Abstract
Distal occlusion is one of the most common dentoalveolar anomalies and can be the reason for the obstructive sleep apnea (OSA) syndrome development among children. The aim of the study was to investigate the relationship between cephalometric and OSA parameters in the pediatric population. Methods: The cohort study included 39 children with OSA symptoms. Orthodontic examination consisted of a cephalometric analysis of 39 linear and angular variables. Patients underwent a sleep diagnostic study. Statistical analysis was performed using SPSS 19.0.0. Results: Of the general sample, 53.8% were mouth breathers and 46.2% had a mixed type of breathing. Moreover, 30.8% of patients had bruxism. The mean apnea-hypopnea index and oxygen desaturation index were 4.6/h and 3.9/h, respectively. A 1.06 times increase in the SNA index indicated the anterior position of the upper jaw. The MnPLSN° exceeded the norm by 1.3 times, which indicated the posterior position of the lower jaw and vertical type of the growth. An increase in ANSPNSSPT° by 1.1 times indicated an inclination of the upper jaw in the posterior position and a narrowing of the nasal passages. Patients with pediatric OSA had a significantly smaller lower airway space and MPH parameter. Conclusion: Systematic orthodontic monitoring of children with pediatric OSA is important for diagnosis and timely treatment.
1. Introduction
Distal occlusion is one of the most common dentoalveolar anomalies, accounting for 30–50% of other anomalies of the dentoalveolar system [1,2]. In the classification of dentoalveolar abnormalities, distal occlusion belongs to class II anomalies, depending on the location of the upper anterior teeth. Class II is divided into two subclasses: the first is characterized by a vestibular deviation of the anterior teeth of the upper jaw with crowding or tremas. The second subclass is typical retrusion of the upper jaw incisors. It is characterized not only by morphological and functional disorders of the dentoalveolar system which cause aesthetic disturbances in the harmony of the facial part of the cranium, but can also be the cause for obstructive sleep apnea development among children (pediatric OSA) [3,4,5,6]. Among the multiple risk factors, the presence of alterations in craniofacial growth has recently been associated with OSA among children [7]. The most frequent craniofacial alterations reported about OSA among children are retrognathia, midface hypoplasia, upper jaw contraction, relative macroglossia, and anterior open bite [8,9,10,11]. Pediatric OSA is a common sleep disorder characterized by repetitive obstructions of the upper airways. This disrupts the normal architecture of sleep and causes intermittent hypoxemia, which is associated with excessive daytime sleepiness and an increased incidence of cardiovascular, neuropsychiatric, and endocrinological disorders [12,13,14]. The sleep bruxism (SB) problem seems to be significant due to the high prevalence of OSA and SB. According to the American Academy of Sleep Medicine (AASM), bruxism is a repetitive jaw-muscle activity characterized by clenching or grinding of the teeth and/or by bracing or thrusting of the mandible [15]. Factors such as age, sex, higher body mass index (BMI), tonsils, adenoids, alcohol and sedative intake are well known to worsen the severity of OSAS [16,17,18,19,20]. In addition to the risk factors for pediatric OSA, more scientific articles have been published about the role of body position during sleep in the case of pediatric OSA and effective approaches to avoid those sleep positions that worsen the severity of pediatric OSA [21]. Patients with pediatric OSA are more likely to experience obstructive events in the supine position than in other non-lying positions. The body position also affects not only the frequency but also the duration of sleep apnea. It is generally known that patients with positional pediatric OSA are younger and less obese; implying the possibility that such patients may have a less severe respiratory phenotype than non-positional patients [22,23]. Taking into consideration the authors’ debate about the fact that posterior upper airway obstruction causes abnormal facial growth, it will be useful to have a reliable diagnostic method to assess the treatment need. However, the diagnosis of posterior airway obstruction is complicated because of the observation difficulties connected with localization [24,25,26,27]. Several tools were used for diagnosis, including nasal breathing and airflow resistance testing, apnea-hypopnea index (AHI) calculation, nasoendoscopy, lateral cephalometry, and 3D imaging [28,29]. Each has positive and negative properties. However, there is no consensus on the gold standard for posterior airway obstruction diagnosis. Most often, nasal airway patency is not clearly studied in dental offices and a referral to an ENT doctor is required to establish it. A simple, economical, affordable, and most commonly used method for diagnosing upper airway obstruction is the lateral cephalogram [30].
