Feasibility of a Patient-Specific Bolus Using the Life-Casting Method for Radiation Therapy
Abstract
1. Introduction
2. Materials and Methods
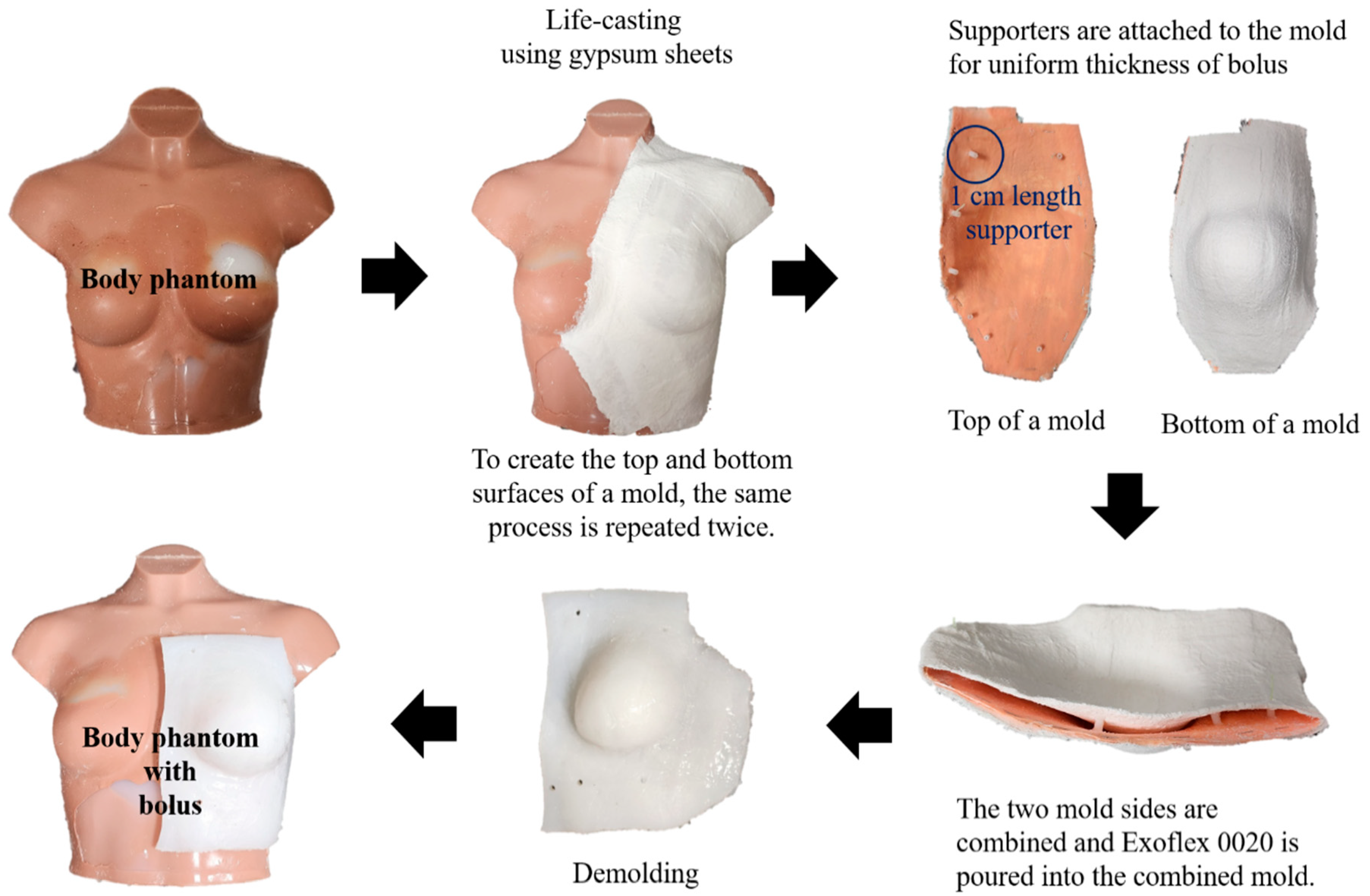
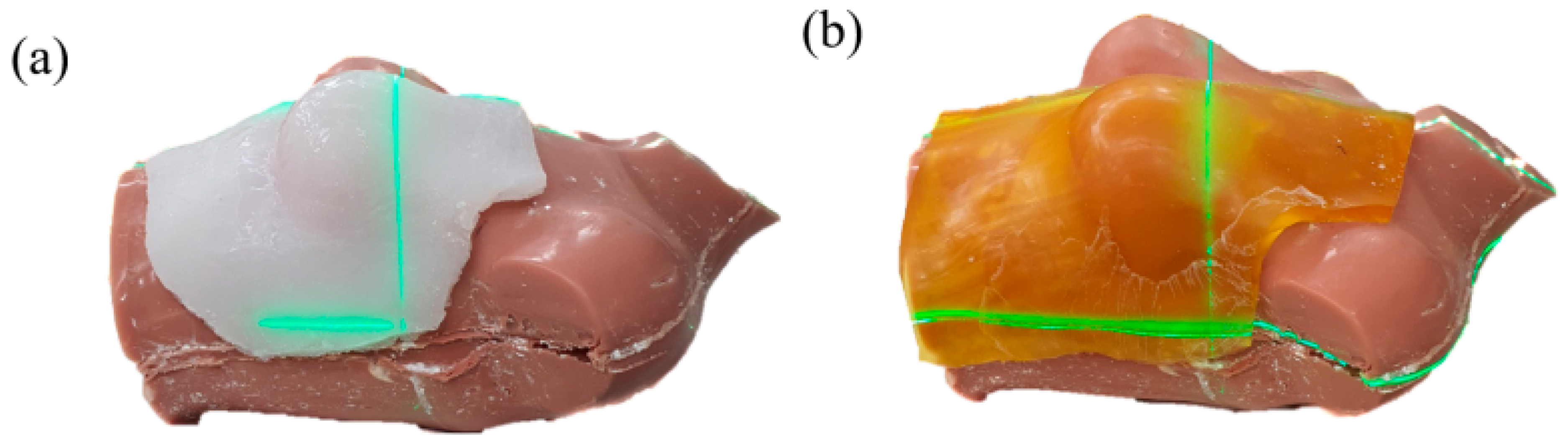
2.1. Fabrication of a Patient-Specific Bolus Using the LC Method
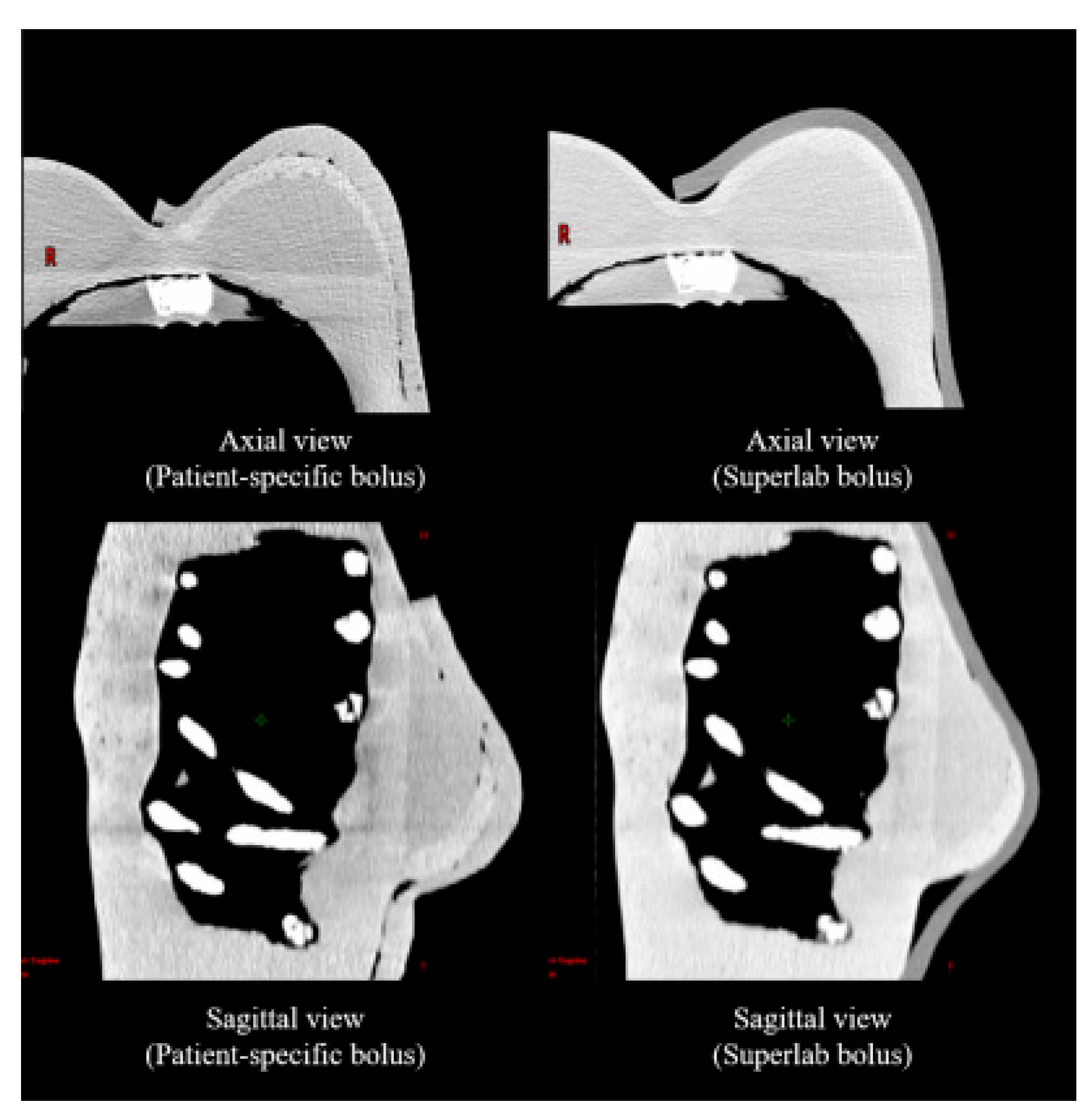
2.2. Air-Gap Quantification
2.3. Treatment Planning
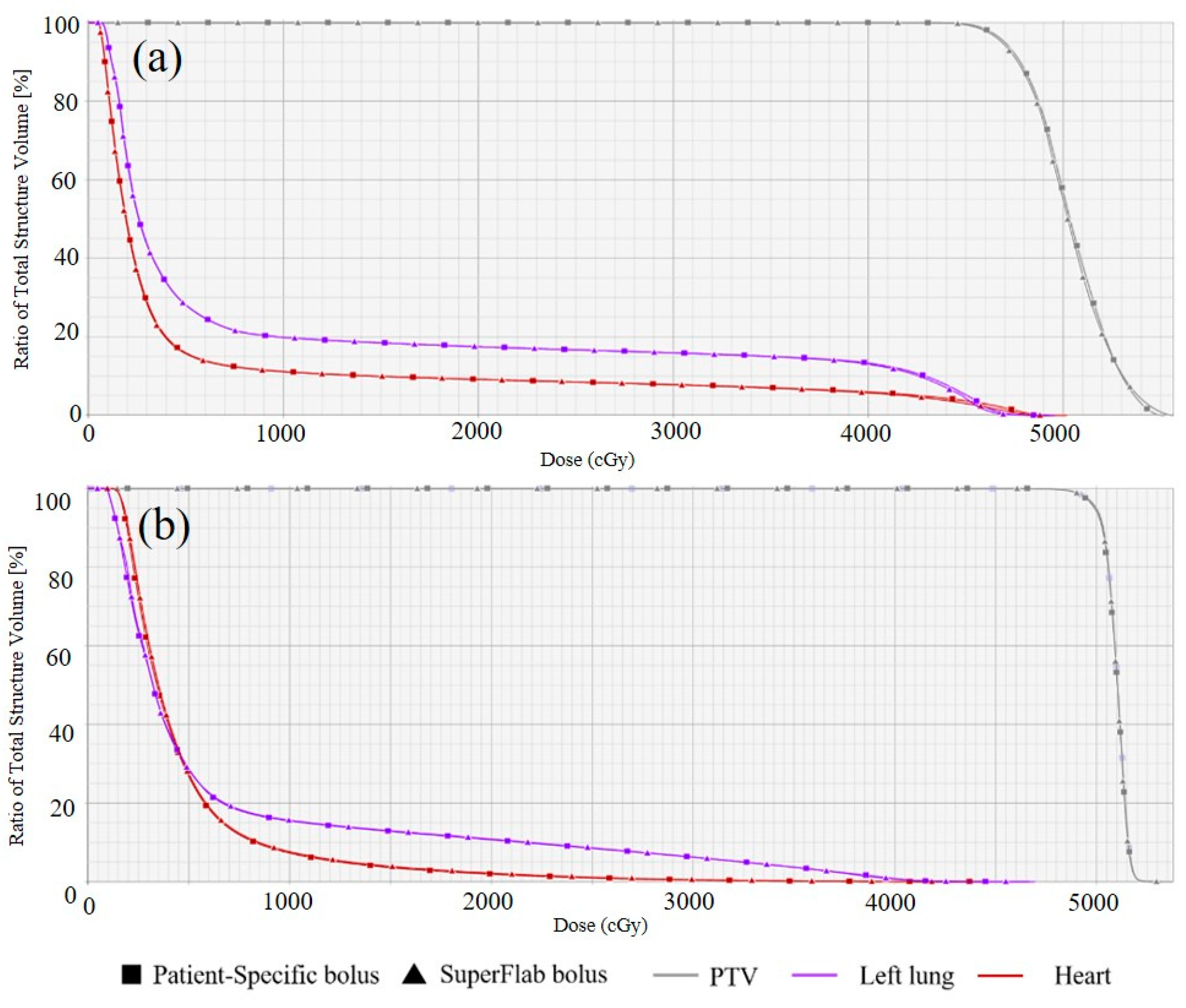
2.4. Dosimetric Evaluation
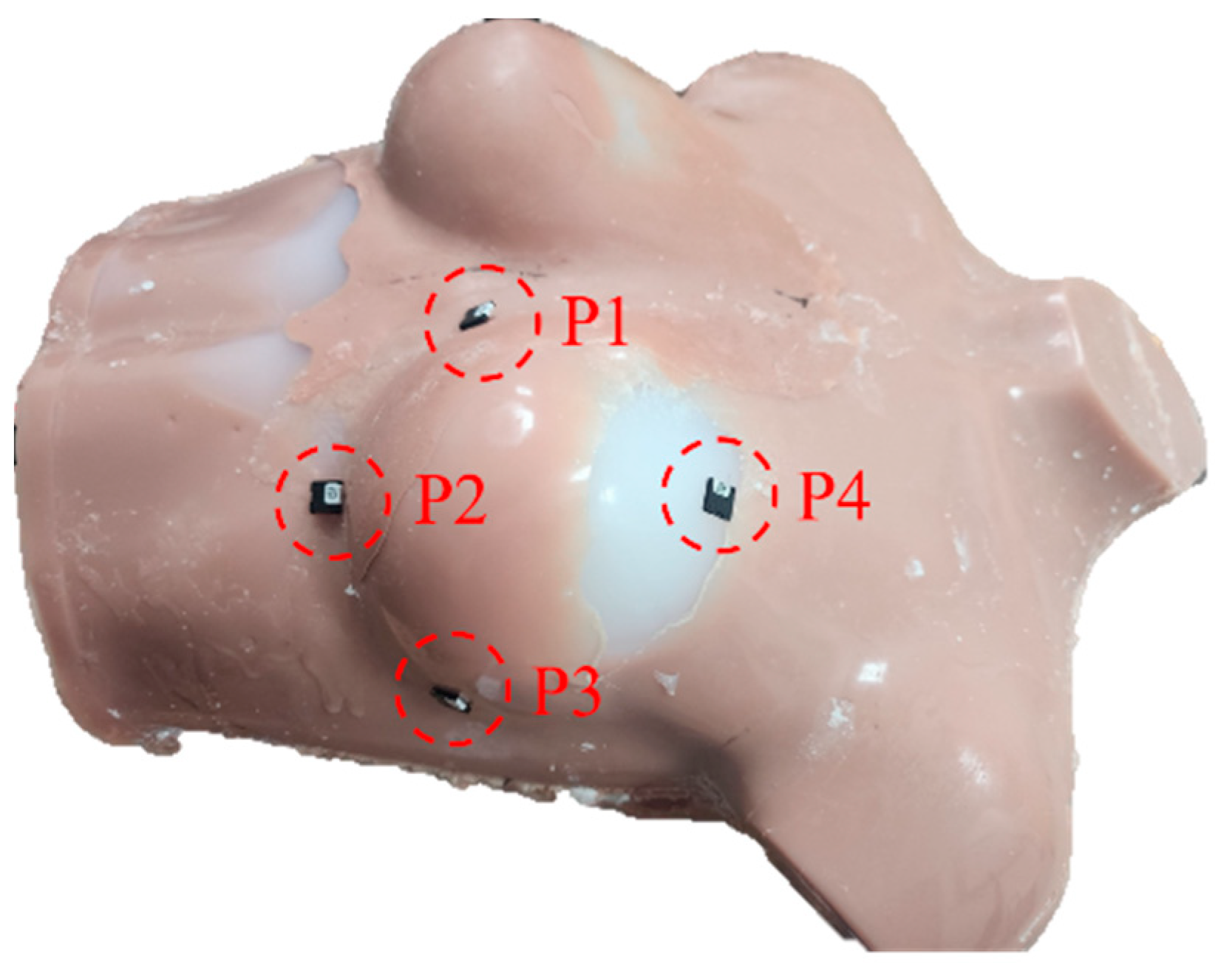
2.5. In Vivo Dosimetry Using Optically Stimulated Luminescence Dosimetry (OSLD)
3. Results
3.1. Air Gap Quantification
3.2. Dosimetric Evaluation
3.3. In Vivo Dosimetry Using OSLD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Song, J.; Yao, X.; An, M.; Shi, Q.; Huang, X. 3D printing polymer-based bolus used for radiotherapy. Int. J. Bioprint. 2021, 7, 414. [Google Scholar] [CrossRef] [PubMed]
- Hodapp, N. The ICRU Report 83: Prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT). Strahlenther. Onkol. 2012, 188, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Dipasquale, G.; Poirier, A.; Sprunger, Y.; Uiterwijk, J.W.E.; Miralbell, R. Improving 3D-printing of megavoltage X-rays radiotherapy bolus with surface scanner. Radiat. Oncol. 2018, 13, 203. [Google Scholar] [CrossRef]
