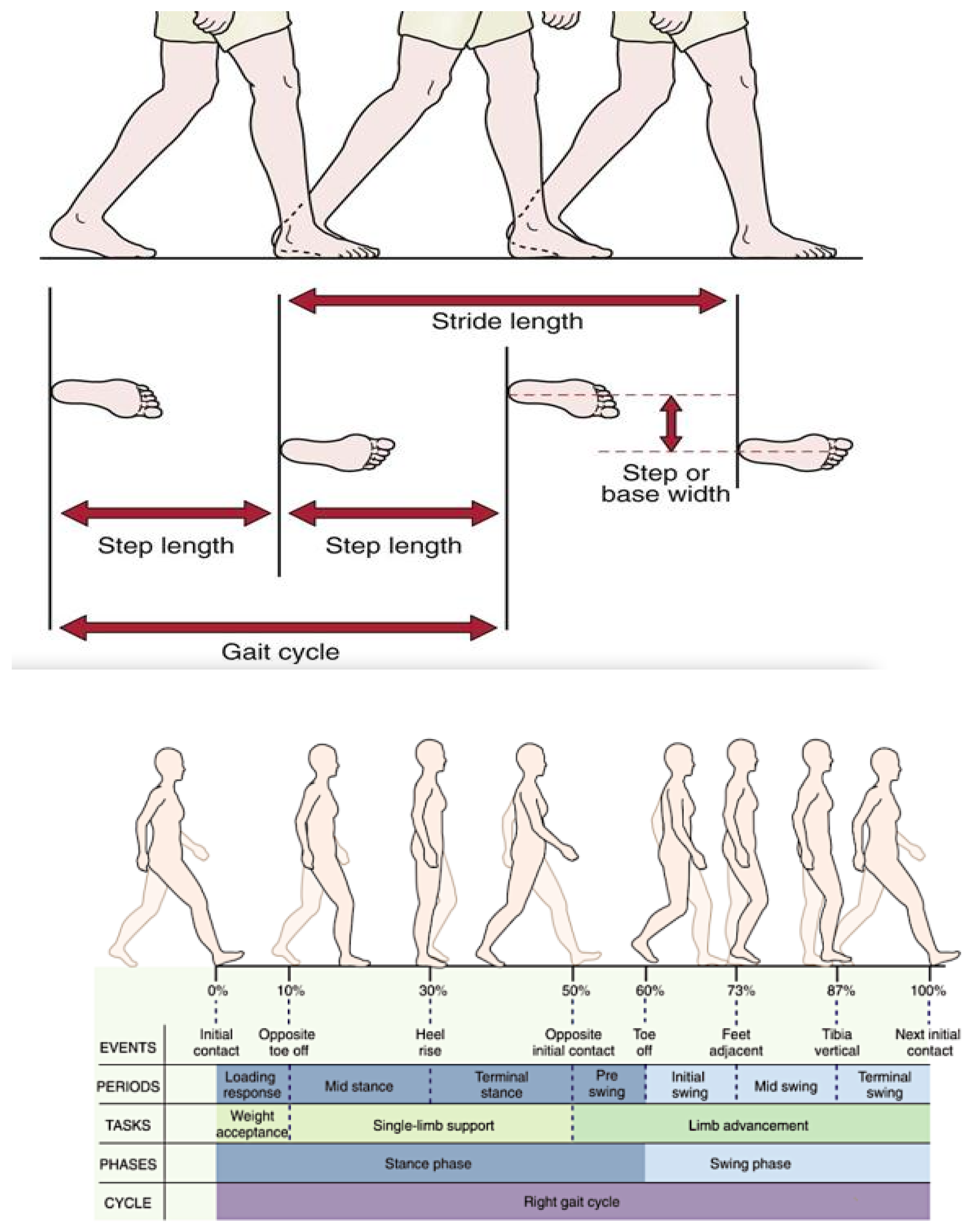
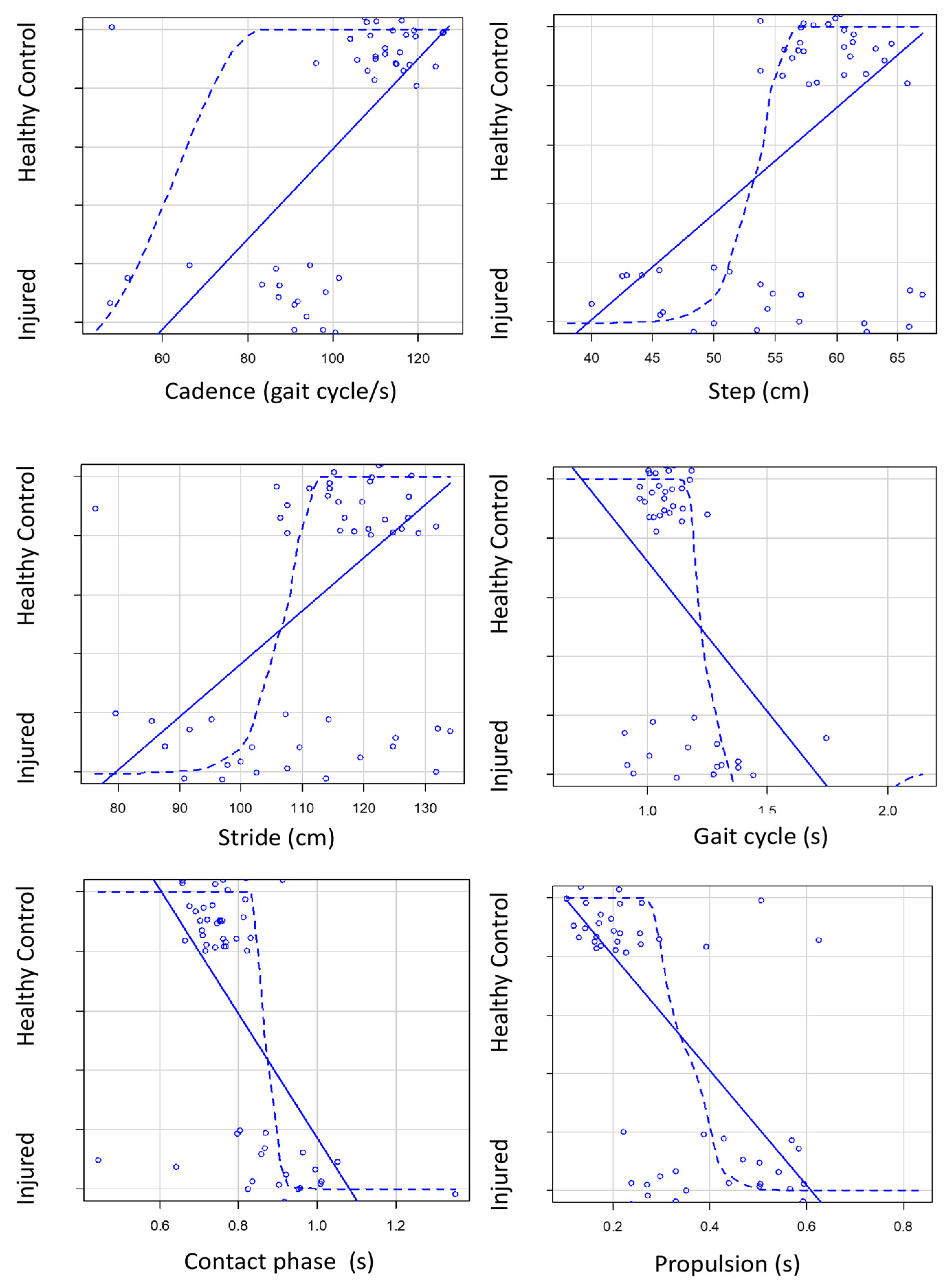
| Gait cycle [s] | 1.14 (0.19) | 1.23 (0.26) | 1.07 (0.06) | <0.001 | 0.16 (0.06–0.26) | 0.0029 |
| Cadence [gait cycle/s] | 99.29 (20.95) | 83.30 (20.31) | 110.81 (11.99) | < 0.001 | 27.51 (19.29–35.73) | <0.0001 |
| Stride [cm] | 112.05 (13.63) | 104.2 (14.84) | 117.7 (9.34) | <0.001 | 13.5 (7.42–19.58) | <0.0001 |
| Step time [s] | 0.55 (0.11) | 0.57 (0.16) | 0.53 (0.04) | 0.002 | 0.06 (0.02–0.10) | 0.133 |
| Step lenght [cm] | 56.03 (6.8) | 52.1 (7.37) | 58.85 (4.67) | <0.001 | 6.75 (3.72–9.78) | <0.0001 |
| Double support [s] | 0.49 (0.13) | 0.58 (0.14) | 0.42 (0.07) | <0.001 | 0.16 (0.10–0.22) | <0.0001 |
| Unipodal support [s] | 0.34 (0.09) | 0.33 (0.013) | 0.35 (0.02) | 0.28 | 0.05 (0.02–0.08) | 0.262 |
| Speed [m/s] | 0.94 (0.26) | 0.73 (0.25) | 1.08 (0.12) | <0.001 | 0.35 (0.25–0.45) | <0.0001 |
| Acceleration [m/s2] | 0.0003 (0.004) | 0.00032 (0.004) | 0.00023 (0.002) | 1.0 | 0.0019 (0.0020–0.0018) | 0.925 |