This study aimed to investigate the relationship between cephalometric parameters and the severity of pediatric OSA.
2. Materials and Methods
The study was conducted in the period from 2017 to 2022 at “Somnus” Neurology Clinic and University Dental Polyclinic N1 of Yerevan State Medical University after Mkhitar Heratsi (YSMU).
The cohort study included 39 children (71.8% boys and 28.2% girls, mean age 9.45 years, standard deviation 1.86, range 7–13 years) with sleep-related breathing disorder symptoms. The research was conducted by the Helsinki Declaration and ethical clearance has been guaranteed from the Ethical committee of the YSMU (N. 10-15; 19 June 2014). Participation in this study was voluntary and participants signed an informed consent. The STROBE guides were followed during preparation of this article.
Inclusion criteria
- Nasal breathing disturbance
- Dentofacial anomalies (malocclusion)
Exclusion criteria
- Bad habits (tongue, lip, finger sucking, subject sucking)
- Refusal of the examination (conducting a cephalometric or polysomnographic/polygraphic study),
- Patients undergoing orthodontic treatment at the time of recruitment
Orthodontic examination consisted of a preliminary assessment of the maxillofacial features onsite and referral for cephalometric X-ray examination using a Planmeca ProMax Type 3D+ (Planmeca OY, Helsinki, Finland) adjusted to 12 mA, 90 kV and a time exposure of 0.30 ms and analysis of the results using Romexis Viewer 5.4.1. software (Planmeca OY, Helsinki, Finland). All children were oriented in a natural head position (NHP). Cephalometric analysis was performed manually twice by different others to exclude the factors of human mistakes, and included linear and angular parameters.
For the sagittal analysis, SNA°, SNB°, and ANB° angles were used to determine the maxilla and mandible positions relative to the anterior part of the cranial base and the corresponding skeletal class. For the vertical analysis, we determined the ArGoGn° and Ba-S-PNS° angles. The position of the hyoid bone was assessed using AH-C3 (mm). It shows the horizontal distance from AH to C3, where AH is the most anterior and superior point on the body of the hyoid bone and represents the underside of the tongue, and C3 is the third cervical vertebra. EbTt is the distance from the tip of the epiglottis to the tip of the tongue. SNP-Eb is the distance between the posterior nasal spine and the tip of the epiglottis. The MnPl value is the lower jaw length in mm. The MxPl value is the upper jaw length in mm. The size of the soft tissues and oropharynx was measured by PAS1, PAS2 (pharyngeal width measured between the posterior pharyngeal wall at its narrowest point and the most anterior airway point on the soft palate), PAS3, and PAS min (lower airway space between the posterior inferior pharyngeal wall and the most anterior airway point on the tongue) [31,32,33].
Patients were then referred to the sleep disorders center for a diagnostic study of sleep using portable respiratory polygraphy (Embletta X10) or night polysomnography (Embla N7000). Sleep parameters were calculated using Somnologica 5.1.1. software. Immediately before the PSG examination, a comprehensive physical examination was performed, which included anthropometric measurements such as height and weight to calculate body mass index. SB was assessed by bilateral masseter electromyography (EMG), and the audio and video evaluation bruxism episodes were scored according to the standards of the AASM in three forms: phasic, tonic, and mixed. For the consideration of SB, EMG bursts should not be separated by >3 s to be considered part of the same episode, and EMG activity has to be at least twice the amplitude of the background EMG [15]. The apnea-hypopnea index (AHI), oxygen desaturation index (ODI), average saturation, obstructive sleep apnea index (OSAI), AHI in the supine and non-supine positions, central sleep apnea index (CSAI), nonrapid eye movement sleep (NREM) AHI, rapid eye movement sleep (REM) AHI were all measured and recorded. Based on polysomnographic data, the subjects were further divided into subgroups according to the severity of pediatric OSA [34,35,36]. Pediatric OSA among the children was diagnosed and classified according to the following classification:
mild, i.e., when the AHI is between 1 and 4 per hour and the minimum saturation index is between 85% and 92%; or
moderate-to-severe pediatric OSA, defined as being when the AHI is above 4 and the minimum saturation index is less than 85% depending on the overall analysis of polysomnography and clinical evaluation [37,38,39].