- Vyas, V.; Palmer, L.; Mudge, R.; Jiang, R.; Fleck, A.; Schaly, B.; Osei, E.; Charland, P. On bolus for megavoltage photon and electron radiation therapy. Med. Dosim. 2013, 38, 268–273. [Google Scholar] [CrossRef]
- Aras, S.; Tanzer, İ.O.; İkizceli, T. Dosimetric comparison of superflab and specially prepared bolus materials used in radiotherapy practice. Eur. J. Breast Health 2020, 16, 167–170. [Google Scholar] [CrossRef]
- Khan, Y.; Villarreal-Barajas, J.E.; Udowicz, M.; Sinha, R.; Muhammad, W.; Abbasi, A.N.; Hussain, A. Clinical and dosimetric implications of air gaps between bolus and skin surface during radiation therapy. J. Cancer Ther. 2013, 4, 1251–1255. [Google Scholar] [CrossRef]
- Fujimoto, K.; Shiinoki, T.; Yuasa, Y.; Hanazawa, H.; Shibuya, K. Efficacy of patient-specific bolus created using three-dimensional printing technique in photon radiotherapy. Phys. Med. 2017, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Butson, M.J.; Cheung, T.; Yu, P.; Metcalfe, P. Effects on skin dose from unwanted air gaps under bolus in photon beam radiotherapy. Radiat. Meas. 2000, 32, 201–204. [Google Scholar] [CrossRef]
