Dosimetric Evaluation of 177Lu Peptide Receptor Radionuclide Therapy Using GATE and Planet Dose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Image Acquisition
2.3. Planet® Dose
2.4. Pre-Processing
2.5. GATE Toolkit
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- PDQ Adult Treatment Editorial Board. Gastrointestinal Neuroendocrine Tumors Treatment (PDQ®): Health Professional Version. 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK65769/ (accessed on 30 July 2023).
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA A Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Panagiotidis, E.; Bomanji, J. Role of 18F-Fluorodeoxyglucose PET in the Study of Neuroendocrine Tumors. PET Clin. 2014, 9, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Zaknun, J.J.; Bodei, L.; Mueller-Brand, J.; Pavel, M.E.; Baum, R.P.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisiol, T.M.; Howe, J.R.; Cremonesi, M.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef]
- Dash, A.; Chakraborty, S.; Pillai, M.R.A.; Knapp, F.F. Peptide Receptor Radionuclide Therapy: An Overview. Cancer Biother. Radiopharm. 2015, 30, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Papadimitroulas, P. Dosimetry applications in GATE Monte Carlo toolkit. Phys. Medica 2017, 41, 136–140. [Google Scholar] [CrossRef]
- Chatzipapas, K.P.; Papadimitroulas, P.; Emfietzoglou, D.; Kalospyros, S.A.; Hada, M.; Georgakilas, A.G.; Kagadis, G.C. Ionizing Radiation and Complex DNA Damage: Quantifying the Radiobiological Damage Using Monte Carlo Simulations. Cancers 2020, 12, 799. [Google Scholar] [CrossRef]
- Eleftheriadis, V.; Savvidis, G.; Paneta, V.; Chatzipapas, K.; Kagadis, G.C.; Papadimitroulas, P. A framework for prediction of personalized pediatric nuclear medical dosimetry based on machine learning and Monte Carlo techniques. Phys. Med. Biol. 2023, 68, 084004. [Google Scholar] [CrossRef]