Pediatric OSA stratification for all children is described in Table 1.

Table 1.
Classification of SDB severity by age group using apnea-hypopnea index (ICSD-3, 2014 and Katz & Marcus 2014 [37,39]).
The participants who had in-laboratory polysomnographic study were described for sleep phases and stages and presence of sleep bruxism according to the rhythmic masticatory muscle activity pattern appearing on the chin EMG correlated with an audio signal.
Statistical analysis. We performed statistical analysis using the Statistical Package for Social Sciences, SPSS Statistics for Windows, version 19.0.0.0 (Armonk, NY, USA: IBM Corp). Categorical variables were described using absolute and relative frequencies and continuous variables were described using summary statistics (mean, standard deviation, minimal and maximal indices). Comparisons between continuous variables in the mild, moderate and severe OSA groups were performed using Student’s t-test. Ratios of each variable of interest were estimated with the respective 95%. Tests were performed with a 5% significance level. Pearson’s correlation test was used for statistical analysis. Cephalometric and PSG parameters were evaluated statistically to select and validate the cephalometric parameters reflecting OSA severity.
3. Results
3.1. Cephalometric Parameters
Table 2 shows the cephalometric analysis of 39 linear and angular variables. All parameters were statistically significant (p < 0.001).

Table 2.
Descriptive statistics of cephalometric analysis.
According to the data obtained, the SNA parameter, which characterizes the position of the upper jaw relative to the anterior cranial base, was 87.5° ± 0.23; the ANB angle, on average, and was in the range of 9.2° ± 0.27. The SNB was 74.1° ± 0.33. The MnPl value was 1.24 times less and amounted to 58.65 ± 0.93, while the MxPl value was practically within the normal range –49.26 ± 0.72. The SNPP angle (palatal plane) exceeded the norm twice and when averaged was 15.59° ± 0.51. The angle of the lower jaw relative to the anterior cranial base (MnPLSN) was 41.4° ± 0.35, exceeding the norm by 1.3 times.
The L1Nb angle averaged 21.7° ± 1.16 and was indicative of Angle’s class II subclass 2. The U1NA indicator was calculated in mm and in degrees: in mm the indicator was within the minimum normal range and amounted to 3.09 mm ± 0.64; when calculated in degrees it was less than the norm –18.22° ± 1.2.
3.2. Polysomnographic Characteristics
After an initial quality check of the data, the analysis showed that the common and routinely used polysomnographic measures covered a large data set that could be stored and thus allow us to proceed with the subsequent unbiased analysis. The mean AHI and ODI values were 4.6/h and 3.9/h (p < 0.001), respectively.
In the sample, 46.2% had mild OSA, 25% had moderate OSA, and 10.3% had severe OSA. The supine AHI was 5.67/h ± 8.8, while the non-supine AHI was 3.695/h ± 5.77; the central apnea index was 0.287/h ± 0.3071 (p < 0.001). The index of obstructive sleep apnea was 1.4/h ± 4.45 (p = 0.043). The saturation averaged at 96.12% ± 1.5 (p < 0.05). Two children (5.13%) had average saturation below 92%.
When analyzing the questionnaire parameters, a significant positive correlation was revealed between the weight of the examined patients and the BMI (r = 0.844 (p < 0.01)). The average weight was 36.03 kg ± 13.2. The average height was 1.31 m ± 0.15. According to the survey, 53.8% were mouth breathers; the remaining 46.2% had a mixed type of breathing. The pulse averaged at 78.8 bpm ± 9.58 (p < 0.001).
Among patients with OSA, 66.7% had in-hospital full polysomnographic studies. The data obtained revealed the following parameters: the average sleep time of patients was 443.65 ± 59.98 min and the sleep efficiency averaged 90.87% ± 8.8% (p < 0.001).
Of the polysomnographically examined children, 30.8% had sleep bruxism. REM sleep averaged at 19.23% ± 6.3, NREM1 was 4.01% ± 4.64, NREM2 sleep phase was 35.5% ± 11.1, and NREM3 was 41.25% ± 12.27 (Table 3).

Table 3.
Data of polysomnographic examination in a hospital.