| Contact phase [s] | 0.81 (0.13) | 0.88 (0.15) | 0.74 (0.05) | <0.001 | 0.14 (0.08–0.20) | <0.0001 |
| Load response [s] | 0.25 (0.06) | 0.29 (0.06) | 0.21 (0.03) | <0.001 | 0.08 (0.05–0.11) | <0.0001 |
| Phase flatfoot [s] | 0.39 (0.13) | 0.33 (0.16) | 0.44 (0.09) | 0.0023 | 0.11 (0.05–0.17) | 0.0011 |
| Preswing [s] | 0.24 (0.06) | 0.29 (0.06) | 0.21 (0.03) | <0.001 | 0.08 (0.05–0.11) | <0.0001 |
| Propulsion [s] | 0.31 (0.17) | 0.45 (0.15) | 0.21 (0.1) | <0.001 | 0.23 (0.17–0.29) | <0.0001 |
| Swing phase [s] | 0.34 (0.09) | 0.35 (0.13) | 0.32 (0.02) | 0.28 | 0.05 (0.02–0.08) | 0.262 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervera-Garvi, P.; Aguilar-Núñez, D.; Páez-Moguer, J.; Jerez, J.M.; Navarro-Ledesma, S. Differences in the Impact of Plantar Fasciopathy on the Spatio-Temporal Gait Parameters between Participants with Bilateral Plantar Fasciopathy and Healthy Subjects: A Cross-Sectional Study. Appl. Sci. 2023, 13, 2133. https://doi.org/10.3390/app13042133
