Ab-Initio Investigations on Hydrogen Dissociation and Cross-Linking of Hydrocarbon Chains of Self-Assembled Monolayers of Alkanes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joseph, S.; Varandas, A.J.C. Ab initio Based DMBE Potential Energy Surface for the Ground Electronic State of the C2H Molecule. J. Phys. Chem. A 2010, 114, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Daugulis, O.; Roane, J.; Tran, L.D. Bidentate, Monoanionic Auxiliary-Directed Functionalization of Carbon−Hydrogen Bonds. Acc. Chem. Res. 2015, 48, 1053–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizollahi, H.; García-López, J.-A. Recent Advances on Synthetic Methodology Merging C–H Functionalization and C–C Cleavage. Molecules 2020, 25, 5900. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, T.J.; Alty, J.W.; Neidhart, E.K.; Miller, A.S.; Leibfarth, F.A.; Alexanian, E.J. Diversification of aliphatic C–H bonds in small molecules and polyolefins through radical chain transfer. Science 2022, 375, 545–550. [Google Scholar] [CrossRef]
- Lazar, P.; Schollmeyer, H.; Riegler, H. Spreading and Two-Dimensional Mobility of Long-Chain Alkanes at Solid/Gas Interfaces. Phys. Rev. Lett. 2005, 94, 116101. [Google Scholar] [CrossRef]
- Knüfing, L.; Schollmeyer, H.; Riegler, H.; Mecke, K. Fractal analysis methods for solid alkane monolayer domains at SiO2/air interfaces. Langmuir 2005, 21, 992–1000. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nozaki, K.; Yamaguchi, A.; Urakami, N. Molecular simulation of crystallization in n-alkane ultrathin films: Effects of film thickness and substrate attraction. J. Chem. Phys. 2007, 127, 154704. [Google Scholar] [CrossRef]