- Hippeläinen, E.; Tenhunen, M.; Sohlberg, A. Fast voxel-level dosimetry for 177Lu labelled peptide treatments. Phys. Med. Biol. 2015, 60, 6685–6700. [Google Scholar] [CrossRef]
- Kost, S.D.; Dewaraja, Y.K.; Abramson, R.G.; Stabin, M.G. VIDA: A Voxel-Based Dosimetry Method for Targeted Radionuclide Therapy Using Geant4. Cancer Biother. Radiopharm. 2015, 30, 16–26. [Google Scholar] [CrossRef]
- Lee, M.S.; Hwang, D.; Kim, J.H.; Lee, J.S. Deep-dose: A voxel dose estimation method using deep convolutional neural network for personalized internal dosimetry. Sci. Rep. 2019, 9, 10308. [Google Scholar] [CrossRef]
- Ligonnet, T.; Pistone, D.; Auditore, L.; Italiano, A.; Amato, E.; Campennì, A.; Schaefer, N.; Boughdad, S.; Baldari, S.; Gnesin, S. Simplified patient-specific renal dosimetry in 177Lu therapy: A proof of concept. Phys. Medica 2021, 92, 75–85. [Google Scholar] [CrossRef]
- Gosewisch, A.; Ilhan, H.; Tattenberg, S.; Mairani, A.; Parodi, K.; Brosch, J.; Kaiser, L.; Gildehaus, F.J.; Todica, A.; Ziegler, S.; et al. 3D Monte Carlo bone marrow dosimetry for Lu-177-PSMA therapy with guidance of non-invasive 3D localization of active bone marrow via Tc-99m-anti-granulocyte antibody SPECT/CT. EJNMMI Res. 2019, 9, 76. [Google Scholar] [CrossRef]
- Goetz, T.I.; Lang, E.W.; Prante, O.; Maier, A.; Cordes, M.; Kuwert, T.; Ritt, P.; Schmidkonz, C. Three-dimensional Monte Carlo-based voxel-wise tumor dosimetry in patients with neuroendocrine tumors who underwent 177Lu-DOTATOC therapy. Ann. Nucl. Med. 2020, 34, 244–253. [Google Scholar] [CrossRef]
- Papadimitroulas, P.; Erwin, W.D.; Iliadou, V.; Kostou, T.; Loudos, G.; Kagadis, G.C. A personalized, Monte Carlo-based method for internal dosimetric evaluation of radiopharmaceuticals in children. Med. Phys. 2018, 45, 3939–3949. [Google Scholar] [CrossRef]
