Comparison of Key Nutrient Content of Commercial Puppy Foods with Canine Dietary Requirements
Abstract
1. Introduction
2. Materials and Methods
2.1. Proximate Analyses
2.2. Energy Value
2.3. Fatty Acid Analyses
2.4. Label Evaluation
2.5. Statistical Analyses
3. Results
3.1. Composition–Ingredients List
3.2. Proximate Composition and Energy Value
3.3. Fatty Acids
3.4. Labelling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FEDIAF. Annual Report; The European Pet Food Industry: Bruxelles, Belgium, 2022. [Google Scholar]
- Van Herwijnen, I.R. Educating dog owners: How owner—Dog interactions can benefit from addressing the human caregiving system and dog–directed parenting styles. Behaviour 2021, 158, 1449–1470. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 767/2009 on the Placing on the Market and Use of Feed, Amending Regulation (EC) No 1831/2003 of the European Parliament and of the Council, and Repealing Council Directive 79/373/EEC, Commission Directive 80/511/EEC, Council Directives 82/471/EEC, 83/228/EEC, 93/74/EEC, 93/113/EC, and 96/25/EC, and Commission Decision 2004/217/EC. Off. J. Eur. Union 2009, L189, 1–52. [Google Scholar]
- Zicker, S.C. Evaluating pet foods: How confident are you when you recommend a commercial pet food? Top. Companion Anim. Med. 2008, 23, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Carrión, P.A. Chapter 18–Pet Food. In Food Safety Management, 2nd ed.; Andersen, V., Lelieveld, H., Motarjemi, Y., Eds.; Academic Press: San Diego, CA, USA, 2023; pp. 363–384. [Google Scholar]
- Statista. Pet Food Report 2023; Statista—The Statistics Portal: New York, NY, USA, 2023. [Google Scholar]
- FEDIAF. Nutritional Guidelines for Complete and Complementary Pet Food for Cats and Dogs; The European Pet Food Industry Federation: Bruxelles, Belgium, 2021. [Google Scholar]
- Sarrazin, J.F.; Comeau, G.; Daleau, P.; Kingma, J.; Plante, I.; Fournier, D.; Molin, F. Reduced incidence of vagally induced atrial fibrillation and expression levels of connexins by n−3 polyunsaturated fatty acids in dogs. J. Am. Coll. Cardiol. 2007, 50, 1505–1512. [Google Scholar] [CrossRef]
- Dillon, G.P.; Keegan, J.D.; Wallace, G.; Yiannikouris, A.; Moran, C.A. The validation & verification of an LC/MS method for the determination of total docosahexaenoic acid concentrations in canine blood serum. Regul. Toxicol. Pharmacol. 2018, 95, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Hadley, K.B.; Bauer, J.; Milgram, N.W. The oil–rich alga Schizochytrium sp. as a dietary source of docosahexaenoic acid improves shape discrimination learning associated with visual processing in a canine model of senescence. Prostaglandins Leukot. Essent. Fat. Acids 2017, 118, 10–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kitajka, K.; Puskás, L.G.; Zvara, Á.; Hackler, L., Jr.; Barceló–Coblijn, G.; Yeo, Y.K.; Farkas, T. The role of n−3 polyunsaturated fatty acids in brain: Modulation of rat brain gene expression by dietary n−3 fatty acids. Proc. Natl. Acad. Sci. USA 2002, 99, 2619–2624. [Google Scholar] [CrossRef]
- Kawashima, A.; Harada, T.; Kami, H.; Yano, T.; Imada, K.; Mizuguchi, K. Effects of eicosapentaenoic acid on synaptic plasticity, fatty acid profile and phosphoinositide 3–kinase signaling in rat hippocampus and differentiated PC12 cells. J. Nutr. Biochem. 2010, 21, 268–277. [Google Scholar] [CrossRef]
- Sharma, S.; Zhuang, Y.; Gomez-Pinilla, F. High–fat diet transition reduces brain DHA levels associated with altered brain plasticity and behaviour. Sci. Rep. 2012, 2, 431. [Google Scholar] [CrossRef]
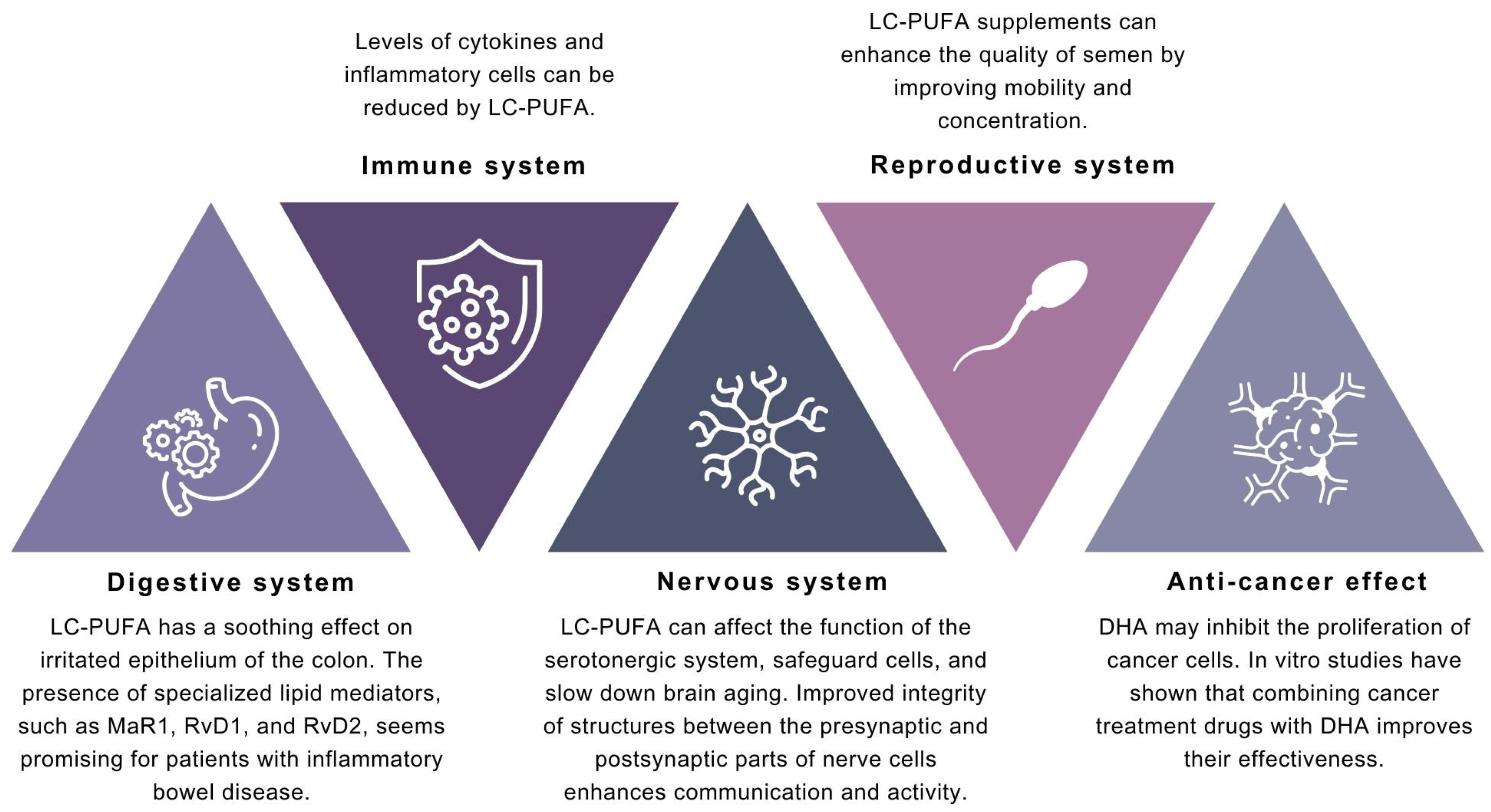
- Lenox, C.E. Role of dietary fatty acids in dogs & cats. Today Vet. Pract. 2016, 6, 83–88. [Google Scholar]
- Cook, H.W. Fatty acid desaturation and chain elongation in eukaryotes. In New Comprehensive Biochemistry; Vance, D.E., Vance, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 31, pp. 129–152. [Google Scholar]
- Innis, S.M. Essential fatty acid metabolism during early development. In Biology of Growing Animals, 1st ed.; Burrin, D.G., Mersmann, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 3, pp. 235–274. [Google Scholar]
- Buddhachat, K.; Siengdee, P.; Chomdej, S.; Soontornvipart, K.; Nganvongpanit, K. Effects of different omega–3 sources, fish oil, krill oil, and green–lipped mussel against cytokine–mediated canine cartilage degradation. Vitr. Cell. Dev. Biol. Anim. 2017, 53, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Combarros, D.; Castilla-Castaño, E.; Lecru, L.A.; Pressanti, C.; Amalric, N.; Cadiergues, M.C. A prospective, randomized, double blind, placebo–controlled evaluation of the effects of an n−3 essential fatty acids supplement (Agepi® ω3) on clinical signs, and fatty acid concentrations in the erythrocyte membrane, hair shafts and skin surface of dogs with poor quality coats. Prostaglandins Leukot. Essent. Fatty Acids 2020, 159, 102140. [Google Scholar] [CrossRef] [PubMed]
- Pasławski, R.; Kurosad, A.; Ząbek, A.; Pasławska, U.; Noszczyk-Nowak, A.; Michałek, M.; Młynarz, P. Effect of 6–month feeding with a diet enriched in EPA+ DHA from fish meat on the blood metabolomic profile of dogs with myxomatous mitral valve disease. Animals 2021, 11, 3360. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.; Rostaher, A.; Fischer, N.M.; Favrot, C. A novel therapeutic diet can significantly reduce the medication score and pruritus of dogs with atopic dermatitis during a nine—Month controlled study. Vet. Dermatol. 2022, 33, 55-e18. [Google Scholar] [CrossRef]
- Maulucci, G.; Cohen, O.; Daniel, B.; Sansone, A.; Petropoulou, P.I.; Filou, S.; Spyridonidis, A.; Pani, G.; De Spirito, M.; Chatgilialoglu, C.; et al. Fatty acid–related modulations of membrane fluidity in cells: Detection and implications. Free Radic. Res. 2016, 50 (Suppl. S1), S40–S50. [Google Scholar] [CrossRef]