- Nie, H.Y. Self-assembled monolayers of octadecylphosphonic acid and polymer films: Surface chemistry and chemical structures studied by time-of-flight secondary ion mass spectrometry. Surf. Interface Anal. 2017, 49, 1431–1441. [Google Scholar] [CrossRef]
- Woon-Ming, L. From surface science research to high-impact knowhows Exemplars of Surface Science Western. Surf. Interface Anal. 2017, 49, 1292–1297. [Google Scholar]
- Nie, H.Y. Negative hydrocarbon species C2nH−: How useful can they be? J. Vac. Sci. Technol. B 2016, 34, 030603. [Google Scholar] [CrossRef]
- Du, W.L.; Shao, H.; He, Z.K.; Tang, C.Y.; Liu, Y.; Shen, T.; Zhu, Y.; Lau, W.M.; Hui, D. Cross-Linking Poly(lactic acid) Film Surface by Neutral Hyperthermal Hydrogen Molecule Bombardment. J. Agric. Food Chem. 2015, 63, 10604–10610. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, D.Q.; Nie, H.Y.; Lau, W.M.; Yang, J. Study of a hydrogen-bombardment process for molecular cross-linking within thin films. J. Chem. Phys. 2011, 134, 074704. [Google Scholar] [CrossRef] [PubMed]
- Patten, T.E.; Xia, J.H.; Abernathy, T.; Matyjaszewski, K. Polymers with Very Low Polydispersities from Atom Transfer Radical Polymerization. Science 1996, 272, 866–868. