Abstract
Desquamative gingivitis is a clinical condition with a chronic course, not specific to a particular disease, characterized by intense erythema, scaling, vesicles, and/or blisters that may involve both the marginal free gingiva (MG) and the neighboring adherent gingiva (AG). This scoping review aimed to investigate whether there is a correlation between oral hygiene and gingival lesions induced by autoimmune diseases of the oral cavity and whether periodontal disease can negatively influence a clinical picture of desquamative gingivitis due to an immune disorder of the oral cavity. Case series studies and randomized controlled trials were considered for this scoping review; studies that did not comply with the inclusion criteria were excluded. A total of seven studies were selected for this review. The PRISMA-ScR (preferred reporting items for scoping reviews) consensus has been followed. Based on the included studies, it is possible to state that improvement in disease and patient-reported outcomes may be the result of appropriate oral hygiene education when patients are found to have autoimmune diseases with gingival manifestations.
1. Introduction
The term “chronic desquamative gingivitis” was first mentioned in a text in 1894 by authors J. Tomes and G. Tomes [1], and it was not until several years later, in 1932, that the term “diffuse chronic desquamative gingivitis” was introduced by Prinz [2], which was used to describe a clinical manifestation characterized by intense erythema, desquamation, and vesicles and/or bullae, subsequently resulting in erosions and/or ulcers, that could involve both the marginal free gingiva (MG) and the neighboring adherent gingiva (AG). Subsequently, following McCarthy’s 1960 hypothesis, Glickman and Smulow illustrated in 1964 that desquamative gingivitis (DG) is a nonspecific clinical manifestation that can be observed in a large number of pathological conditions [3,4]. Most cases in which DG is found in the clinical setting occur when a mucocutaneous pathology is present, such as oral lichen planus (OLP), mucous membrane pemphigoid (MMP), and pemphigus vulgaris (PV), which are the diseases most frequently associated with DG [5,6].
Lichen planus (LP) is a chronic recurrent immune-mediated inflammatory dermatosis of unknown etiology that can affect skin, mucosa, and nails. It most frequently affects Caucasian women between 50 and 70 years of age, and the oral cavity’s sites experiencing the greatest impact, in descending order of frequency, are the posterior buccal mucosa, the gingiva, the dorsum and lingual margins, the palate, and the lips, almost always with a tendency to produce bilateral and symmetrical lesions [7,8,9]. OLP lesions are characterized by altered immune regulation mediated by CD8+ T lymphocytes, resulting in damage at the level of the keratinocytes, with a specific target of the pathology being basement membrane keratinocytes. At the histopathological level, different degrees of hyper-, ortho-, and parakeratosis, liquefaction of basal cells, basal membrane thickening, and atrophy of the epithelial ridges can be observed in such lesions; at the level of the lamina propria, however, a banded infiltrate formed predominantly by T lymphocytes is observed [10,11,12]. The worldwide prevalence of LP lesions at the level of the oral cavity is estimated to be between 1% and 2%: almost half of these lesions see gingival tissue involved in an obvious manner, and about 10% of patients with OLP have only gingival manifestations in the form of an erythematous lesion, which is clinically referred to as “desquamative gingivitis” [13,14,15]. OLP occurring at the gum level can be identified in clinical terms by the existence of redness (atrophic lichen planus), erosions, and/or ulcerations (erosive lichen planus) of bullae/blisters (bullous lichen planus). In each of these gingival manifestations, OLP lesions vary in the clinical form, extent, and degree of gingival involvement [16,17,18].
Mucous membrane pemphigoid (MMP) and pemphigus vulgaris (PV) represent the main subtypes of autoimmune bullous diseases that most commonly and predominantly affect the oral mucosa; the former most frequently affects female subjects aged 50–70 years, electively in the oral mucosa (90% of cases) and conjunctival mucosa (65% of cases); the latter, on the other hand, predominantly affects skin and oral mucosa, having precisely the latter as the site of first appearance in 50–70% of cases, without, however, presenting a clear predilection for either sex [19]. MMP localizes electively at the gingival tissue level, causing erythema, edema, scaling, blistering, and ulceration; this frequently leads it to be mistaken for periodontal disease. Other less frequent localizations are the buccal mucosa, tongue, and palate. MMP lesions are characterized by the linear deposition of IgG, IgA, or C3 along the epithelial basement membrane zone and the presence of autoantibodies directed against an antigen present at the basement membrane level, BPAG-2. This leads to a clear separation of the epithelial lamina from the underlying lamina propria, with the formation of a subepithelial blister [20,21]. Instead, oral PV lesions are due to the presence of autoantibodies directed against a component of the desmosome–plasma membrane complex, namely a transmembrane protein called desmoglein-3 (Dsg-3), and this results in the creation of an intracellular blister due to acantholysis, with a process likely related in this formation to the release of a plasminogen activator and proteinase that is followed by damage to the desmosomes. Once more, it is feasible to visually detect the accumulation of IgG or C3 in proximity to the basement membrane using direct immunofluorescence [22,23,24].