- Souza, C.M.M.; de Lima, D.C.; Bastos, T.S.; de Oliveira, S.G.; Beirão, B.C.B.; Félix, A.P. Microalgae Schizochytrium sp. as a source of docosahexaenoic acid (DHA): Effects on diet digestibility, oxidation and palatability and on immunity and inflammatory indices in dogs. Anim. Sci. J. 2019, 90, 1567–1574. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, X.; Cheng, J.; Guo, M. Analysis and comparison of nutrition profiles of canine milk with bovine and caprine milk. Foods 2022, 11, 472. [Google Scholar] [CrossRef]
- Heinze, C.R.; Freeman, L.M.; Martin, C.R.; Power, M.L.; Fascetti, A.J. Comparison of the nutrient composition of commercial dog milk replacers with that of dog milk. J. Am. Vet. Med. Assoc. 2014, 244, 1413–1422. [Google Scholar] [CrossRef]
- Heinemann, K.M.; Bauer, J.E. Docosahexaenoic acid and neurologic development in animals. J. Am. Vet. Med. Assoc. 2006, 228, 700–705. [Google Scholar] [CrossRef]
- Stoeckel, K.; Bachmann, L.; Dobeleit, G.; Fuhrmann, H. Response of plasma fatty acid profiles to changes in dietary n−3 fatty acids and its correlation with erythrocyte fatty acid profiles in dogs. J. Anim. Physiol. Anim. Nutr. 2013, 97, 1142–1151. [Google Scholar] [CrossRef]
- Mehler, S.J.; May, L.R.; King, C.; Harris, W.S.; Shah, Z. A prospective, randomized, double blind, placebo–controlled evaluation of the effects of eicosapentaenoic acid and docosahexaenoic acid on the clinical signs and erythrocyte membrane polyunsaturated fatty acid concentrations in dogs with osteoarthritis. Prostaglandins Leukot. Essent. Fatty Acids 2016, 109, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Brockman, J.A.; Davidson, S.J.; MacLeay, J.M.; Jewell, D.E. Increased dietary long–chain polyunsaturated fatty acids alter serum fatty acid concentrations and lower risk of urine stone formation in cats. PLoS ONE 2017, 12, e0187133. [Google Scholar] [CrossRef] [PubMed]
- Adler, N.; Schoeniger, A.; Fuhrmann, H. Polyunsaturated fatty acids influence inflammatory markers in a cellular model for canine osteoarthritis. J. Anim. Physiol. Anim. Nutr. 2018, 102, e623–e632. [Google Scholar] [CrossRef]
- Che, H.; Li, H.; Song, L.; Dong, X.; Yang, X.; Zhang, T.; Wang, Y.; Xie, W. Orally administered DHA–enriched phospholipids and DHA–enriched triglyceride relieve oxidative stress, improve intestinal barrier, modulate inflammatory cytokine and gut microbiota, and meliorate inflammatory responses in the brain in dextran sodium sulfate induced colitis in mice. Mol. Nutr. Food Res. 2021, 65, 2000986. [Google Scholar] [CrossRef]
- de Viteri, S.M.; Hernandez, M.; Bilbao-Malavé, V.; Fernandez-Robredo, P.; González-Zamora, J.; Garcia-Garcia, L.; Ispizua, N.; Recalde, S.; Garcia-Layana, A. A higher proportion of eicosapentaenoic acid (EPA) when combined with docosahexaenoic acid (DHA) in omega–3 dietary supplements provides higher antioxidant effects in human retinal cells. Antioxidants 2020, 9, 828. [Google Scholar] [CrossRef]
- Paixão, E.M.S.; Oliveira, O.A.C.M.; Pizato, N.; Muniz-Junqueira, M.I.; Magalhães, K.G.; Nakano, N.E.Y.; Ito, M.K. The effects of EPA and DHA enriched fish oil on nutritional and immunological markers of treatment natïve breast cancer patients: A randomized double–blind controlled trial. Nutr. J. 2017, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Landsberg, G.; Mougeot, I.; Kelly, S.; Xu, H.; Bhatnagar, S.; Gardner, C.L.; Milgram, N.W. Efficacy of a therapeutic diet on dogs with signs of cognitive dysfunction syndrome (CDS): A prospective double blinded placebo controlled clinical study. Front. Nutr. 2018, 5, 127. [Google Scholar] [CrossRef]
- Dahms, I.; Bailey-Hall, E.; Sylvester, E.; Parenteau, A.; Yu, S.; Karagiannis, A.; Roos, F.; Wilson, J. Safety of a novel feed ingredient, Algal Oil containing EPA and DHA, in a gestation–lactation–growth feeding study in Beagle dogs. PLoS ONE 2019, 14, e0217794. [Google Scholar] [CrossRef]
- Pedrinelli, V.; Lima, D.M.; Duarte, C.N.; Teixeira, F.A.; Porsani, M.; Zarif, C.; Amaral, A.R.; Vendramini, T.H.A.; Kogika, M.M.; Brunetto, M.A. Nutritional and laboratory parameters affect the survival of dogs with chronic kidney disease. PLoS ONE 2020, 15, e0234712. [Google Scholar] [CrossRef]
- Souza, C.M.M.; de Lima, D.C.; Bastos, T.S.; Komarcheuski, A.S.; de Oliveira, S.G.; Félix, A.P. The effect of supplementation of microalgae Schizochytrium sp. as a source of docosahexaenoic acid (DHA) on dogs with naturally occurring gingivitis. Arch. Vet. Sci. 2020, 25, 80–86. [Google Scholar] [CrossRef]
- VanderSluis, L.; Mazurak, V.C.; Damaraju, S.; Field, C.J. Determination of the relative efficacy of eicosapentaenoic acid and docosahexaenoic acid for anti–cancer effects in human breast cancer models. Int. J. Mol. Sci. 2017, 18, 2607. [Google Scholar] [CrossRef]
- Zhang, Z.; Xue, Z.; Yang, H.; Zhao, F.; Liu, C.; Chen, J.; Lu, S.; Zou, Z.; Zhou, Y.; Zhang, X. Differential effects of EPA and DHA on DSS–Induced colitis in mice and possible mechanisms involved. Food Funct. 2021, 12, 1803–1817. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Madrigal, R.; Gil-Iturbe, E.; de Calle, M.L.; Moreno-Aliaga, M.J.; Lostao, M.P. DHA and its derived lipid mediators MaR1, RvD1, and RvD2 block TNF–α inhibition of intestinal sugar and glutamine uptake in Caco–2 cells. J. Nutr. Biochem. 2020, 76, 108264. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Bilotto, S.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; Nabavi, S.M. Omega–3 polyunsaturated fatty acids and cancer: Lessons learned from clinical trials. Cancer Metastasis Rev. 2015, 34, 359–380. [Google Scholar] [CrossRef]
- Bauer, J.E. Facilitative and functional fats in diets of cats and dogs. J. Am. Vet. Med. Assoc. 2006, 229, 680–684. [Google Scholar] [CrossRef]
- Magalhaes, T.R.; Lourenco, A.L.; Gregorio, H.; Queiroga, F.L. Therapeutic effect of EPA/DHA supplementation in neoplastic and non–neoplastic companion animal diseases: A systematic review. In Vivo 2021, 35, 1419–1436. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, G.K.; Fettman, M.J.; Mallinckrodt, C.H.; Walton, J.A.; Hansen, R.A.; Davenport, D.J.; Gross, K.L.; Richardson, K.L.; Rogers, Q.; Hand, M.S. Effect of fish oil, arginine, and doxorubicin chemotherapy on remission and survival time for dogs with lymphoma: A double–blind, randomized placebo–controlled study. Cancer 2000, 88, 1916–1928. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Harvey, K.A.; Xu, Z.; Bammerlin, E.M.; Walker, C.; Altenburg, J.D. Docosahexaenoic acid: A natural powerful adjuvant that improves efficacy for anticancer treatment with no adverse effects. Biofactors 2011, 37, 399–412. [Google Scholar] [CrossRef]
