Analysis of TihxOy Films Produced by Physical Vapor Deposition Method
Abstract
1. Introduction
2. Materials and Methods
3. Results
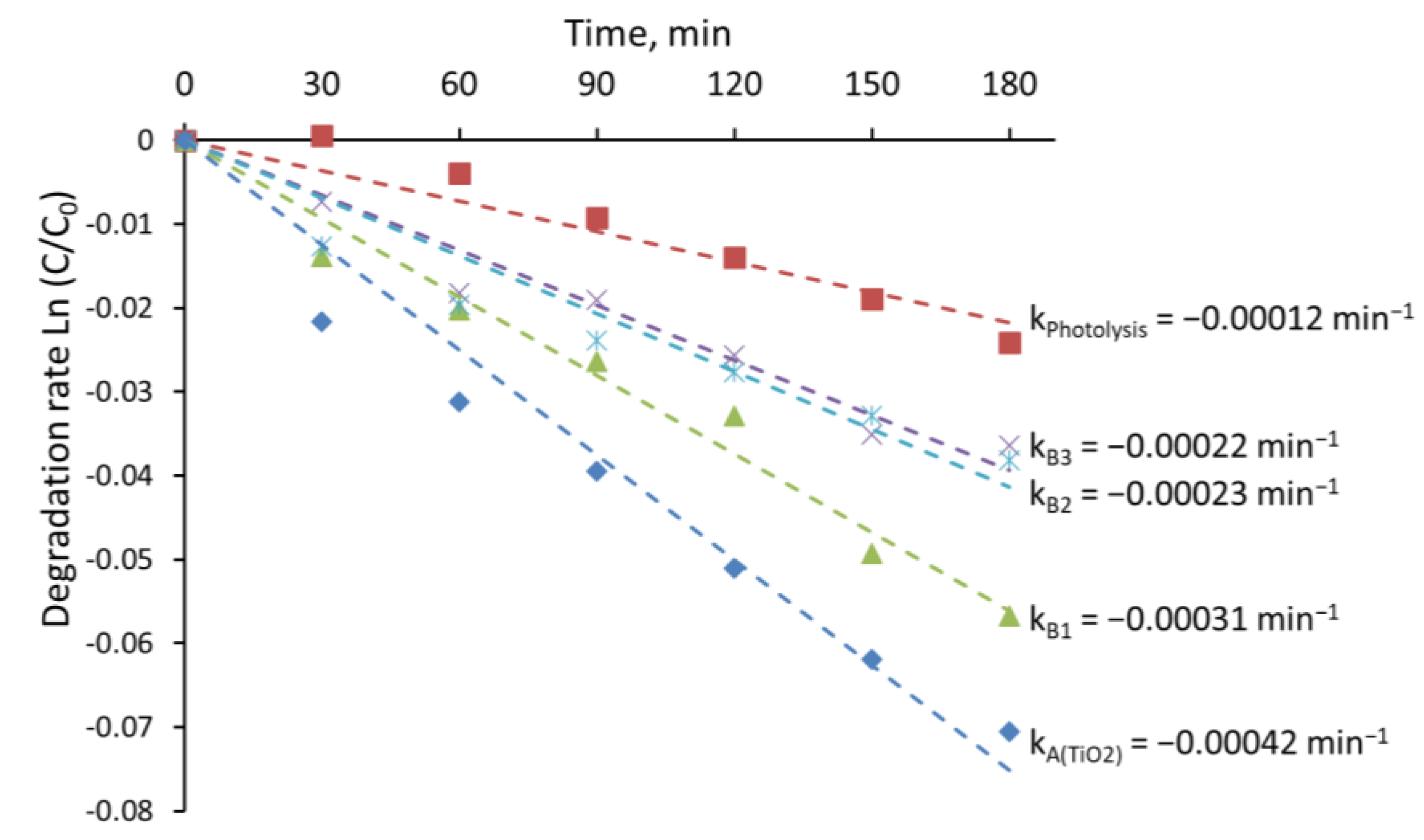
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pishtshev, A.; Strugovshchikov, E.; Karazhanov, S. Conceptual Design of Yttrium Oxyhydrides: Phase Diagram, Structure, and Properties. Cryst. Growth Des. 2019, 19, 2574–2582. [Google Scholar] [CrossRef]
- Brice, J.F.; Moreau, A. Synthèse et conductivité anionique des hydruro-oxydes de lanthane de formule LaHO, LaH1+2xO1−x et LaH1+yO1−x (y < 2x). Ann. Chim. 1982, 7, 623–634. [Google Scholar]
- Norby, T.; Widerøe, M.; Glöckner, R.; Larring, Y. Hydrogen in oxides. Dalton Trans. 2004, 19, 3012–3018. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Hernandez, O.; Tassel, C.; Kageyama, H. New chemistry of transition metal oxyhydrides. Sci. Technol. Adv. Mater. 2017, 18, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Malaman, B.; Brice, J.F. Etude structurale de l’hydruro-oxyde LaHO par diffraction des rayons X et par diffraction des neutrons. J. Solid State Chem. 1984, 53, 44–54. [Google Scholar] [CrossRef]
- Montero, J.; Martinsen, F.A.; Lelis, M.; Karazhanov, S.Z.; Hauback, B.C.; Marstein, E.S. Preparation of yttrium hydride-based photochromic films by reactive magnetron sputtering. Sol. Energy Mater. Sol. Cells 2018, 177, 106–109. [Google Scholar] [CrossRef]
- Moldarev, D.; Moro, M.V.; You, C.C.; Baba, E.M.; Karazhanov, S.Z.; Wolff, M.; Primetzhofer, D. Yttrium oxyhydrides for photochromic applications: Correlating composition and optical response. Phys. Rev. Mater. 2018, 2, 115203. [Google Scholar] [CrossRef]
- Rotella, F.J.; Flotow, H.E.; Gruen, D.M.; Jorgensen, J.D. Deuterium site occupation in the oxygen-stabilized η-carbides Zr3V3ODx. I. Preparation and neutron powder diffraction. J. Chem. Phys. 1983, 79, 4522–4531. [Google Scholar] [CrossRef]