- Papadimitroulas, P.; Kostou, T.; Chatzipapas, K.; Visvikis, D.; Mountris, K.A.; Jaouen, V.; Katsanos, K.; Diamantopoulos, A.; Apostolopoulos, D.; Balomenos, A.; et al. A Review on Personalized Pediatric Dosimetry Applications Using Advanced Computational Tools. IEEE Trans. Radiat. Plasma Med. Sci. 2019, 3, 607–620. [Google Scholar] [CrossRef]
- Alsadi, R.; Djekidel, M.; Bouhali, O.; Doherty, J.O. Towards Routine Clinical Use of Dosimetry in [177Lu]Lu-PSMA Prostate Cancer Radionuclide Therapy: Current Efforts and Future Perspectives. Front. Phys. 2022, 10, 618. [Google Scholar] [CrossRef]
- Villoing, D.; Marcatili, S.; Garcia, M.-P.; Bardiès, M. Internal dosimetry with the Monte Carlo code GATE: Validation using the ICRP/ICRU female reference computational model. Phys. Med. Biol. 2017, 62, 1885–1904. [Google Scholar] [CrossRef] [PubMed]
- Tsougos, I.; Loudos, G.; Georgoulias, P.; Theodorou, K.; Kappas, C. Patient-specific internal radionuclide dosimetry. Nucl. Med. Commun. 2010, 31, 97–106. [Google Scholar] [CrossRef]
- Papadimitroulas, P.; Loudos, G.; Nikiforidis, G.C.; Kagadis, G.C. A dose point kernel database using GATE Monte Carlo simulation toolkit for nuclear medicine applications: Comparison with other Monte Carlo codes. Med. Phys. 2012, 39, 5238–5247. [Google Scholar] [CrossRef] [PubMed]
- Della Gala, G.; Bardiès, M.; Tipping, J.; Strigari, L. Overview of commercial treatment planning systems for targeted radionuclide therapy. Phys. Medica 2021, 92, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Mora-Ramirez, E.; Santoro, L.; Cassol, E.; Ocampo-Ramos, J.C.; Clayton, N.; Kayal, G.; Chouaf, S.; Trauchessec, D.; Pouget, J.; Kotzki, P.; et al. Comparison of commercial dosimetric software platforms in patients treated with 177 Lu-DOTATATE for peptide receptor radionuclide therapy. Med. Phys. 2020, 47, 4602–4615. [Google Scholar] [CrossRef]