- Jreije, A.; Keshelava, L.; Ilickas, M.; Laurikaitiene, J.; Urbonavicius, B.G.; Adliene, D. Development of patient specific conformal 3D-printed devices for dose verification in radiotherapy. Appl. Sci. 2021, 11, 8657. [Google Scholar] [CrossRef]
- Asfia, A.; Novak, J.I.; Mohammed, M.I.; Rolfe, B.; Kron, T. A review of 3D printed patient specific immobilisation devices in radiotherapy. Phys. Imaging Radiat. Oncol. 2020, 13, 30–35. [Google Scholar] [CrossRef]
- Sheykholeslami, N.; Parwaie, W.; Vaezzadeh, V.; Babaie, M.; Farzin, M.; Geraily, G.; Karimi, A.H. Dual application of polyvinyl alcohol glutaraldehyde methylthymol blue Fricke hydrogel in clinical practice: Surface dosimeter and bolus. Appl. Radiat. Isot. 2023, 197, 110827. [Google Scholar] [CrossRef] [PubMed]
- Hilts, M.; Jirasek, A.; Duzenli, C. Effects of gel composition on the radiation induced density change in PAG polymer gel dosimeters: A model and experimental investigations. Phys. Med. Biol. 2004, 49, 2477–2490. [Google Scholar] [CrossRef] [PubMed]
- Derda, A.; Nadolski, M.; Muskala, K. Lifecasitng in artistic casting. Metall. Foundry Eng. 2011, 11, 63–72. [Google Scholar] [CrossRef][Green Version]
- Wang, K.M.; Rickards, A.J.; Bingham, T.; Tward, J.D.; Price, R.G. Technical note: Evaluation of a silicone-based custom bolus for radiation therapy of a superficial pelvic tumor. J. Appl. Clin. Med. Phys. 2022, 23, e13538. [Google Scholar] [CrossRef]