- Clark, N.J.; Wu, E. Hydrogen absorption by M5X3 phase Zr-Al compounds. J. Less Common Met. 1988, 142, 145–154. [Google Scholar] [CrossRef]
- Lavrenko, V.A.; Shemet, V.Z.; Petrov, L.A.; Teplov, O.A.; Dolukhanyan, S.K. High-temperature oxidation of titanium-hydride powders. Oxid. Met. 1990, 33, 177–189. [Google Scholar] [CrossRef]
- Lang, P.F.; Smith, B.C. Ionic radii for Group 1 and Group 2 halide, hydride, fluoride, oxide, sulfide, selenide and telluride crystals. Dalton Trans. 2010, 39, 7786–7791. [Google Scholar] [CrossRef] [PubMed]
- You, C.C.; Mongstad, T.; Maehlen, J.P.; Karazhanov, S. Engineering of the band gap and optical properties of thin films of yttrium hydride. Appl. Phys. Lett. 2014, 105, 31910. [Google Scholar] [CrossRef]
- Baba, E.M.; Montero, J.; Moldarev, D.; Moro, M.V.; Wolff, M.; Primetzhofer, D.; Sartori, S.; Zayim, E.; Karazhanov, S. Preferential Orientation of Photochromic Gadolinium Oxyhydride Films. Molecules 2020, 25, 3181. [Google Scholar] [CrossRef] [PubMed]
- Strugovshchikov, E.; Pishtshev, A.; Karazhanov, S. Theoretical Design of Effective Multilayer Optical Coatings Using Oxyhydride Thin Films. Phys. Status Solidi 2021, 258, 2100179. [Google Scholar] [CrossRef]
- Fokin, V.N.; Malov, Y.I.; Fokina, E.E.; Troitskaya, S.L.; Shilkin, S.P. Investigation of interactions in the TiH2-O2 system. Int. J. Hydrogen Energy 1995, 20, 387–389. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- ISO 10678:2010; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Photocatalytic Activity of Surfaces in an Aqueous Medium by Degradation of Methylene Blue. International Organization for Standardization: Geneva, Switzerland, 2010.