- Zhang, J.L.; Wang, Z.; Hu, W.; Chen, S.S.; Lou, X.E.; Zhou, H.J. DHA regulates angiogenesis and improves the efficiency of CDDP for the treatment of lung carcinoma. Microvasc. Res. 2013, 87, 14–24. [Google Scholar] [CrossRef]
- Esmaeili, V.; Shahverdi, A.H.; Moghadasian, M.H.; Alizadeh, A.R. Dietary fatty acids affect semen quality: A review. Andrology 2015, 3, 450–461. [Google Scholar] [CrossRef]
- Da Rocha, A.A.; Da Cunha, I.C.N.; Ederli, B.B.; Albernaz, A.P.; Quirino, C.R. Effect of daily food supplementation with essential fatty acids on canine semen quality. Reprod. Domest. Anim. 2009, 44, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Risso, A.; Pellegrino, F.J.; Relling, A.E.; Corrada, Y. Effect of long–term fish oil supplementation on semen quality and serum testosterone concentrations in male dogs. Int. J. Fertil. Steril. 2016, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Singla, A.; Singh, S.; Shilwant, S.; Kaur, R. Role of omega–3 fatty acids in canine health: A review. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2283–2293. [Google Scholar] [CrossRef]
- Echeverría, F.; Valenzuela, R.; Hernandez-Rodas, M.C.; Valenzuela, A. Docosahexaenoic acid (DHA), a fundamental fatty acid for the brain: New dietary sources. Prostaglandins Leukot. Essent. Fatty Acids 2017, 124, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, H.; Kontani, M.; Kawashima, H.; Kiso, Y.; Shibata, H.; Osumi, N. Differential effect of arachidonic acid and docosahexaenoic acid on age–related decreases in hippocampal neurogenesis. Neurosci. Res. 2014, 88, 58–66. [Google Scholar] [CrossRef]
- Farooqui, A.A. Recent Development on the Neurochemistry of Docosanoids. In Lipid Mediators and Their Metabolism in the Brain, 1st ed.; Springer: New York NY, USA, 2011; pp. 51–88. [Google Scholar]
- Morelli, G.; Stefanutti, D.; Ricci, R. A survey among dog and cat owners on pet food storage and preservation in the households. Animals 2021, 11, 273. [Google Scholar] [CrossRef]
- Hill, R.C.; Choate, C.J.; Scott, K.C.; Molenberghs, G. Comparison of the guaranteed analysis with the measured nutrient composition of commercial pet foods. J. Am. Vet. Med. Assoc. 2009, 234, 347–351. [Google Scholar] [CrossRef]
- Rolinec, M.; Bíro, D.; Gálik, B.; Šimko, M.; Juráček, M.; Tvarožková, K.; Ištoková, A. The nutritive value of selected commercial dry dog foods. Acta Fytotech. Zootech. 2016, 19, 25–28. [Google Scholar] [CrossRef][Green Version]
- Alvarenga, I.C.; Ou, Z.; Thiele, S.; Alavi, S.; Aldrich, C.G. Effects of milling sorghum into fractions on yield, nutrient composition, and their performance in extrusion of dog food. J. Cereal Sci. 2018, 82, 121–128. [Google Scholar] [CrossRef]
- Meineri, G.; Peiretti, P.G.; Tassone, S.; Candellone, A.; Longato, E.; Russo, N.; Pattono, D.; Prola, L. Nutritional value of extruded dog food with mechanically separated chicken meat or meat by–products. Biology 2019. [Google Scholar] [CrossRef]
- Stercova, E.; Strakova, E.; Tsponova, J.; Grmelova, M.; Janacova, K.; Muchova, K. Nutritional evaluation of commercial dry dog foods available on the Czech market. J. Anim. Physiol. Anim. Nutr. 2022, 106, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Kazimierska, K.; Biel, W.; Witkowicz, R. Mineral composition of cereal and cereal–free dry dog foods versus nutritional guidelines. Molecules 2020, 25, 5173. [Google Scholar] [CrossRef] [PubMed]
- Sgorlon, S.; Sandri, M.; Stefanon, B.; Licastro, D. Elemental composition in commercial dry extruded and moist canned dog foods. Anim. Feed Sci. Technol. 2022, 287, 115287. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Jiang, B.; Tsao, R.; Li, Y.; Miao, M. Food Safety: Food Analysis Technologies/Techniques. In Encyclopedia of Agriculture and Food Systems; Van Alfen, N.K., Ed.; Academic Press: Oxford, UK, 2014; pp. 273–288. ISBN 978-0-08-093139-5. [Google Scholar]
- NRC. Nutrient Requirements of Dogs and Cats; National Research Council: Washington, DC, USA, 2006.
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.S. Advances in lipid extraction methods—A review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef]
- Szczuko, M.; Kotlęga, D.; Palma, J.; Zembroń-Łacny, A.; Tylutka, A.; Gołąb-Janowska, M.; Drozd, A. Lipoxins, RevD1 and 9, 13 HODE as the most important derivatives after an early incident of ischemic stroke. Sci. Rep. 2020, 10, 12849. [Google Scholar] [CrossRef]
- FEDIAF. Code of Good Labelling Practice for Pet Food; The European Pet Food Industry Federation: Bruxelles, Belgium, 2019. [Google Scholar]
- Cohen, J. A profile similarity coefficient invariant over variable reflection. Psychol. Bull. 1996, 71, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Markovich, J.E.; Heinze, C.R.; Freeman, L.M. Thiamine deficiency in dogs and cats. J. Am. Vet. Med. Assoc. 2013, 243, 649–656. [Google Scholar] [CrossRef]
- Sechi, S.; Chiavolelli, F.; Spissu, N.; Di Cerbo, A.; Canello, S.; Guidetti, G.; Fiore, F.; Cocco, R. An antioxidant dietary supplement improves brain–derived neurotrophic factor levels in serum of aged dogs: Preliminary results. J. Vet. Med. 2015, 2015, 412501. [Google Scholar] [CrossRef]
- Davies, M.; Alborough, R.; Jones, L.; Davis, C.; Williams, C.; Gardner, D.S. Mineral analysis of complete dog and cat foods in the UK and compliance with European guidelines. Sci. Rep. 2017, 7, 17107. [Google Scholar] [CrossRef]
- Freeman, L.M.; Stern, J.A.; Fries, R.; Adin, D.B.; Rush, J.E. Diet–associated dilated cardiomyopathy in dogs: What do we know? J. Am. Vet. Med. Assoc. 2018, 253, 1390–1394. [Google Scholar] [CrossRef]
- Paulelli, A.C.C.; Martins, A.C.; de Paula, E.S.; Souza, J.M.O.; Carneiro, M.F.H.; Júnior, F.B.; Batista, B.L. Risk assessment of 22 chemical elements in dry and canned pet foods. J. Consum. Prot. Food Saf. 2018, 13, 359–365. [Google Scholar] [CrossRef]