Desquamative gingivitis (DG) is a clinical condition that occurs more frequently in female subjects than in male subjects—a ratio of about 4:1—and in most cases, it appears after the age of 30, although it is observable at any age, starting from puberty and forward. It generally presents a chronic type of manifestation, with periods of remission and exacerbation: gingivitis may heal after a few months or, in some cases, DG may persist for several years [25,26]. Oral manifestations of desquamative gingivitis can vary in both size and seriousness, spanning from minor, restricted lesions to larger affected regions and occasionally accompanied by episodes of spontaneous bleeding [27]. Generally, when the first clinical signs of DG appear, erythema and minimal desquamation of the mucosa initially predominate; only later does the appearance of vesiculobullous lesions occur. There have been several observed cases of extensive areas of ulceration [28]. Less severe cases, characterized by limited symptoms, can result in heightened sensitivity to spicy and/or acidic foods and discomfort when using specific toothpaste. In contrast, more extreme cases with widespread symptoms typically cause significant and adverse effects on the patient’s overall quality of life [18,29]. Painful gum and mouth lesions can deter patients from carrying out thorough and efficient tooth surface brushing, potentially raising the risk of long-term periodontal tissue damage due to the buildup of plaque at particular locations. Additionally, they may intensify gum inflammation in regions adjacent to the lesions caused by DG [30,31]. Other indirect consequences of plaque accumulation on the dental elements may be the occurrence of dental caries and halitosis [32,33,34,35]. The direct effects, on the other hand, that DG might have on periodontitis seem to be plausible, as there is some sharing of both pathogenetic mechanisms and mediators of inflammation at the base [36]. Certainly, the development of periodontal disease is indeed connected to a localized inflammatory response and the activation of the immune system triggered by bacterial components [37]. This process heavily relies on the stimulation of pro-inflammatory molecules and the activation of cytokine networks, particularly interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α) [38]. Conversely, autoimmune–inflammatory mechanisms are also pivotal in the development of most diseases associated with DG, often involving shared cytokine molecules/networks (for example, TNF-α in the case of OLP) [18,39]. Consequently, one could speculate that the simultaneous presence of bacterial-induced inflammation and autoimmune-related inflammation might exacerbate the harm inflicted on the tissues supporting the teeth.
To date, however, there is only limited systematic information on the impact and potential negative influence that DG has on the development and/or progression of plaque-related periodontal disease, and vice versa, and on the correlation between a patient’s home oral hygiene and the progression of desquamative gingivitis. Therefore, this scoping review aims to investigate whether there is a correlation between oral hygiene and gingival lesions due to an immune disorder of the oral cavity and whether periodontal disease can negatively influence a clinical picture of desquamative gingivitis attributable to an immune disease of the oral cavity.
2. Materials and Methods
2.1. Focused Questions
Is there a correlation between oral hygiene and gingival lesions induced by autoimmune oral cavity diseases? Can periodontal disease worsen the clinical picture of desquamative gingivitis attributable to an immune disease of the oral cavity?
2.2. Eligibility Criteria
The inclusion criteria for the preparation of this scoping review were (I) study design: clinical trials, case-control studies, case series studies, cross-sectional studies, and cohort studies; (II) participants: patients with autoimmune diseases of the oral cavity and with the presence of periodontal disease in the forms of both gingivitis and periodontitis; (III) interventions: professional oral hygiene by tartar ablation and/or scaling and root planing and home oral hygiene instruction.
Studies that did not satisfy the aforementioned inclusion criteria were not taken into account for the following article. In addition, the exclusion criteria applied in writing this review were (I) abstracts of articles published in languages other than English, (II) duplicate studies, (III) irrelevant duplicate studies, (III) in vitro or animal clinical studies, (IV) lack of ethics committee approval, (V) studies that did not meet all the inclusion criteria of ethics committee approval, and (VI) narrative reviews, scoping reviews, systematic reviews, and meta-analyses.
