Isolation and Screening of Odor-Reducing Microbes from Swine Manure and Its Role in Reducing Ammonia Release in Combination with Surfactant Foam
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of Odor-Degrading Microbial Consortium (Isolation and Screening)
2.3. Selection of an Optimal Ratio of AOS/Gelatin Mixture for Stable Foam
2.4. Characterization of Surfactant Foam
2.5. Lab-Scale Odor Reduction Test
3. Results and Discussion
3.1. Isolation and Screening of Odor Degrading Microorganisms
3.2. Optimal AOS/Gelatin Concentration for Stable Foam Generation
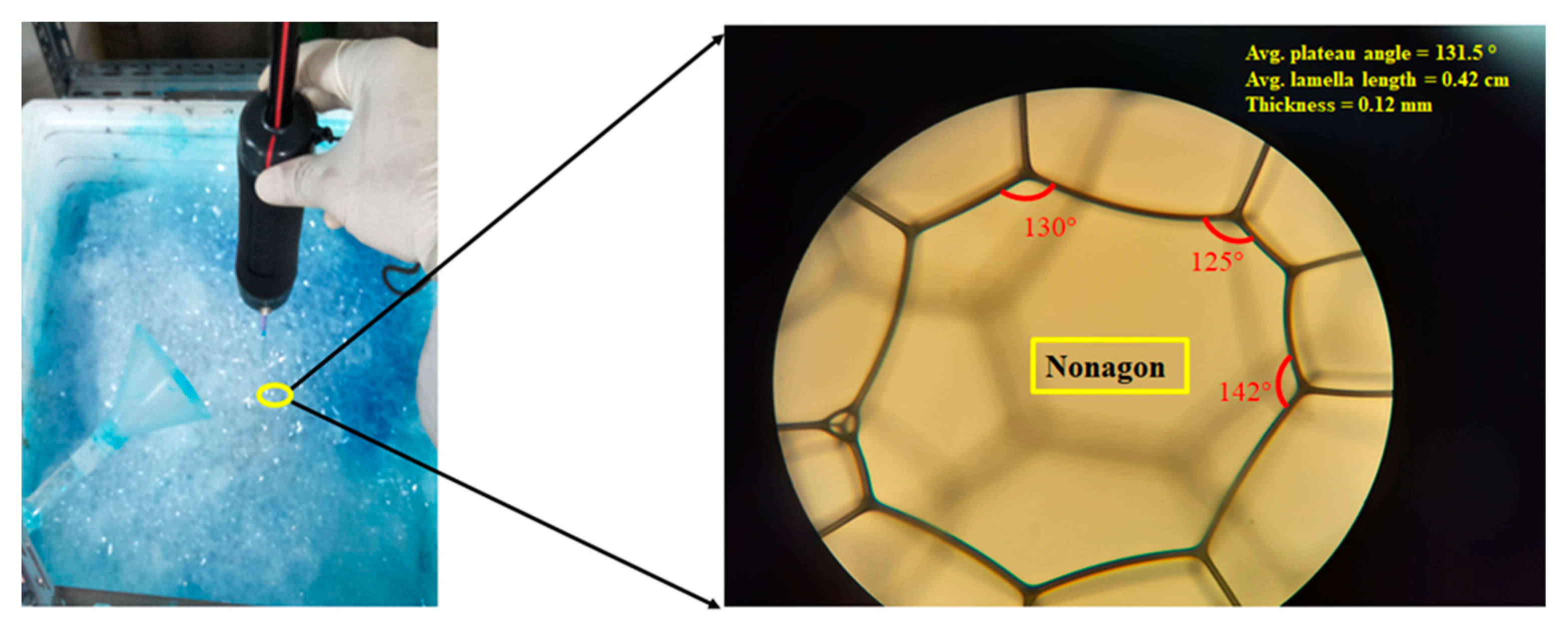
3.3. Characterization of Surfactant Foam
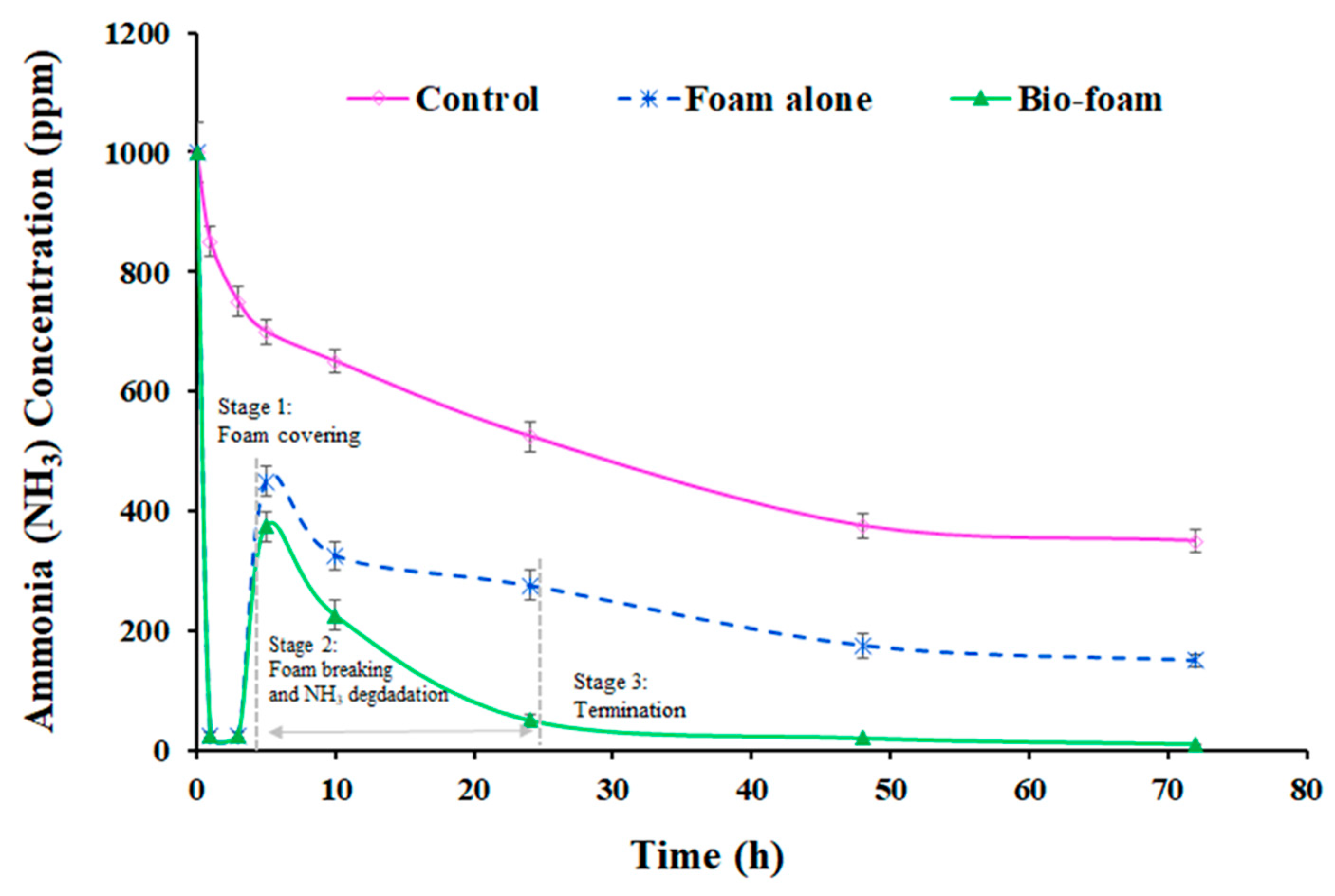
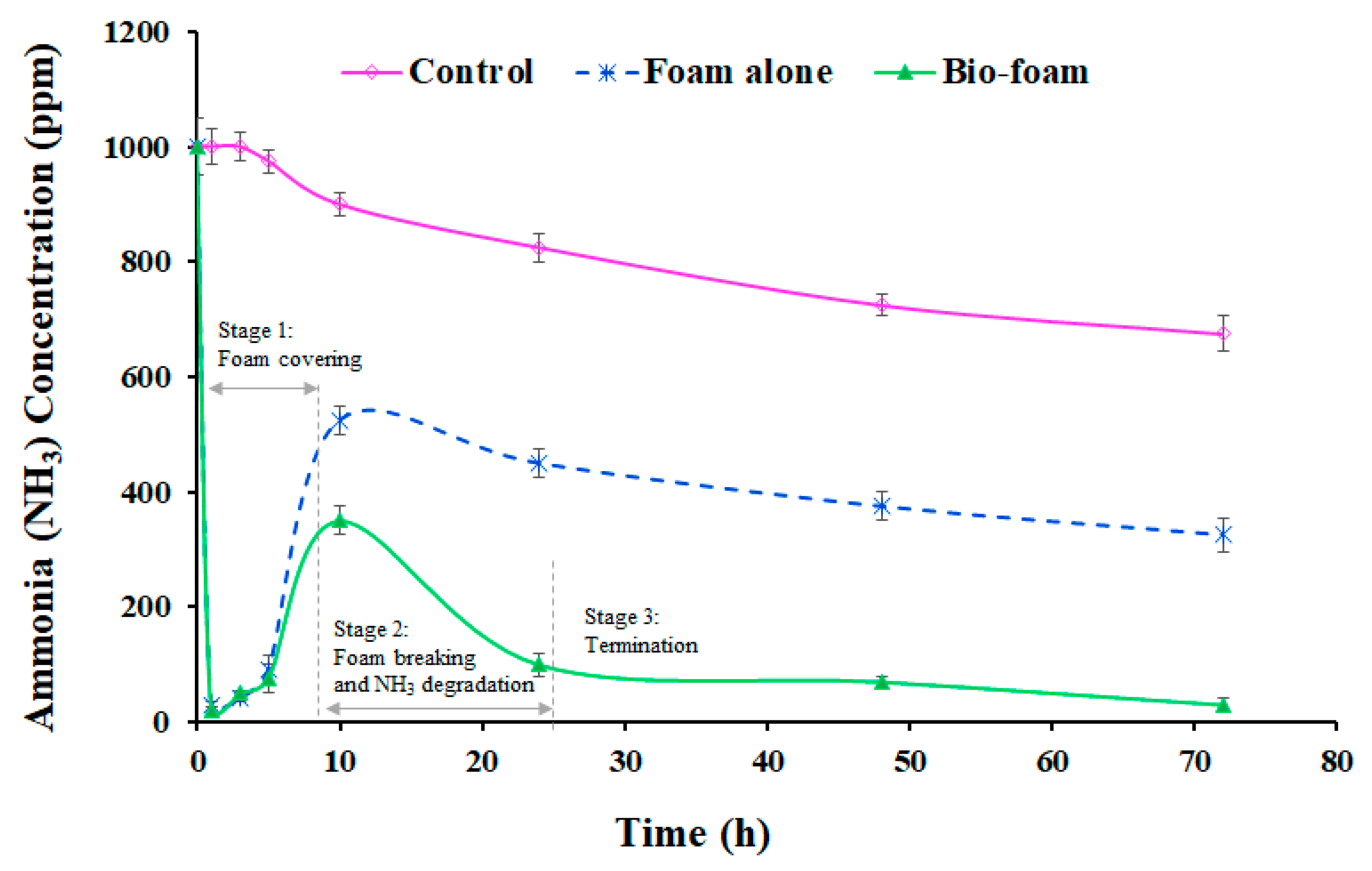
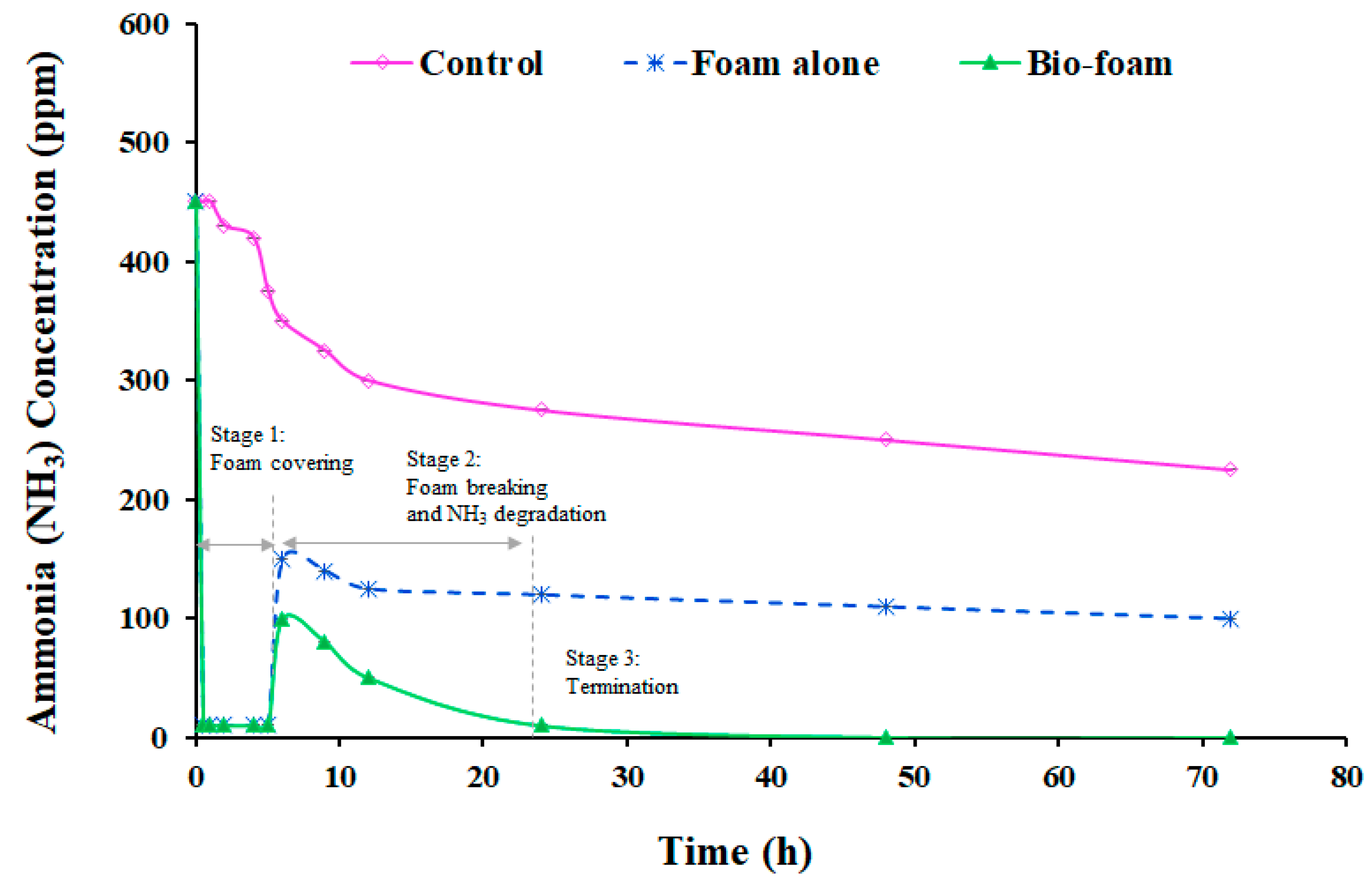
3.4. Odor Reduction Using Surface Foam-Covering
3.4.1. Open Soil Reactor System
3.4.2. Closed Soil Reactor System
3.4.3. Open Swine Manure Reactor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pig Farming—Wikipedia. Available online: https://en.wikipedia.org/wiki/Pig_farming (accessed on 5 January 2022).