- Sarrut, D.; Halty, A.; Badel, J.-N.; Ferrer, L.; Bardiès, M. Voxel-based multimodel fitting method for modeling time activity curves in SPECT images. Med. Phys. 2017, 44, 6280–6288. [Google Scholar] [CrossRef]
- Zhao, W.; Esquinas, P.L.; Frezza, A.; Hou, X.; Beauregard, J.-M.; Celler, A. Accuracy of kidney dosimetry performed using simplified time activity curve modelling methods: A 177Lu-DOTATATE patient study. Phys. Med. Biol. 2019, 64, 175006. [Google Scholar] [CrossRef]
- Rinscheid, A.; Kletting, P.; Eiber, M.; Beer, A.J.; Glatting, G. Influence of sampling schedules on [177Lu]Lu-PSMA dosimetry. EJNMMI Phys. 2020, 7, 41. [Google Scholar] [CrossRef]
- Marin, G.; Vanderlinden, B.; Karfis, I.; Guiot, T.; Wimana, Z.; Reynaert, N.; Vandenberghe, S.; Flamen, P. A dosimetry procedure for organs-at-risk in 177Lu peptide receptor radionuclide therapy of patients with neuroendocrine tumours. Phys. Medica 2018, 56, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Santoro, L.; Pitalot, L.; Trauchessec, D.; Mora-Ramirez, E.; Kotzki, P.O.; Bardiès, M.; Deshayes, E. Clinical implementation of PLANET® Dose for dosimetric assessment after [177Lu]Lu-DOTA-TATE: Comparison with Dosimetry Toolkit® and OLINDA/EXM® V1.0. EJNMMI Res. 2021, 11, 1. [Google Scholar] [CrossRef]
- Huizing, D.M.V.; Peters, S.M.B.; Versleijen, M.W.J.; Martens, E.; Verheij, M.; Sinaasappel, M.; Stokkel, M.P.M.; Veen, B.J.d.W.-V.d. A head-to-head comparison between two commercial software packages for hybrid dosimetry after peptide receptor radionuclide therapy. EJNMMI Phys. 2020, 7, 36. [Google Scholar] [CrossRef]