2.3. Search Strategy
In line with the JBI approach to scoping reviews, a three-step search procedure was contemplated, comprising the following stages: (i) limited preliminary search on PubMed (MEDLINE) and Scopus; (ii) selection of key terms from the retrieved articles to elaborate upon the search strategy; and (iii) a reference list search of all included articles for further research. Furthermore, the PCC model was implemented, founded on the subsequent trio of components: population (people with autoimmune diseases of the oral cavity and with the presence of periodontal disease in the forms of gingivitis and periodontitis), concept (worsening of autoimmune disease of the oral cavity as a result of poor oral hygiene conditions), and context (in this instance, the review was not constrained by any particular cultural elements or context). Abstracts of studies that evaluated and analyzed correlations between the worsening course of autoimmune diseases of the oral cavity following conditions of poor oral hygiene were reviewed.
During the examination of existing literature, the preferred reporting items for scoping reviews (PRISMA-ScR) consensus guidelines [40] were adhered to; see Table S1 (Supplementary Material).
2.4. Research
The Medical Subject Heading (MeSH) terms used for the search included autoimmune disease, oral lichen planus, benign mucous membrane pemphigoid, pemphigus vulgaris, periodontal disease, and oral hygiene. An electronic search was conducted on PubMed (MEDLINE), Scopus, and Web of Science databases, focusing on articles published between 1980 and 2023. The process of extracting data occurred between March 2023 and May 2023, culminating in the final search conducted on 14 May 2023.
Three reviewers (G.L.V., F.P., and M.P.) carried out the search, and any conflicts or variations were addressed by reaching a consensus, with input from two additional reviewers (A.S. and F.S.). Titles and abstracts of the initially retrieved articles were meticulously examined, and studies that lacked relevance were excluded. The complete contents of all pertinent articles were thoroughly examined and analyzed, with results recorded, and analogous studies that met the inclusion criteria were identified.
The present protocol has been registered on the Open Science Framework platform (Registration DOI: https://doi.org/10.17605/OSF.IO/V9FMS).
The detailed strategies employed for each electronic database search are presented in Table S2 (Supplementary Material).
2.5. Quality Assessment
This review was conducted by assessing the risk of bias by quality analysis of clinical studies through JBI Quality Assessment of case reports, case series, case-controlled and randomized controlled trials, observational cohort, and cross-sectional studies.
3. Results
In total, 236 articles were recognized using MeSH terms. Subsequently, 164 articles were eliminated from PubMed and 54 articles from Scopus because the abstracts were not published in English, and/or duplicate, and/or in vitro or animal clinical studies, and/or irrelevant, and/or lacking ethics committee approval. Accordingly, 18 articles underwent a screening process and were assessed for eligibility. Subsequently, eight articles were excluded due to their nature as narrative reviews and scoping reviews. In total, seven relevant articles were included and evaluated in this review. Figure 1 shows the flowchart of the review process.
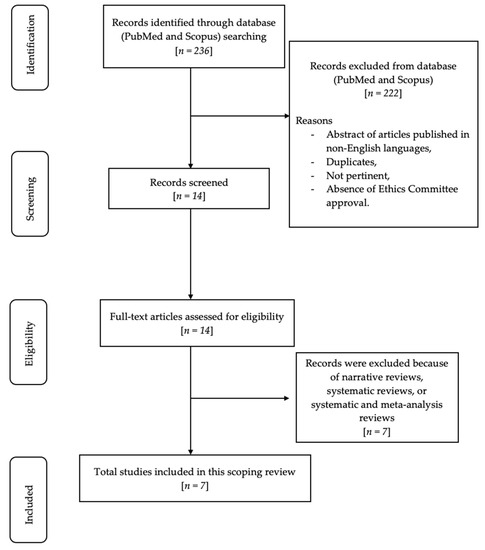
Figure 1.
Flow chart of the review process.
Table S3 (Supplementary Materials) displays the research articles not considered in this review, along with the rationales for their exclusion [27,39,41,42,43,44,45].
The studies belonged to two categories: case-series studies [46,47,48,49] and randomized controlled trials [50,51,52].
Risk of Bias
The JBI Quality Assessment approach was employed to evaluate the potential for bias in the articles incorporated into this review (Table 1 and Table 2), using evaluation criteria for bias risk as provided in Tables S4 and S5 (Supplementary Materials). A low risk of bias was observed in this review.

Table 1.
Risk of bias of the case series studies included in this review: the green symbol represents a low risk of bias, while the yellow symbol represents a high risk of bias.

Table 2.
Risk of bias of the RCT studies included in this review: the green symbol represents a low risk of bias, while the yellow symbol represents a high risk of bias.