Cervera-Garvi P, Aguilar-Núñez D, Páez-Moguer J, Jerez JM, Navarro-Ledesma S. Differences in the Impact of Plantar Fasciopathy on the Spatio-Temporal Gait Parameters between Participants with Bilateral Plantar Fasciopathy and Healthy Subjects: A Cross-Sectional Study. Applied Sciences. 2023; 13(4):2133. https://doi.org/10.3390/app13042133
Chicago/Turabian StyleCervera-Garvi, Pablo, Daniel Aguilar-Núñez, Joaquin Páez-Moguer, Jose M. Jerez, and Santiago Navarro-Ledesma. 2023. "Differences in the Impact of Plantar Fasciopathy on the Spatio-Temporal Gait Parameters between Participants with Bilateral Plantar Fasciopathy and Healthy Subjects: A Cross-Sectional Study" Applied Sciences 13, no. 4: 2133. https://doi.org/10.3390/app13042133
APA StyleCervera-Garvi, P., Aguilar-Núñez, D., Páez-Moguer, J., Jerez, J. M., & Navarro-Ledesma, S. (2023). Differences in the Impact of Plantar Fasciopathy on the Spatio-Temporal Gait Parameters between Participants with Bilateral Plantar Fasciopathy and Healthy Subjects: A Cross-Sectional Study. Applied Sciences, 13(4), 2133. https://doi.org/10.3390/app13042133