- Grunert, K.G.; Sonntag, W.I.; Glanz-Chanos, V.; Forum, S. Consumer interest in environmental impact, safety, health and animal welfare aspects of modern pig production: Results of a cross-national choice experiment. Meat Sci. 2018, 137, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Krajnc, B.; Bontempo, L.; Luis Araus, J.; Giovanetti, M.; Alegria, C.; Lauteri, M.; Augusti, A.; Atti, N.; Smeti, S.; Taous, F.; et al. Selective Methods to investigate authenticity and geographical origin of mediterranean food products. Food Rev. Int. 2020, 37, 656–682. [Google Scholar] [CrossRef]
- Ko, J.; Park, G.; Bae, J.; Oh, G.; Chung, S. A study on the odor characteristics and identification of microbial in biological swine manure treatment process by bioreactor. J. Korean Soc. Environ. Eng. 2015, 37, 526–532. [Google Scholar] [CrossRef]
- Nie, E.; Zheng, G.; Ma, C. Characterization of odorous pollution and health risk assessment of volatile organic compound emissions in swine facilities. Atmos. Environ. 2020, 223, 117233. [Google Scholar] [CrossRef]
- NIOSH Preventing Deaths of Farm Workers in Manure Pits|NIOSH|CDC. Available online: https://www.cdc.gov/niosh/updates/upd-07-03-07.html (accessed on 5 December 2021).