- Raditic, D.M. Insights into commercial pet foods. Vet. Clin. Small Anim. Pract. 2021, 51, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.C.; Cummins, K.A.; Hayek, M.G.; Davenport, G.M. Effects of dietary protein on whole–body protein turnover and endocrine function in young–adult and aging dogs. J. Anim. Sci. 2001, 79, 3128–3136. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, D.P. Pet food safety: Dietary protein. Top. Companion Anim. Med. 2008, 23, 154–157. [Google Scholar] [CrossRef]
- Gagné, J.W.; Wakshlag, J.J.; Center, S.A.; Rutzke, M.A.; Glahn, R.P. Evaluation of calcium, phosphorus, and selected trace mineral status in commercially available dry foods formulated for dogs. J. Am. Vet. Med. Assoc. 2013, 243, 658–666. [Google Scholar] [CrossRef]
- Olivindo, R.F.G.; Zafalon, R.V.A.; Teixeira, F.A.; Vendramini, T.H.A.; Pedrinelli, V.; Brunetto, M.A. Evaluation of the nutrients supplied by veterinary diets commercialized in Brazil for obese dogs undergoing a weight loss program. J. Anim. Physiol. Anim. Nutr. 2022, 106, 355–367. [Google Scholar] [CrossRef]
- Kępińska-Pacelik, J.; Biel, W.; Witkowicz, R.; Podsiadło, C. Mineral and heavy metal content in dry dog foods with different main animal components. Sci. Rep. 2023, 13, 6082. [Google Scholar] [CrossRef]
- Lewis, G. Musculoskeletal development of the puppy. Anim. Ther. Mag. 2019, 15, 41–44. [Google Scholar]
- Pereira, A.M.; Pinto, E.; Matos, E.; Castanheira, F.; Almeida, A.A.; Baptista, C.S.; Segundo, M.A.; Fonseca, A.J.; Cabrita, A.R. Mineral composition of dry dog foods: Impact on nutrition and potential toxicity. J. Agric. Food Chem. 2018, 66, 7822–7830. [Google Scholar] [CrossRef]
- Shao, M.; Li, L.; Gu, Z.; Yao, M.; Xu, D.; Fan, W.; Yan, L.; Song, S. Mycotoxins in commercial dry pet food in China. Food Addit. Contam. Part B 2018, 11, 237–245. [Google Scholar] [CrossRef]
- Kazimierska, K.; Biel, W.; Witkowicz, R.; Karakulska, J.; Stachurska, X. Evaluation of nutritional value and microbiological safety in commercial dog food. Vet. Res. Commun. 2021, 45, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Geicu, O.I.; Bilteanu, L.; Stanca, L.; Ionescu Petcu, A.; Iordache, F.; Pisoschi, A.M.; Serban, A.I. Composition–based risk estimation of mycotoxins in dry dog foods. Foods 2022, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Brazis, P.; Serra, M.; Sellés, A.; Dethioux, F.; Biourge, V.; Puigdemont, A. Evaluation of storage mite contamination of commercial dry dog food. Vet. Dermatol. 2008, 19, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Witaszak, N.; Stępień, Ł.; Bocianowski, J.; Waśkiewicz, A. Fusarium species and mycotoxins contaminating veterinary diets for dogs and cats. Microorganisms 2019, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Ricci, R.; Berlanda, M.; Tenti, S.; Bailoni, L. Study of the chemical and nutritional characteristics of commercial dog foods used as elimination diet for the diagnosis of canine food allergy. Ital. J. Anim. Sci. 2009, 8, 328–330. [Google Scholar] [CrossRef]
- Glodde, F.; Günal, M.; Kinsel, M.E.; AbuGhazaleh, A. Effects of natural antioxidants on the stability of omega–3 fatty acids in dog food. J. Vet. Res. 2018, 62, 103. [Google Scholar] [CrossRef]
- Martinez, N.; McDonald, B. A study into the fatty acid content of selected veterinary diets, supplements and fish oil capsules in Australia. Vet. Dermatol. 2021, 32, 256-e69. [Google Scholar] [CrossRef]
- Kearns, R.J.; Hayek, M.G.; Turek, J.J.; Meydani, M.; Burr, J.R.; Greene, R.J.; Marshall, C.A.; Adams, S.M.; Borgert, R.C.; Reinhart, G.A. Effect of age, breed and dietary omega–6 (n−6): Omega–3 (n−3) fatty acid ratio on immune function, eicosanoid production, and lipid peroxidation in young and aged dogs. Vet. Immunol. Immunopathol. 1999, 69, 165–183. [Google Scholar] [CrossRef]
- Stehle, M.E.; Hanczaruk, M.; Schwarz, S.C.N.; Göbel, T.W.; Mueller, R.S. Effects of polyunsaturated fatty acids on isolated canine peripheral blood mononuclear cells and cytokine expression (IL–4, IFN–γ, TGF–β) in healthy and atopic dogs. Vet. Dermatol. 2010, 21, 113–118. [Google Scholar] [CrossRef]
- Dodd, S.A.S.; Shoveller, A.K.; Fascetti, A.J.; Yu, Z.Z.; Ma, D.W.L.; Verbrugghe, A. A comparison of key essential nutrients in commercial plant–based pet foods sold in Canada to American and European canine and feline dietary recommendations. Animals 2021, 11, 2348. [Google Scholar] [CrossRef]
- Szlinder-Richert, J.; Usydus, Z.; Wyszyński, M.; Adamczyk, M. Variation in fat content and fatty–acid composition of the Baltic herring Clupea harengus membras. J. Fish Biol. 2010, 77, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.E.; da Silva Vasconcelos, M.A.; de Almeida Ribeiro, M.; Sarubbo, L.A.; Andrade, S.A.C.; de Melo Filho, A.B. Nutritional and lipid profiles in marine fish species from Brazil. Food Chem. 2014, 160, 67–71. [Google Scholar] [CrossRef]
- Linhartová, Z.; Krejsa, J.; Zajíč, T.; Másílko, J.; Sampels, S.; Mráz, J. Proximate and fatty acid composition of 13 important freshwater fish species in central Europe. Aquac. Int. 2018, 26, 695–711. [Google Scholar] [CrossRef]
- Molversmyr, E.; Devle, H.M.; Naess-Andresen, C.F.; Ekeberg, D. Identification and quantification of lipids in wild and farmed Atlantic salmon (Salmo salar), and salmon feed by GC–MS. Food Sci. Nutr. 2022, 10, 3117–3127. [Google Scholar] [CrossRef]
- West, A.L.; Miles, E.A.; Lillycrop, K.A.; Han, L.; Sayanova, O.; Napier, J.A.; Calder, P.C.; Burdge, G.C. Postprandial incorporation of EPA and DHA from transgenic Camelina sativa oil into blood lipids is equivalent to that from fish oil in healthy humans. Br. J. Nutr. 2019, 121, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Betancor, M.B.; MacEwan, A.; Sprague, M.; Gong, X.; Montero, D.; Han, L.; Napier, J.A.; Norambuena, F.; Izquierdo, M.; Tocher, D.R. Oil from transgenic Camelina sativa as a source of EPA and DHA in feed for European sea bass (Dicentrarchus labrax L.). Aquaculture 2021, 530, 735–759. [Google Scholar] [CrossRef] [PubMed]