- Jackson, P.; McIntosh, L.; Hofman, M.S.; Kong, G.; Hicks, R.J. Technical Note: Rapid multiexponential curve fitting algorithm for voxel-based targeted radionuclide dosimetry. Med. Phys. 2020, 47, 4332–4339. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Agostinelli, S.; Allison, J.; Amako, K.; Apostolakis, J.; Araujo, H.; Arce, P.; Asai, M.; Axen, D.; Banerjee, S.; Barrand, G.; et al. Geant4—A simulation toolkit. Nucl. Instrum. Methods Phys. Res. Sect. A 2003, 506, 250–303. [Google Scholar] [CrossRef]
- Jan, S.; Benoit, D.; Becheva, E.; Carlier, T.; Cassol, F.; Descourt, P.; Frisson, T.; Grevillot, L.; Guigues, L.; Maigne, L.; et al. GATE V6: A major enhancement of the GATE simulation platform enabling modelling of CT and radiotherapy. Phys. Med. Biol. 2011, 56, 881–901. [Google Scholar] [CrossRef]
- European Medicines Agency. Lutathera: EPAR—Product Information. Updated 25 October 2022. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lutathera (accessed on 6 July 2023).
- Dash, A.; Pillai, M.R.A.; Knapp, F.F., Jr. Production of 177Lu for Targeted Radionuclide Therapy: Available Options. Nucl. Med. Mol. Imaging 2010, 49, 85–107. [Google Scholar] [CrossRef]
- Moshkbar-Bakhshayesh, K.; Mohtashami, S. Developing an approach for fast estimation of range of ion in interaction with material using the Geant4 toolkit in combination with the neural network. Nucl. Eng. Technol. 2022, 54, 4209–4214. [Google Scholar] [CrossRef]
- Wellisch, J.P.; Kossov, M.; Degtyarenko, P. Electro and Gamma Nuclear Physics in Geant4. June 2003. Available online: http://arxiv.org/abs/nucl-th/0306012 (accessed on 30 July 2023).
- Geant4 Collaboration. Physics Reference Manual. GEANT4 A Simulation Toolkit, Volume 1, pp. 1–554. 2019. Available online: https://geant4-userdoc.web.cern.ch/UsersGuides/PhysicsReferenceManual/fo/PhysicsReferenceManual.pdf (accessed on 30 July 2023).