- Pfost, D.; Fulhage, C.; Hoene, J. Odors from Livestock Operations: Causes and Possible Cures; University of Missouri: Columbia, MO, USA, 2018. [Google Scholar]
- Wang, Y.C.; Han, M.F.; Jia, T.P.; Hu, X.R.; Zhu, H.Q.; Tong, Z.; Lin, Y.T.; Wang, C.; Liu, D.Z.; Peng, Y.Z.; et al. Emissions, measurement, and control of odor in livestock farms: A review. Sci. Total Environ. 2021, 776, 145735. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Bacenetti, J.; Coppola, G.; Orsi, L.; Ganzaroli, A.; Guarino, M. Improvement of human health and environmental costs in the European Union by air scrubbers in intensive pig farming. J. Clean. Prod. 2020, 275, 124007. [Google Scholar] [CrossRef]
- Douglas, P.; Robertson, S.; Gay, R.; Hansell, A.L.; Gant, T.W. A systematic review of the public health risks of bioaerosols from intensive farming. Int. J. Hyg. Environ. Health 2018, 221, 134–173. [Google Scholar] [CrossRef] [PubMed]
- Sattler, M.L.; Rosenberk, R.S. Removal of carbonyl sulfide using activated carbon adsorption. J. Air Waste Manag. Assoc. 2012, 56, 219–224. [Google Scholar] [CrossRef][Green Version]
- Abdulrasheed, A.A.; Jalil, A.A.; Triwahyono, S.; Zaini, M.A.A.; Gambo, Y.; Ibrahim, M. Surface modification of activated carbon for adsorption of SO2 and NOX: A review of existing and emerging technologies. Renew. Sustain. Energy Rev. 2018, 94, 1067–1085. [Google Scholar] [CrossRef]
- Burgess, J.E.; Parsons, S.A.; Stuetz, R.M. Developments in odour control and waste gas treatment biotechnology: A review. Biotechnol. Adv. 2001, 19, 35–63. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, F.; Huang, W.; Huang, W.; Li, F.; Lei, Z.; Zhang, Z. Enhanced anaerobic digestion of ammonia-rich swine manure by zero-valent iron: With special focus on the enhancement effect on hydrogenotrophic methanogenesis activity. Bioresour. Technol. 2018, 270, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Liu, T.T.; Song, J.L.; Lv, J.H.; Jiang, J.S. Effects of chemical additives on emissions of ammonia and greenhouse gas during sewage sludge composting. Process Saf. Environ. Prot. 2020, 143, 129–137. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, L. Effects of altruism and burnout on driving behavior of bus drivers. Accid. Anal. Prev. 2017, 102, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Onogi, A. Effects of ascorbic acid on the deodorizing activity of polyphenols against methanethiol. Biosci. Biotechnol. Biochem. 1996, 60, 1703–1704. [Google Scholar] [CrossRef]
- Kolar, P.; Kastner, J.R. Low-temperature catalytic oxidation of aldehyde mixtures using wood fly ash: Kinetics, mechanism, and effect of ozone. Chemosphere 2010, 78, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Narengerile; Watanabe, T. Acetone decomposition by water plasmas at atmospheric pressure. Chem. Eng. Sci. 2012, 69, 296–303. [Google Scholar] [CrossRef]
- Lebrero, R.; Rodríguez, E.; Martin, M.; García-Encina, P.A.; Muñoz, R. H2S and VOCs abatement robustness in biofilters and air diffusion bioreactors: A comparative study. Water Res. 2010, 44, 3905–3914. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, S.R.; Han, J.K.; Nam, K. Mitigation of ammonia and hydrogen sulfide emissions by stable aqueous foam-microbial media. Environ. Sci. Technol. 2006, 40, 3030–3035. [Google Scholar] [CrossRef]
- Gautam, P.S.; Mohanty, K.K. Novel aqueous foams for suppressing VOC emission. Environ. Sci. Technol. 2004, 38, 2721–2728. [Google Scholar] [CrossRef]