- West, A.L.; Miles, E.A.; Lillycrop, K.A.; Napier, J.A.; Calder, P.C.; Burdge, G.C. Genetically modified plants are an alternative to oily fish for providing n−3 polyunsaturated fatty acids in the human diet: A summary of the findings of a Biotechnology and Biological Sciences Research Council funded project. Nutr. Bull. 2021, 46, 60–68. [Google Scholar] [CrossRef]
- Ghidoli, M.; Ponzoni, E.; Araniti, F.; Miglio, D.; Pilu, R. Genetic improvement of Camelina sativa (L.) Crantz: Opportunities and challenges. Plants 2023, 12, 570. [Google Scholar] [CrossRef]
- Barros de Medeiros, V.P.; da Costa, W.K.A.; da Silva, R.T.; Pimentel, T.C.; Magnani, M. Microalgae as source of functional ingredients in new–generation foods: Challenges, technological effects, biological activity, and regulatory issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 4929–4950. [Google Scholar] [CrossRef]
- Martínez-Ruiz, M.; Martínez-González, C.A.; Kim, D.-H.; Santiesteban-Romero, B.; Reyes-Pardo, H.; Villaseñor-Zepeda, K.R.; Meléndez-Sánchez, E.R.; Ramírez-Gamboa, D.; Díaz-Zamorano, A.L.; Sosa-Hernández, J.E.; et al. Microalgae bioactive compounds to topical applications products—A review. Molecules 2022, 27, 3512. [Google Scholar] [CrossRef]
- Chan, K.Y.; Gao, Q.F.; Yip, K.M.; Wong, W.H.; Shin, P.K.S.; Cheung, S.G. Lipid content and fatty acid composition in the green–lipped mussel Perna viridis (L.). J. Food Lipids 2004, 11, 123–130. [Google Scholar] [CrossRef]
- Chakraborty, K.; Chakkalakal, S.J.; Joseph, D.; Asokan, P.K.; Vijayan, K.K. Nutritional and antioxidative attributes of green mussel (Perna viridis L.) from the southwestern coast of India. J. Aquat. Food Prod. Technol. 2016, 25, 968–985. [Google Scholar] [CrossRef]
- Taylor, A.G.; Savage, C. Fatty acid composition of New Zealand green–lipped mussels, Perna canaliculus: Implications for harvesting for n−3 extracts. Aquaculture 2006, 261, 430–439. [Google Scholar] [CrossRef]
- Miller, M.R.; Tian, H. Changes in proximate composition, lipid class and fatty acid profile in Greenshell™ mussels (Perna canaliculus) over an annual cycle. Aquacult. Res. 2018, 49, 1153–1165. [Google Scholar] [CrossRef]
- Anstiss, L.; Weber, C.C.; Baroutian, S.; Shahbaz, K. Menthol–based deep eutectic solvents as green extractants for the isolation of omega–3 polyunsaturated fatty acids from Perna canaliculus. J. Chem. Technol. Biotechnol. 2023, 98, 1791–1802. [Google Scholar] [CrossRef]
- Pettersen, A.K.; Turchini, G.M.; Jahangard, S.; Ingram, B.A.; Sherman, C.D.H. Effects of different dietary microalgae on survival, growth, settlement and fatty acid composition of blue mussel (Mytilus galloprovincialis) larvae. Aquaculture 2010, 309, 115–124. [Google Scholar] [CrossRef]
- Peycheva, K.; Panayotova, V.; Stancheva, R.; Makedonski, L.; Merdzhanova, A.; Cammilleri, G.; Ferrantelli, V.; Calabrese, V.; Cicero, N.; Fazio, F. Effect of steaming on chemical composition of Mediterranean mussel (Mytilus galloprovincialis): Evaluation of potential risk associated with human consumption. Food Sci. Nutr. 2022, 10, 3052–3061. [Google Scholar] [CrossRef]
- De Swaaf, M.E.; Sijtsma, L.; Pronk, J.T. High–cell–density fed–batch cultivation of the docosahexaenoic acid producing marine alga Crypthecodinium cohnii. Biotechnol. Bioeng. 2003, 81, 666–672. [Google Scholar] [CrossRef]
- Martins, D.A.; Custódio, L.; Barreira, L.; Pereira, H.; Ben-Hamadou, R.; Varela, J.; Abu-Salah, K.M. Alternative sources of n−3 long–chain polyunsaturated fatty acids in marine microalgae. Mar. Drugs 2013, 11, 2259–2281. [Google Scholar] [CrossRef]
- Barta, D.G.; Coman, V.; Vodnar, D.C. Microalgae as sources of omega–3 polyunsaturated fatty acids: Biotechnological aspects. Algal Res. 2021, 58, 102410. [Google Scholar] [CrossRef]
- Zinnai, A.; Sanmartin, C.; Taglieri, I.; Andrich, G.; Venturi, F. Supercritical fluid extraction from microalgae with high content of LC–PUFAs. A case of study: Sc–CO2 oil extraction from Schizochytrium sp. J. Supercrit. Fluids 2016, 116, 126–131. [Google Scholar] [CrossRef]
- Samuelsen, T.A.; Oterhals, Å.; Kousoulaki, K. High lipid microalgae (Schizochytrium sp.) inclusion as a sustainable source of n−3 long–chain PUFA in fish feed–Effects on the extrusion process and physical pellet quality. Anim. Feed Sci. Technol. 2018, 236, 14–28. [Google Scholar] [CrossRef]
- Ledesma-Amaro, R.; Nicaud, J.M. Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids. Prog. Lipid Res. 2016, 61, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Sharpe, P.L.; Hong, S.-P.; Yadav, N.S.; Xie, D.; Short, D.R.; Damude, H.G.; Rupert, R.A.; Seip, J.E.; Wang, J.; et al. Production of omega–3 eicosapentaenoic acid by metabolic engineering of Yarrowia lipolytica. Nat. Biotechnol. 2013, 31, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Miller, E.; Sharpe, P.; Jackson, E.; Zhu, Q. Omega–3 production by fermentation of Yarrowia lipolytica: From fed–batch to continuous. Biotechnol. Bioeng. 2017, 114, 798–812. [Google Scholar] [CrossRef]
- Park, Y.K.; Nicaud, J.M. Metabolic engineering for unusual lipid production in Yarrowia lipolytica. Microorganisms 2020, 8, 1937. [Google Scholar] [CrossRef]
- Tavares, S.; Grotkjær, T.; Obsen, T.; Haslam, R.P.; Napier, J.A.; Gunnarsson, N. Metabolic engineering of Saccharomyces cerevisiae for production of eicosapentaenoic acid, using a novel Δ5–desaturase from Paramecium tetraurelia. Appl. Environ. Microbiol. 2011, 77, 1854–1861. [Google Scholar] [CrossRef]
- Shi, T.; Yu, A.; Li, M.; Ou, X.; Xing, L.; Li, M. Identification of a novel C22–Δ4–producing docosahexaenoic acid (DHA) specific polyunsaturated fatty acid desaturase gene from Isochrysis galbana and its expression in Saccharomyces cerevisiae. Biotechnol. Lett. 2012, 34, 2265–2274. [Google Scholar] [CrossRef]
- Cao, Y.; Cao, Y.; Zhao, M. Biotechnological production of eicosapentaenoic acid: From a metabolic engineering point of view. Process Biochem. 2012, 47, 1320–1326. [Google Scholar] [CrossRef]