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.F.; Schmidt, B.V.K.J.; Pfeifer, S. Tailored polymer microstructures prepared by atom transfer radical copolymerization of styrene and N-substituted maleimides. Macromol. Rapid Commun. 2011, 32, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zorvaryan, A.; Inceoglu, S.; Acar, M. Alkylation of polyethyleneimine for homogeneous ligands in ATRP. Polymer 2011, 52, 617–621. [Google Scholar] [CrossRef]
- Santonicola, M.G.; Groot, G.W.; Memesa, M.; Meszyńska, A.; Vancso, G.J. Reversible pH-controlled switching of poly (methacrylic acid) grafts for functional biointerfaces. Langmuir 2010, 26, 17513–17519. [Google Scholar] [CrossRef]
- Fabio, d.L.; Matyjaszewski, K. Transition metal catalysts for controlled radical polymerization. Prog. Polym. Sci. 2010, 35, 959–1021. [Google Scholar]
- Andersen, C.A.; Roden, H.J.; Robinson, C.F. Negative Ion Bombardment of Insulators to Alleviate Surface Charge-Up. J. Appl. Phys. 1969, 40, 3419–3420. [Google Scholar] [CrossRef]
- Zhu, Z.; Stevie, F.A.; Griffis, D.P. Model study of electron beam charge compensation for positive secondary ion mass spectrometry using a positive primary ion beam. Appl. Surf. Sci. 2008, 254, 2708–2711. [Google Scholar] [CrossRef]
- Arnold, J.C.; Sawin, H.H. Charging of pattern features during plasma etching. J. Appl. Phys. 1991, 70, 5314–5317. [Google Scholar] [CrossRef]
- Bao, J.; Shi, H.; Huang, H.; Ho, P.S. Oxygen plasma damage to blanket and patterned ultralow-κ surfaces. J. Vac. Sci. Technol. 2010, 28, 207–215. [Google Scholar] [CrossRef]