- Gleisner, K.S.; Chouin, N.; Gabina, P.M.; Cicone, F.; Gnesin, S.; Stokke, C.; Konijnenberg, M.; Cremonesi, M.; Verburg, F.A.; Bernhardt, P.; et al. EANM dosimetry committee recommendations for dosimetry of 177Lu-labelled somatostatin-receptor- and PSMA-targeting ligands. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1778–1809. [Google Scholar] [CrossRef]
- Santoro, L.; Mora-Ramirez, E.; Trauchessec, D.; Chouaf, S.; Eustache, P.; Pouget, J.-P.; Kotzki, P.-O.; Bardiès, M.; Deshayes, E. Implementation of patient dosimetry in the clinical practice after targeted radiotherapy using [177Lu-[DOTA0, Tyr3]-octreotate. EJNMMI Res. 2018, 8, 103. [Google Scholar] [CrossRef]
- Vergnaud, L.; Giraudet, A.-L.; Moreau, A.; Salvadori, J.; Imperiale, A.; Baudier, T.; Badel, J.-N.; Sarrut, D. Patient-specific dosimetry adapted to variable number of SPECT/CT time-points per cycle for 177Lu-DOTATATE therapy. EJNMMI Phys. 2022, 9, 37. [Google Scholar] [CrossRef]
- Svensson, J.; Berg, G.; Wängberg, B.; Larsson, M.; Forssell-Aronsson, E.; Bernhardt, P. Renal function affects absorbed dose to the kidneys and haematological toxicity during 177Lu-DOTATATE treatment. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 947–955. [Google Scholar] [CrossRef]
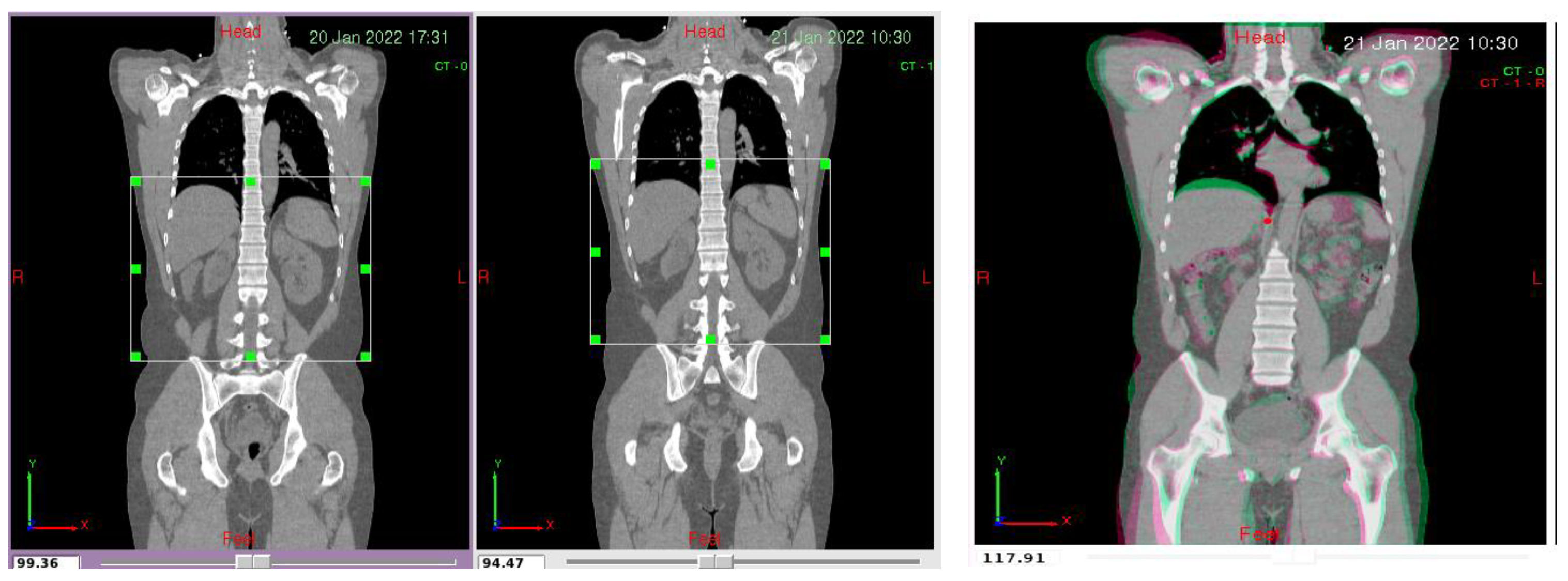

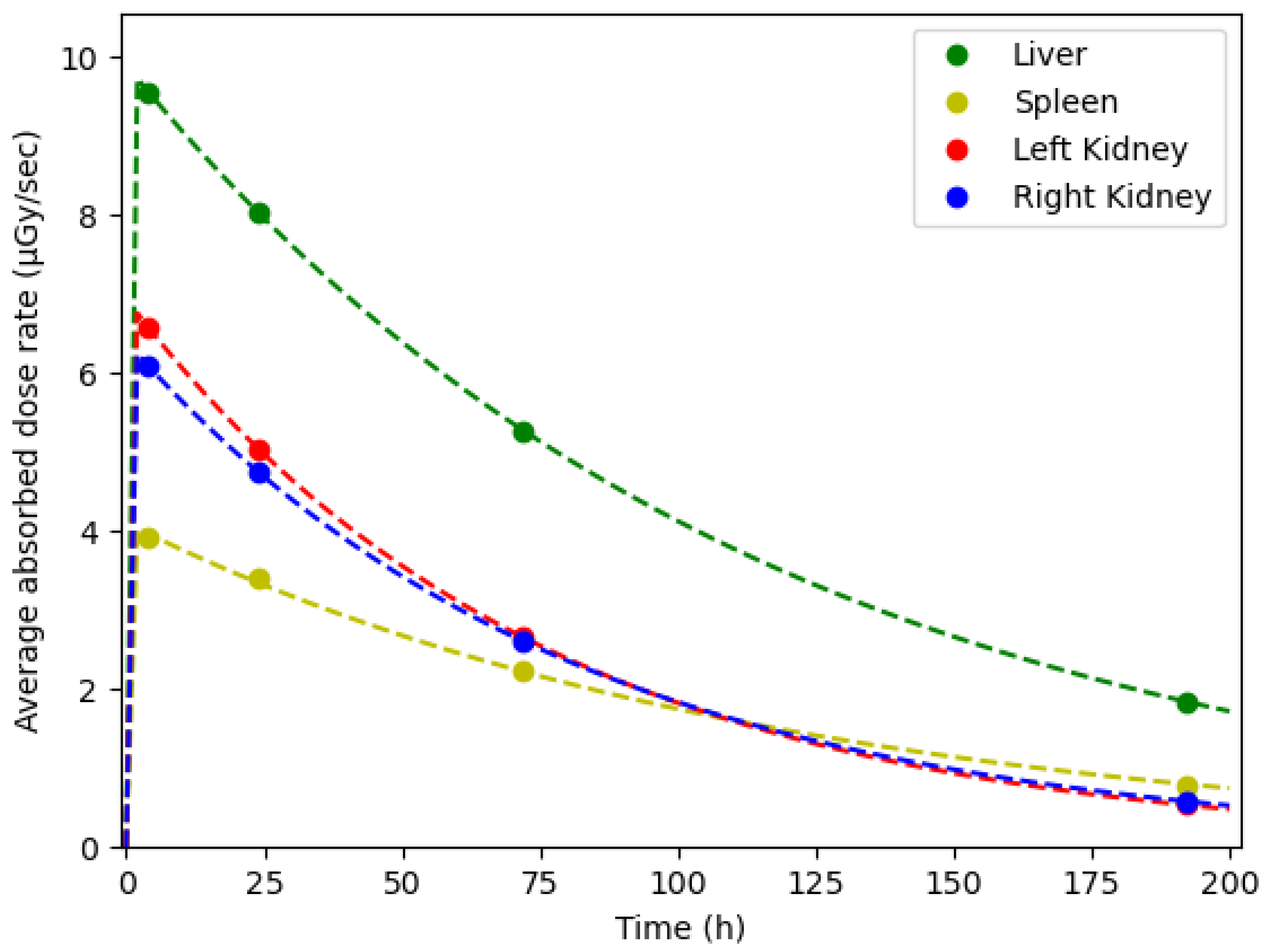

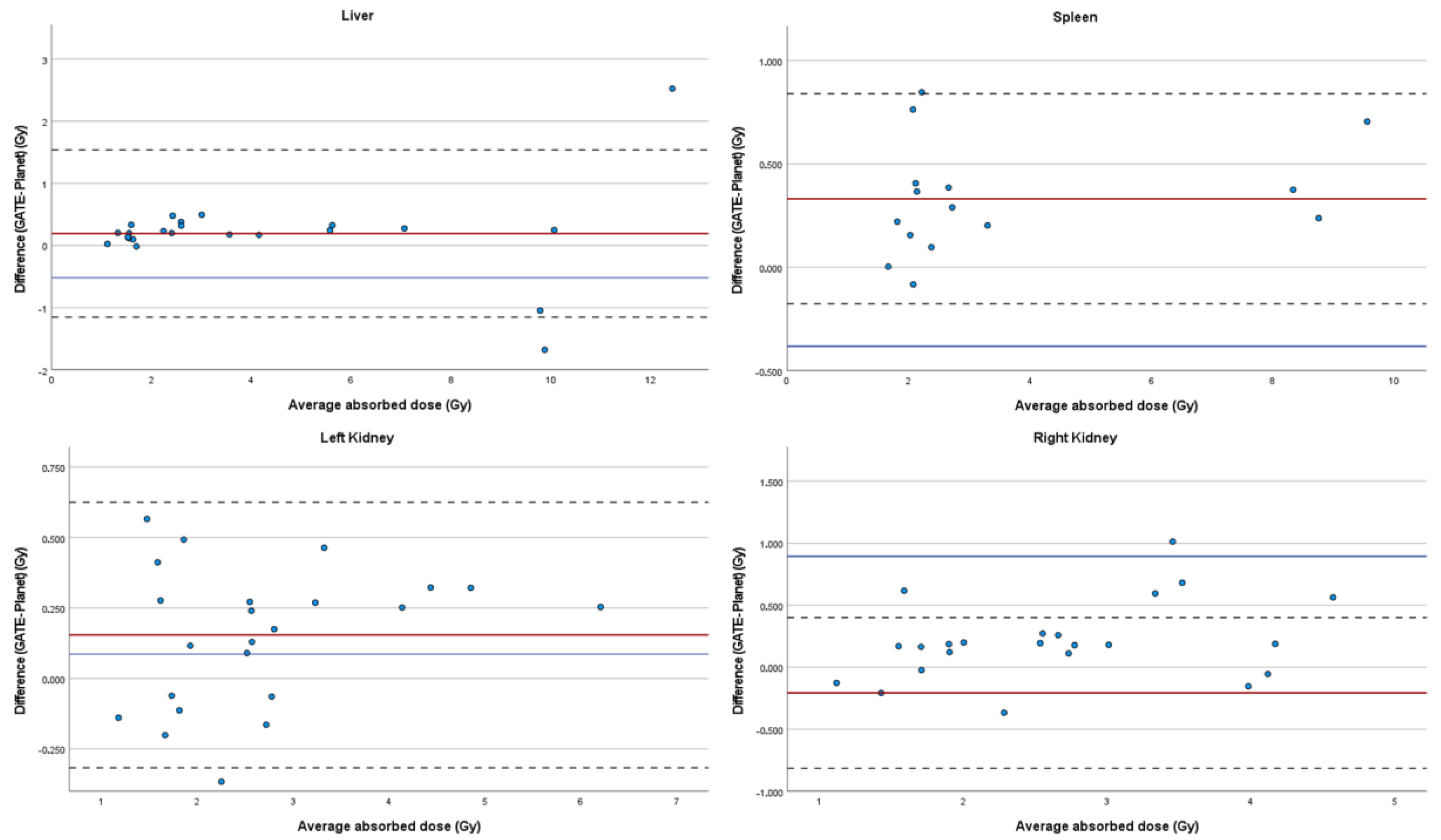
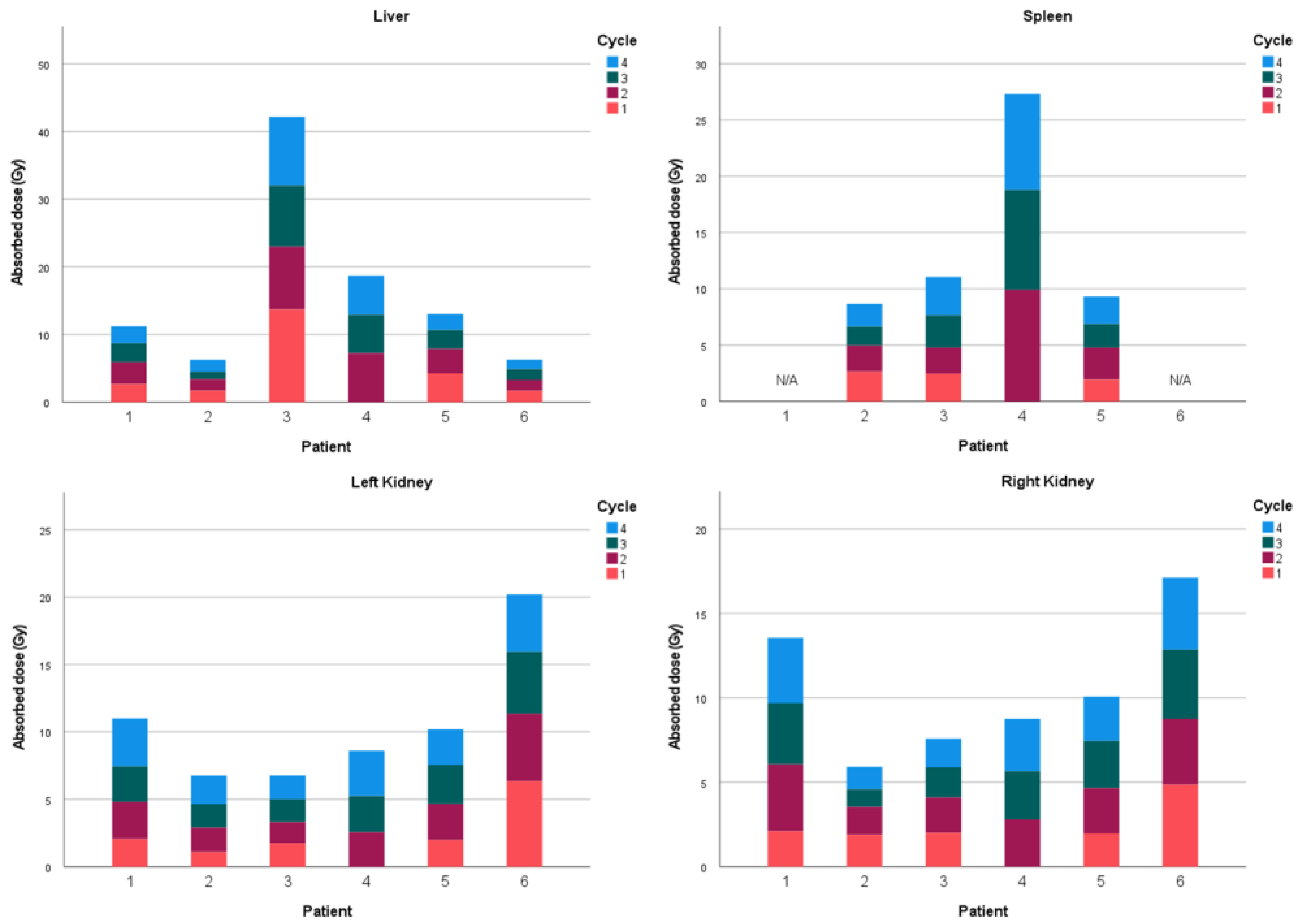
| Modality | Resolution | Pixel Spacing | Slice Thickness |
|---|---|---|---|
| CT | 527 × 527 | 0.977 × 0.977 mm2 | 2.5 mm |
| SPECT | 128 × 128 | 5.474 × 5.474 mm2 | 5.474 mm |
| Dose Map | 128 × 128 | 5.474 × 5.474 mm2 | 5.474 mm |
| Patient No. | Cycle No. | Liver (Difference %) | Spleen (Difference %) | Right Kidney (Difference %) | Left Kidney (Difference %) | Average Difference (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bi vs. Tri | Bi vs. Gate | Tri vs. Gate | Bi vs. Tri | Bi vs. Gate | Tri vs. Gate | Bi vs. Tri | Bi vs. Gate | Tri vs. Gate | Bi vs. Tri | Bi vs. Gate | Tri vs. Gate | Bi vs. Tri | Bi vs. Gate | Tri vs. Gate | ||