- Jensen, K.N.; Jacobsen, C.; Nielsen, H.H. Fatty acid composition of herring (Clupea harengus L.): Influence of time and place of catch on n−3 PUFA content. J. Sci. Food Agric. 2007, 87, 710–718. [Google Scholar] [CrossRef]
- Domiszewski, Z. Effect of heating fatty fish: Baltic herring (Clupea harengus membras), European sprat (Sprattus sprattus), and rainbow trout (Oncorhynchus mykiss) on lipid oxidation and contents of eicosapentaenoic and docosahexaenoic acids. Int. J. Food Sci. Technol. 2013, 48, 786–793. [Google Scholar] [CrossRef]
- Biton-Porsmoguer, S.; Bou, R.; Lloret, E.; Alcaide, M.; Lloret, J. Fatty acid composition and parasitism of European sardine (Sardina pilchardus) and anchovy (Engraulis encrasicolus) populations in the northern Catalan Sea in the context of changing environmental conditions. Conserv. Physiol. 2020, 8, coaa121. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Chen, C.; Shi, Z.; Wang, L. Amino acid and fatty acid composition of the muscle tissue of yellowfin tuna (Thunnus albacares) and bigeye tuna (Thunnus obesus). J. Food Nutr. Res. 2013, 1, 42–45. [Google Scholar]
- Srichan, R.; Worawattanameteekul, W.; Tepwong, P. Seasonal variation and regression prediction of fatty acid compositions in tuna oil from three tuna species (Katsuwonus pelamis, Thunnus tonggol and Euthynnus affinis). Food Appl. Biosci. J. 2018, 6, 53–64. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Jiang, W.; Yan, X. Proximate composition and nutritional profile of rainbow trout (Oncorhynchus mykiss) heads and skipjack tuna (Katsuwonus pelamis) heads. Molecules 2019, 24, 3189. [Google Scholar] [CrossRef]
- Sprague, M.; Fawcett, S.; Betancor, M.B.; Struthers, W.; Tocher, D.R. variation in the nutritional composition of farmed Atlantic Salmon (Salmo salar L.) fillets with emphasis on EPA and DHA contents. J. Food Compos. Anal. 2020, 94, 103618. [Google Scholar] [CrossRef]
- Devadason, C.; Jayasinghe, C.; Sivakanesan, R.; Senarath, S.; Beppu, F.; Gotoh, N. Comparative analysis of lipid content and fatty acid composition of commercially important fish and shellfish from Sri Lanka and Japan. J. Oleo Sci. 2016, 65, 543–556. [Google Scholar] [CrossRef][Green Version]
- Shulgina, L.V.; Davletshina, T.A.; Pavlovskii, A.M.; Pavel, K.G. Lipid and fatty–acid compositions of muscle tissue from Sardinops melanostictus. Chem. Nat. Compd. 2020, 56, 305–308. [Google Scholar] [CrossRef]
- Mkadem, H.; Kaanane, A. Seasonal changes in chemical composition and fatty acids of sardines (Sardina pilchardus) from the Dakhla coast (Morocco). Moroccan J. Agric. Sci. 2020, 1, 161–170. [Google Scholar]
- Betancor, M.B.; Sprague, M.; Sayanova, O.; Usher, S.; Metochis, C.; Campbell, P.J.; Napier, J.A.; Tocher, D.R. nutritional evaluation of an EPA–DHA oil from transgenic camelina sativa in feeds for post–smolt Atlantic Salmon (Salmo salar L.). PLoS ONE 2016, 11, e0159934. [Google Scholar] [CrossRef]
- Petrie, J.R.; Shrestha, P.; Belide, S.; Kennedy, Y.; Lester, G.; Liu, Q.; Divi, U.K.; Mulder, R.J.; Mansour, M.P.; Nichols, P.D.; et al. Metabolic engineering Camelina sativa with fish oil–like levels of DHA. PLoS ONE 2014, 9, e85061. [Google Scholar] [CrossRef] [PubMed]
- Usher, S.; Haslam, R.P.; Ruiz-Lopez, N.; Sayanova, O.; Napier, J.A. Field trial evaluation of the accumulation of omega–3 long chain polyunsaturated fatty acids in transgenic Camelina sativa: Making fish oil substitutes in plants. Metab. Eng. Commun. 2015, 2, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.L.; Huang, Y.-S.; Bobik, E.G.; Kinney, A.J.; Stecca, K.L.; Packer, J.C.L.; Mukerji, P. A novel omega3–fatty acid desaturase involved in the biosynthesis of eicosapentaenoic acid. Biochem. J. 2004, 378, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J. Oxy radicals, lipid peroxidation and DNA damage. Toxicology 2002, 181, 219–222. [Google Scholar] [CrossRef]
- Scott, D.W.; Miller, W.H., Jr.; Reinhart, G.A.; Mohammed, H.O.; Bagladi, M.S. Effect of an omega–3/omega–6 fatty acid–containing commercial lamb and rice diet on pruritus in atopic dogs: Results of a single–blinded study. Can. J. Vet. Res. 1997, 61, 145. [Google Scholar] [PubMed]
- Hall, J.A.; Picton, R.A.; Skinner, M.M.; Jewell, D.E.; Wander, R.C. The (n−3) fatty acid dose, independent of the (n−6) to (n−3) fatty acid ratio, affects the plasma fatty acid profile of normal dogs. J. Nutr. 2006, 136, 2338–2344. [Google Scholar] [CrossRef]
- Popović, T.; Martaćić, J.D.; Pokimica, B.; Ravić, B.; Ranković, S.; Glibetić, M.; Stepanović, P. Phospholipid fatty acid profiles of plasma and erythrocyte membranes in dogs fed with commercial granulated food. Acta Vet. 2023, 73, 119–132. [Google Scholar] [CrossRef]
- Boretti, F.S.; Burla, B.; Deuel, J.; Gao, L.; Wenk, M.R.; Liesegang, A.; Sieber-Ruckstuhl, N.S. Serum lipidome analysis of healthy beagle dogs receiving different diets. Metabolomics 2020, 16, 1–12. [Google Scholar] [CrossRef]
- Jackson, M.I.; Jewell, D.E. Feeding of fish oil and medium–chain triglycerides to canines impacts circulating structural and energetic lipids, endocannabinoids, and non–lipid metabolite profiles. Front. Vet. Sci. 2023, 10, 1168703. [Google Scholar] [CrossRef]
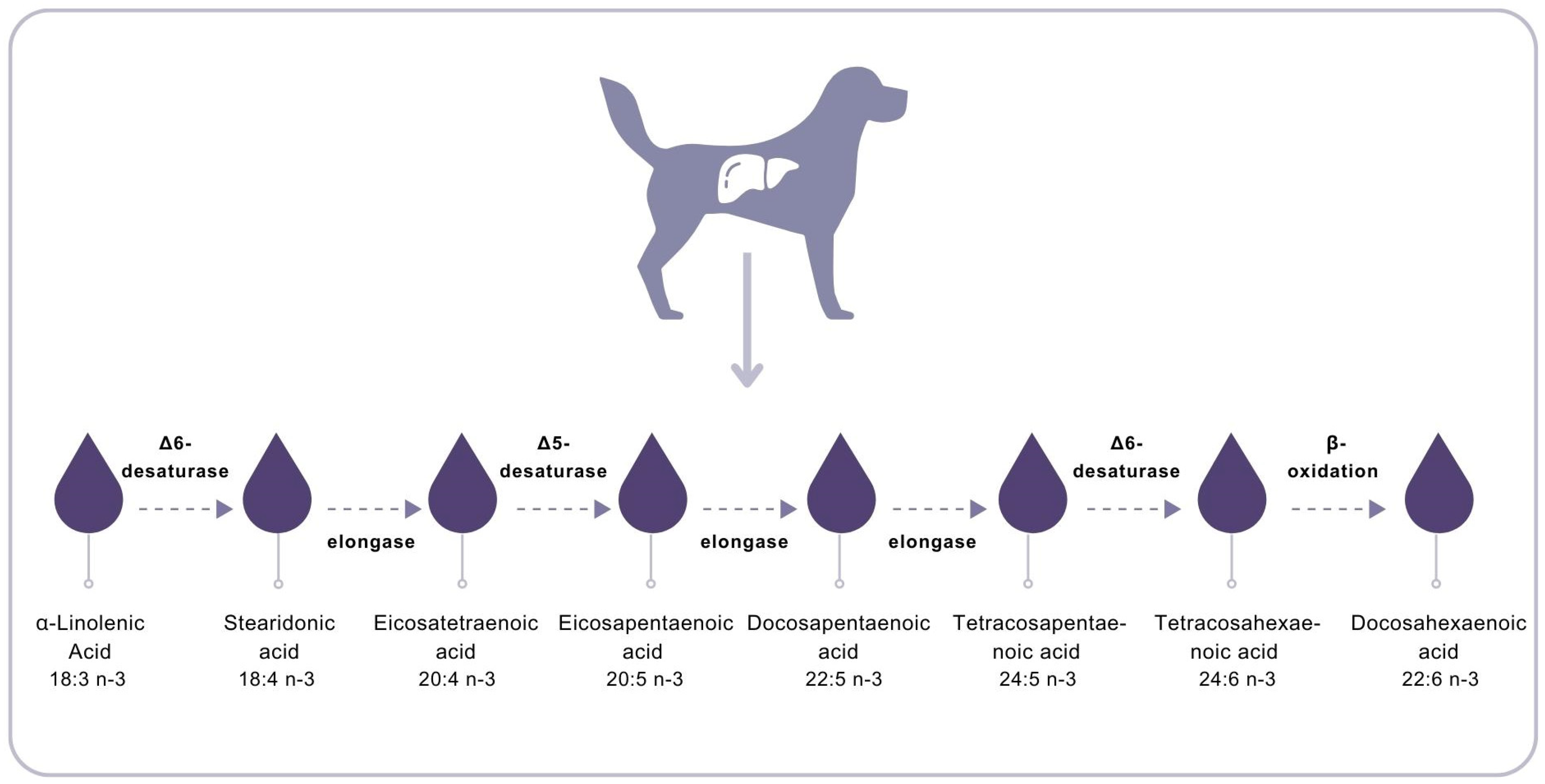
- Dunbar, B.L.; Bauer, J.E. Conversion of essential fatty acids by delta 6–desaturase in dog liver microsomes. J. Nutr. 2002, 132, 1701S–1703S. [Google Scholar] [CrossRef]
- Mueller, R.S.; Fieseler, K.V.; Fettman, M.J.; Zabel, S.; Rosychuk, R.A.W.; Ogilvie, G.K.; Greenwalt, T.L. Effect of omega–3 fatty acids on canine atopic dermatitis. J. Small Anim. Pract. 2004, 45, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Ecker, J. The opposing affects of n−3 and n−6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Waldron, M.K.; Hannah, S.S.; Bauer, J.E. Plasma phospholipid fatty acid and ex vivo neutrophil responses are differentially altered in dogs fed fish– and linseed–oil containing diets at the same n−6:n−3 fatty acid ratio. Lipids 2012, 47, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Tooley, K.A.; Gradin, J.L.; Jewell, D.E.; Wander, R.C. Effects of dietary n−6 and n−3 fatty acids and vitamin E on the immune response of healthy geriatric dogs. Am. J. Vet. Res. 2003, 64, 762–772. [Google Scholar] [CrossRef]