- Despiau-Pujo, E.; Chabert, P. Velocity distribution function of sputtered gallium atoms during inductively coupled argon plasma treatment of a GaAs surface. J. Vac. Sci. Technol. A 2010, 28, 356–361. [Google Scholar] [CrossRef]
- Vozniy, O.V.; Yeom, G.Y. High-energy negative ion beam obtained from pulsed inductively coupled plasma for charge-free etching process. Appl. Phys. Lett. 2009, 94, 231502. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Xu, X.D.; Fan, X.L.; Lau, W.M.; Kwok, R.W.M. Ultrathin polymer film formation by collision-induced cross-linking of adsorbed organic molecules with hyperthermal protons. J. Am. Chem. Soc. 2004, 126, 12336–12342. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Kwok, W.M.; Lau, W.M. A new cross-linking route via the unusual collision kinematics of hyperthermal protons in unsaturated hydrocarbons: The case of poly(trans-isoprene). Chem. Commun. 2006, 29, 3122–3124. [Google Scholar] [CrossRef]
- Zheng, Z.; Wong, K.W.; Lau, W.C.; Kwok, R.W.M.; Lau, W.M. Unusual kinematics-driven chemistry: Cleaving C–H but not COO–H bonds with hyperthermal protons to synthesize tailor-made molecular films. Chem. Eur. J. 2007, 13, 3187–3192. [Google Scholar] [CrossRef]
- Trebicky, T.; Crewdson, P.; Paliy, M.; Bello, I.; Nie, H.Y.; Zheng, Z.; Fan, X.L.; Yang, J.; Gillies, E.R.; Tang, C.Y.; et al. Cleaving C–H bonds with hyperthermal H2: Facile chemistry to cross-link organic molecules under low chemical- and energy-loads. Green Chem. 2014, 16, 1316–1325. [Google Scholar] [CrossRef]