| 1 | 1 | 3.3 | 18.0 | 15.3 | N/A | 88.4 | 17.5 | 121.3 | 0.0 | 17.7 | 17.7 | 8.53 | 14.27 | 21.93 | ||
| 2 | 4.5 | 15.2 | 11.5 | 0.3 | 25.6 | 25.3 | 0.0 | 2.3 | 2.3 | |||||||
| 3 | 1.5 | 13.7 | 12.3 | 0.0 | 16.4 | 16.4 | 0.1 | 6.2 | 6.3 | |||||||
| 4 | 4.2 | 8.0 | 4.1 | 0.0 | 17.6 | 17.6 | 0.0 | 13.0 | 13.0 | |||||||
| 2 | 1 | 14.4 | 0.9 | 15.5 | 0.0 | 32.1 | 32.1 | 39.0 | 32.5 | 6.2 | 33.6 | 12.5 | 50.3 | 35.42 | 15.21 | 37.36 |
| 2 | 0.0 | 5.8 | 5.8 | 444.1 | 17.5 | 348.9 | 3.3 | 10.3 | 7.4 | 0.0 | 23.0 | 23.0 | ||||
| 3 | 3.9 | 2.3 | 6.1 | 0.0 | 0.2 | 0.2 | 1.4 | 11.9 | 10.3 | 2.7 | 32.2 | 34.0 | ||||
| 4 | 16.0 | 18.9 | 5.9 | 6.3 | 4.1 | 10.6 | 2.1 | 15.7 | 18.1 | 0.1 | 23.4 | 23.4 | ||||
| 3 | 1 | 22.9 | 18.4 | 0.3 | 14.2 | 31.1 | 21.3 | 1218.5 | 9.3 | 1095.5 | 0.1 | 15.8 | 15.7 | 88.11 | 11.35 | 83.79 |
| 2 | 27.8 | 11.3 | 42.3 | 23.4 | 15.8 | 3.9 | 0.2 | 9.5 | 9.4 | 6.3 | 12.9 | 20.0 | ||||
| 3 | 3.8 | 18.6 | 23.1 | 2.7 | 10.1 | 7.7 | 5.2 | 9.2 | 4.4 | 1.1 | 3.6 | 4.8 | ||||
| 4 | 80.1 | 2.4 | 75.7 | 0.3 | 5.9 | 5.6 | 0.0 | 1.4 | 1.4 | 3.2 | 6.4 | 9.8 | ||||
| 4 | 1 | N/A | 2.19 | 5.80 | 4.03 | |||||||||||
| 2 | 3.6 | 3.8 | 0.4 | 3.9 | 7.1 | 3.5 | 4.3 | 9.3 | 5.4 | 2.3 | 3.5 | 1.3 | ||||
| 3 | 6.1 | 4.3 | 1.5 | 3.1 | 2.7 | 0.3 | 0.1 | 6.2 | 6.1 | 0.0 | 8.9 | 8.9 | ||||
| 4 | 1.1 | 5.6 | 4.6 | 1.8 | 4.4 | 2.6 | 0.0 | 5.8 | 5.8 | 0.0 | 8.0 | 8.0 | ||||
| 5 | 1 | 6.7 | 4.1 | 2.3 | 11.7 | 11.5 | 1.1 | 0.1 | 6.2 | 6.1 | 2.9 | 5.8 | 3.1 | 5.35 | 7.59 | 5.64 |
| 2 | 17.2 | 4.9 | 11.4 | 6.9 | 13.5 | 7.6 | 6.1 | 10.1 | 4.6 | 16.6 | 10.1 | 4.8 | ||||
| 3 | 0.0 | 11.6 | 11.6 | 1.3 | 7.4 | 8.6 | 0.9 | 4.0 | 4.8 | 0.0 | 6.1 | 6.1 | ||||
| 4 | 7.9 | 9.8 | 2.8 | 7.4 | 4.0 | 3.1 | 0.0 | 7.4 | 7.3 | 0.0 | 4.9 | 4.9 | ||||
| 6 | 1 | 5.8 | 12.0 | 6.9 | N/A | 3.5 | 11.6 | 8.5 | 4.4 | 4.0 | 0.2 | 2.04 | 7.22 | 5.68 | ||
