Electrochemical Synthesis and Structural Characteristics of New Carbon-Based Materials Generated in Molten Salts
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
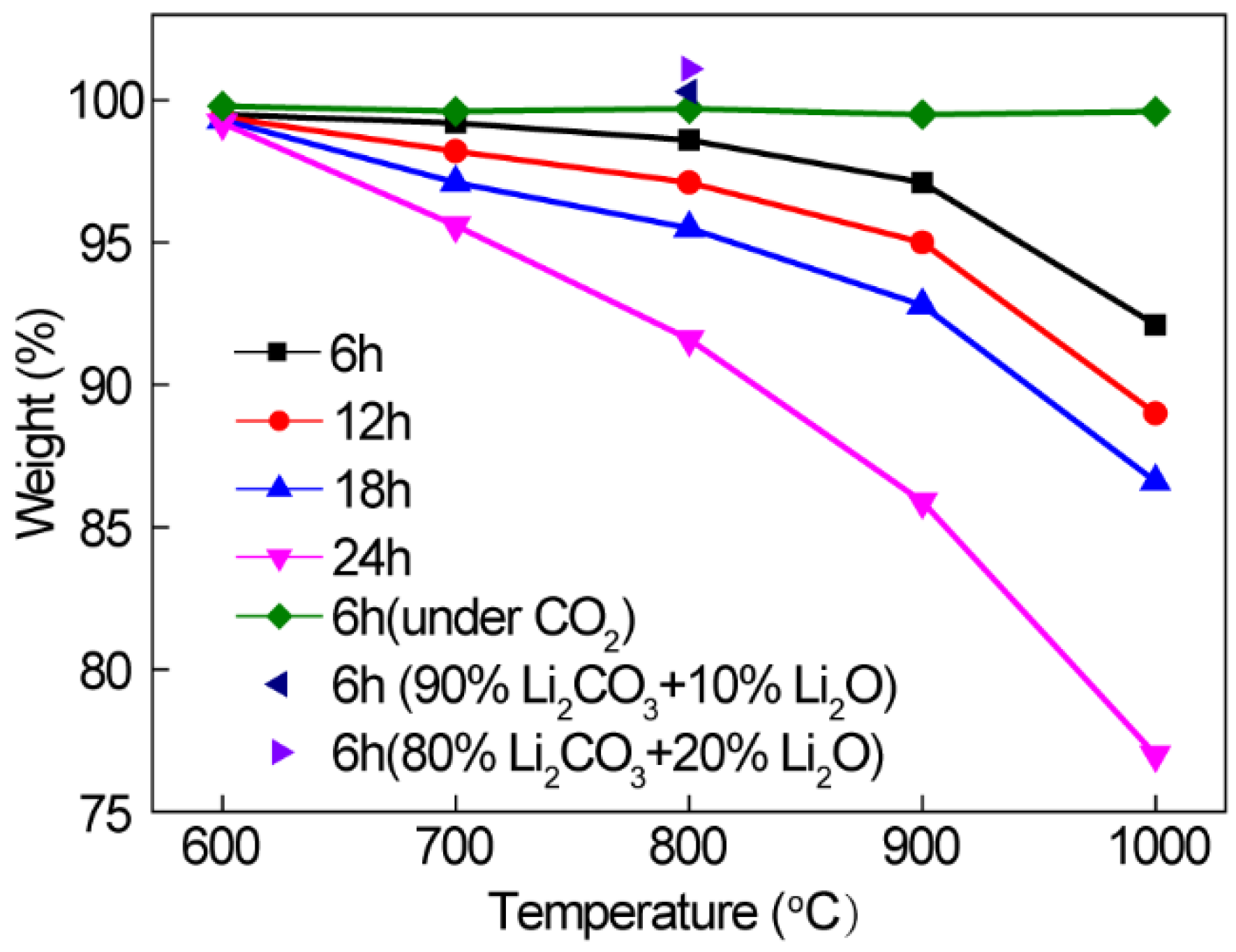
3.1. Li2CO3 Splitting and CO2 Transformation
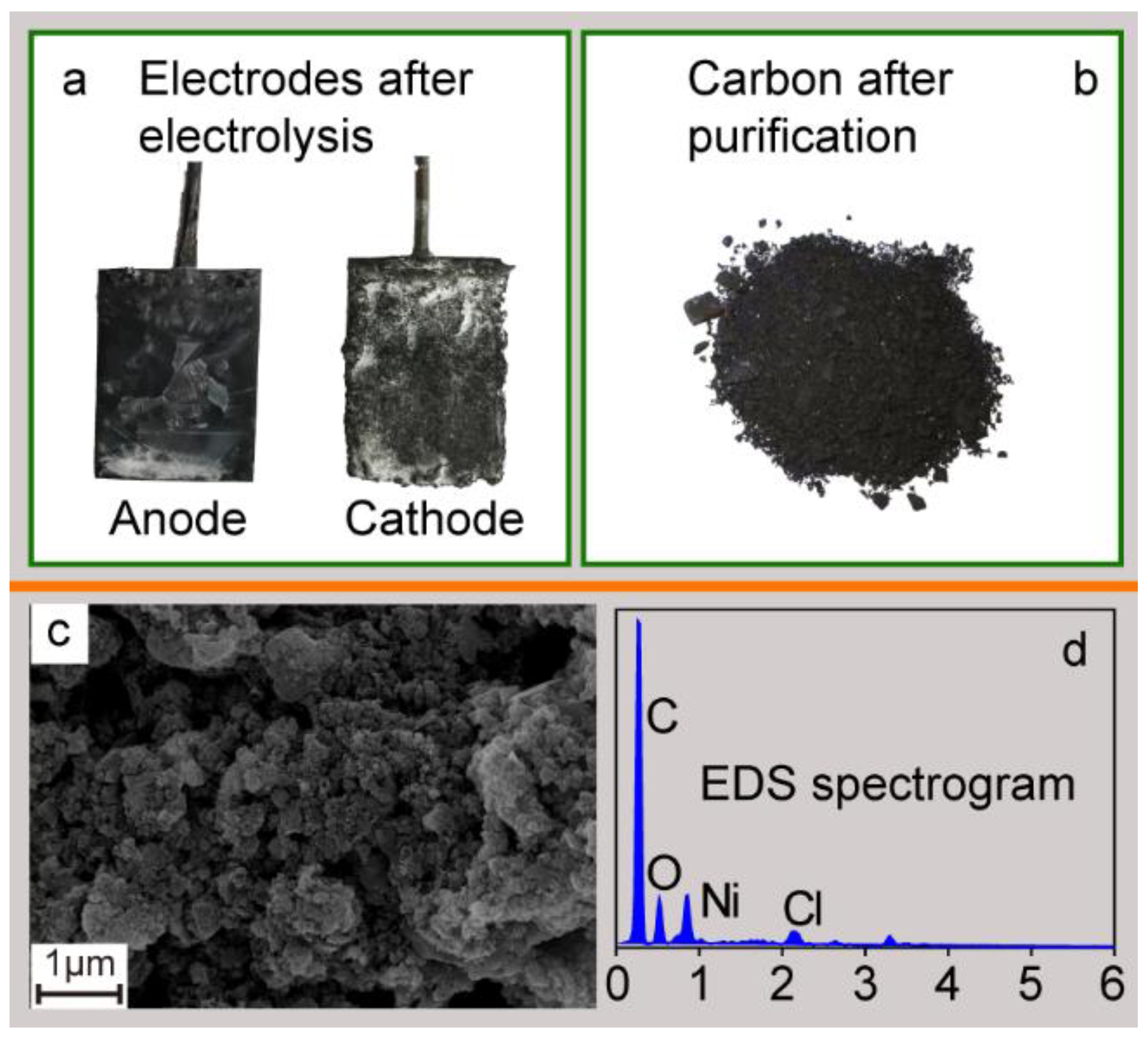
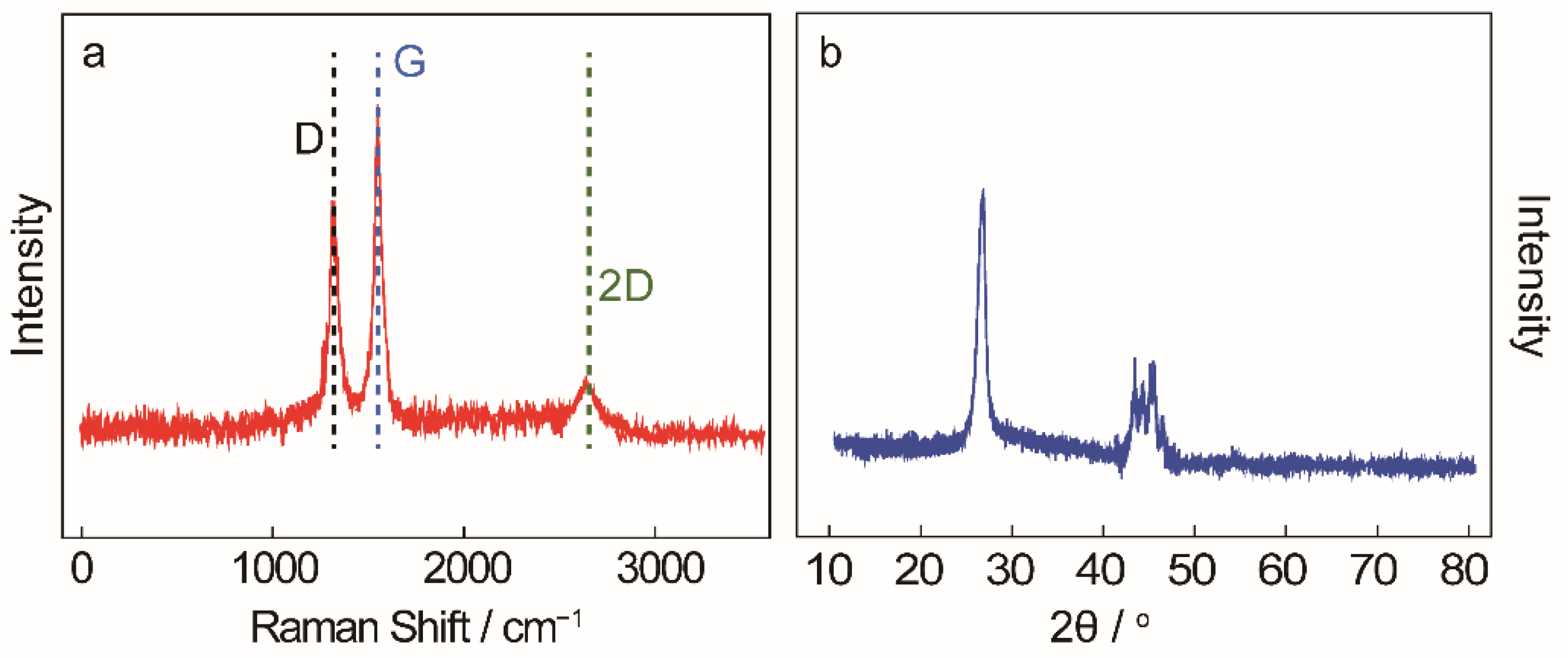
3.2. Amorphous Carbon
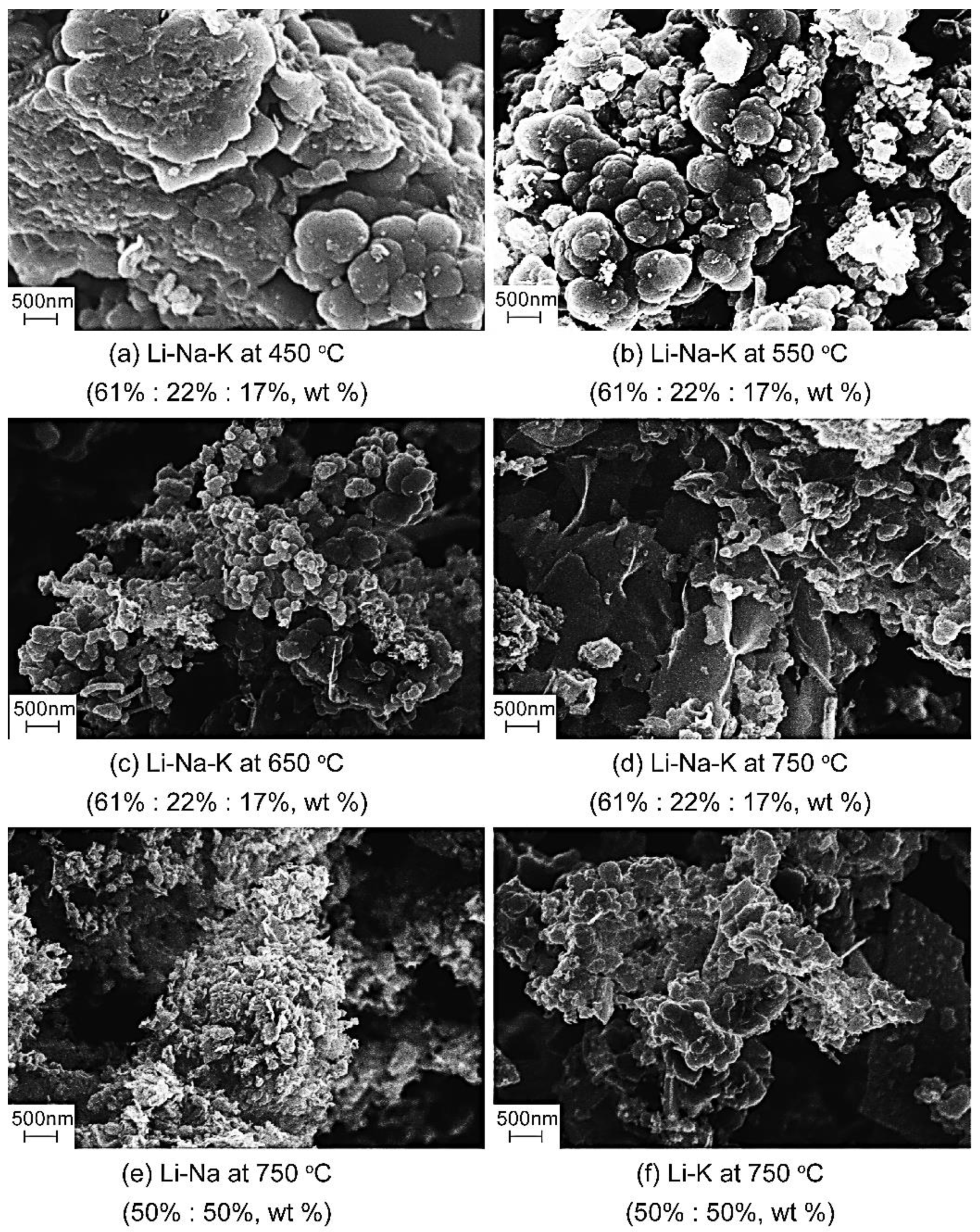
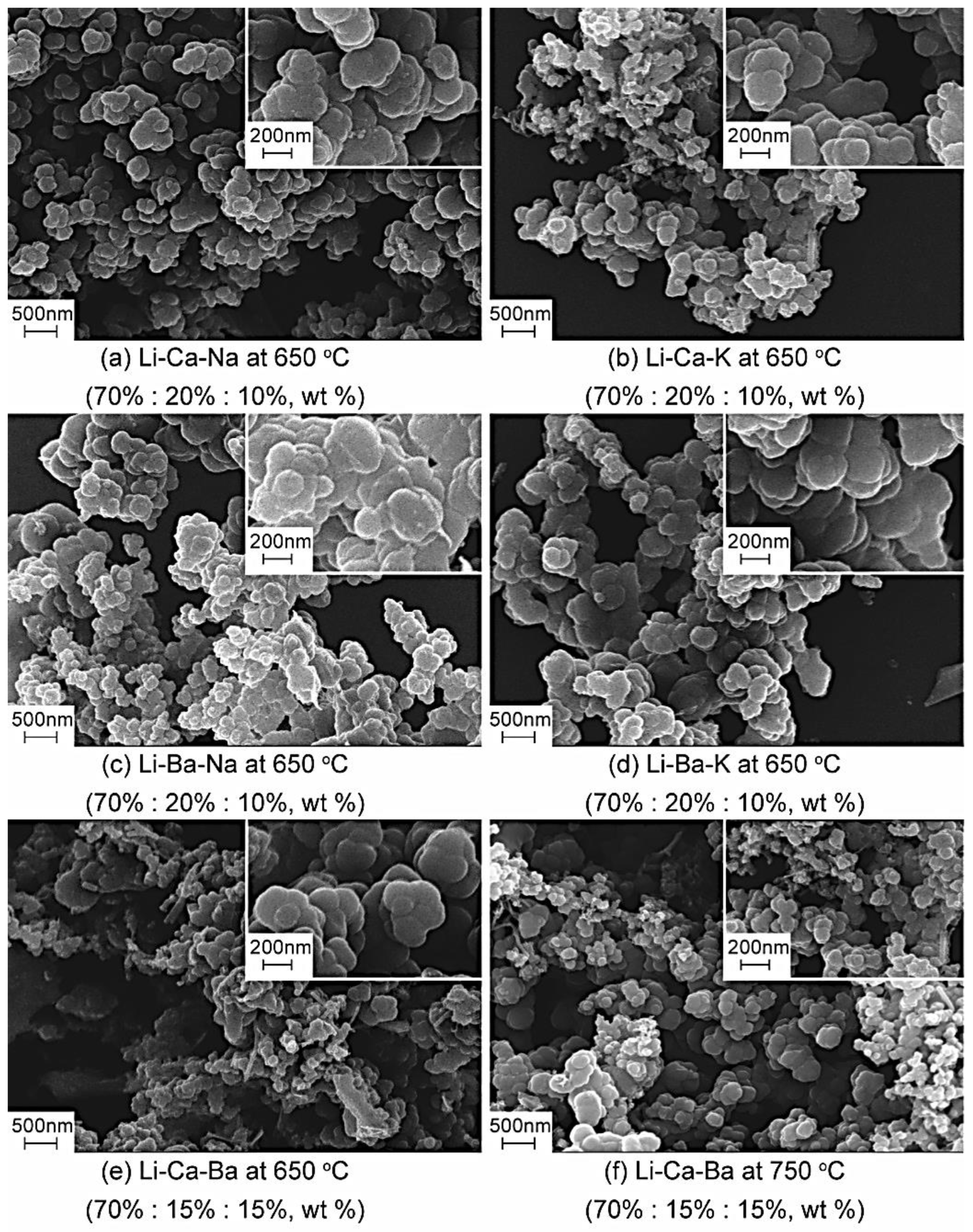
3.3. Spherical Carbon
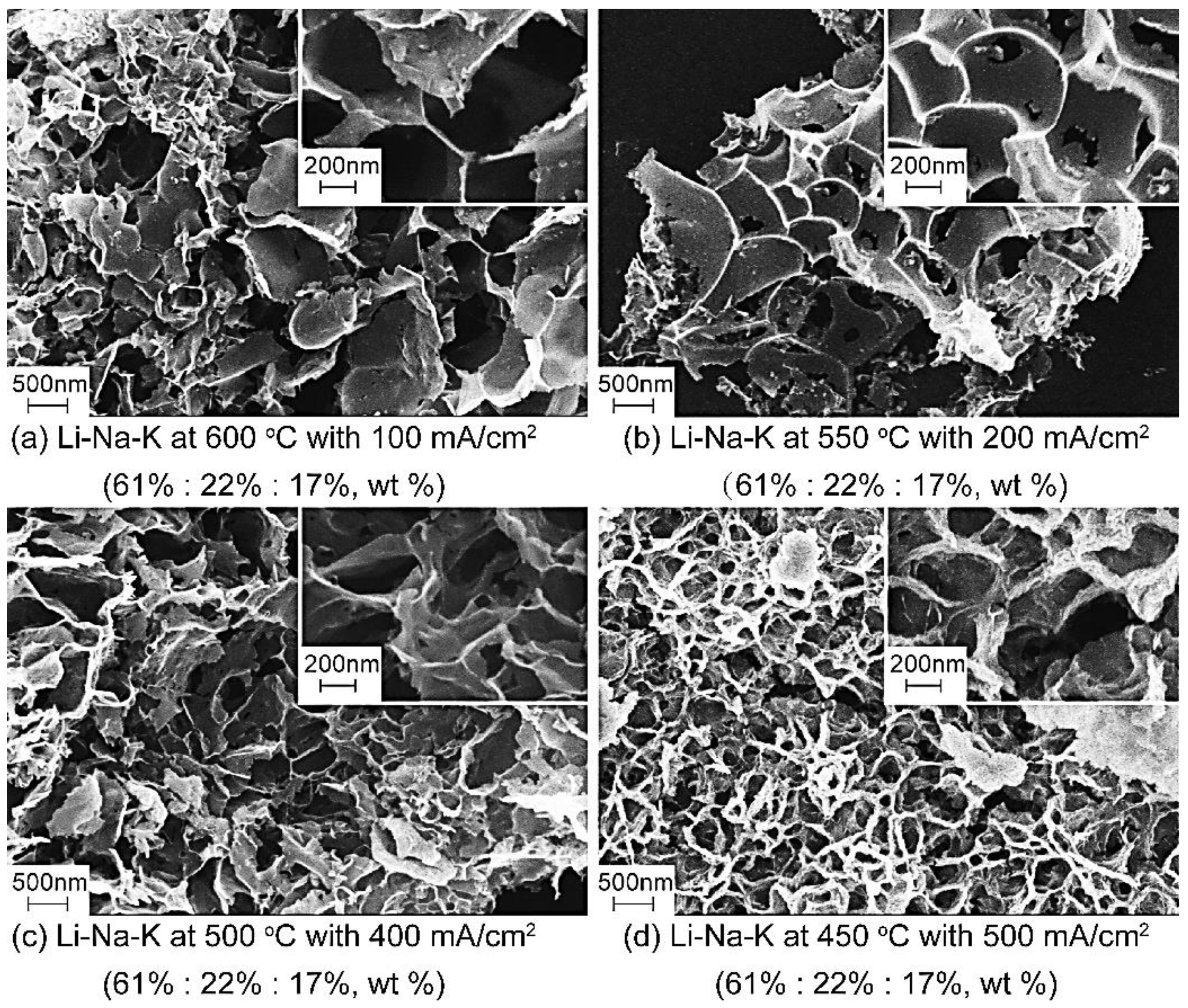
3.4. Cellular Porous Carbon
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Salehizadeh, H.; Yan, N.; Farnood, R. Recent advances in microbial CO2 fixation and conversion to value-added products. Chem. Eng. J. 2020, 390, 124584. [Google Scholar] [CrossRef]
- Sen, R.; Goeppert, A.; Kar, S.; Prakash, G.S. Hydroxide based integrated CO2 capture from air and conversion to methanol. J. Am. Chem. Soc. 2020, 142, 4544–4549. [Google Scholar] [CrossRef] [PubMed]
- Dindi, A.; Quang, D.V.; Vega, L.F.; Nashef, E.; Abu-Zahra, M.R. Applications of fly ash for CO2 capture, utilization, and storage. J. CO2 Util. 2019, 29, 82–102. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Z.; Lu, X.; Liang, Y.; Wang, H. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature 2019, 575, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Zhao, H.; Li, T.; Luo, Y.; Fan, G.; Chen, G.; Gao, S.; Shi, X.; Lu, S.; Sun, X. Metal-based electrocatalytic conversion of CO2 to formic acid/formate. J. Mater. Chem. A 2020, 8, 21947–21960. [Google Scholar] [CrossRef]
- Yang, Y.; Li, F.; Chen, J.; Fan, J.; Xiang, Q. Single Au atoms anchored on amino-group-enriched graphitic carbon nitride for photocatalytic CO2 reduction. ChemSusChem 2020, 13, 1979–1985. [Google Scholar] [CrossRef]
- Burakova, E.A.; Dyachkova, T.P.; Rukhov, A.V.; Tugolukov, E.N.; Galunin, E.V.; Tkachev, A.G.; Ali, I. Novel and economic method of carbon nanotubes synthesis on a nickel magnesium oxide catalyst using microwave radiation. J. Mol. Liq. 2018, 253, 340–346. [Google Scholar] [CrossRef]
- Zhou, H.; He, D.; Saana, A.I.; Yang, J.; Wang, Z.; Zhang, J.; Liang, Q.; Yuan, S.; Zhu, J.; Mu, S. Mesoporous-silica induced doped carbon nanotube growth from metal–organic frameworks. Nanoscale 2018, 10, 6147–6154. [Google Scholar] [CrossRef]
- de Jesús Barraza-García, F.; Caballero-Briones, F.; Morelos-Gómez, A.; Martínez-Villegas, N.; Hernández-Martínez, J.L.; Endo, M.; López-Urías, F.; Muñoz-Sandoval, E. The synthesis of sponge-type nitrogen-doped multiwall carbon nanotubes using ball-milled natural red-leptosol as catalyst precursor: A cycle voltammetry study. Carbon 2022, 196, 510–524. [Google Scholar] [CrossRef]