- Kumar Paul, P.; Arshad Hussain, S.; Bhattacharjee, D.-A.; Gau, C.; Chen, S.-Y.; Tsai, H.-L.; Hosseini, S.; Kheawhom, S.; Chiong Yuh Tiong, A. A mechanistic study of surfactants, particles, and polymers on Foam stabilization. IOP Conf. Ser. Mater. Sci. Eng. 2019, 495, 012058. [Google Scholar] [CrossRef]
- Jeong, S.W.; Jeong, J.; Kim, J. Simple surface foam application enhances bioremediation of oil-contaminated soil in cold conditions. J. Hazard. Mater. 2015, 286, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Bajagain, R.; Gautam, P.; Jeong, S.W. Improved delivery of remedial agents using surface foam spraying with vertical holes into unsaturated diesel-contaminated soil for total petroleum hydrocarbon removal. Appl. Sci. 2021, 11, 781. [Google Scholar] [CrossRef]
- Bajagain, R.; Gautam, P.; Jeong, S.-W. Degradation of petroleum hydrocarbons in unsaturated soil and effects on subsequent biodegradation by potassium permanganate. Environ. Geochem. Health 2019, 42, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Huang, C.; Tseng, C.P. Reduction of H2S/NH3 Production From Pig Feces by Controlling Environmental Conditions. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 2008, 31, 139–155. [Google Scholar] [CrossRef]
- Hirai, M.; Kamamoto, M.; Yani, M.; Shoda, M. Comparison of the Biological NH3 Removal Characteristics Among Four Inorganic Packing Materials. J. Biosci. Bioeng. 2001, 91, 428–430. [Google Scholar] [CrossRef]
- Chaudhary, D.K.; Bajagain, R.; Jeong, S.-W.; Kim, J. Biodegradation of diesel oil and n-alkanes (C18, C20, and C22) by a novel strain Acinetobacter sp. K-6 in unsaturated soil. Environ. Eng. Res. 2020, 25, 290–298. [Google Scholar] [CrossRef]
- Kuroda, K.; Osada, T.; Yonaga, M.; Kanematu, A.; Nitta, T.; Mouri, S.; Kojima, T. Emissions of Malodorous Compounds and Greenhouse Gases From Composting Swine Feces. Bioresour. Technol. 1996, 56, 265–271. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Heber, A.J.; Lim, T.-T. Ammonia and hydrogen sulfide in swine production. In Air Quality and Livestock Farming; CRC Press: Boca Raton, FL, USA, 2018; pp. 29–47. ISBN 9781315738338. [Google Scholar]
- Ninh, H.P.; Tanaka, Y.; Nakamoto, T.; Hamada, K. A Bad-Smell Sensing Network Using Gas Detector Tubes and Mobile Phone Cameras. Sens. Actuators B Chem. 2007, 125, 138–143. [Google Scholar] [CrossRef]
- Osada, T.; Kuroda, K.; Yonaga, M. Determination of Nitrous Oxide, Methane, and Ammonia Emissions From a Swine Waste Composting Process. J. Mater. Cycles Waste Manag. 2000, 2, 51–56. [Google Scholar] [CrossRef]
- Goddard, E.D.; Ananthapadmanabhan, K.P. Interactions of Surfactants with Polymers and Proteins; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Richtering, W. The colloidal domain—Where physics, chemistry, biology and technology meet. Appl. Rheol. 2001, 11, 177. [Google Scholar] [CrossRef]
- Alinezhad, E.; Haghighi, M.; Rahmani, F.; Keshizadeh, H.; Abdi, M.; Naddafi, K. Technical and economic investigation of chemical scrubber and bio-filtration in removal of H2S and NH3 from wastewater treatment plant. J. Environ. Manag. 2019, 241, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, T.; Jinsiriwanit, S.; Hattori, T.; Deshusses, M.A. Removal of ammonia from contaminated air in a biotrickling filter—Denitrifying bioreactor combination system. Water Res. 2008, 42, 4507–4513. [Google Scholar] [CrossRef] [PubMed]