3.3. Correlations of Polysomnographic and Cephalometric Parameters
In this age group of children, Pearson’s correlation analysis was carried out between the polygraphic and cephalometric parameters. BMI was significantly correlated with average saturation, r = −0.317 (p = 0.049).
Between angle measurements, a notable positive correlation with the AHI was observed for the angle of the mandible relative to the base of the skull (MnPLSN); r = 0.319 (p = 0.048, Figure 1), while the SNPP angle (palatal plane) showed no relationship. The angle MnPLSN had a moderate correlation with the AHI in the supine position, but this correlation was not statistically significant (r = 0.308, p = 0.057).
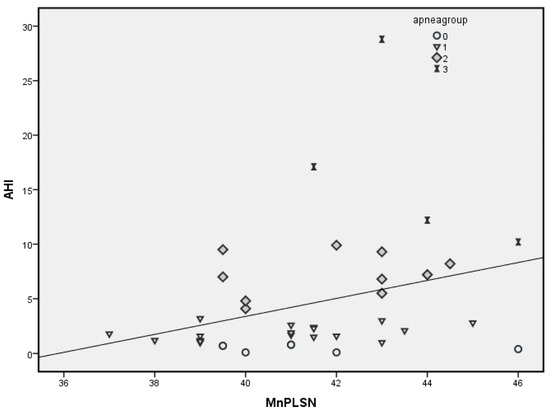
Figure 1.
Correlation of AHI and MnPLSN parameter.
The BaSPNS parameter (the angle between the foramen magnum (Ba), the sella turcica (S), and the posterior nasal spine (PNS) which characterizes the bony boundaries of the nasopharyngeal space) was reduced compared to the norm (137.05 ± 0.25), but no correlation was found for this parameter with the AHI. Average oxygen saturation was associated with maximum uvula thickness (MSPT), r = −0.461 (p < 0.01).
The supine AHI position was negatively moderately correlated with MnPl and MxPl: r = −0.319 (p = 0.048) and r = −0.354 (p = 0.027), respectively.
A significant negative correlation was observed between the anteroposterior pharyngeal size index at the PAS2 level and the AHI and supine AHI (r = −0.338 (p = 0.036) and r = −0.318 (p = 0.048), respectively). PAS2 also correlated with oxygen desaturation index (ODI): r = −0.395 (p = 0.013).
A significant negative correlation was also observed between the anteroposterior pharyngeal size index at the level of the mandibular plane: PAS3 and the AHI and supine AHI (r = −0.386 (p = 0.015) and r = −0.317 (p = 0.05), respectively). PAS3 also correlated with the ODI: r = −0.366 (p = 0.022).
The SNA angle was negatively moderately correlated with the PAS3 indices and PAS min (r = −0.426 (p = 0.007) and r = −0.359 (p = 0.025), respectively) as well as with the MPH parameter (distance from the plane of the base of the lower jaw to the hyoid bone): r = −0.394 (p = 0.013).
Among children undergoing study in a hospital, REM sleep had significant positive correlation with AHI (r = 0.428; p = 0.029); correlations were also observed between MSPT and average saturation (r = −0.74; p < 0.01) and AHI (r = 0.385; p = 0.05).
The ad1 cephalometric parameter was positively correlated with PAS1 and PAS min (r = 0.747 (p < 0.001) and r = 0.39 (p = 0.049), respectively) in children who underwent polysomnographic study in a hospital. Parameter ad2 was positively correlated with PAS1 (r = 0.642 (p < 0.001)), PAS2 (r = 0.452 (p = 0.02)), PAS3 (r = 0.427 (p = 0.03)), and PAS min (r = 0.479 (p = 0.013)) parameters.
4. Discussion
This is the first study to analyze polysomnographic and cephalometric characteristics and contributors on positional and REM dependency in patients with mixed dentition and varying degrees of severity of OSA. A correlation analysis was carried out between the parameters of the sleep cycles (REM sleep) and the airway space at the level of PAS1, PAS2, and MPH (the distance between the mandibular plane and the hyoid).
According to night polygraphy data, the subjects were divided into subgroups based on the severity of pediatric OSA. Snoring was detected in 7 children, severe OSA was diagnosed among 4 children, moderate OSA among 10 children, and mild OSA among 18 children.