- Shin, W.G.; Lee, S.Y.; Jin, H.; Kim, J.; Kang, S.; Kim, J.I.; Jung, S. Development and evaluation of a thimble-like head bolus shield for hemi-body electron beam irradiation technique. J. Radiat. Prot. Res. 2022, 47, 152–157. [Google Scholar] [CrossRef]
- Son, J.; Jung, S.; Park, J.M.; Wu, H.G.; Kim, J.I. Evaluation of platinum-catalyzed silicones for fabrication of biocompatible patient-specific elastic bolus. 2020. Preprint (Version 1). Available online: https://www.researchsquare.com/article/rs-48004/v1 (accessed on 1 September 2023).
- Kim, S.W.; Shin, H.J.; Kay, C.S.; Son, S.H. A customized bolus produced using a 3-dimensional printer for radiotherapy. PLoS ONE 2014, 9, e110746. [Google Scholar] [CrossRef]
- Lukowiak, M.; Boehlke, M.; Lewocki, M.; Kot, W.; Matias, D.; Piątek- Hnat, M.; El Fray, M.; Jezierska, K.; Podraza, W. Use of a 3D printer to create a bolus for patients undergoing tele-radiotherapy. Int. J. Radiat. Res. 2016, 14, 287–295. [Google Scholar] [CrossRef]