Table 3 displays the fundamental characteristics of the patients encompassed within the chosen research papers. Findings from the research considered in this review (study design and aim, methods, results, and conclusions) is shown in Table S6 (Supplementary Materials).

Table 3.
Baseline characteristics of patients included in the selected studies.
4. Discussion
Desquamative gingivitis (DG) is a clinical nonspecific condition distinguished by intense erythema, scaling, blistering, and/or vesicles and may involve both the marginal free gingiva (MG) and the nearby adherent gingiva (AG) [2,3,4]. This peculiar form of gingivitis, with a chronic course, has a higher frequency of onset in the female sex—M:F ratio of about 1:4—and in most cases tends to appear after the age of 30 years, peaking between 50 and 70 years of age, with local clinical manifestations commonly linked with oral lichen planus (OLP), mucosal pemphigoid (MMP), and pemphigus vulgaris (PV) [5,6,25,26]. Oral manifestations of DG can vary in both extent and severity: one can have mild, localized lesions, which can lead to sensitivity to certain types of particularly acidic and/or spicy foods and to some particular toothpastes; or one can have more extensive, sometimes bleeding and painful lesions, which can worsen both their lifestyle and their overall oral health [18,27,29,30,31].
While painful gum and oral lesions can impede effective oral hygiene routines and raise the likelihood of dental plaque buildup, there is currently limited research available on at-home oral care for individuals with DG and its potential impact on the development or continuation of periodontal disease in this population.
In 1985 and 1990, Erpenstein and Holmstrup et al. were the first to emphasize the significance of periodontal treatment and plaque management in individuals with ulcerative or atrophic lesions associated with OLP. Specifically, Holmstrup et al. gave their patients as atraumatic an oral hygiene protocol as possible, modifying their normal habits and including—when necessary—rinses with chlorhexidine 0.12% to further improve plaque control. Patients were observed over a span of 12 months, with follow-ups approximately every 3 months; this enabled us to showcase that post-treatment monitoring plays a crucial role in treatment plan formulation, as it can both facilitate enhancements in the patient’s oral hygiene and alleviate painful symptoms concurrently. It was immediately apparent from these early studies that dental plaque control led to an elimination of the disorder’s underlying cause; nonetheless, under certain circumstances, there was a positive change in the symptoms and seriousness of gingival lesions [46].
After these two articles, few other uncontrolled studies have demonstrated similar results in a small sample of patients. Guiglia et al., in a 2007 article, reported positive clinical effects by assigning, to a group of 30 patients with OLP and suffering from DG, both a professional and autonomous oral hygiene protocol and combining it with the application of topical steroids. The protocol included oral hygiene instructions, supra- and subgingival scaling, and the administration of chlorhexidine mouthwash at a concentration of 0.12% for a duration of 7 days alongside the topical application of steroids. Patients were evaluated for about 3 months with follow-ups every month, in which the importance of proper home oral hygiene was reinforced to them [47].
In 2010, López-Jornet and Camacho-Alonso released a study with the objective of assessing the efficiency of a protocol designed to motivate oral hygiene, which included scaling, supragingival polishing of dental elements, and administration of a topical corticosteroid, in a group of 40 individuals with gingival lichen planus. The protocol included follow-ups after 4 and 8 weeks in which home oral hygiene instructions were reinforced; only when necessary were scaling and polishing of the teeth repeated. There was both a reduction in bacterial plaque and a reduction in gingival bleeding [48].
Salgado et al., in a 2013 publication, demonstrated that the reduction of dental plaque had the strongest correlation with enhancements in both the clinical presentation and symptom relief in a cohort of 20 patients suffering from OLP, specifically within the gingival tissues affected by the lesions [49].
Unlike prior research, indeed, despite the occurrence of an immune–inflammatory response associated with a chronic disease, they showed that the key to maintaining gingival health and improving painful symptomatology and gingival lesion severity is imperative to achieve adequate plaque control through the setting of proper supportive periodontal therapy. In 2015, Stone et al. were the first with their study to offer compelling proof of the significance of maintaining plaque control when treating patients with OLP gingival lesions. The randomized controlled trial involved a total of 82 patients, divided into two groups: the first received home oral hygiene instruction with sonic-type electric toothbrushes and interdental brushes; the second, on the other hand, was instructed to continue with their usual oral hygiene maneuvers. Both groups underwent follow-up visits at 4 and 20 weeks, and it was shown that the intervention group, compared with the control group, had statistically significant (p < 0.05) reductions in perceived pain, functional limitation, psychological discomfort, and plaque indices [50].