- Jacobs, D.C. Reactive Collisions of Hyperthermal Energy Molecular Ions with Solid Surfaces. Annu. Rev. Phys. Chem. 2002, 53, 379–407. [Google Scholar] [CrossRef]
- Ocko, B.M.; Wu, X.Z.; Sirota, E.B.; Sinha, S.K.; Gang, O.; Deutsch., M. Surface freezing in chain molecules: Normal alkanes. Phys. Rev. E 1997, 55, 3164. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, D.J.; Weiss, R.G. n-Alkanes Gel n-alkanes (and many other organic liquids). Langmuir 2000, 16, 352–355. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J. Phys. Condens. Matter 1994, 6, 8245–8257. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Mills, G.; Jonsson, H.; Schenter, G.K. Reversible work transition state theory: Application to dissociative adsorption of hydrogen. Surf. Sci. 1995, 324, 305. [Google Scholar] [CrossRef] [Green Version]
- Van, Z.B.; Utterback, N.G.; Amme, R.C. Charged-particle production in low-energy H+H2 and H+He collisions. Rev. Sci. Instrum. 1976, 47, 314–323. [Google Scholar]
- Hughes, P.P.; Coplan, M.A.; DeFazio, J.N.; Chornay, D.J.; Collier, M.R.; Ogilvie, K.W.; Shappirio, M.D. Scattering of neutral hydrogen at energies less than 1 keV from tungsten and diamondlike carbon surfaces. J. Vac. Sci. Technol. A 2009, 27, 1188–1195. [Google Scholar] [CrossRef]
- de Vries, A.E.; Pedrys, R.; Haring, R.A.; Haring, A.; Saris, F.W. Emission of large hydrocarbons from frozen CH4 by keV proton irradiation. Nature 1984, 311, 39–40. [Google Scholar] [CrossRef]