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Cronemeyer, D.C. Infrared Absorption of Reduced Rutile TiO2 Single Crystals. Phys. Rev. 1959, 113, 1222–1226. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Lopez, V.H. The decomposition behavior of as-received and oxidized TiH2 foaming-agent powder. Mater. Sci. Eng. A 2003, 357, 258–263. [Google Scholar] [CrossRef]
- Barros, H.W.S.; Duarte, D.A.; Sagás, J.C. Optical and electrical properties of Ti suboxides grown by reactive grid-assisted magnetron sputtering. Thin Solid Film. 2020, 696, 137762. [Google Scholar] [CrossRef]
- Stryhalski, J.; Duarte, D.A.; Rebouta, L.M.; Sagas, J.C.; Tavares, C.J.; Fontana, L.C. Nb-doped Ti2O3 Films Deposited through Grid-Assisted Magnetron Sputtering on Glass Substrate: Electrical and Optical Analysis. Mater. Res. 2019, 22, e20180524. [Google Scholar] [CrossRef]
- Godoy Junior, A.; Pereira, A.; Gomes, M.; Fraga, M.; Pessoa, R.; Leite, D.; Petraconi, G.; Nogueira, A.; Wender, H.; Miyakawa, W.; et al. Black TiO2 Thin Films Production Using Hollow Cathode Hydrogen Plasma Treatment: Synthesis, Material Characteristics and Photocatalytic Activity. Catalysts 2020, 10, 282. [Google Scholar] [CrossRef]
- Xiu, Z.; Guo, M.; Zhao, T.; Pan, K.; Xing, Z.; Li, Z.; Zhou, W. Recent advances in Ti3+ self-doped nanostructured TiO2 visible light photocatalysts for environmental and energy applications. Chem. Eng. J. 2020, 382, 123011. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A. Reduced and n-type doped TiO2: Nature of Ti3+ species. J. Phys. Chem. C 2009, 113, 20543–20552. [Google Scholar] [CrossRef]
- Sarkar, A.; Khan, G.G. The formation and detection techniques of oxygen vacancies in titanium oxide-based nanostructures. Nanoscale 2019, 11, 3414–3444. [Google Scholar] [CrossRef]