Subsequently, in 2018, Bianco et al. published a randomized controlled trial of a total sample of 32 patients diagnosed with desquamative gingivitis, in which the efficacy of intensive plaque control programs with a sonic toothbrush and with a manual toothbrush were compared: patients were divided into two separate groups, with the first receiving oral hygiene instruction with a sonic toothbrush and the second, on the other hand, receiving instruction with a manual one, and both clinical outcomes and matrix metalloproteinase (MMP) levels within the gingival crevicular fluid (GCF) were assessed. While there were no statistically significant variances observed between the utilization of a sonic toothbrush and a manual toothbrush, the group that employed the sonic toothbrush exhibited a statistically notable decrease in MMP-1 and MMP-9 levels [51].
Mergoni et al. in 2019 conducted a study of 60 patients—divided into an intervention group and a control group—with OLP with gingival lesions. This study was very similar in setting and results to the study by Stone et al. but with the difference that the intervention group was provided with manual soft brushes. The rationale for this choice was that this type of toothbrush appears to be more widespread in Italy to date [52].
Although there are—albeit few—findings in the literature inherent in the relationship between desquamative gingivitis and plaque control, to date there are still few published studies that have directed their focus on the role that periodontal pathogenic bacteria, and thus overt periodontal disease, have in influencing the course of gingival lesions ascribable to the term desquamative gingivitis. In 2017, a study conducted by Arduino et al. examined 11 bacterial species found in lesions associated with desquamative gingivitis related to oral lichen planus (OLP) and in cases of plaque-induced gingivitis. The research revealed a higher occurrence of bacterial species like Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, and Eikenella corrodens in specimens from OLP patients compared to those without OLP [53]. Further insights into the bacterial role in the pathogenesis of OLP were provided by Choi et al. who, in patients with OLP lesions, found that resident oral cavity bacteria can (1) compromise the epithelial physical barrier, (2) be internalized into epithelial cells or T cells, and (3) promote T-cell chemokine release. Furthermore, they have demonstrated that the existence of pathogens in both the lamina propria and epithelium is closely linked to elevated T-cell levels in OLP tissues, implying that intracellular bacteria can have a substantial impact on the influx of immune cells [54].
Limits of the Study and Future Perspectives
There is currently limited literature that considers home oral hygiene in patients with desquamative gingivitis and the possible role that the onset or persistence of periodontal disease may play in these individuals. In addition, most of these studies focus more on investigating the clinical manifestations of DG in patients with PLO, rather than extending the area of interest more decisively to patients with MMP and PV as well. Therefore, it would be desirable and necessary for research to focus more on investigating the role of DG in patients with periodontal disease and for the importance of the oral microbiome in disease evolution to be explored in this context.
Future research prospects could include further case-control studies, case series studies, and randomized controlled trials. Laboratory studies on cell lines could be useful to determine whether there is a possible relationship between desquamative gingivitis and periodontal disease.
5. Conclusions
Adequate control of bacterial plaque and consistent follow-up over time in patients with clinical manifestations attributable to desquamative gingivitis (DG) may play a key role in reducing painful symptoms and the extent of oral lesions while also bringing improvement in overall oral health and its impact on lifestyle. However, given the limited number of studies in the literature on the topic, the authors of this scoping review believe that further studies need to be conducted to strengthen the conclusions reported so far and to further investigate the relationship (and/or coexistence) between desquamative gingivitis, the microbial component, and overt periodontal disease. In addition, the relationship between DG and mucous membrane pemphigoid (MMP) and pemphigus vulgaris (PV) should be further investigated.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app131810535/s1. Table S1: PRISMA-ScR Checklist; Table S2: Search strategies for electronic databases; Table S3. Summary table of studies excluded in this scoping review. Table S4: JBI Critical Appraisal Checklist for Case Series; Table S5: JBI Critical Appraisal Checklist for RCTs; Table S6: Evidence of studies included in this scoping review.