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for carbon nanotubes synthesis. J. Mater. Chem. 2011, 21, 15872–15884. [Google Scholar] [CrossRef]
- Baddour, C.E.; Fadlallah, F.; Nasuhoglu, D.; Mitra, R.; Vandsburger, L.; Meunier, J.-L. A simple thermal CVD method for carbon nanotube synthesis on stainless steel 304 without the addition of an external catalyst. Carbon 2009, 47, 313–318. [Google Scholar] [CrossRef]
- Kumar, A. Natural Materials—Interesting Candidates for Carbon Nanomaterials. Physchem 2021, 1, 4–25. [Google Scholar] [CrossRef]
- Song, Q.; Xu, Q.; Wang, Y.; Shang, X.; Li, Z. Electrochemical deposition of carbon films on titanium in molten LiCl–KCl–K2CO3. Thin Solid Films 2012, 520, 6856–6863. [Google Scholar] [CrossRef]
- Massot, L.; Chamelot, P.; Bouyer, F.; Taxil, P. Studies of carbon nucleation phenomena in molten alkaline fluoride media. Electrochim. Acta 2003, 48, 465–471. [Google Scholar] [CrossRef]
- Hu, L.; Song, Y.; Jiao, S.; Liu, Y.; Ge, J.; Jiao, H.; Zhu, J.; Wang, J.; Zhu, H.; Fray, D.J. Direct conversion of greenhouse gas CO2 into graphene via molten salts electrolysis. ChemSusChem 2016, 9, 588–594. [Google Scholar] [CrossRef]