- Vaughn, D.M.; Reinhart, G.A.; Swaim, S.F.; Lauten, S.D.; Garner, C.A.; Boudreaux, M.K.; Spano, J.S.; Hoffman, C.E.; Conner, B. Evaluation of effects of dietary n−6 to n−3 fatty acid ratios on leukotriene B synthesis in dog skin and neutrophils. Vet. Dermatol. 1994, 5, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, N.; Kagiono, S.; Yoshinaga, K.; Mizobe, H.; Nagai, T.; Yoshida, A.; Beppu, F.; Nagao, K. Study of trans fatty acid formation in oil by heating using model compounds. J. Oleo Sci. 2018, 67, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, C.; Crapiste, G.H.; Carrín, M.E. Study of acyl migration during enzymatic interesterification of liquid and fully hydrogenated soybean oil. J. Mol. Catal. B Enzym. 2015, 122, 117–124. [Google Scholar] [CrossRef]
- Yepez, X.V.; Keener, K.M. High–voltage atmospheric cold plasma (HVACP) hydrogenation of soybean oil without trans–fatty acids. Innov. Food Sci. Emerg. Technol. 2016, 38, 169–174. [Google Scholar] [CrossRef]
- Monguchi, T.; Hara, T.; Hasokawa, M.; Nakajima, H.; Mori, K.; Toh, R.; Irino, Y.; Ishida, T.; Hirata, K.; Shinohara, M. Excessive intake of trans fatty acid accelerates atherosclerosis through promoting inflammation and oxidative stress in a mouse model of hyperlipidemia. J. Cardiol. 2017, 70, 121–127. [Google Scholar] [CrossRef]
- Ohmori, H.; Fujii, K.; Kadochi, Y.; Mori, S.; Nishiguchi, Y.; Fujiwara, R.; Kishi, S.; Sasaki, T.; Kuniyasu, H. Elaidic acid, a trans–fatty acid, enhances the metastasis of colorectal cancer cells. Pathobiology 2017, 84, 144–151. [Google Scholar] [CrossRef]
- Cassagno, N.; Palos-Pinto, A.; Costet, P.; Breilh, D.; Darmon, M.; Bérard, A.M. Low amounts of trans 18:1 fatty acids elevate plasma triacylglycerols but not cholesterol and alter the cellular defence to oxidative stress in mice. Br. J. Nutr. 2005, 94, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.W.; Zhao, L.; Yuan, L.H.; Yu, H.L.; Wang, H.; Gong, X.Y.; Wei, F.; Xiao, R. Elaidic acid induces cell apoptosis through induction of ROS accumulation and endoplasmic reticulum stress in SH–SY5Y cells. Mol. Med. Rep. 2017, 16, 9337–9346. [Google Scholar] [CrossRef] [PubMed]
- Plötz, T.; Krümmel, B.; Laporte, A.; Pingitore, A.; Persaud, S.J.; Jörns, A.; Elsner, M.; Mehmeti, I.; Lenzen, S. The monounsaturated fatty acid oleate is the major physiological toxic free fatty acid for human beta cells. Nutr. Diabetes 2017, 7, 305. [Google Scholar] [CrossRef] [PubMed]

| Common Name | Structural Formula | IUPAC * | n−x ** |
|---|---|---|---|
| Saturated Fatty Acids (SFAs) | |||
| Myristic Acid | C13H27COOH | C14:0 | - |
| Palmitic Acid | C15H31COOH | C16:0 | - |
| Stearic Acid | C17H35COOH | C18:0 | - |
| Arachidic Acid | C19H39COOH | C20:0 | - |
| Behenic Acid | C21H43COOH | C22:0 | - |
| Tricosylic Acid | C22H45COOH | C23:0 | - |
| Monounsaturated Fatty Acids (MUFAs) | |||
| Palmitoleic Acid | C15H29COOH | C16:1 (9) | n−7 |
| Oleic Acid | C17H33COOH | C18:1 (9) | n−9 |
| Elaidic Acid | C17H33COOH | C18:1 (9t) | n−9 |
| Polyunsaturated Fatty Acids (PUFAs) | |||
| Linoleic Acid | C17H31COOH | C18:2 (9, 12) | n−6 |
| γ−Linolenic Acid | C17H29COOH | C18:3 (6, 9, 12) | n−6 |
| Arachidonic Acid | C19H31COOH | C20:4 (5, 8, 11, 14) | n−6 |
| α−Linolenic Acid | C17H29COOH | C18:3 (9, 12, 15) | n−3 |
| Eicosapentaenoic Acid | C19H29COOH | C20:5 (5, 8, 11, 14, 17) | n−3 |
| Docosahexaenoic Acid | C21H31COOH | C22:6 (4, 7, 10, 13, 16, 19) | n−3 |
| Item | Labelling/Component Claims | Animal Sources of Nutrients | Plant Sources of Nutrients | Source of Fat/Oil | Other Ingredients |
|---|---|---|---|---|---|
| DEPF_1 | with salmon | sea fish, salmon | potatoes, peas, sweet potatoes, tomatoes | salmon oil, canola oil | chicory, yucca schidigera |
| DEPF_2 | salmon | salmon | beetroot, potatoes, rice | animal fat | ginger, flaxseed, yucca schidigera |
| DEPF_3 | with herring, mackerel, flounder, hake and rockfish | herring, mackerel, flounder, blue whiting, hake, rockfish | chickpeas, chicory, beans | sunflower oil | apples, beets, blueberries, burdock root, carrots, chicory, cranberries, holy thistle, kale, lavender, pears, pumpkin, rosehips, seaweed, spinach, turmeric, turnip greens, valerian root, zucchini |
| DEPF_4 | chicken | chicken, eggs | rice, sorghum | animal fat (poultry and pork), krill, salmon oil | algae, calendula, flaxseed, grape seeds, green tea, lecithin, rosemary |
| DEPF_5 | chicken | chicken | apples buckwheat, beetroot, oats, potatoes | chicken fat, salmon oil | algae, black currant, calendula, collagen, dandelion, flaxseed, green–lipped mussel, parsley, rosemary, thyme |
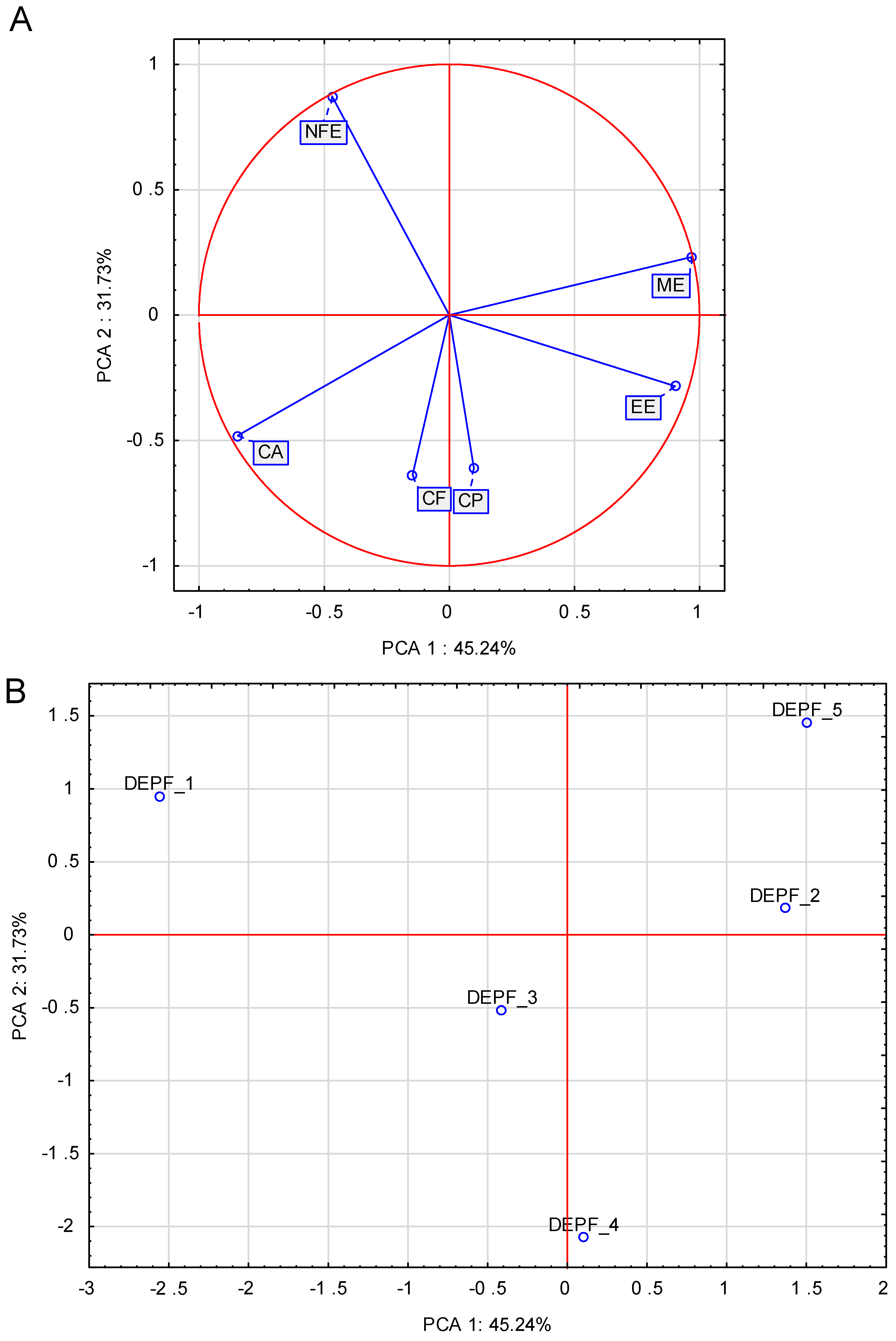
| Item | DEPF_1 | DEPF_2 | DEPF_3 | DEPF_4 | DEPF_5 | Recommendations * | |
|---|---|---|---|---|---|---|---|
| Early Growth | Late Growth | ||||||
| Dry matter (g/100 g fresh food) | 95.4 b | 95.8 b | 92.7 a | 95.4 b | 96.6 b | NR ** | NR |
| Nutrient (unit/100 g DM) | |||||||
| Crude protein (g) | 30.1 a | 31.7 ab | 35.3 c | 32.8 bc | 30.8 ab | 25.0 | 20.0 |
| Crude fat (g) | 11.7 a | 19.2 c | 14.5 b | 19.5 c | 19.1 c | 8.5 | 8.5 |
| Crude fiber (g) | 6.1 a | 6.2 a | 4.5 a | 7.8 a | 4.7 a | NR | NR |
| Crude ash (g) | 7.7 c | 6.4 a | 7.2 b | 7.6 c | 6.4 a | NR | NR |
| NFE (g) | 39.8 c | 32.3 ab | 31.3 ab | 27.7 a | 35.7 bc | NR | NR |
| ME (kcal) | 355.9 a | 397.6 b | 373.6 ab | 379.0 ab | 407.8 b | NR | NR |
| Item | DEPF_1 | DEPF_2 | DEPF_3 | DEPF_4 |
|---|---|---|---|---|
| DEPF_2 | −0.682 | − | − | − |
| DEPF_3 | −0.145 | −0.356 | − | − |
| DEPF_4 | −0.255 | 0.062 | −0.250 | − |
| DEPF_5 | −0.374 | 0.816 | −0.155 | −0.347 |
 —x ≥ +0.75 (high similarity);
—x ≥ +0.75 (high similarity);  —+0.75 > x > +0.30 (moderate similarity);
—+0.75 > x > +0.30 (moderate similarity);  —+0.30 ≥ x ≥ −0.30 (no similarity);
—+0.30 ≥ x ≥ −0.30 (no similarity);  —−0.30 > x > −0.75 (moderate dissimilarity);
—−0.30 > x > −0.75 (moderate dissimilarity);  —x ≤ −0.75 (high dissimilarity).