Author Contributions
Conceptualization, F.S.; methodology, F.S.; software, M.P. and A.S.; validation, A.S. and F.S.; formal analysis, A.S. and F.S.; investigation, G.L.V. and F.S.; resources, F.P. and F.S.; data curation, F.P. and M.P.; writing—original draft preparation, G.L.V., F.P. and M.P.; writing—review and editing, G.L.V., F.P. and M.P.; visualization, A.S. and F.S.; supervision, A.S. and F.S.; project administration, F.S.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Upon request to the corresponding author, the data are available for use.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tomes, J.; Tomes, G. Dental Surgery, 4th ed.; J and A Churchill Ltd.: London, UK, 1894. [Google Scholar]
- Prinz, H. Chronic diffuse desquamative gingivitis. Dent Cosm. 1932, 74, 332–333. [Google Scholar]
- McCarthy, F.P.; McCarthy, P.L.; Shklar, G. Chronic desquamative gingivitis: A reconsideration. Oral Surg. Oral Med. Oral Pathol. 1960, 13, 1300–1313. [Google Scholar] [CrossRef]
- Glickman, I.; Smulow, J.B. Chronic desquamative gingivitis—Its nature and treatment. J. Periodontol. 1964, 35, 397–405. [Google Scholar] [CrossRef]
- Russo, L.L.; Fierro, G.; Guiglia, R.; Compilato, D.; Testa, N.F.; Muzio, L.L.; Campisi, G. Epidemiology of desquamative gingivitis: Evaluation of 125 patients and review of the literature. Int. J. Dermatol. 2009, 48, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Leao, J.; Ingafou, M.; Khan, A.; Scully, C.; Porter, S. Desquamative gingivitis: Retrospective analysis of disease associations of a large cohort. Oral Dis. 2008, 14, 556–560. [Google Scholar] [CrossRef]
- Alrashdan, M.S.; Cirillo, N.; McCullough, M. Oral lichen planus: A literature review and update. Arch. Dermatol. Res. 2016, 308, 539–551. [Google Scholar] [CrossRef]
- Li, C.-L.; Ren, X.-M.; Fang, X.; Luo, H.-Y.; Hua, H. Clinical, histological and direct immunofluorescence features in oral mucosal patches striae diseases with malignant potential. J. Dent. Sci. 2023, 18, 1008–1015. [Google Scholar] [CrossRef]
- Rotaru, D.; Chisnoiu, R.; Picos, A.M.; Picos, A.; Chisnoiu, A. Treatment trends in oral lichen planus and oral lichenoid lesions (Review). Exp. Ther. Med. 2020, 20, 198. [Google Scholar] [CrossRef]
- El-Howati, A.; Thornhill, M.H.; Colley, H.E.; Murdoch, C. Immune mechanisms in oral lichen planus. Oral Dis. 2023, 29, 1400–1415. [Google Scholar]
- Payeras, M.R.; Cherubini, K.; Figueiredo, M.A.; Salum, F.G. Oral lichen planus: Focus on etiopathogenesis. Arch. Oral Biol. 2013, 58, 1057–1069. [Google Scholar] [CrossRef]
- Farhi, D.; Dupin, N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: Facts and controversies. Clin. Dermatol. 2010, 28, 100–108. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Warnakulasuriya, S.; González-Ruiz, I.; González-Ruiz, L.; Ayén, Á.; Lenouvel, D.; Ruiz-Ávila, I.; Ramos-García, P. Worldwide Prevalence of Oral Lichen Planus: A Systematic Review and Meta-analysis. Oral Dis. 2020, 4, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, W.C.; Chi, A.C.; Neville, B.W. Common oral lesions: Part I. Superficial mucosal lesions. Am. Fam. Physician 2007, 75, 501–507. [Google Scholar] [PubMed]
- Mignogna, M.D.; Russo, L.L.; Fedele, S. Gingival involvement of oral lichen planus in a series of 700 patients. J. Clin. Periodontol. 2005, 32, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.P.; Yu-Fong Chang, J.; Wang, Y.P.; Wu, Y.H.; Lu, S.Y.; Sun, A. Oral lichen planus—Differential diagnoses, serum autoantibodies, hematinic deficiencies, and management. J. Formos. Med. Assoc. 2018, 117, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Carrozzo, M.; Porter, S.; Mercadante, V.; Fedele, S. Oral lichen planus: A disease or a spectrum of tissue reactions? Types, causes, diagnostic algorhythms, prognosis, management strategies. Periodontol. 2000 2019, 80, 105–125. [Google Scholar] [CrossRef]
- Boccellino, M.; Di Stasio, D.; Romano, A.; Petruzzi, M.; Lucchese, A.; Serpico, R.; Frati, L.; Di Domenico, M. Lichen planus: Molecular pathway and clinical implications in oral disorders. J. Biol. Regul. Homeost. Agents 2018, 32, 135–138. [Google Scholar]