During comparison of polysomnographic parameters in children with different severities of OSA, significant differences in AHI, ODI, and non-supine AHI were revealed in almost all groups.
There were no differences in these parameters between subgroups of children with severe OSA and moderate OSA.
The REM sleep was significantly higher in the subgroup of children with severe OSA compared with patients with mild OSA.
Cephalometric parameters showed no significant differences between the groups of patients with snoring and moderate OSA, and mild and moderate OSA. However, between groups of patients with snoring and mild OSAS, there were significant differences between MnPl and MxPl. Significant differences were observed between BaSN values (the angle of the posterior cranial base) between the subgroups of patients with moderate OSAS and mild OSAS with the group of patients with severe OSAS and ANSPNSSSPT in the same subgroups.
In the comparison of cephalometric parameters, a significant difference between PAS 2 (posterior airway space at the level of the occlusal plane), PAS 3 (anterior-posterior pharyngeal size at the level of the mandibular plane), SNA, MnPLSN, and ad2 (distance from the PNS point to the nearest point of the adenoid tissue) was observed in subgroups of children with mild OSA and severe OSA. The difference between PAS 2, PAS 3, and ad2 was also observed between groups of children with mild OSAS and severe OSAS. Obtained data were comparable with the study results of Mislik B. et al. [26] and Pirilä-Parkkinen K. et al. [27].
The PAS1, PAS2, and ad2 parameters were negatively correlated with the REM sleep phase, and ad1 was positively correlated with NREM1 (the parameter correlation was statistically significant).
A moderate correlation was found between the MPH (the distance between the mandibular plane and the hyoid) and the NREM2 and NREM3 sleep stages (r = −0.457; p = 0.019; r = 0.409; p = 0.038, respectively).
A 1.06 times increase in the SNA index indicated the anterior position of the upper jaw. Angle’s class II skeletal form was also evidenced by an increase in the ANB angle by almost 2.3 times [8]. The SNB index was 1.05 times less than normal and indicated a more posterior position of the lower jaw. The angle of the lower jaw relative to the base of the skull exceeded the norm by 1.3 times, which also indicated the posterior position of the lower jaw and the vertical type of growth of the facial skull. The decrease in the U1NA angle was almost 1.2 times. According to the literature, a decrease in this angle below 22° also indicates II class 2nd subclass according to the angle. An increase in the ANSPNSSPT angle by 1.1 times from the norm indicated an inclination of the upper jaw in the posterior position and a decrease in the nasal passages.
The MSPT data indicated a normal soft palate thickness. A decrease in EbTt by 1.4 times indicated a decrease in the airway space. Thus, patients with pediatric OSA had a significantly smaller lower airway space and MPH parameter.
The main limitation of this study was the small number of participants in each group. The race of participants was Armenian.
5. Conclusions
OSA is closely associated with anatomical deformities of the craniofacial anomalies and pharyngeal soft tissues. Lateral cephalometry may reveal important predictors of pediatric OSA among children. Our results showed that patients with pediatric OSA have a significantly longer vertical airway length, a retrognathic mandible, a normal uvula thickness, and an elongated average face length. Thus, it is important to have pharynx measurement data. The position of the hyoid bone tended to be located posteriorly. The data obtained showed a moderate correlation of the main parameters of pediatric OSA with many cephalometric parameters, such as the vertical length of the airways and the distance between the epiglottis and the highest point of the dorsum of the tongue. The significant role of dentoalveolar measurements showing the distal position of the mandible has clinically useful correlations in pediatric OSA. We can suggest an important role of various craniofacial measurements in pediatric OSA. Pediatric OSA affects the development of the craniofacial skeleton in children, so systematic orthodontic monitoring of patients with pediatric OSA is necessary. Taking standardized cephalometric radiographs can be helpful in determining appropriate treatment. It is mandatory to measure cephalometric parameters which show high correlation in accounting for anomalies during orthodontic training. This will help to define and prevent obstructive sleep apnea and provide effective treatment for, and systematic orthodontic monitoring of, patients with pediatric OSA.