- Canters, R.A.; Lips, I.M.; Wendling, M.; Kusters, M.; van Zeeland, M.; Gerritsen, R.M.; Poortmans, P.; Verhoef, C.G. Clinical implementation of 3D printing in the construction of patient specific bolus for electron beam radiotherapy for non-melanoma skin cancer. Radiother. Oncol. 2016, 121, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.; Tan, J.; Brenner, M.; Gu, X.; Yang, M.; Westover, K.; Strom, T.; Sher, D.; Jiang, S.; Zhao, B. Three-dimensional printer aided casting of soft, custom silicone boluses (SCSBs) for head and neck radiation therapy. Pract. Radiat. Oncol. 2018, 8, e167–e174. [Google Scholar] [CrossRef]
- An, H.J.; Kim, M.S.; Kim, J.; Son, J.; Choi, C.H.; Park, J.M.; Kim, J. Geometric evaluation of patient-specific 3D bolus from 3D printed mold and casting method for radiation therapy. Prog. Med. Phys. 2019, 30, 32–38. [Google Scholar] [CrossRef]
- Sprowls, C.J.; Shah, A.P.; Kelly, P.; Burch, D.R.; Mathews, R.S.; Swanick, C.W.; Meeks, S.L. Whole brain radiotherapy with hippocampal sparing using Varian HyperArc. Med. Dosim. 2021, 46, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Choi, C.H.; Park, J.M.; Chun, M.S.; Han, J.H.; Kim, J.I. A patient-specific polylactic acid bolus made by a 3D printer for breast cancer radiation therapy. PLoS ONE 2016, 11, e0168063. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Park, S.; Jeon, M.J.; Choi, J.; Kim, J.W.; Cho, Y.J.; Jang, W.S.; Keum, Y.S.; Lee, I.J. Clinical application of 3D-printed-step-bolus in post-total mastectomy electron conformal therapy. Oncotarget 2017, 8, 25660–25668. [Google Scholar] [CrossRef] [PubMed][Green Version]
| 3D-CRT | VMAT | |||
|---|---|---|---|---|
| Dosimetric Parameter | Patient-Specific Bolus | SuperFlab Bolus | Patient-Specific Bolus | SuperFlab Bolus |
| CI_PTV | 0.31 | 0.31 | 0.94 | 0.93 |
| HI_PTV | 0.16 | 0.17 | 0.05 | 0.05 |
| V95%_PTV (%) | 92.2 | 91.3 | 100.0 | 99.8 |
| V20Gy_Lung (%) | 17.5 | 17.5 | 10.8 | 10.7 |
| Dmean_Lung (Gy) | 9.72 | 9.66 | 6.84 | 6.79 |
| V25Gy_Heart (%) | 8.50 | 8.45 | 1.13 | 0.97 |
| Dmean_Heart (Gy) | 5.92 | 5.82 | 4.84 | 4.71 |
| 3D-CRT | |||||||
| Patient-Specific Bolus | SuperFlab Bolus | ||||||
| Point | Plan (cGy) | OSLD (cGy, Mean ± SD) | %Diff (%) | Point | Plan (cGy) | OSLD (cGy, Mean ± SD) | %Diff (%) |
| P1 | 189 | 189 ± 1 | 0.31 | P1 | 194 | 192 | 0.88 |
| P2 | 202 | 206 ± 3 | 1.69 | P2 | 209 | 213 ± 7 | 2.00 |
| P3 | 202 | 202 ± 1 | 0.20 | P3 | 198 | 201 ± 2 | 1.19 |
| P4 | 204 | 206 ± 2 | 1.08 | P4 | 203 | 202 ± 4 | 0.32 |
| VMAT | |||||||
| Patient-Specific Bolus | SuperFlab Bolus | ||||||