- Rashid, H.; Lamberts, A.; Diercks, G.F.H.; Pas, H.H.; Meijer, J.M.; Bolling, M.C.; Horváth, B. Oral lesions in autoimmune bullous diseases: An overview of clinical characteristics and diagnostic algorithm. Am. J. Clin. Dermatol. 2019, 20, 847–861. [Google Scholar] [CrossRef]
- Schmidt, E.; Zillikens, D. Pemphigoid diseases. Lancet 2013, 381, 320–332. [Google Scholar] [CrossRef]
- Tricamo, M.B.; Rees, T.D.; Hallmon, W.W.; Wright, J.M.; Cueva, M.A.; Plemons, J.M. Periodontal status in patients with gingival mucous membrane pemphigoid. J. Periodontol. 2006, 77, 398–405. [Google Scholar] [CrossRef]
- Rehman, A.; Huang, Y.; Wan, H. Evolving Mechanisms in the Pathophysiology of Pemphigus Vulgaris: A Review Emphasizing the Role of Desmoglein 3 in Regulating p53 and the Yes-Associated Protein. Life 2021, 11, 621. [Google Scholar] [CrossRef] [PubMed]
- Nili, A.; Farid, A.S.; Asgari, M.; Tavakolpour, S.; Mahmoudi, H.; Daneshpazhooh, M. Current status and prospects for the diagnosis of pemphigus vulgaris. Expert Rev. Clin. Immunol. 2021, 17, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Pollmann, R.; Schmidt, T.; Eming, R.; Hertl, M. Pemphigus: A comprehensive review on pathogenesis, clinical presentation and novel therapeutic approaches. Clin. Rev. Allergy Immunol. 2018, 54, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Bhat, R.M.; Madhumita, M.; Jaganathan, P. Desquamative gingivitis in dermatological disorders. Indian J. Dermatol. Venereol. Leprol. 2021, 87, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Maderal, A.D.; Lee Salisbury, P., 3rd; Jorizzo, J.L. Desquamative gingivitis: Diagnosis and treatment. J. Am. Acad. Dermatol. 2018, 78, 851–861. [Google Scholar] [CrossRef]
- Sciuca, A.M.; Toader, M.P.; Stelea, C.G.; Maftei, G.A.; Ciurcanu, O.E.; Stefanescu, O.M.; Onofrei, B.-A.; Popa, C. Desquamative Gingivitis in the Context of Autoimmune Bullous Dermatoses and Lichen Planus—Challenges in the Diagnosis and Treatment. Diagnostics 2022, 12, 1754. [Google Scholar] [CrossRef]
- Tofan, E.; Părlătescu, I.; Ţovaru, Ş.; Nicolae, C.; Preda, A.; Funieru, C. Desquamative Gingivitis—A Clinicopathological Review. Curr. Health Sci. J. 2018, 44, 331–336. [Google Scholar]
- Cheng, S.; Kirtschig, G.; Cooper, S.; Thornhill, M.; Leonardi-Bee, J.; Murphy, R. Interventions for erosive lichen planus affecting mucosal sites. Cochrane Database Syst. Rev. 2012, 2, CD008092. [Google Scholar] [CrossRef]
- Erpenstein, H. Periodontal and prosthetic treatment in patients with oral lichen planus. J. Clin. Periodontol. 1985, 12, 104–112. [Google Scholar] [CrossRef]
- Lim, H.-D.; Kang, J.-K.; Lee, Y.-M.; Shim, Y.-J. The correlation between desquamative gingivitis associated-diseases and plaque-induced periodontal disease. J. Oral Med. Pain 2015, 40, 135–139. [Google Scholar] [CrossRef]
- Hampelska, K.; Jaworska, M.M.; Babalska, Z.Ł.; Karpiński, T.M. The Role of Oral Microbiota in Intra-Oral Halitosis. J. Clin. Med. 2020, 9, 2484. [Google Scholar] [CrossRef]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontol. 2000 2020, 83, 14–25. [Google Scholar] [CrossRef] [PubMed]
- De Geest, S.; Laleman, I.; Teughels, W.; Dekeyser, C.; Quirynen, M. Periodontal diseases as a source of halitosis: A review of the evidence and treatment approaches for dentists and dental hygienists. Periodontol. 2000 2016, 71, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Cassiano, L.S.; Abdullahi, F.; Leite, F.R.M.; López, R.; Peres, M.A.; Nascimento, G.G. The association between halitosis and oral-health-related quality of life: A systematic review and meta-analysis. J. Clin. Periodontol. 2021, 48, 1458–1469. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.L.; Guiglia, R.; Pizzo, G.; Fierro, G.; Ciavarella, D.; Muzio, L.L.; Campisi, G. Effect of desquamative gingivitis on periodontal status: A pilot study. Oral Dis. 2010, 16, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontol. 2000 2022, 89, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, E.; Conti, P.; Carinci, F.; Lauritano, D.; Theoharides, T. IL-1 Superfamily Members and Periodontal Diseases. J. Dent. Res. 2020, 99, 1425–1434. [Google Scholar] [CrossRef]