- Tang, D.; Yin, H.; Mao, X.; Xiao, W.; Wang, D. Effects of applied voltage and temperature on the electrochemical production of carbon powders from CO2 in molten salt with an inert anode. Electrochim. Acta 2013, 114, 567–573. [Google Scholar] [CrossRef]
- Ji, D.; Li, Z.; Li, W.; Yuan, D.; Wang, Y.; Yu, Y.; Wu, H. The optimization of electrolyte composition for CH4 and H2 generation via CO2/H2O co-electrolysis in eutectic molten salts. Int. J. Hydrogen Energy 2019, 44, 5082–5089. [Google Scholar] [CrossRef]
- Wu, H.; Ji, D.; Li, L.; Yuan, D.; Zhu, Y.; Wang, B.; Zhang, Z.; Licht, S. A new technology for efficient, high yield carbon dioxide and water transformation to methane by electrolysis in molten salts. Adv. Mater. Technol. 2016, 1, 1600092. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, D.; Ji, D.; Li, Z.; Zhang, Z.; Wang, B.; Wu, H. Syngas production: Diverse H2/CO range by regulating carbonates electrolyte composition from CO2/H2O via co-electrolysis in eutectic molten salts. RSC Adv. 2017, 7, 52414–52422. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Ji, D.; Li, Z.; Yi, G.; Yuan, D.; Wang, B.; Zhang, Z.; Wang, P. Renewable and high efficient syngas production from carbon dioxide and water through solar energy assisted electrolysis in eutectic molten salts. J. Power Sources 2017, 362, 92–104. [Google Scholar] [CrossRef]