Author Contributions
G.E.M.: Conceptualization, investigation, formal analysis, data curation, writing—original draft, visualization; S.G.K.: conceptualization, formal analysis, data curation, writing—review and editing; M.M.M.: conceptualization, investigation, writing—review and editing, supervision; H.Y.T.-P.: conceptualization, methodology, writing—original draft preparation; A.G.H.: writing—original draft preparation, writing—review and editing, methodology; M.E.M.: investigation, methodology, writing—review and editing; I.F.V.: data curation, formal analysis, writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of YSMU (N.10-15, 19 June 2014).
Informed Consent Statement
Participation in this study was voluntary and participants signed an informed consent.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. The data are not publicly available due to privacy.
Acknowledgments
To all children who participated in this study. To the staff of “Somnus” Neurology Clinic, who performed all polysomnography exams.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, Y.; Zhao, T.; Ngan, P.; Qin, D.; Hua, F.; He, H. The dental and craniofacial characteristics among children with obstructive sleep apnoea: A systematic review and meta-analysis. Eur. J. Orthod. 2023, 45, 346–355. [Google Scholar] [CrossRef]
- Pawar, R.O.; Mane, D.R.; Patil, C.h.D.; Bhalerao, S.n.V.; Parkar, A.F.; Agarwa, S.h. To Check the Reliability of Various Cephalometric Parameters used for Predicting the Type of Malocclusions and Growth Patterns. J. Pharm. Bioallied Sci. 2022, 14 (Suppl. S1), S808–S811. [Google Scholar] [CrossRef] [PubMed]
- Behrents, R.G.; Shelgikar, A.V.; Conley, R.S.; Flores-Mir, C.; Hans, M.; Levine, M.; McNamara, J.A.; Palomo, J.M.; Pliska, B.; Stockstill, J.W.; et al. Obstructive sleep apnea and orthodontics: An American Association of Orthodontists White Paper. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, R.; Krishnaswamy, N.; Ravikumar, A. Evaluation of craniofacial morphology in patients with obstructive sleep apnea using lateral cephalometry and dynamic MRI. Indian J. Dent. Res. 2011, 22, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Bittar, R.F.; Duailibi, S.E.; Prado, G.P.R.; Ferreira, L.M.; Pereira, M.D. Cephalometric measures correlate with polysomnography parameters in individuals with midface deficiency. Sci. Rep. 2021, 11, 7949. [Google Scholar] [CrossRef] [PubMed]
- Juliano, M.L.; Machado, M.A.C.; de Carvalho, L.B.C.; Zancanella, E.; Santos, G.M.S.; Prado, L.B.F.D.; Prado, G.F.D. Polysomnographic Findings are Associated with Cephalometric Measurements in Mouth-Breathing Children. J. Clin. Sleep Med. 2009, 5, 554–561. [Google Scholar] [CrossRef]
- Di Francesco, R.; Monteiro, R.; Paulo, M.L.; Buranello, F.; Imamura, R. Craniofacial morphology and sleep apnea in children with obstructed upper airways: Differences bet-ween genders. Sleep Med. 2012, 13, 616–620. [Google Scholar] [CrossRef]
- Autar, R.; Chauhan, A.; Pradhan, K.L.; Yadav, V. Comparison of pharyngeal airway dimension, tongue and hyoid bone position based on ANB angle. Natl. J. Maxillofac. Surg. 2015, 6, 42–51. [Google Scholar] [CrossRef]
- Hultcrantz, E.; Tideström, B.L. The development of sleep disordered breathing from 4 to 12 years and dental arch morphology. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1234–1241. [Google Scholar] [CrossRef]
- Neagos, A.; Dumitru, M.; Neagos, C.M.; Mitroi, M.; Vrinceanu, D. Correlations between Morphology, the Functional Properties of Upper Airways, and the Severity of Sleep Apnea. J. Clin. Med. 2022, 11, 5347. [Google Scholar] [CrossRef]