- Nunes, G.P.; Pirovani, B.O.; Nunes, L.P.; Silva, A.N.A.; Morábito, M.J.S.D.; Nunes-Júnior, N.A.; Delbem, A.C.B.; Ferrisse, T.M. Does oral lichen planus aggravate the state of periodontal disease? A systematic review and meta-analysis. Clin. Oral. Investig. 2022, 26, 3357–3371. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Sanadi, R.; Khandekar, P.; Chaudhari, S.; Javali, M.; Gurav, N. Association of periodontal disease with oral lichen planus: A systematic review and meta analysis. J. Oral Maxillofac. Pathol. 2023, 27, 173–180. [Google Scholar] [CrossRef]
- Garcia-Pola, M.; Rodriguez-Lopez, S.; Fernanz-Vigil, A.; Bagan, L.; Garcia-Martin, J. Oral hygiene instructions and professional control as part of the treatment of desquamative gingivitis. Systematic review. Med. Oral. Pathol. Oral. Cir. Bucal. 2019, 24, e136–e144. [Google Scholar] [CrossRef]
- Albaghli, F.; Zhou, Y.; Hsu, C.-C.; Nibali, L. The effect of plaque control in the treatment of Oral Lichen Planus with gingival manifestations: A Systematic Review. Community Dent. Health 2021, 28, 112–118. [Google Scholar]
- Peacock, M.; Arce, R.; Cutler, C. Periodontal and other oral manifestations of immunodeficiency diseases. Oral Dis. 2017, 23, 866–888. [Google Scholar] [CrossRef]
- Jascholt, I.; Lai, O.; Zillikens, D.; Kasperkiewicz, M. Periodontitis in oral pemphigus and pemphigoid: A systematic review of published studies. J. Am. Acad. Dermatol. 2017, 76, 975–978. [Google Scholar] [CrossRef]
- Holmstrup, P.; Schiøtz, A.W.; Westergaard, J. Effect of dental plaque control on gingival lichen planus. Oral Surg. Oral Med. Oral Pathol. 1990, 69, 585–590. [Google Scholar] [CrossRef]
- Guiglia, R.; Di Liberto, C.; Pizzo, G.; Picone, L.; Muzio, L.L.; Gallo, P.D.; Campisi, G.; D’Angelo, M. A combined treatment regimen for desquamative gingivitis in patients with oral lichen planus. J. Oral Pathol. Med. 2007, 36, 110–116. [Google Scholar] [CrossRef]
- López-Jornet, P.; Camacho-Alonso, F. Application of a motivation-behavioural skills protocol in gingival lichen planus: A short-term study. J. Periodontol. 2010, 8, 1449–1454. [Google Scholar] [CrossRef]
- Salgado, D.S.; Jeremias, F.; Capela, M.V.; Onofre, M.A.; Massucato, E.M.S.; Orrico, S.R.P. Plaque control improves the painful symptoms of oral lichen planus gingival lesions. A short-term study. J. Oral Pathol. Med. 2013, 42, 728–732. [Google Scholar] [CrossRef]
- Stone, S.J.; Heasman, P.A.; Staines, K.S.; McCracken, G.I. The impact of structured plaque control for patients with gingival manifestations of oral lichen planus: A randomized controlled study. J. Clin. Periodontol. 2015, 42, 356–362. [Google Scholar] [CrossRef]
- Bianco, L.; Romano, F.; Maggiora, M.; Bongiovanni, L.; Guzzi, N.; Curmei, E.; Arduino, P.G.; Aimetti, M. Effect of sonic versus manual supervised toothbrushing on both clinical and biochemical profiles of patients with desquamative gingivitis associated with oral lichen planus: A randomized controlled trial. Int. J. Dent. Hyg. 2019, 17, 161–169. [Google Scholar] [CrossRef]
- Mergoni, G.; Magnani, V.; Goldoni, M.; Vescovi, P.; Manfredi, M. Effects of oral healthcare motivation in patients with gingival oral lichen planus: A randomized controlled trial. Oral Dis. 2019, 25, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Arduino, P.; Broccoletti, R.; Sciannameo, V.; Scully, C. A practical clinical recording system for cases of desquamative gingivitis. Br. J. Dermatol. 2017, 177, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Kim, Y.; Yoon, H.-J.; Baek, K.J.; Alam, J.; Park, H.K.; Choi, Y. The presence of bacteria within tissue provides insights into the pathogenesis of oral lichen planus. Sci. Rep. 2016, 6, 29186. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