- Ren, J.; Yu, A.; Peng, P.; Lefler, M.; Li, F.-F.; Licht, S. Recent advances in solar thermal electrochemical process (STEP) for carbon neutral products and high value nanocarbons. Acc. Chem. Res. 2019, 52, 3177–3187. [Google Scholar] [CrossRef] [PubMed]
- Licht, S. Efficient Solar-Driven Synthesis, Carbon Capture, and Desalinization, STEP: Solar Thermal Electrochemical Production of Fuels, Metals, Bleach. Adv. Mater. 2011, 23, 5592–5612. [Google Scholar] [CrossRef] [PubMed]
- Licht, S.; Cui, B.; Wang, B. STEP carbon capture–the barium advantage. J. CO2 Util. 2013, 2, 58–63. [Google Scholar] [CrossRef]
- Licht, S. STEP (solar thermal electrochemical photo) generation of energetic molecules: A solar chemical process to end anthropogenic global warming. J. Phys. Chem. C 2009, 113, 16283–16292. [Google Scholar] [CrossRef]
- Ramesh, P.; Sampath, S. Electrochemical characterization of binderless, recompressed exfoliated graphite electrodes: Electron-transfer kinetics and diffusion characteristics. Anal. Chem. 2003, 75, 6949–6957. [Google Scholar] [CrossRef]
- Gao, F.; Guo, X.; Yin, J.; Zhao, D.; Li, M.; Wang, L. Electrocatalytic activity of carbon spheres towards NADH oxidation at low overpotential and its applications in biosensors and biofuel cells. RSC Adv. 2011, 1, 1301–1309. [Google Scholar] [CrossRef]