| Point | Plan (cGy) | OSLD (cGy, Mean ± SD) | %Diff (%) | Point | Plan (cGy) | OSLD (cGy, Mean ± SD) | %Diff (%) |
| P1 | 148 | 144 ± 7 | 2.33 | P1 | 145 | 138 ± 9 | 4.85 |
| P2 | 50 | 52 ± 8 | 2.86 | P2 | 51 | 56 ± 14 | 10.1 |
| P3 | 188 | 181 ± 5 | 3.46 | P3 | 180 | 192 ± 5 | 6.83 |
| P4 | 195 | 185 ± 4 | 5.03 | P4 | 185 | 172 ± 7 | 6.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Park, J.; Park, B.; Park, B.; Kim, T.-G. Feasibility of a Patient-Specific Bolus Using the Life-Casting Method for Radiation Therapy. Appl. Sci. 2023, 13, 9977. https://doi.org/10.3390/app13179977
Kim J, Park J, Park B, Park B, Kim T-G. Feasibility of a Patient-Specific Bolus Using the Life-Casting Method for Radiation Therapy. Applied Sciences. 2023; 13(17):9977. https://doi.org/10.3390/app13179977
Chicago/Turabian StyleKim, Jeongho, Jeehoon Park, Beomjun Park, Byungdo Park, and Tae-Gyu Kim. 2023. "Feasibility of a Patient-Specific Bolus Using the Life-Casting Method for Radiation Therapy" Applied Sciences 13, no. 17: 9977. https://doi.org/10.3390/app13179977
APA StyleKim, J., Park, J., Park, B., Park, B., & Kim, T.-G. (2023). Feasibility of a Patient-Specific Bolus Using the Life-Casting Method for Radiation Therapy. Applied Sciences, 13(17), 9977. https://doi.org/10.3390/app13179977