- Tanellari, O.; Toti, C.; Baruti Papa, E.; Ghanim, S.; Savin, C.; Romanec, C.; Balcoș, C.; Zetu, I.N. The Link between Obstructive Sleep Apnea Syndrome and Cephalometric Assessment of Upper Airways and Hyoid Bone Position. Medicina 2022, 58, 1213. [Google Scholar] [CrossRef] [PubMed]
- Cauby, M.C., Jr.; Araújo, V.M.A.; Estanislau, I.M.G.; Candéa, J.d.J.; Moro, A.; de Bruin, V.M.S.; Bruin, P.F.C.; Fonteles, C.S. A retrospective study of the influence of obesity on polysomnography and cephalometric parameters in males with obstructive sleep apnea. Cranio® 2021, 1–7. [Google Scholar] [CrossRef]
- Khositseth, A.; Chokechuleekorn, J.; Kuptanon, T.; Leejakpai, A. Rhythm disturbances in childhood obstructive sleep apnea during apnea-hypopnea episodes. Ann. Pediatr. Cardiol. 2013, 6, 39–42. [Google Scholar] [CrossRef]
- Thomas, S.; Patel, S.; Gummalla, P.; Tablizo, M.A.; Kier, C. You Cannot Hit Snooze on OSA: Sequelae of Pediatric Obstructive Sleep Apnea. Children 2022, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Martynowicz, H.; Gac, P.; Brzecka, A.; Poreba, R.; Wojakowska, A.; Mazur, G.; Smardz, J.; Wieckiewicz, M. The Relationship between Sleep Bruxism and Obstructive Sleep Apnea Based on Polysomnographic Findings. J. Clin. Med. 2019, 8, 1653. [Google Scholar] [CrossRef]
- Feres, M.F.; Hermann, J.S.; Pignatari, S.S. Cephalometric evaluation of adenoids: An analysis of current methods and a propediatric OSAl of a new assessment tool. Am. J. Orthod. Dentofac. Orthop. 2012, 142, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Schwengel, D.A.; Sterni, L.M.; Tunkel, D.E.; Heitmiller, E.S. Perioperative Management of Children with Obstructive Sleep Apnea. Anesth. Analg. 2009, 109, 60–75. [Google Scholar] [CrossRef]
- Wysocki, J.; Krasny, M.; Skarżyński, P.H. Patency of nasopharynx and a cephalometric image in the children with orthodontic problems. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1803–1809. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Y.; Tai, J.; Feng, G.; Ge, W.; Zheng, L.; Zhou, Z.h.; Ni, X. Risk factors of obstructive sleep apnea syndrome in children. J. Otolaryngol. Head Neck Surg. 2020, 49, 111–118. [Google Scholar] [CrossRef]
- Zicari, A.M.; Duse, M.; Occasi, F.; Luzzi, V.; Ortolani, E.; Bardanzellu, F.; Bertin, S.; Polimeni, A. Cephalometric Pattern and Nasal Patency in Children with Primary Snoring: The Evidence of a Direct Correlation. PLoS ONE 2014, 9, e111675. [Google Scholar] [CrossRef]
- Selvadurai, S.; Voutsas, G.; Massicotte, C.; Kassner, A.; Katz, S.L.; Propst, E.J.; Narang, I. Positional obstructive sleep apnea in an obese pediatric population. J. Clin. Sleep Med. 2020, 16, 1295–1301. [Google Scholar] [CrossRef]
- Huang, W.; Wang, X.; Xu, C.; Xu, H.; Zhu, H.; Liu, S.; Zou, J.; Guan, J.; Yi, H.; Yin, S. Prevalence, characteristics, and respiratory arousal threshold of positional obstructive sleep apnea in China: A large scale study from Shanghai Sleep Health Study cohort. Respir. Res. 2022, 23, 240. [Google Scholar] [CrossRef]
- Jo, J.-H.; Kim, S.-H.; Jang, J.-H.; Park, J.-W.; Chung, J.-W. Comparison of polysomnographic and cephalometric parameters based on positional and rapid eye movement sleep dependency in obstructive sleep apnea. Sci. Rep. 2022, 12, 9828. [Google Scholar] [CrossRef] [PubMed]
- Camañes-Gonzalvo, S.; Marco-Pitarch, R.; Plaza-Espín, A.; Puertas-Cuesta, J.; Agustín-Panadero, R.; Fons-Font, A.; Fons-Badal, C.; García-Selva, M. Correlation between Polysomnographic Parameters and Tridimensional Changes in the Upper Airway of Obstructive Sleep Apnea Patients Treated with Mandibular Advancement Devices. J. Clin. Med. 2021, 10, 5255. [Google Scholar] [CrossRef] [PubMed]