- Huan, C.; Fang, J.; Tong, X.; Zeng, Y.; Liu, Y.; Jiang, X.; Ji, G.; Xu, L.; Lyu, Q.; Yan, Z. Simultaneous elimination of H2S and NH3 in a biotrickling filter packed with polyhedral spheres and best efficiency in compost deodorization. J. Clean. Prod. 2021, 284, 124708. [Google Scholar] [CrossRef]
- Bouzid, I.; Maire, J.; Ahmed, S.I.; Fatin-Rouge, N. Enhanced remedial reagents delivery in unsaturated anisotropic soils using surfactant foam. Chemosphere 2018, 210, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Koziel, J.A.; Banik, C.; Ma, H.; Lee, M.; O’brien, S.C.; Li, P.; Andersen, D.S.; Białowiec, A.; Brown, R.C. Mitigation of Gaseous Emissions from Stored Swine Manure with Biochar: Effect of Dose and Reapplication on a Pilot-Scale. Atmosphere 2021, 12, 96. [Google Scholar] [CrossRef]
- Dougherty, B.; Gray, M.; Johnson, M.G.; Kleber, M. Can Biochar Covers Reduce Emissions From Manure Lagoons While Capturing Nutrients? J. Environ. Qual. 2017, 46, 659. [Google Scholar] [CrossRef] [PubMed]
| Time of Deodorization (h) | Samples | NH3 Concentration (ppm) in the Open Jar with a Consortium of TP1:TP3:TP5 (1:1:1) | ||
|---|---|---|---|---|
| Concentration (ppm) | Reduction (%) in Comparison to the Control | Reduction (%) in Comparison to the Initial | ||
| 0 | Control | 1000 (±0.0) A | - | - |
| Experimental | 1000 (±0.0) Aa | - | - | |
| after bacteria spraying * | Control | 320 (±34.6) B | ||
| Experimental | 3.33 (±5.77) Gd | 98.96 | 99.6 | |
| 12 | Control | 226.7 (±30.6) C | ||
| Experimental | 70 (±10.0) EFb | 69.12 | 93 | |
| 24 | Control | 160 (±20.0) D | ||
| Experimental | 30 (±10.0) FGc | 81.25 | 97 | |
| 36 | Control | 80 (± 20.0) E | ||
| Experimental | 3.33 (±5.77) Gd | 95.83 | 99.7 | |
| 48 | Control | 60 (±10.0) EF | ||
| Experimental | 0 (±0.0) Gd | 100 | 100 | |
| Foam Characteristics | Unit | Value |
|---|---|---|
| Foamability (in 1 L cylinder) | s | 2.4 |
| Foam quality (FQ) | % | 98.5–99.0 |
| Foam stability (half-life) | h | 2.0–2.1 |
| Foam density Foam bubble density Foam expansion ratio Foam lamella thickness Bubble shape Plateau angle Liquid content in the bubble | g/cm3 No./cm2 Foam/liquid vol. mm Degree (°) | 0.025–0.026 1 110–112 0.12 Nonagon 131.5° (Avg.) Very less |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajagain, R.; Gautam, P.; Le, T.T.N.; Dahal, R.H.; Kim, J.; Jeong, S.-W. Isolation and Screening of Odor-Reducing Microbes from Swine Manure and Its Role in Reducing Ammonia Release in Combination with Surfactant Foam. Appl. Sci. 2022, 12, 1806. https://doi.org/10.3390/app12041806
Bajagain R, Gautam P, Le TTN, Dahal RH, Kim J, Jeong S-W. Isolation and Screening of Odor-Reducing Microbes from Swine Manure and Its Role in Reducing Ammonia Release in Combination with Surfactant Foam. Applied Sciences. 2022; 12(4):1806. https://doi.org/10.3390/app12041806
Chicago/Turabian StyleBajagain, Rishikesh, Prakash Gautam, Thi Tuyet Nhan Le, Ram Hari Dahal, Jaisoo Kim, and Seung-Woo Jeong. 2022. "Isolation and Screening of Odor-Reducing Microbes from Swine Manure and Its Role in Reducing Ammonia Release in Combination with Surfactant Foam" Applied Sciences 12, no. 4: 1806. https://doi.org/10.3390/app12041806
APA StyleBajagain, R., Gautam, P., Le, T. T. N., Dahal, R. H., Kim, J., & Jeong, S.-W. (2022). Isolation and Screening of Odor-Reducing Microbes from Swine Manure and Its Role in Reducing Ammonia Release in Combination with Surfactant Foam. Applied Sciences, 12(4), 1806. https://doi.org/10.3390/app12041806