- Novoselova, I.; Oliinyk, N.; Volkov, S.; Konchits, A.; Yanchuk, I.; Yefanov, V.; Kolesnik, S.; Karpets, M. Electrolytic synthesis of carbon nanotubes from carbon dioxide in molten salts and their characterization. Phys. E Low-Dimens. Syst. Nanostruct. 2008, 40, 2231–2237. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Y.; Li, W.; Wang, G.; Peng, L.; Li, J.; Gu, D.; Yuan, D.; Wu, H. Carbon dioxide electrolysis and carbon deposition in alkaline-earth-carbonate-included molten salts electrolyzer. New J. Chem. 2018, 42, 15663–15670. [Google Scholar] [CrossRef]
- Singh, G.; Ismail, I.S.; Bilen, C.; Shanbhag, D.; Sathish, C.; Ramadass, K.; Vinu, A. A facile synthesis of activated porous carbon spheres from d-glucose using a non-corrosive activating agent for efficient carbon dioxide capture. Appl. Energy 2019, 255, 113831. [Google Scholar] [CrossRef]
- Wan, X.K.; Wu, H.B.; Guan, B.Y.; Luan, D.; Lou, X.W. Confining sub-nanometer Pt clusters in hollow mesoporous carbon spheres for boosting hydrogen evolution activity. Adv. Mater. 2020, 32, 1901349. [Google Scholar] [CrossRef]