| 2 | 0.0 | 7.4 | 7.4 | 1.6 | 3.9 | 5.6 | 4.2 | 6.4 | 2.5 | |||||||
| 3 | 0.0 | 8.5 | 8.5 | 0.0 | 1.3 | 1.3 | 0.0 | 7.0 | 7.0 | |||||||
| 4 | 4.8 | 14.2 | 10.0 | 0.1 | 4.4 | 4.3 | 0.0 | 5.9 | 5.9 | |||||||
| Average Relative Difference (%) | ||
|---|---|---|
| Organ | GATE vs. Planet® (bi-exp) | GATE vs. Planet® (tri-exp) |
| Liver | 9.6 | 12.4 |
| Spleen | 11.1 | 30.5 |
| Right Kidney | 10.7 | 60.6 |
| Left Kidney | 10.4 | 11.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamouli, I.; Nanos, T.; Chatzipapas, K.; Papadimitroulas, P.; Zoglopitou, L.-A.; Kalathas, T.; Katsakiori, P.F.; Makridou, A.; Kagadis, G.C. Dosimetric Evaluation of 177Lu Peptide Receptor Radionuclide Therapy Using GATE and Planet Dose. Appl. Sci. 2023, 13, 9836. https://doi.org/10.3390/app13179836
Stamouli I, Nanos T, Chatzipapas K, Papadimitroulas P, Zoglopitou L-A, Kalathas T, Katsakiori PF, Makridou A, Kagadis GC. Dosimetric Evaluation of 177Lu Peptide Receptor Radionuclide Therapy Using GATE and Planet Dose. Applied Sciences. 2023; 13(17):9836. https://doi.org/10.3390/app13179836
Chicago/Turabian StyleStamouli, Ioanna, Thomas Nanos, Konstantinos Chatzipapas, Panagiotis Papadimitroulas, Lydia-Aggeliki Zoglopitou, Theodoros Kalathas, Paraskevi F. Katsakiori, Anna Makridou, and George C. Kagadis. 2023. "Dosimetric Evaluation of 177Lu Peptide Receptor Radionuclide Therapy Using GATE and Planet Dose" Applied Sciences 13, no. 17: 9836. https://doi.org/10.3390/app13179836
APA StyleStamouli, I., Nanos, T., Chatzipapas, K., Papadimitroulas, P., Zoglopitou, L.-A., Kalathas, T., Katsakiori, P. F., Makridou, A., & Kagadis, G. C. (2023). Dosimetric Evaluation of 177Lu Peptide Receptor Radionuclide Therapy Using GATE and Planet Dose. Applied Sciences, 13(17), 9836. https://doi.org/10.3390/app13179836