- Moore, M.H.; Hudson, R.L. Infrared Study of Ion-Irradiated Water-Ice Mixtures with Hydrocarbons Relevant to Comets. Icarus 1998, 135, 518–527. [Google Scholar] [CrossRef] [Green Version]
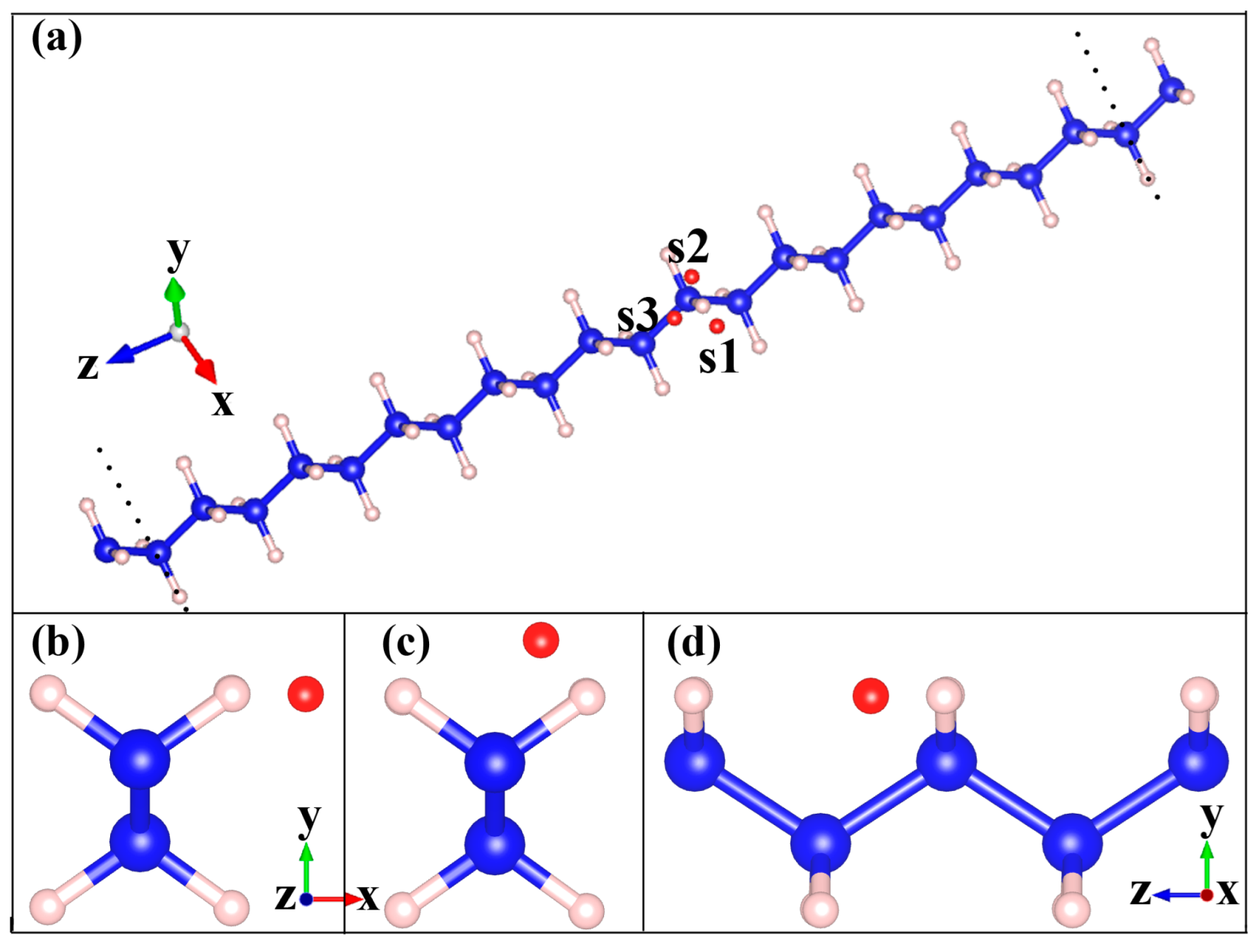

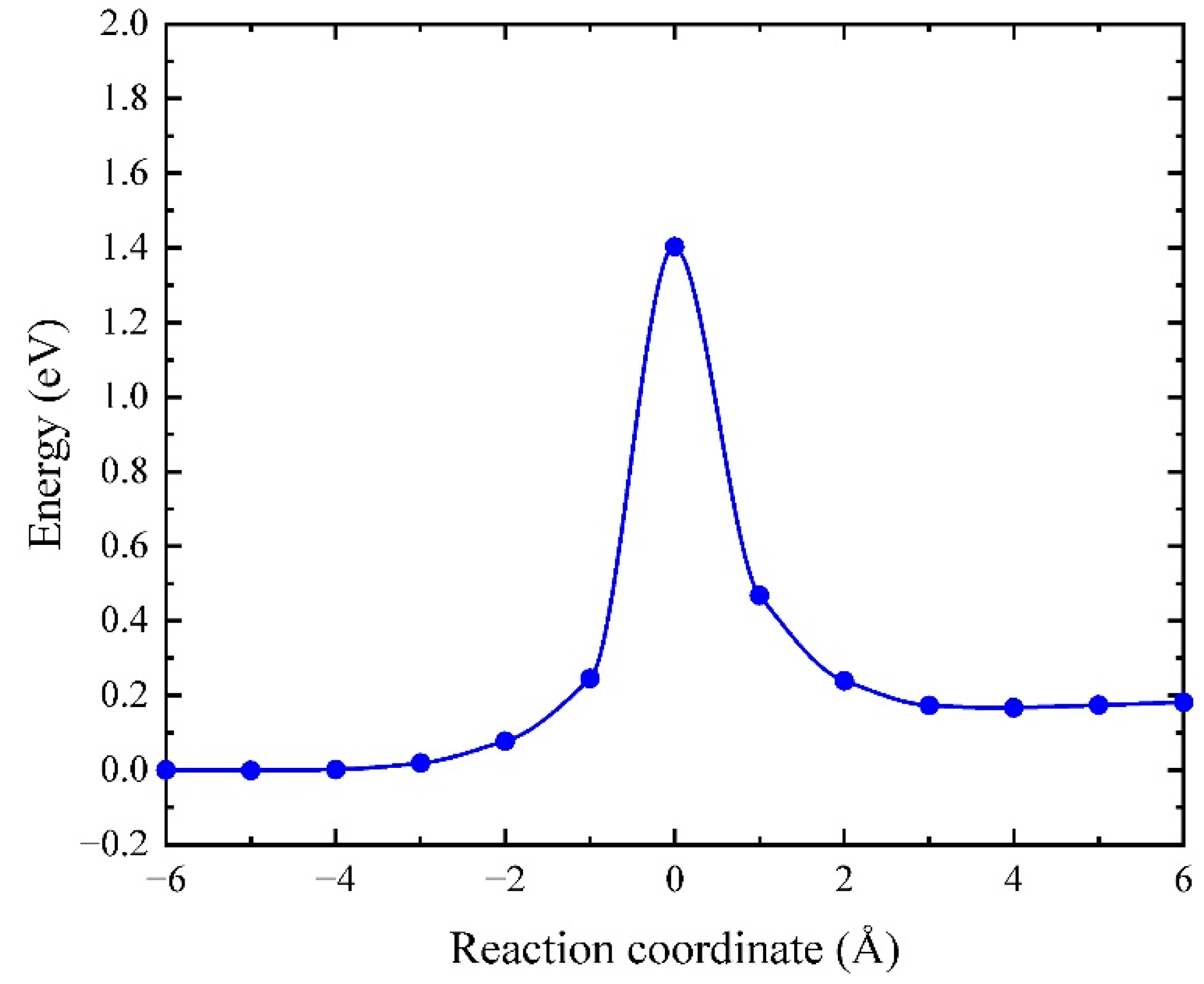
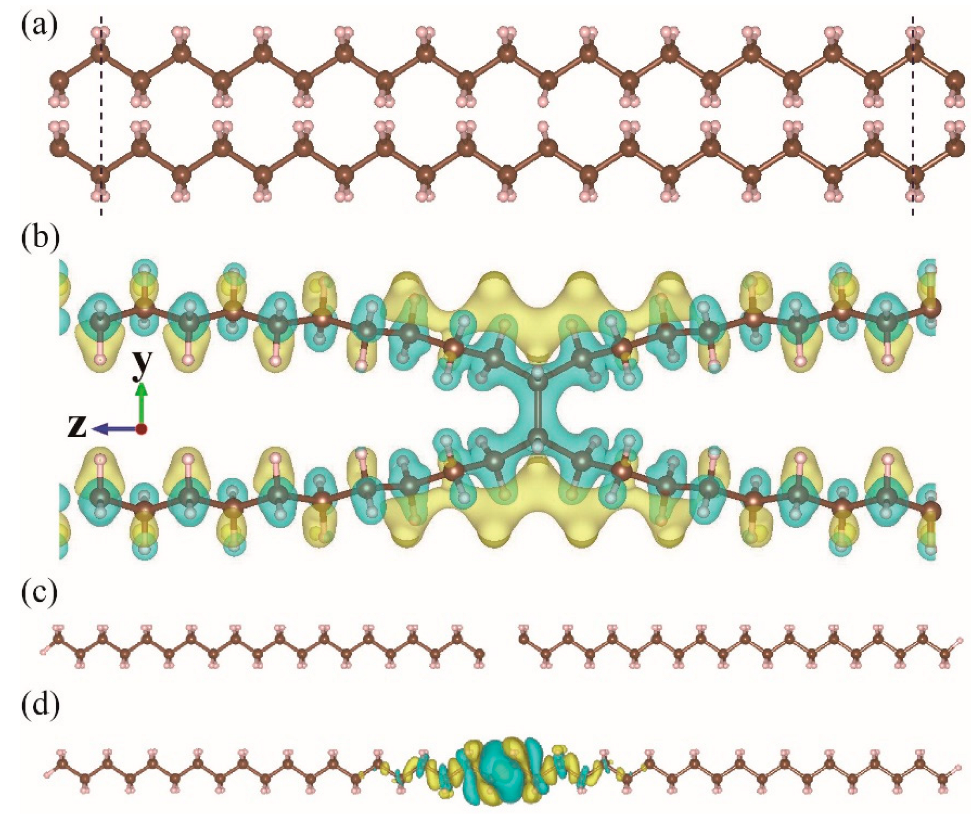
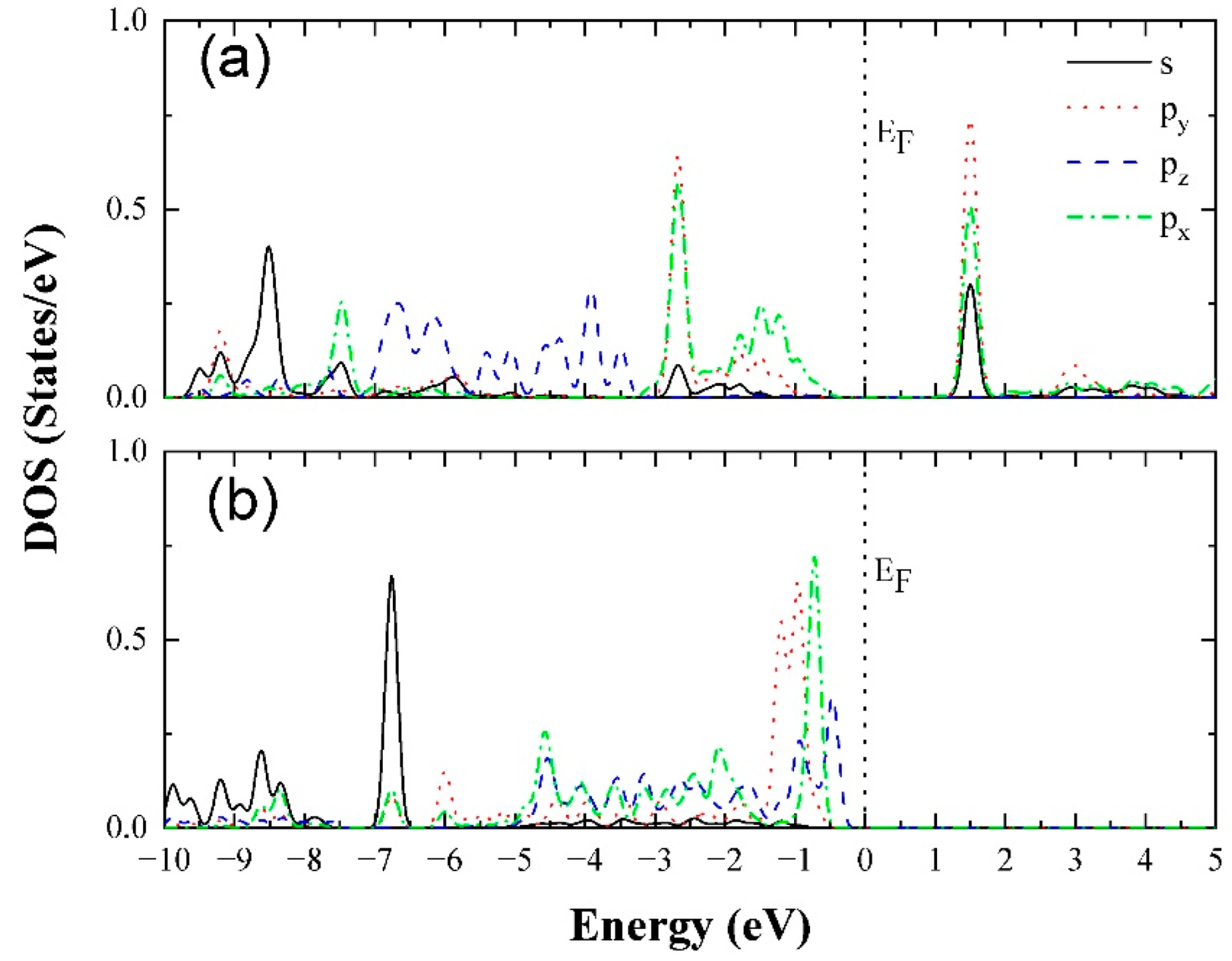
| Ea (eV) | |||
|---|---|---|---|
| s1 | s2 | s3 | |
| H | 1.19 | 2.87 | 2.86 |
| H2 | 2.39 | 4.61 | 5.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Wei, X.-Y.; Xu, R. Ab-Initio Investigations on Hydrogen Dissociation and Cross-Linking of Hydrocarbon Chains of Self-Assembled Monolayers of Alkanes. Appl. Sci. 2022, 12, 6020. https://doi.org/10.3390/app12126020
Zhu Y, Wei X-Y, Xu R. Ab-Initio Investigations on Hydrogen Dissociation and Cross-Linking of Hydrocarbon Chains of Self-Assembled Monolayers of Alkanes. Applied Sciences. 2022; 12(12):6020. https://doi.org/10.3390/app12126020
Chicago/Turabian StyleZhu, Yan, Xin-Yuan Wei, and Run Xu. 2022. "Ab-Initio Investigations on Hydrogen Dissociation and Cross-Linking of Hydrocarbon Chains of Self-Assembled Monolayers of Alkanes" Applied Sciences 12, no. 12: 6020. https://doi.org/10.3390/app12126020
APA StyleZhu, Y., Wei, X.-Y., & Xu, R. (2022). Ab-Initio Investigations on Hydrogen Dissociation and Cross-Linking of Hydrocarbon Chains of Self-Assembled Monolayers of Alkanes. Applied Sciences, 12(12), 6020. https://doi.org/10.3390/app12126020