- Gisbert-Garzaran, M.; Berkmann, J.C.; Giasafaki, D.; Lozano, D.; Spyrou, K.; Manzano, M.; Steriotis, T.; Duda, G.N.; Schmidt-Bleek, K.; Charalambopoulou, G. Engineered pH-responsive mesoporous carbon nanoparticles for drug delivery. ACS Appl. Mater. Interfaces 2020, 12, 14946–14957. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yuan, D.; Wu, H.; Li, W.; Gu, D. A novel route to synthesize carbon spheres and carbon nanotubes from carbon dioxide in a molten carbonate electrolyzer. Inorg. Chem. Front. 2018, 5, 208–216. [Google Scholar] [CrossRef]
- Licht, S.; Douglas, A.; Ren, J.; Carter, R.; Lefler, M.; Pint, C.L. Carbon nanotubes produced from ambient carbon dioxide for environmentally sustainable lithium-ion and sodium-ion battery anodes. ACS Cent. Sci. 2016, 2, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.i.; Mitsushima, S.; Kato, S.; Asano, S.; Yoshitake, H.; Kamiya, N. Solubilities of nickel oxide in molten carbonate. J. Electrochem. Soc. 1992, 139, 667. [Google Scholar] [CrossRef]

| No. | Electrolyte (Weight Ratio) | Temperature (°C) | Current Density (mA/cm2) | Specific Surface Area (m2/g) |
|---|---|---|---|---|
| 1 | Li2CO3 + Na2CO3 + K2CO3 (61: 22:17) | 450 | 100 | 127 |
| 2 | Li2CO3 + Na2CO3 + K2CO3 (61: 22:17) | 550 | 100 | 89.5 |
| 3 | Li2CO3 + Na2CO3 + K2CO3 (61: 22:17) | 650 | 100 | 56.3 |
| 4 | Li2CO3 + Na2CO3 + K2CO3 (61: 22:17) | 750 | 100 | 37.1 |
| 5 | Li2CO3 + Na2CO3 + K2CO3 (61: 22:17) | 750 | 50 | 28.2 |