- Andronic, L.; Lelis, M.; Enesca, A.; Karazhanov, S. Photocatalytic activity of defective black-titanium oxide photocatalysts towards pesticide degradation under UV/VIS irradiation. Surf. Interfaces 2022, 32, 102123. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-Ray Photoelectron Spectroscopy Database, NIST Standard Reference Database 20; Version 4.1; On-Line Database, Updated on 2012; NIST: Gaithersburg, MD, USA, 2012.
- An, H.-R.; Park, S.Y.; Kim, H.; Lee, C.Y.; Choi, S.; Lee, S.C.; Seo, S.; Park, E.C.; Oh, Y.-K.; Song, C.-G.; et al. Advanced nanoporous TiO2 photocatalysts by hydrogen plasma for efficient solar-light photocatalytic application. Sci. Rep. 2016, 6, 29683. [Google Scholar] [CrossRef]
- Liu, H.; Liu, S.; Zhang, Z.; Dong, X.; Liu, T. Hydrothermal etching fabrication of TiO2@graphene hollow structures: Mutually independent exposed {001} and {101} facets nanocrystals and its synergistic photocaltalytic effects. Sci. Rep. 2016, 6, 33839. [Google Scholar] [CrossRef]
- Krishnan, P.; Liu, M.; Itty, P.A.; Liu, Z.; Rheinheimer, V.; Zhang, M.-H.; Monteiro, P.J.M.; Yu, L.E. Characterization of photocatalytic TiO2 powder under varied environments using near ambient pressure X-ray photoelectron spectroscopy. Sci. Rep. 2017, 7, 43298. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Wakim, F.G.; Addiss, R.R. Photoelectronic Processes in Rutile. Phys. Rev. 1969, 184, 979–988. [Google Scholar] [CrossRef]
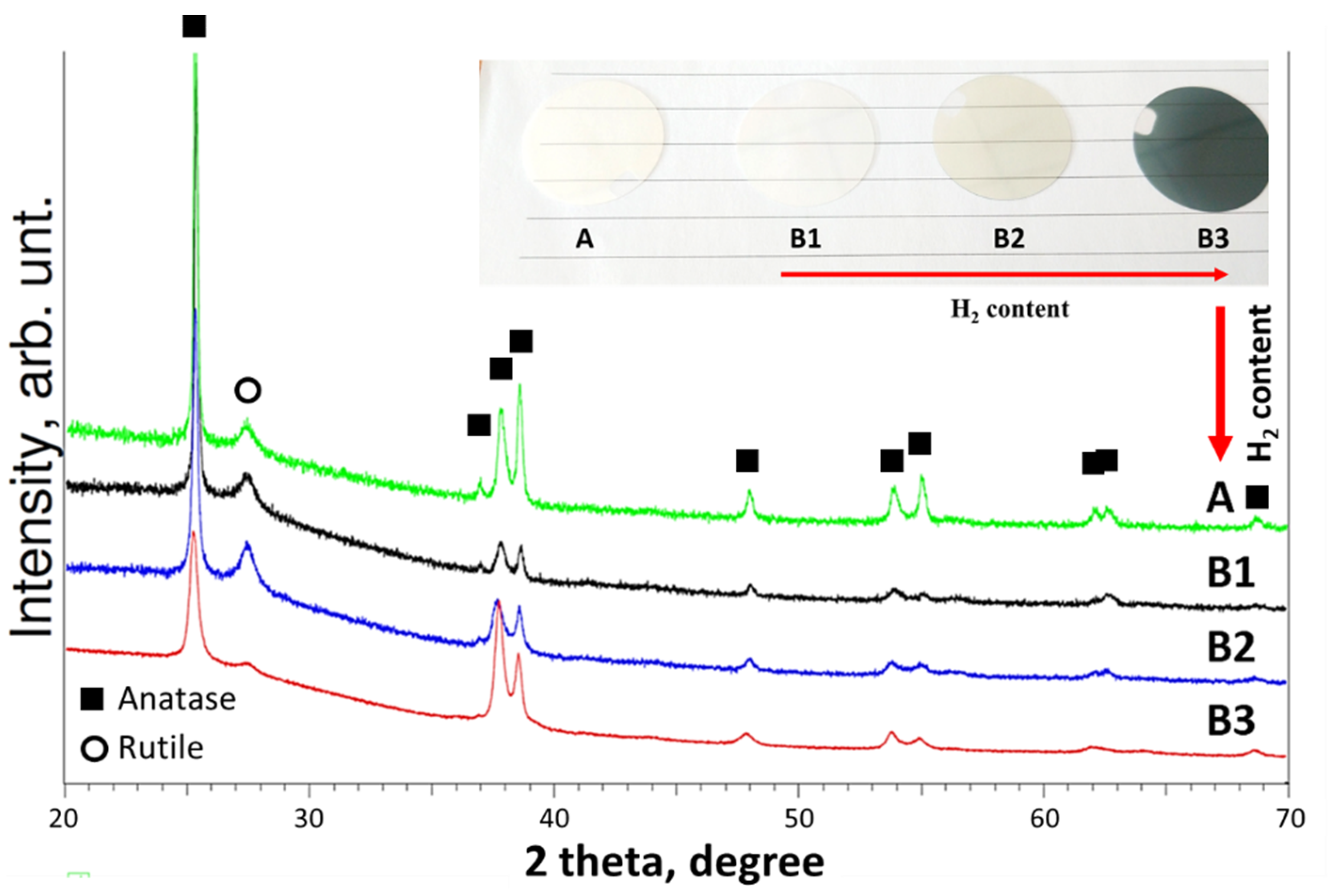
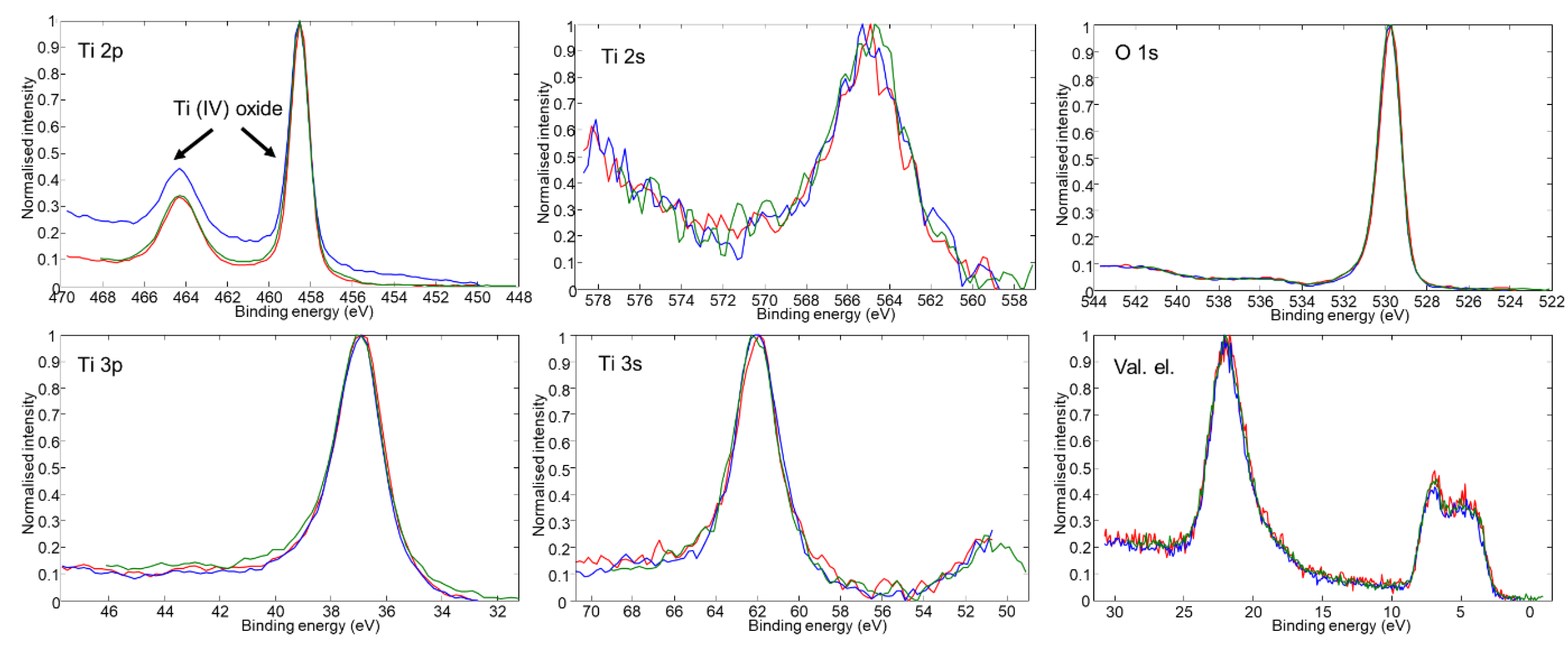
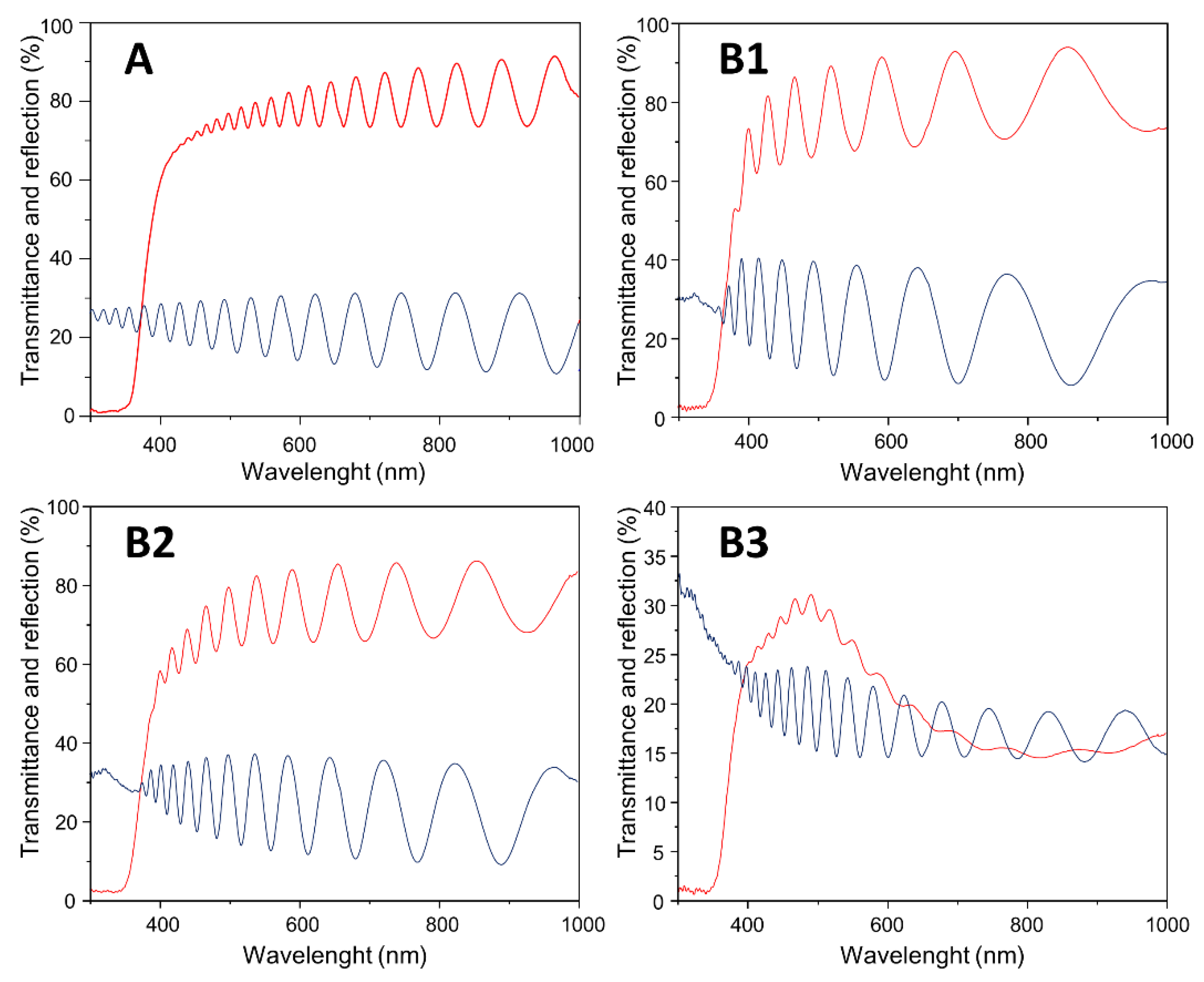
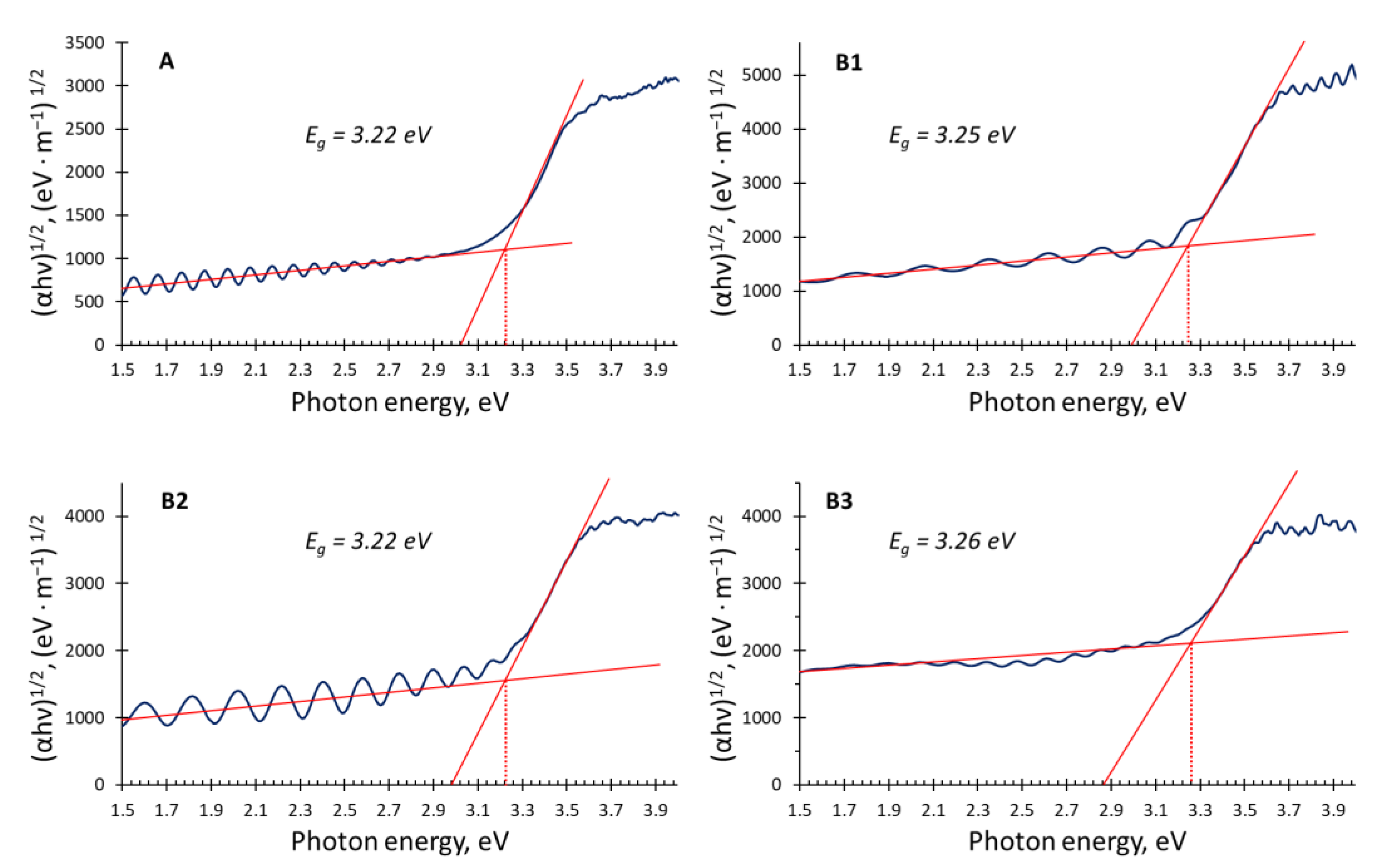
| Samples | Ar Flux | O2 Flux | H2 Flux | Ar:O2:H2 Flux Ratio | Total Pressure | Power Source |
|---|---|---|---|---|---|---|
| A | 4.5 sccm | 1.2 sccm | - | 3.78:1:- | 6 × 10−3 mbar | 300 W p-DC |
| B1 | 2.7 sccm | 6.4 sccm | 15.1 sccm | 0.42:1:2.36 | 6 × 10−3 mbar | 300 W DC |
| B2 | 2.8 sccm | 4.2 sccm | 20.1 sccm | 0.67:1:4.78 | 6 × 10−3 mbar | 300 W DC |
| B3 | 2.8 sccm | 3.6 sccm | 22.5 sccm | 0.78:1:6.25 | 6 × 10−3 mbar | 300 W DC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbonavicius, M.; Tuckute, S.; Karazhanov, S.; Lelis, M. Analysis of TihxOy Films Produced by Physical Vapor Deposition Method. Appl. Sci. 2022, 12, 10811. https://doi.org/10.3390/app122110811
Urbonavicius M, Tuckute S, Karazhanov S, Lelis M. Analysis of TihxOy Films Produced by Physical Vapor Deposition Method. Applied Sciences. 2022; 12(21):10811. https://doi.org/10.3390/app122110811
Chicago/Turabian StyleUrbonavicius, Marius, Simona Tuckute, Smagul Karazhanov, and Martynas Lelis. 2022. "Analysis of TihxOy Films Produced by Physical Vapor Deposition Method" Applied Sciences 12, no. 21: 10811. https://doi.org/10.3390/app122110811
APA StyleUrbonavicius, M., Tuckute, S., Karazhanov, S., & Lelis, M. (2022). Analysis of TihxOy Films Produced by Physical Vapor Deposition Method. Applied Sciences, 12(21), 10811. https://doi.org/10.3390/app122110811