—x ≤ −0.75 (high dissimilarity).| Item | DEPF_1 | DEPF_2 | DEPF_3 | DEPF_4 |
|---|---|---|---|---|
| DEPF_2 | −0.516 | − | − | − |
| DEPF_3 | 0.352 | 0.175 | − | − |
| DEPF_4 | 0.167 | −0.401 | −0.046 | − |
| DEPF_5 | −0.045 | −0.666 | −0.105 | 0.657 |
 —x ≥ +0.75 (high similarity);
—x ≥ +0.75 (high similarity);  —+0.75 > x > +0.30 (moderate similarity);
—+0.75 > x > +0.30 (moderate similarity);  —+0.30 ≥ x ≥ −0.30 (no similarity);
—+0.30 ≥ x ≥ −0.30 (no similarity);  —−0.30 > x > −0.75 (moderate dissimilarity);
—−0.30 > x > −0.75 (moderate dissimilarity);  —x ≤ −0.75 (high dissimilarity).
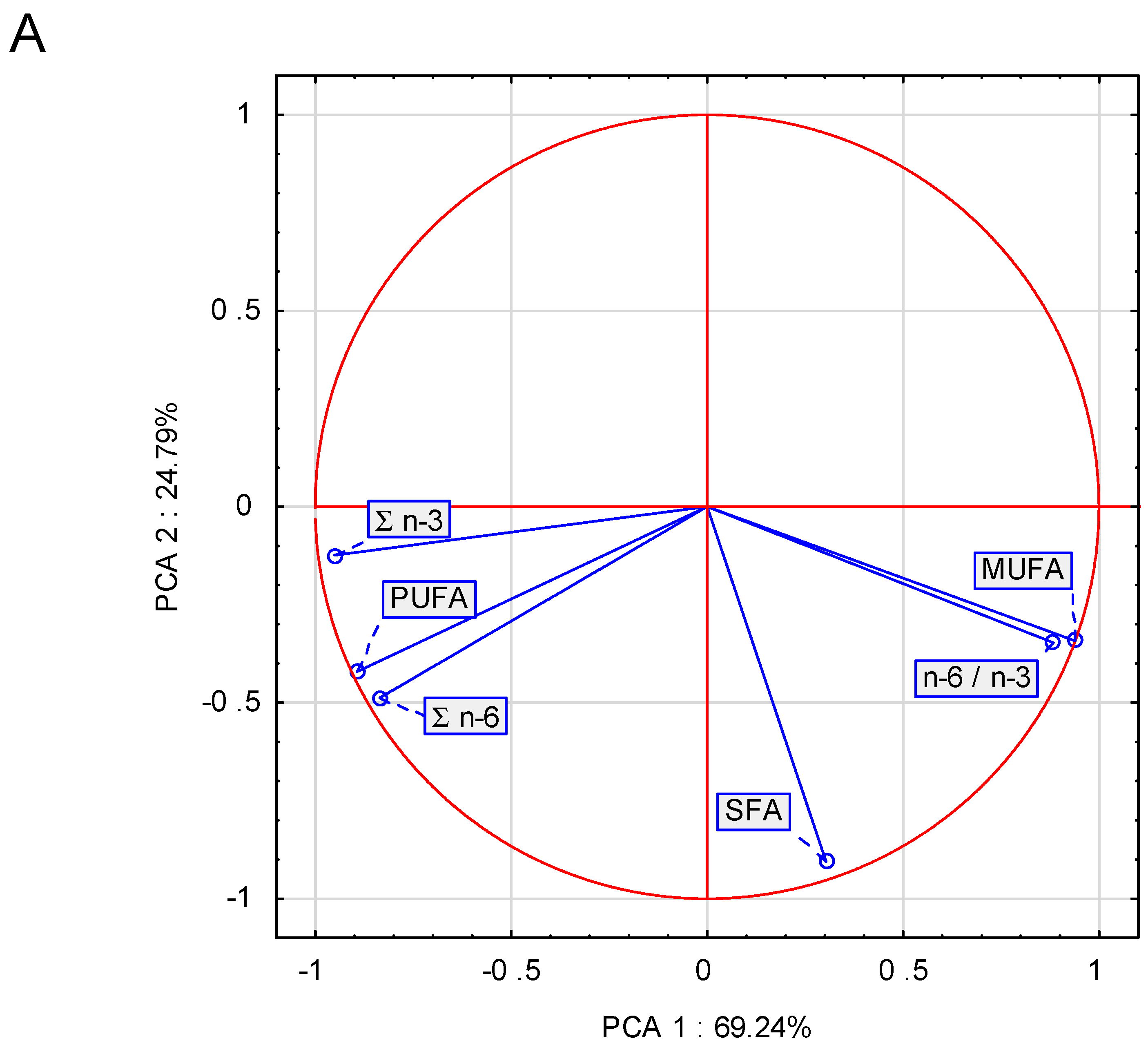
—x ≤ −0.75 (high dissimilarity).| Nutrients | Unit | Content | Recommendations * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DEPF_1 | DEPF_2 | DEPF_3 | DEPF_4 | DEPF_5 | Early Growth | Late Growth | Adult | ||
| n−6: | |||||||||
| LA | g | 2.10 | 0.55 | 1.18 | 2.85 | 3.37 | 1.30 | 1.30 | 1.32 |
| AA | g | 0.01 | 0.06 | 0.05 | 0.04 | 0.03 | 0.03 | 0.03 | NR * |
| n−3: | |||||||||
| ALA | g | 0.58 | 0.03 | 0.23 | 0.74 | 0.38 | 0.08 | 0.08 | NR |
| EPA | g | 0.03 | 0.01 | 0.02 | 0.05 | 0.07 | NR | NR | NR |
| DHA | g | 0.03 | 0.02 | 0.04 | 0.05 | 0.09 | NR | NR | NR |
| EPA + DHA | g | 0.06 | 0.03 | 0.06 | 0.10 | 0.16 | 0.05 ** | 0.05 ** | NR |
| label EPA + DHA | g | 0.1 | 0.24 | 1.82 | 0.22 | 0.94 | – | – | – |
| Item | Moisture | Crude Protein | Crude Fat | Crude Fiber | Energy | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | |
| DEPF_1 | ↓ 48.7% | 9–11 | WPT | 27–33 | ↓ 19.2% | 14.5–18.9 | WPT | 4.7–6.4 | ↓ 6.3% | 380–420 |
| DEPF_2 | ↓ 48.1% | 8.1–9.9 | WPT | 31.1–37.1 | WPT | 19.8–24.2 | ↑ 63.7% | 2.17–3.8 | ↓ 7.2% | 428.4–473.6 |
| DEPF_3 | ↓ 32% | 10.8–13.2 | ↓ 3.9% | 36.8–38.8 | ↓ 15.5% | 17.1–21.5 | ↓ 23% | 5.8–7.8 | ↓ 10.2% | 416.1–459.9 |
| DEPF_4 | ↓ 36.4% | 7.2–8.8 | WPT | 31.8–37.8 | ↓ 10% | 21.7–26.1 | ↑ 93% | 2.5–4.1 | ↓ 16.6% | 454.1–501.9 |
| DEPF_5 | ↓ 62.7% | 9–11 | ↓ 2.1% | 31.4–37.4 | ↓ 9.7% | 21.1–25.5 | ↑ 54.3% | 1.4−3.0 | ↓ 5.2% | 430.4–475.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacuńska, W.; Biel, W.; Witkowicz, R.; Maciejewska-Markiewicz, D.; Piątkowska, E. Comparison of Key Nutrient Content of Commercial Puppy Foods with Canine Dietary Requirements. Appl. Sci. 2023, 13, 11791. https://doi.org/10.3390/app132111791
Jacuńska W, Biel W, Witkowicz R, Maciejewska-Markiewicz D, Piątkowska E. Comparison of Key Nutrient Content of Commercial Puppy Foods with Canine Dietary Requirements. Applied Sciences. 2023; 13(21):11791. https://doi.org/10.3390/app132111791
Chicago/Turabian StyleJacuńska, Weronika, Wioletta Biel, Robert Witkowicz, Dominika Maciejewska-Markiewicz, and Ewa Piątkowska. 2023. "Comparison of Key Nutrient Content of Commercial Puppy Foods with Canine Dietary Requirements" Applied Sciences 13, no. 21: 11791. https://doi.org/10.3390/app132111791
APA StyleJacuńska, W., Biel, W., Witkowicz, R., Maciejewska-Markiewicz, D., & Piątkowska, E. (2023). Comparison of Key Nutrient Content of Commercial Puppy Foods with Canine Dietary Requirements. Applied Sciences, 13(21), 11791. https://doi.org/10.3390/app132111791