| 6 | Li2CO3 + Na2CO3 + K2CO3 (61: 22:17) | 750 | 200 | 51.7 |
| 7 | Li2CO3 + Na2CO3 + K2CO3 (33: 33:33) | 750 | 100 | 412 |
| 8 | Li2CO3 + Na2CO3 (50:50) | 750 | 100 | 37.6 |
| 9 | Li2CO3 + K2CO3 (50:50) | 750 | 100 | 13.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, D.; Zhang, F.; Qiao, Z.; Zhang, J.; Wu, H.; Wang, G. Electrochemical Synthesis and Structural Characteristics of New Carbon-Based Materials Generated in Molten Salts. Appl. Sci. 2022, 12, 9923. https://doi.org/10.3390/app12199923
Ji D, Zhang F, Qiao Z, Zhang J, Wu H, Wang G. Electrochemical Synthesis and Structural Characteristics of New Carbon-Based Materials Generated in Molten Salts. Applied Sciences. 2022; 12(19):9923. https://doi.org/10.3390/app12199923
Chicago/Turabian StyleJi, Deqiang, Fajin Zhang, Zhiqiang Qiao, Jing Zhang, Hongjun Wu, and Guanzhong Wang. 2022. "Electrochemical Synthesis and Structural Characteristics of New Carbon-Based Materials Generated in Molten Salts" Applied Sciences 12, no. 19: 9923. https://doi.org/10.3390/app12199923
APA StyleJi, D., Zhang, F., Qiao, Z., Zhang, J., Wu, H., & Wang, G. (2022). Electrochemical Synthesis and Structural Characteristics of New Carbon-Based Materials Generated in Molten Salts. Applied Sciences, 12(19), 9923. https://doi.org/10.3390/app12199923