- Daraze, A.; Delatte, M.; Liistro, G.; Majzoub, Z. Cephalometrics of Pharyngeal Airway Space in Lebanese Adults. Int. J. Dent. 2017, 2017, 3959456. [Google Scholar] [CrossRef] [PubMed]
- Mislik, B.; Hänggi, M.P.; Signorelli, L.; Peltomäki, T.A.; Patcas, R. Pharyngeal airway dimensions: A cephalometric, growth-study-based analysis of physiological variations in children aged 6–17. Eur. J. Orthod. 2014, 36, 331–339. [Google Scholar] [CrossRef]
- Pirilä-Parkkinen, K.; Pirttiniemi, P.; Pääkkö, E.; Tolonen, U.; Nieminen, P.; Löppönen, H. Pharyngeal airway in children with sleep-disordered breathing in relation to head posture. Sleep Breath. 2012, 16, 737–746. [Google Scholar] [CrossRef]
- Naoko, S.; Ayako, I.; Shoko, S.; Satomi Sh Nanako Sh Fusae, K.; Yo, S.; Fumihiko, M.; Katsuhisa, I.; Takatoshi, K. Clinical, polysomnographic, and cephalometric features of obstructive sleep apnea with AHI over 100. Sleep Breath. 2021, 25, 1379–1387. [Google Scholar]
- Tam, S.; Woodson, B.T.; Rotenberg, B. Outcome measurements in obstructive sleep apnea: Beyond the apnea-hypopnea index. Laryngoscope 2013, 124, 337–343. [Google Scholar] [CrossRef]
- Barata, A.R.; Kizi, G.; Alves, V.; Proença, L.; Delgado, A. Association between mouth-breathing and atypical swallowing in young orthodontic patients at Egas Moniz Dental Clinic. Ann. Med. 2021, 53 (Suppl. S1), S71–S72. [Google Scholar] [CrossRef]
- Banhiran, W.; Wanichakorntrakul, P.; Metheetrairut, C.; Chiewvit, P.; Planuphap, W. Lateral cephalometric analysis and the risks of moderate to severe obstructive sleep-disordered breathing in Thai patients. Sleep Breath. 2013, 17, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.-H.; Kim, C.-H.; Cheon, S.-M.; Bae, W.-Y.; Kim, S.-H.; Koo, S.-K.; Kim, M.-S.; Kim, B.-J. The usefulness of cephalometric measurement as a diagnostic tool for obstructive sleep apnea syndrome: A retrospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 119, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Y.; Zhang, Z.; Wang, H.; Xu, R.; Feng, X.; Man, R. Application research of polysomnography and lateral cephalometric radiographs in the diagnosis and treatment of sleep-disordered breathing in children. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi J. Clin. Otorhinolaryngol. Head Neck Surg. 2014, 28, 1982–1985. [Google Scholar]
- Aurora, R.N.; Lamm, C.I.; Zak, R.S.; Kristo, D.A.; Bista, S.R.; Rowley, J.A.; Casey, K.R. Practice Parameters for the Non-Respiratory Indications for Polysomnography and Multiple Sleep Latency Testing for Children. Sleep 2012, 35, 1467–1473. [Google Scholar] [CrossRef]
- DeHaan, K.L.; Seton, C.; Fitzgerald, D.A.; Waters, K.A.; MacLean, J.E. Polysomnography for the diagnosis of sleep disordered breathing in children under 2 years of age. Pediatr. Pulmonol. 2015, 50, 1346–1353. [Google Scholar] [CrossRef]
- Rundo, J.V.; Downey, R. , 3rd. Polysomnography. Handb. Clin. Neurol. 2019, 160, 381–392. [Google Scholar]
- American Academy of Sleep Medicine (AASM). International Classification of Sleep Disorders, 3rd ed.; AASM: Darien, IL, USA, 2014. [Google Scholar]
- Sateia, M.J. International Classification of Sleep Disorders. Third Edition (ICSD 3). Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- Katz, E.; Marcus, C. Diagnosis of Obstructive Sleep Panea. In Principles and Practice of Pediatric Sleep Medicine E-Book; Sheldon, S.H., Kryger, M.H., Gozal, D., Ferber, R., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).